Abstract
Mast cells (MCs) are cells of hematopoietic origin that normally reside in mucosal tissues, often near epithelial cells, glands, smooth muscle cells, and nerves. Best known for their contributions to pathology during IgE-associated disorders such as food allergy, asthma, and anaphylaxis, MCs are also thought to mediate IgE-associated effector functions during certain parasite infections. However, various MC populations also can be activated to express functional programs – such as secreting pre-formed and/or newly synthesized biologically active products – in response to encounters with products derived from diverse pathogens, other host cells (including leukocytes and structural cells), damaged tissue, or the activation of the complement or coagulation systems, as well as by signals derived from the external environment (including animal toxins, plant products, and physical agents). In this review, we will discuss evidence suggesting that MCs can perform diverse effector and immunoregulatory roles that contribute to homeostasis or pathology in mucosal tissues.
Keywords: Anaphylaxis, asthma, basophils, complement system, food allergy, innate immunity, infection, parasites, pattern recognition receptors, pathogen-associated molecular patterns, Toll-like receptors, IgE, immunoglobulin E
Introduction
Mast cells (MCs) are normal residents of mucosal tissues, but their numbers and anatomical location can change markedly during immune responses, infections, and other disorders affecting such sites, in humans, mice, and other species1–5. MCs stimulated via the high affinity receptor for IgE (FcεRI) or by any of multiple other mechanisms can release a diverse spectrum of biologically active mediators, and such products, individually or in aggregate, can have many different effects on immune or structural cells present in mucosal tissues4, 6–8. As a result, there is no lack of ideas about the potential effector or immunoregulatory functions MCs might have during mucosal immune responses3, 4, 8.
However, it can be quite challenging to prove that MCs can perform such proposed functions in vivo, and even more difficult, in light of the potential redundancy of effector and immunoregulatory mechanisms, to assess the biological importance of such MC functions in particular settings. In this review, we will outline some basic principles of MC biology and then consider evidence that implicates MCs in physiological, immunological and pathologic processes affecting mucosal sites. We will particularly focus on findings derived from studies in mice, a species in which biological responses can be analyzed in animals that lack MCs or specific MC-associated products.
Mast cell development, phenotype, tissue distribution and plasticity
MCs are tissue-resident cells that arise from hematopoietic progenitors9. Unlike other immune cells, MCs normally do not mature before leaving the bone marrow but circulate through the vascular system as immature progenitors that complete their development peripherally within connective or mucosal tissues, or in serosal cavities, in a process potentially regulated by multiple local factors3–5, 10, 11.
The KIT ligand stem cell factor (SCF) plays a critical role in MC biology by regulating the development, migration, growth, survival and local activation of MCs12–14. Various other factors also can modulate MC growth and survival, including IL-315, IL-416–19, IL-920, 21, IL-1022–24, IL-3325–27, CXCL1228, 29, TGF-β30 and NGF31.
MCs reside in almost all vascularized tissues, and can be especially numerous in those exposed to the external environment, such as the skin and mucosal sites3. MCs are therefore well positioned to respond to various allergens, pathogens, and other agents which can be ingested, inhaled or encountered after disruption of the epithelial barrier8. Moreover, many phenotypic and functional characteristics of MCs, such as their proliferation, survival, and ability to store and/or secrete various products, can be modulated or “tuned” by many genetic and environmental factors, including changes in the cytokine milieu associated with inflammatory or immune responses8.
Despite their potential phenotypic “plasticity”, MCs are often sub-classified based on certain of their “baseline” phenotypic characteristics and their anatomic locations (Table 1). In mice, two types of MCs have been described: “connective tissue-type” MCs (CTMCs) and mucosal MCs (MMCs)4, 5, 8. CTMCs are often located around venules and near nerves, and reside in serosal cavities, while MMCs occupy the mucosae of the gut and respiratory mucosa5. MMCs are found at relatively low numbers in most mucosal tissues (in mice, they are normally present in higher numbers in the glandular stomach mucosa than in the intestines), but expansion of MMC populations can be induced in a T cell-dependent manner5, 32. CTMCs and MMCs often are distinguished based on their protease content (Table 1). Mouse intestinal MMCs elicited during parasite infection express the chymase mouse MC protease-1 (MCPT1) but not the elastase MCPT5, whereas CTMCs do not express MCPT1 but express MCPT5, the chymase MCPT4, and the tryptases MCPT6 and MCPT75, 33, 34 (notably, C57BL/6 mice don’t express MCPT7 because of a point mutation in the exon/intron 2 splice of the Mcpt7 gene35). However, the plasticity of MC phenotype can make such classification challenging, as features of the cells, including their protease content, may vary during the course of immune responses5, 8, 33, 36, 37.
Table 1. Major mast cell “subtypes”, and some of their phenotypic features, in mice and humans.
Many aspects of the phenotype of MC populations can vary based on whether the MCs are present at sites of inflammatory or immune responses, their exposure to various growth factors and other cytokines, and/or the history of their activation for secretion175. Accordingly, the features listed in the table are primarily those of MC populations at “baseline” in the tissues mentioned. These MC subpopulations can vary in features not covered in the table, such as in their sensitivity to pathogen-associated molecular patterns and in the spectrum of cytokines, chemokines and growth factors that they can secrete or respond to. However, much of the evidence for such differences is based on in vitro rather than in vivo studies, and these features also may be subject to variation in vivo depending on the biological setting.
CTMC, connective tissue-type mast cell; FcγR, receptor for IgG; FcεRI, high affinity receptor for IgE; MCPT, mast cell protease; MMC, mucosal mast cell; MCT, tryptase-expressing mast cell, MCTC, tryptase and chymase-expressing mast cell.
| Mouse | Human | |||
|---|---|---|---|---|
| Classic “subtypes”* | MMCs | CTMCs | MCT | MCTC |
| T cell dependence | T cells required for baseline populations and for expansion (e.g., in response to parasites)32 | Do not require T cells for baseline populations or for expansion | Reduced numbers in subjects with congenital or acquired deficiencies in T cells41 | Normal numbers in subjects with congenital or acquired deficiencies in T cells41. |
| Anatomical distribution (at baseline) | Glandular stomach mucosa (many at baseline), small intestinal and colonic mucosae (very few at baseline), and respiratory mucosa344–346 | Skin, serosal cavities, tongue, submucosa and muscularis of the stomach, trachea & around large airways155, 346 | Preponderance of MCs in nasal and bronchial mucosae, lung alveoli, conjunctiva, gastric, small intestinal and colonic mucosae40, 42 | Preponderance of MCs in skin, gastric, small intestinal and colonic submucosa and muscularis, bronchial submucosa, smooth muscle of major bronchi, conjunctiva, breast parenchyma and axillary lymph nodes40, 42 |
| Proteoglycan content | Little or no heparin347, 348; Chondroitin sulfate di-B, A, E349, 350 | Heparin347, 348; Chondroitin sulfate E349, 350 | Heparin351; Chondroitin sulfate A, E349, 350 | Heparin351; Chondroitin sulfate A, E349, 350 |
| Protease content | Express predominantly the chymases MCPT-1 and 25, 118, 175, 352 | Express predominantly the chymase MCPT-4, the elastase MCPT-5, the tryptases MCPT-6 and 7 and Carboxypeptidase A35, 118, 175, 352 | Tryptase (α, β[I–III], and γ)353–355**; Little or no chymase, carboxypeptidase A3 and cathepsin G38, 356, 357 | Tryptase (α, β[I–III], and γ)353, 354, 358**; Chymase38; Carboxypeptidase A3357; Cathepsin G356 |
| Bioactive amine content | Low levels of histamine359 ; Serotonin360 | High levels of histamine359; Serotonin361 | Histamine; No evidence of serotonin | Histamine; Low level of serotonin detected in MCs derived from CD34+ peripheral blood progenitors362 |
| Response to cationic compounds | Little or none (?)*** | Yes | Little or none363 | Yes363 |
| Adenosine receptors364 | A2a365; A2b365; A3365 | A3366 | A2a367; A3368 | Skin-derived cultured MCs express low levels of A3 receptor368 |
| Ig receptors100 | FcεRI; FcγRIIb369; FcγRIII (?)**** | FcεRI; FcγRIIb370; FcγRIII371, 372 | FcεRI373; Human intestinal MCs express FcγRI, FcγRIIa, FcγRIIb but not FcγRIII374 | FcεRI375; MCs derived from CD34+ peripheral blood progenitors110 and some skin MCs in patients with psoriasis376 express FcγRI; skin-derived MCs express FcγRIIa but not FcγRIIb377 |
Not all features listed have been reported for MCs in each anatomic site.
In humans, α tryptases have no or minimal catalytic activity and are absent in many people, whereas β tryptases are active (βI and βII) or are predicted to be active (βIII); tryptase deficiency alleles are common, and different populations of humans differ in whether they inherit 2 versus 4 active alleles378. The more recently described γ-tryptases are expressed at the mRNA level in multiple MC populations but less is known about the distribution of the protein in various MC populations353, 355.
This point is well established in the rat, based on studies of purified MMCs379. However, much less is known about the responsiveness of mouse MMCs to such compounds.
BMCMCs (which have been used by some groups as a “model” for MMCs) express functional FcγRIII when stimulated with SCF380. However these cells only resemble MMCs in some respects and, to our knowledge, FcγRIII expression on mouse MMCs has not been reported in vivo.
In humans, MCs can be subcategorized into MCT, which express high levels of the MC-specific protease tryptase but little or no chymase (these therefore are thought to resemble rodent MMCs), and MCTC, which express both tryptase and chymase (and in that respect resemble rodent CTMCs)38, 39 (Table 1). MCC (which express chymase but little or no tryptase) also have been described, but they appear to be infrequent40. Clinical evidence suggests that human MCT (like mouse MMCs) may be dependent on T-cells, at least in part, to maintain normal numbers in mucosal sites41. The majority of human lung MCs ordinarily are MCT (~ 90%), and these cells are found in the bronchial/bronchiolar lamina propria and alveoli42. MCTC typically are located beneath the epithelium in the lamina propria and submocosa, in close proximity to submucosal glands, and some MCTC are found within and around the airway smooth muscle layers of major bronchi43. The lamina propria of the human intestinal mucosa normally contains ~1.5–3% MCs44, 45. In the human small intestine, MCT represent about 98% of all MCs in the mucosa and ~13% of MCs in the submucosa are MCT42. In naïve mice, relatively low numbers of MCs are found in the lung, and these cells are located around the larger airways and blood vessels. As noted above, in naïve mice few MCs are found in the mucosa of the gastrointestinal tract except for the glandular stomach, and small numbers can be found in the submucosa and muscularis propria.
However, MC numbers at mucosal sites can increase in both humans and mice in pathological settings such as inflammatory bowel disease (IBD)46, 47, food allergy48, 49, parasite infections50, 51, asthma52–56 or various types of lung fibrosis57–60. Such increases in MC numbers could reflect, at least in part, the division of mature MCs at mucosal sites. Although MCs are often considered terminally differentiated cells which can’t divide, we and others have provided evidence that at least certain “mature” mast cells, i.e., those which can be identified morphologically based on their abundant cytoplasmic granules, retain some proliferative ability61–64. Increased MC numbers in such settings also may reflect the maturation of increased numbers of MC progenitors, whose numbers in tissues may increase due to their increased recruitment and/or survival in such tissues, and/or via the local proliferation of such progenitors5, 65. While it is not yet clear to what extent MC progenitors can proliferate in tissues, increased numbers of such progenitors have been observed at mucosal sites under various pathological conditions. For example, Arinobu et al. observed a four-fold expansion of MC progenitors in the intestine following sensitization and challenge of mice with the antigen ovalbumin66. Antigen-dependent expansion of MC progenitors also was observed in mouse lung following sensitization and challenge with aerosolized ovalbumin, and IL-9 and CD4+ T cells were found to contribute to such expansion67. Finally, it has been reported that certain MC progenitors can proliferate in vitro66, however, whether they can also proliferate within mucosal tissues remains to be proven.
Mast cell activation
MCs ordinarily express on their surface large numbers of the high affinity IgE receptor, FcεRI. During IgE-dependent immune responses, the antigen-dependent cross-linking of antigen-specific IgE bound to FcεRI induces the aggregation of FcεRI, promoting the activation of downstream signaling events that lead to the secretion of biologically active products implicated in allergic reactions6, 68, 69. The IgE-dependent stimulation of MCs has been extensively reviewed6, 69–72. It was recently reported that perivascular MCs can “sample” circulating IgE directly in the blood by extending cell processes across the vessel wall73. Moreover, MC FcεRI were shown to be able to distinguish between high- or low-affinity stimuli, permitting the MCs to respond differentially to such signals by releasing distinct spectra of secretory products in vitro and by orchestrating distinct in vivo outcomes74.
Our group recently reported a beneficial role for IgE, FcεRIα, and FcεRIγ in defense against honeybee venom-induced mortality in mice75. Together with evidence that expression of the FcεRIα chain is important for full expression of acquired resistance to the hypothermia-inducing effect of honeybee venom-derived phospholipase A276, these findings support the hypothesis that IgE, which contributes to allergic disorders, also has an important function in protection of the host against noxious substances77, 78.
MCs can respond to many stimuli beside IgE. MCs can respond to various pathogens though activation of TLRs, including TLR-2 and TLR-479, 80 and, via GPCRs, to certain peptides found in venoms81–83, or can be activated by various complement peptides84, 85 and platelet-activation factor86. There is evidence that MCs also can be directly or indirectly activated by some plant products, including aqueous pollen extracts from birch87, and by products of the coagulation system, including Factor Xa88 and thrombin receptor activating peptide (TRAP)89. MCs also can respond to certain chemokines and cytokines (including IL-3325–27, 90, 91 and TSLP92), or be activated through the aryl hydrocarbon receptor93, 94, the CD40 ligand95 or the OX40 ligand96–98 or by immune complexes of IgG99, 100. MC activation (e.g., via the FcεRI) can also be modulated by various mechanisms, including interactions with other cells such as granulocytes101, regulatory T cells102 and other lymphocytes103, via a variety of negative regulatory receptors expressed on their surface8, 104–106, or by exposure to certain cytokines, including the KIT ligand SCF8, 12–14, 107, 108, IL-3325–27, 109 and IFN-γ56, 110.
Mast cell-derived mediators
MCs store preformed mediators in their granules and can release some of them almost instantly upon degranulation. These stored mediators include vasoactive amines such as histamine111, 112 (although MCs are considered the main source of histamine outside of the CNS, other cells also can produce histamine, including basophils113 and neutrophils114, 115), and, in rodents, serotonin112. MC granules also contain many neutral proteases (tryptases, chymases, and carboxypeptidase A3 [CPA3])42, 116–122 (Table 1). As noted above, MC protease content can vary depending on the cells’ tissue location and microenvironment. Only one chymase is expressed in human MCs but there are 13 known mouse chymase genes123. Among those, the β-chymase MC protease 4 (MCPT4) appears to be the most functionally similar to human chymase124, 125. MC granules also contain some preformed cytokines and growth factors, including TNF in both humans126, 127 and mice128, 129. MCs can also synthesize and secrete certain lipid mediators, such as prostaglandins and leukotrienes130, 131. Finally, MCs are also able to synthesize and secrete a large number of cytokines, chemokines, and growth factors, including TNF128, 132–134, IL-1135–137, IL-6135, 138, 139, IL-10140–142, IL-17143–145, VEGF and other vascular growth factors146–148, SCF149, 150 and many others. Release of lipid mediators typically occurs within 1–2 hours after MC activation and is associated with immediate responses, whereas synthesis and secretion of cytokines and chemokines characteristically occurs over a longer time frame, associated with the development of late phase or more chronic responses8, 151.
Mouse models to study mast cell functions in vivo
Pharmacological agents thought to target MC activation or MC proteases have been used in vivo to assess the functions of MCs. However, none of the drugs or antibodies described to date is fully specific for MCs or for particular MC proteases70, 152, 153. Therefore, we favor using genetic approaches to gain insights into MCs functions in vivo.
c-kit mutant mast cell-deficient mice and the ‘mast cell knock-in model’
For many years, c-kit mutant MC-deficient mice, such as WBB6F1-KitW/W-v and C57BL/6-KitW-sh/W-sh mice, have been used to analyze the functions of MCs in vivo7, 8, 141, 154–158. These two types of mice are profoundly MC-deficient but also have several other phenotypic abnormalities155, 157–163, including a marked reduction in intestinal cells of Cajal (ICCs), which results in abnormal electrical pacemaker activity in the small intestine155, 164. Abnormalities in biological responses in c-kit mutant mice may reflect their MC deficiency and/or one or more of their other phenotypic abnormalities. However, at many anatomical sites, the deficiency in MCs can be selectively “repaired” by the adoptive transfer of genetically-compatible, in vitro-derived MCs such as bone-marrow-derived cultured MCs (BMCMCs), to create so-called ‘MC knock-in mice’8, 10, 60, 155, 156, 165, 166.
c-kit-independent mast cell-deficient mice and mice deficient for mast cell-associated products
More recently, several groups have generated new strains of mice permitting the constitutive or inducible deletion of MCs independently of mutations affecting c-kit structure or expression60, 167–172. Most of these groups used a strategy consisting of generating transgenic mice expressing the Cre recombinase under the control of promoters for MC proteases, such as those for carboxypeptidase A3 (Cpa3) or MC protease 5 (Mcpt5)167, 168, 172. Such mice then were crossed with mice in which genes of interest have been “floxed” to delete expression of these gene products in the MCs168, 173. Our group mated Cpa3-Cre mice with mice expressing the floxed survival factor Mcl-1: the resulting Cpa3-Cre; Mcl-1fl/fl mice were severely deficient in MCs but had also markedly reduced basophil levels167. Feyerabend et al. reported a severe MC deficiency (and a more modest deficiency in basophils) in another line of Cpa3-Cre mice due to Cre-mediated cytotoxicity172. Mcpt5-Cre mice, which express Cre in connective tissue-type MCs but not mucosal MCs168, 169, were mated with transgenic mice expressing Cre inducible diphtheria toxin A (DT-A) or diphtheria toxin receptor (iDTR) genes to achieve constitutive (in Mcpt5-Cre; DTA+ mice) or inducible (after DT injection in Mcpt5-Cre; iDTR+ mice) ablation of CTMCs168. All of these mice and some additional new types of MC-deficient mice have been recently reviewed in detail152, 174, 175.
Several strains of mice that are deficient for one or multiple MC-associated proteases, or are unable to synthetize histamine (due to a deficiency in histidine decarboxylase) or heparin (due to a deficiency in N-deacetylase/N-sulfotransferase-2) also have been developed. While each of these strains of mice can provide important information concerning the roles of particular products released by MCs, some of them have a complex phenotype and there are a number of considerations that should be kept in mind when interpreting findings obtained with these animals, as reviewed in152, 175, 176.
Role of mast cells in the regulation of intestinal epithelial permeability
The intestinal epithelium forms a selectively permeable barrier against the external environment177. Disruption or dysregulation of this barrier is associated with many intestinal disorders, including bacterial, viral and parasitic infections, inflammatory bowel disease (IBD), and food allergies177, 178. Groschwitz et al. demonstrated that naïve c-kit mutant MC-deficient KitW-sh/W-sh mice and mice deficient for the chymase MCPT4 have altered intestinal barrier structure and function, with decreased intestinal epithelial cell migration along the villus/crypt axis of jejunum, increased crypt depth in the jejunum (without differences in villus length) and increased intestinal permeability as compared to WT mice177. Engraftment of KitW-sh/W-sh mice with WT BMCMCs but not Mcpt4−/− BMCMCs restored these features to levels observed in WT mice, evidence that MCs can contribute to the homeostatic regulation of the intestinal barrier through MCPT4-dependent mechanisms177.
Other studies have provided evidence that MCs can control intestinal epithelial ion transport or permeability during effector phases of inflammatory responses179–181, including during anaphylaxis179. Isolated intestinal preparations from ovalbumin (OVA)-sensitized WT mice displayed increases in short-circuit current (Isc) following ex vivo stimulation with OVA or following electrical transmural stimulation of intestinal neurons. Such responses were significantly diminished in MC-deficient KitW/W-v or WCB6F1-MgfSl/Sl-d mice (Sl-d is a deletion in the transmembrane domain of the Scf gene182 and MgfSl/Sl-d mice don’t express the membrane form of SCF183). Moreover, transfer of BM cells from WT mice to KitW/W-v mice “normalized” the Isc responses to both antigen and transmural stimulation, indicating a role for MCs and/or other BM-derived cell type in this process179. A role for MCs in this setting also was suggested by tests of pharmacological agents which antagonize the factions of certain MC-derived mediators179.
Infection with the parasite T. spiralis increases paracellular permeability of the jejunum and decreases the expression of occludin in the tight junctions of enterocytes181. Treatment of WT mice with a c-kit blocking antibody abrogated MC hyperplasia during T. spiralis infection and blocked parasite-induced increases in intestinal permeability181. Mice deficient in the chymase MCPT1 also exhibited diminished intestinal permeability during T. spiralis infection, even though numbers of intestinal MMCs were higher during infection in Mcpt1−/− mice than in WT mice181.
Injection of the neuropeptide substance P induces intestinal ion secretion with increase in Isc responses. In intestinal preparations from MC-deficient KitW/W-v mice, substance P-induced Isc responses were diminished to about 50% of those observed in WT mice and were normalized by the adoptive transfer of WT BM cells, suggesting that MCs can contribute to substance P-induced changes in intestinal ion secretion184. By contrast, our group demonstrated that MCs can limit the toxicity associated with high concentrations of another neuropeptide, vasoactive intestinal polypeptide (VIP)82. In that setting, our evidence indicated that VIP induced MC degranulation, releasing the chymase MCPT4 which then degraded VIP82.
Roles of mast cells in allergic responses at mucosal sites
Asthma
Asthma is a multifaceted disorder characterized by reversible airway narrowing (in many patients in response to particular allergens), immunologically non-specific airway hyper-responsiveness (AHR), chronic inflammation of the airways, and airway remodeling, including fibrosis, goblet cell hyperplasia/metaplasia, increased mucus production, smooth muscle thickening and increased vascularity185–187. Early manifestations of the disorder can appear in childhood, and both genetic188 and environmental factors189 contribute to the development and progression of asthma. Rather than being a single “disease”, the disorder called asthma is likely comprised of distinct subphenotypes with different clinical characteristics and underlying mechanisms190–192. Analysis of lung epithelial brushes, bronchoalveolar lavage (BAL) fluids, lung biopsies, and autopsies have shown increased numbers of MCs in the airways of some asthmatic subjects54, 193–195 but not others194, 196, 197. One feature more often seen in asthmatic subjects than in those without the disease is the presence of MCs within the bronchial epithelium198–200.
In subjects with asthma, B cell class switching to IgE occurs in the lymph nodes201, as well as locally in the respiratory mucosa202. IgE binds to FcεRI, highly expressed on MCs and basophils, but also, in certain settings by eosinophils and neutrophils; evidence has been reported that FcεRI also can be expressed by airway epithelial and smooth muscle cells and by certain nerves (reviewed in69). IgE not only permits allergen-dependent MC activation, but also enhances the stability of FcεRI on the MC surface, thus increasing the levels of receptor expression of FcεRI, contributing to the maintenance of a positive amplification loop (reviewed in6).
Several mouse models of allergic airway inflammation have been developed to recapitulate many aspects of asthma. Studies using the MC knock-in model in c-kit mutant mice sensitized with an antigen in the absence of artificial adjuvant55, 56, 203–206, or employing relatively low doses of antigen for sensitization or challenge207, 208, have provided evidence that MCs and MC-derived TNF can amplify multiple features of allergic airway inflammation, including airway responsiveness, inflammation, and tissue remodeling55, 56, 203–205, 207, 208. However, contributions of MCs to various features of allergic asthma are not observed (perhaps because they are redundant) in some models of allergic airway inflammation employing strong artificial adjuvants (such as alum) and relatively high doses of antigen for sensitization and challenge203, 209–212.
Genetic background also can influence the contribution of MCs to allergic airway inflammation. Becker et al. confirmed that KitW-sh/W-sh mice on the C57BL/6 background have reduced airway inflammation and AHR in an adjuvant-free model of asthma, but found no significant differences between BALB/c-KitW-sh/W-sh and BALB/c-WT mice in their model213. These findings clearly indicate that roles of MCs in this asthma model that are important in one strain background (the “Th1-biased” C57BL/6 background) may not be important (or may be redundant) in the more “Th2-biased” BALB/c background. These findings are of substantial interest, given the strong evidence that genetic factors have an important role in human asthma.
In a mouse model of chronic allergic airway inflammation, studies in MC knock-in mice indicated that MC expression of the IFN-γR contributes to the development of many features of the model that also require MCs and FcεRIγ for optimal expression, including AHR, neutrophil and eosinophil infiltration in the lung, lung collagen deposition, and increased expression of lung IL-6, IL-13, IL-33, multiple chemokines, arginase-1, and the acute-phase protein serum amyloid A3. However, expression of IFN-γR also contributes to some features of the model which require MCs for optimal expression but that occur relatively independently of FcεRIγ, such as elevations of levels of integrin α7 and the macrophage receptor with collagenous structure (MARCO) in the affected lungs56. In a passive model of OVA-induced allergic airway inflammation, transfer of OVA323–339-peptide-specific, IFN-γ-producing Th1 cells to naive mice primed them to develop airway neutrophilia and AHR that was most prominent in mice challenged with LPS as well as antigen214. It also has been reported that co-stimulation of mouse pulmonary macrophages with LPS and IFN-γ induces the production of IL-27215, that in turn can enhance production of IL-1 and TNF by MCs216. Such studies provide support for the hypothesis that bacterial infections can sustain or enhance inflammation driven by Th1 responses in asthma.
Some patients with severe asthma exhibit enhanced sputum neutrophilia (but not eosinophilia) and enhanced serum and sputum levels of IL-17217. In diseases with a prominent Th17 signature such as atopic dermatitis218, chronic exposure to antigens, such as via epicutaneous sensitization219, can enhance airway inflammation and “local” Th17 inflammation in the lung220. Evidence from our mouse models55, 56 and those of others221 show that chronic airway exposure to OVA can increase BAL neutrophils and lung levels of IL-17 (in addition to Th2 cytokines), and that the presence of MCs is essential for the development of such features. Some mouse or human MCs can produce IL-17 upon non-IgE-dependent stimulation (e.g., with 6-formylindolo(3,2-b)carbazole [FICZ]) or when exposed FICZ in combination with IgE/antigen and, based on immunohistochemical findings, MCs appear to represent a major in vivo source of IL-17 in the chronically inflamed bronchial lamina propria of patients with chronic obstructive pulmonary disease94, and in other settings145, 222, 223.
IL-33 is also thought to contribute to the pathology of asthma100, 224–228. The IL-33 receptor, ST2, is expressed by MCs and basophils229, but not by airway smooth muscle cells or lung fibroblasts230. In mice, IL-33-induced enhanced airway inflammation is partly dependent on IL-33-dependent MC production of IL-13231. IL-33 is considered an alarmin or a pro-inflammatory cytokine232, but its biology might be more complex since it has been reported that chronic exposure of human and mouse MCs to IL-33 in vitro can induce a hypo-responsive MC phenotype, raising the intriguing possibility that IL-33 might actually have certain protective roles in chronic airway inflammation233.
In summary, evidence from studies of human asthma and mouse models of the disorder support the general conclusion that MCs can have critical roles in amplifying acute immunological responses to antigen and in helping to orchestrate the later development of multiple features of the disorder, but also suggest that the roles of MCs in particular sub-phenotypes of asthma may vary, in part due to differences in the cytokines present in those settings (Figure 1). Moreover, recent data raise the interesting possibility that some individual MC mediators may have effects that can restrain the development of certain features of the pathology. For example, Waern and collaborators reported that mice deficient for the chymase MCPT4 exhibit increased pathology (i.e., airway inflammation, AHR and smooth muscle thickening) in two different models of allergic lung inflammation, and that such protective effects might reflect, at least in part, degradation of IL-33 by the chymase234, 235.
Figure 1. Schematic, highly simplified representation of the potential roles of MCs in airway chronic allergic inflammation and remodeling.
Individuals not yet sensitized to environmental allergens do not have specific IgE to such allergens, and few MCs are present within the epithelium (left panel). During allergic sensitization, environmental antigens (Ag) are captured by APCs in the airway lumen or in the epithelium of the airway mucosa, and Ag-activated APCs mature and migrate to regional lymph nodes, where priming of T cells occurs (not shown). The presence of IL-4 or IL-13, which may be derived from a variety of potential cellular sources, induces T cells to become TH2 cells (right panel). In some cases, allergens also can reach APCs in the submucosa through damaged epithelium. Cytokines induced by epithelial damage (such as IL-33 and TSLP) can activate ILC2 cells, which secrete type 2 cytokines, such as IL-4 and IL-13. The Th2 environment promotes heavy-chain class switching from IgM or IgG to IgE for Ag-specific IgE production in B cells. IgE binds to FcεRI on MCs (and basophils) and sensitizes these cells to respond to subsequent Ag exposures. Ag-induced aggregation of IgE-bound FcεRI causes the prompt release of pre-stored MC mediators, including histamine and TNF, which can promote bronchoconstriction and, more slowly, fibroblast proliferation. FcεRI activation also induces the production and the release of de novo synthesized compounds, such as leukotrienes, prostaglandins, and pro-inflammatory cytokines (e.g., IL-5, IL-6, IL-8, IL-13, and TNF) and chemokines (not shown), that contribute to the development of local inflammation.
Both soluble factors, such as IFN-γ, TSLP, IL-33, S1P, LPS (through PRRs) and cells present at the site, such as TH cells and various Treg cells (not shown), which can interact with OX40L on MCs, modulating IgE-dependent MC activation, or B cells, which can interact with CD40L on MCs, which may enhance B cell IgE production. At least one MC-secreted product, MCPT4 (not shown), can negatively regulate the inflammatory environment, in part through the degradation of IL-33.
Repetitive exposure to specific Ag favors persistent inflammation (with large numbers of eosinophils, and with MCs appearing within the epithelium), goblet cell hyperplasia and increased mucus secretion, smooth muscle cell proliferation, increased vascular permeability (and increased numbers of blood vessels) and airway edema, thickening and remodeling. In some asthma subtypes, genetic or environmental factors, including pathogen-derived products, tissue damage, airway pollutants, and oxidative stress, may confer strong TH1 and/or TH17 signatures associated with large numbers of neutrophils at the site of inflammation. Studies in MC–knockin mice have indicated that some actions of MCs, such as increasing the number of epithelial goblet cells, can occur in a model of chronic allergic inflammation by MC–dependent mechanisms that do not require MC signaling through the FcεRIγ chain, whereas MCs must express both the FcεRIγ chain and the IFN-γ receptor 1 (IFN-γR1) to mediate substantial increases in lung eosinophils and neutrophils. Note: down-regulatory mechanisms that can be engaged in this setting, such as co-engagement by multivalent Ag of both FcεRI and FcγRIIb, or effects of regulatory T cell populations, are not shown.
AhR, Aryl hydrocarbon receptor; Baso, basophils; Eos, eosinophils; FcεRI, high affinity receptor for IgE; ILC2, innate lymphoid cells type 2; Neu, neutrophils; PRR, pattern recognition receptor; TH, T helper; TSLP, thymic stromal lymphopoietin.
Food allergy and anaphylaxis
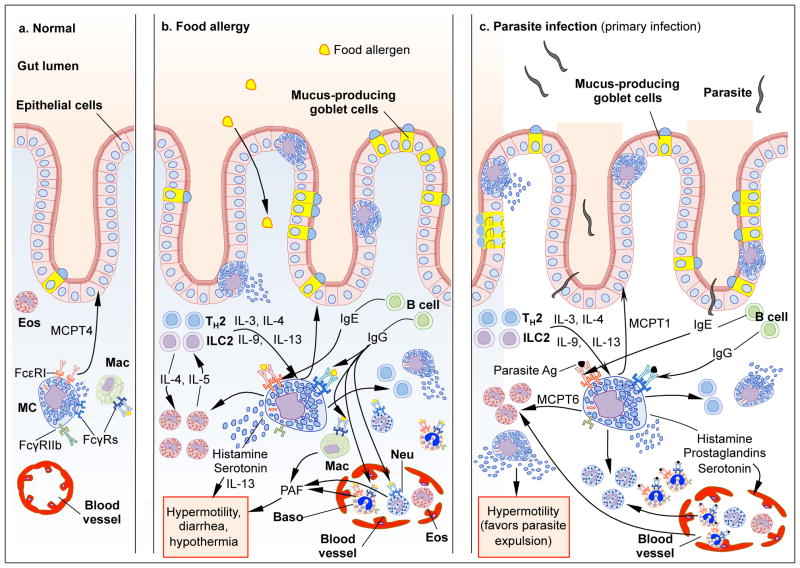
Food allergies are caused by adverse acquired immune responses to food components, primarily proteins236. Their prevalence has recently increased and food allergies now affect ~6% of children and 3–4% of adults in developed countries236. The manifestations of food allergy can range from mild to severe, with the most severe form being anaphylaxis, an acute and potentially life-threatening multisystem reaction to allergen exposure. In the U.S., the majority of cases of food-induced fatal or near-fatal anaphylaxis are caused by peanuts or tree nuts237, 238. Studies in mice indicate that MCs are critical effector cells of both food-induced intestinal inflammation and anaphylaxis (Figure 2).
Figure 2. Schematic, highly simplified representation of the potential roles of MCs in food allergy and parasite infections.
In the normal intestine, MCs can contribute to the homeostatic regulation of the epithelial barrier through chymase- (MCPT4-) dependent mechanisms and few MCs are present within the epithelium (left panel). During sensitization with food allergens (middle panel) or primary infections with parasites (right panel), antigens (Ag) are captured by APCs and Ag-activated APCs mature and migrate to regional lymph nodes, where priming of T cells occurs (not shown). The presence of IL-4 or IL-13, which may be derived from a variety of potential cellular sources, induces T cells to become TH2 cells. TH2 cells and ILC2 cells release IL-3 and IL-9 which promote expansion of mucosal MCs (MMCs), and some of these MMCs are found in the intestinal epithelium. IgE binds to FcεRI on MCs (and basophils) and sensitizes these cells to respond to subsequent Ag exposures. Ag-induced aggregation of IgE-bound FcεRI causes the prompt release of pre-stored MC mediators, including histamine which can promote vasodilatation and increased vascular permeability. FcεRI activation also induces the production and release of de novo synthesized compounds, such as leukotrienes, prostaglandins, and pro-inflammatory cytokines (such as IL-13) and chemokines (not shown). Such MC-derived products contribute to intestinal inflammation (including the recruitment and activation of neutrophils, basophils and eosinophils and other leukocytes), increased intestinal permeability and motility, and, in the case of parasite infections, worm expulsion. During food allergy, the activation of MCs also can promote diarrhea and, in some unfortunate individuals, anaphylaxis (not shown). IgG-Ag immune complexes can potentially modulate MC activation through Fcγ receptors (MCs express the activating receptor FcγRIII and the inhibitory receptor FcγRIIb). Macrophages, basophils and neutrophils are also activated by IgG-Ag immune complexes and release PAF, which is thought to contribute to diarrhea and anaphylaxis in food allergy. Note: down-regulatory mechanisms that can be engaged in these settings, such as co-engagement by multivalent Ag of both FcεRI and FcγRIIb, or effects of regulatory T cell populations, are not shown.
Baso, basophils; Eos, eosinophils; FcεRI, high affinity receptor for IgE; FcγRs: receptors for IgGs; ILC2, innate lymphoid cells type 2; Neu, neutrophils; PAF, platelet-activating factor; PRR, pattern recognition receptor; TH2, T helper 2.
Multiple mouse models of anaphylaxis have been developed to investigate the contribution of MCs and other effector cells. Two main pathways of active anaphylaxis have been described in mice: a “classical” pathway consisting of antigens, IgE, FcεRI, MCs, and histamine, and an “alternative” pathway involving IgG-antigen immune complexes, FcγRIII, platelet-activating factor (PAF), and, depending on the exact model used, macrophages, basophils and/or neutrophils239–245. Several studies using KitW/W-v and/or KitW-sh/W-sh MC-deficient mice have provided evidence that MCs can contribute significantly to peanut-induced active anaphylaxis242, 244, 245. We recently confirmed these findings using c-kit-independent MC-deficient mice, by showing that selective ablation of CTMCs (induced by repeated injections of diphtheria toxin in Mcpt5-Cre; iDTR mice168) significantly diminished the hypothermia induced by peanut challenge in mice sensitized orally with peanut together with the mucosal adjuvant cholera toxin243. However, antigen challenge induced significant hypothermia (albeit less than that in the corresponding WT mice) in Cpa3-Cre+; Mcl-1fl/fl mice, which have a marked MC deficiency and a substantial reduction in basophils243. Antigen-induced elevations in serum histamine were abolished in MC- and basophil-deficient Cpa3-Cre+; Mcl-1fl/fl mice, whereas small but significant increases in PAF levels were still detected in spleen specimens from these mice243. Together, these findings implicate the involvement of both the classical and alternative pathways of anaphylaxis in this mouse model of peanut-induced active anaphylaxis.
The reaction to peanut in some mouse models might be even more complex, since Khodoun et al. found that peanut, but not milk or egg proteins, can induce shock reactions through an innate immune mechanism in mice246. The authors found that this response was almost absent in mice lacking the complement factor C3 or the receptor C3aR, but developed fully in antibody-deficient Rag1 mice (which lack mature T and B cells) and μMT mice (in which the development of conventional B cells is arrested at the pro-B cell stage)246. However, some reports indicate that μMT mice have B1 B cells and can produce IgE and IgG247–249. Macrophages, basophils and PAF contributed to this shock reaction to a greater extent than did MCs and histamine246. Therefore, depending on the model used, innate components might also participate importantly in peanut-induced anaphylaxis, which perhaps accounts for the fact that peanut allergy is more likely than most other forms of food allergy to cause lethal anaphylaxis. However, it is important to recognize that Khodoun et al. increased the sensitivity of the mice to develop shock reactions in these experiments by pretreating the animals i.v. with a long-acting form of IL-4 (consisting on IL-4/anti–IL-4 mAb complexes, which slowly dissociate in vivo to release free IL-4) and with the β-adrenergic antagonist propranolol246.
Although IgE-dependent activation of MCs is widely thought to contribute importantly to anaphylaxis in humans, subjects with food allergy-associated anaphylaxis, unlike those with insect venom-induced anaphylaxis, typically exhibit little or no elevations in blood levels of the MC-associated protease, tryptase250. By contrast, levels of PAF in the serum have been directly correlated with the severity of organ system involvement in patients with acute allergic reactions triggered by foods, medications, or insect stings251, 252. Moreover, the serum activity of PAF acetylhydrolase (an enzyme that converts PAF to the biologically inactive lyso-PAF) was significantly lower in peanut allergic patients with fatal peanut anaphylaxis than in those with mild allergic reactions to peanuts or in control groups251. Although they do not constitute proof, these results are consistent with the possibility that activation of both the “classical” pathway and the “alternative” pathway might be involved in at least some examples of anaphylaxis in humans. The existence of IgG-mediated anaphylaxis in humans is perhaps best supported by the occurrence of anaphylaxis in patients infused with monoclonal antibodies (mAbs), such as the chimeric mouse/human anti-TNF mAb infliximab239, 253. One study showed that 11 out of 165 patients with Crohn disease treated with infliximab developed signs of anaphylaxis. All these patients had IgG antibodies to the mouse immunoglobulin determinants on infliximab. While none of the patients had detectably increased serum levels of total IgE, the authors did not report whether they attempt to measure levels of infliximab-specific IgE. However, none of these patients had increased tryptase levels in blood 20 minutes after the onset of the reaction239, 253.
Anaphylaxis represents the extreme end of a spectrum of responses to food allergens in allergic patients. In most patients, reactions are manifested mainly by local signs and symptoms, and the skin is affected in ~80% of subjects254. Up to 50% of patients also develop gastrointestinal symptoms (abdominal pain, vomiting, diarrhea) and a significant portion of patients also experience respiratory symptoms (cough, chest tightness, wheezing)255, 256. Multiple lines of evidence suggest that IgE-dependent MC activation can play an important role in these local manifestations of food allergy. Cafarelli et al. found elevated numbers of IgE-positive cells (plasma cells, and 2.7% MCs) in duodenal biopsies from children with food allergies, whereas MCs were virtually absent in the control biopsies256, 257. Moreover, when stimulated ex vivo with anti-IgE, intestinal MCs obtained from enzymatically dispersed duodenal biopsies from food allergic patients released more histamine in comparison to cells from non-allergic individuals256, 258.
Brandt et al. developed a mouse model of allergen-induced gastrointestinal inflammation consisting of sensitization with OVA together with alum and repeated oral challenges with OVA48. In this model, sensitized and challenged BALB/c mice (but not C57BL/6 mice) developed large increases in numbers of MMCs in the jejunum, ileum, and colon and increased levels of MCPT1 in the plasma. These mice also exhibited a strong Th2 response in the intestine, with signs of allergy such as diarrhea and increased intestinal permeability, but without hypothermia48. However, systemic (i.v.) OVA challenge of OVA/alum-sensitized mice induced hypothermia that was significantly more severe in animals which had been previously challenged with OVA intra-gastrically compared with those mock-challenged with saline. Notably, lethal anaphylactic shock occurred only in mice that previously had developed gastrointestinal allergy, suggesting that gastrointestinal allergic inflammation can prime mice for more severe anaphylaxis following systemic antigen challenge48. The authors showed that treatment with an anti-KIT antibody (ACK2) abrogated the diarrhea, diminished intestinal permeability, and eliminated MMCs in the jejunum48. These features were also diminished in mice treated with an anti-IgE antibody and in mice deficient for the high affinity IgE receptor FcεRI (but not in mice treated with a blocking antibody against the IgG receptors FcγRII/III). Finally, they demonstrated that treatment with a combination of pharmacological inhibitors of PAF and serotonin blocked diarrhea, while blockade of histamine had no effect on diarrhea48. Wang et al. reported that, in a model of peanut allergy in BALB/c mice, allergen-induced diarrhea and other features of the response were also partially diminished in mice deficient for the FcεRIα chain. Adoptive transfer of WT BMCMCs, but not FcεRIα−/− or Il-13−/− BMCMCs, restored diarrhea in FcεRIα-deficient mice, suggesting that this feature is dependent on IgE-mediated activation of MCs and on the release of IL-13 by MCs259.
Little is known about the mechanism(s) leading to sensitization with food allergens. Forbes et al. showed that transgenic mice which overexpress IL-9 have increased numbers of intestinal MMCs, associated with increased intestinal permeability which can enhance oral sensitization to OVA administered without an adjuvant260. Epidemiologic studies have demonstrated that cutaneous inflammation associated with atopic dermatitis (AD) is a significant risk factor for the development of food allergies261–263. Recently, Noti et al. reported that epicutaneous sensitization of mice to food antigens (OVA or peanut extract) applied to an AD-like skin lesion (which lead to increased levels of TSLP in the skin) followed by oral challenge with the antigen promoted intestinal Th2-driven inflammation and increased numbers of intestinal MMCs263. Such features are much diminished in mice deficient for the TLSP receptor (TSLPR) or IgE, or in mice in which basophils have been depleted (but the authors did not assess responses of MC-deficient mice in this model). These results indicate that a “TSLP-basophil axis” can contribute to the development of IgE-mediated intestinal MMCs expansion and food allergy in mice sensitized epicutaneously with food allergens263.
Burton et al. recently developed an adjuvant-free model of peanut allergy using mice with a disinhibiting mutation in the IL-4 receptor α chain (il4raF709 mice), which results in amplified signaling upon interaction of the receptor with the Th2 cytokines IL-4 or IL-13 but not constitutive activation264. Oral sensitization of il4raF709 mice with peanut, followed by oral challenge with peanut, led to expansion and activation of intestinal MMCs, and the development of diarrhea, intestinal inflammation and hypothermia. The authors used MC-deficient Mcpt5-Cre;DTA mice and IgE-deficient mice to demonstrate that, in this model, both MCs and IgE were required for induction of antibody and Th2-cell-mediated responses to peanut ingestion, as well as for suppression of expansion of regulatory T (Treg) cells. MC-targeted genetic deletion of the FcεRI signaling kinase Syk in Mcpt5-Cre;Sykfl/fl mice also prevented peanut sensitization. Therefore, in addition to their key effector role during many allergic reactions, under certain circumstances MCs and IgE also appear to be able to amplify sensitization to certain food allergens such as peanut, as well as participate in the suppression of tolerance.
Roles of mast cells in defense against mucosal pathogens
MCs are located at sites exposed to invading pathogens, such as the skin, the gut, and the lung and genitourinary mucosa. MCs are therefore likely to be among the first innate cells (together with macrophages and dendritic cells [DCs]) to respond to such pathogens. Studies in mice indicate that MCs can contribute to multiple defense strategies against various pathogens, including parasites (Figure 2), bacteria, and viruses79, 265–268, but that, in certain settings, MCs can contribute to the pathology associated with such infections.
Parasite infections
Parasite infections that involve the intestines and provoke the development of Th2 responses are often associated with a large expansion in MMCs in rodents269–271, and with expansion of mucosal MC populations in monkeys272 and humans273. Space does not permit a comprehensive discussion of the complex innate and adaptive immune mechanisms which are thought to contribute to helminth clearance274–277. Instead, we will review briefly some of the evidence indicating that MCs can influence aspects of these responses. Woodbury et al. demonstrated that, in rats infected with Trichinella spiralis or Nippostrongylus brasiliensis, systemic secretion of the rat MC-associated chymase rMCP-2 coincides with the immune expulsion of these nematodes269. Many groups have assessed the responses of KitW/W-v and/or KitWsh/Wsh mice to primary infection with various parasites, including Nippostrongylus brasiliensis278, 279, Strongyloides ratti280, Strongyloides venezuelensis51, 281, 282, Trichinella spiralis283, 284, and Trichinella muris285, 286. Most of these studies show that such c-kit mutant MC-deficient mice have a delay in intestinal worm clearance during the primary infection. However, due to the inability to engraft intestinal MMCs in such c-kit mutant mice by the systemic adoptive transfer of MCs155, 177, 287, 288, it is not possible to know to what extent the delays in parasite clearance detected in these MC-deficient mice reflect their lack of MMCs vs. one or more of their other phenotypic abnormalities (including their deficiency on intestinal cells of Cajal, which results in abnormal gut motility164).
However, other lines of evidence support an important contribution for MCs in intestinal worm clearance. Ha et al. showed that engraftment with total BM cells accelerated expulsion of T. spiralis in KitW/W-v mice283. Expulsion of T. spiralis was significantly delayed in mice lacking the chymase MCPT1, which suggests an important contribution of intestinal MMCs and MCPT1 in the clearance of this infection271. Although the kinetics of T. spiralis expulsion from the small intestine were similar between MCPT6-deficient and WT mice, the MCPT6-deficient mice had diminished levels of eosinophils in infected skeletal muscle289. Recently, Blankenhaus et al. showed that c-kit-independent MC-deficient BALB/c-Cpa3Cre/+ mice (which, beside their MC deficiency, also have reduced basophil numbers172) exhibited increased parasite burden in the small intestine following infection with S. ratti290.
While results described above suggest potentially important roles for MMCs and some of their associated chymases in worm expulsion, it is possible that in some parasite infections effects of MCs might actually favor the parasite. For example, anti-SCF treatment diminished intestinal MMC hyperplasia in rats infected with N. brasiliensis or T. spiralis, but such anti-SCF treatment decreased parasite egg production during N. brasiliensis infection291. Similarly, during a primary infection with N. brasiliensis, c-kit mutant MC-deficient Ws/Ws rats exhibited reduced numbers of eggs in the feces at day 8 of infection than did the corresponding WT rats292. Neither study proved that MCs were responsible for the observed effects, but the results are intriguing in suggesting that some parasites may have learned how to exploit MC-associated effector mechanisms to their own advantage.
IL-3 can promote expansion of intestinal MMCs in mice51, 283 and treatment with IL-3 accelerates expulsion of S. ratti283. Both KitW/W-v mice and mice lacking IL-3 exhibited a delay in S. venezuelensis expulsion, and this delay was greatly enhanced when these deficiencies were combined (i.e., in Il-3−/−; KitW/W-v mice, in which infection provoked little or no expansion of basophil or MMC populations)51. These findings indicate that one of the functions of IL-3 in this setting is to expand populations of hematopoietic effector cells, and are consistent with the possibility that both MCs and basophils contribute to expulsion of S. venezuelensis during the primary infection.
IL-9 also plays an important role in expansion of intestinal MMCs during parasite infection and transgenic mice which overexpress IL-9 have increased intestinal MMCs and increased efficiency of worm expulsion during infection with T. spilaris181. There is evidence that IL-9-mediated MC activation is also a key mechanism mediating S. ratti expulsion in mice290. This mechanism can’t be generalized to all parasites, since in the case of infection with N. brasiliensis, it appears that neither IL-9 nor MCPT1 influences worm expulsion271, 293.
Most studies of the roles of MCs in parasite infection have focused on the primary responses to the infection (Figure 2). Many parasites induce strong antibody responses, including high levels of antigen non-specific IgE as well as antigen-specific IgE and IgG antibodies, and secondary infections are often associated with a more rapid expulsion of the parasites than occurs in the primary infection294–296. However, it is not yet clear to what extent interactions of such antibodies with MCs importantly contribute to such secondary responses. While there have been few studies of secondary parasite infections in genetically MC-deficient mice, numbers of MMCs and serum MCPT1 levels were significantly higher in BALB/c WT mice at day 3 after secondary vs. primary infections with T. spiralis, and worm burden at that time was significantly less in the secondary than in the primary infection181. Given that co-engagement of FcγRIIB with FcεRI can diminish antigen-dependent MC activation297, 298, it will be important to investigate whether this or other mechanisms can down-regulate or otherwise alter MC responses during secondary parasite infections, as well as to determine whether MCs can confer benefits to the host or the parasite in such settings.
Bacterial infections
Several studies have indicated that MCs can have an important role in enhancing survival during models of experimental bacterial sepsis in mice. Many of these data were obtained using the cecal ligation and puncture (CLP) model in which commensal bacteria are allowed to escape from the cecum into the peritoneum, and most of the studies employing MC-deficient mice have used KitW/W-v and/or KitW-sh/W-sh mice, which have multiple defects in immune responses other than their MC deficiency80, 158, 299–305. Experiments assessing responses of MC-deficient KitW/W-v mice engrafted with WT or various mutant BMCMCs have demonstrated that such MCs are mainly activated through TLR4 (but not TLR2) during polymicrobial sepsis80, and that MC-derived IL-12306, as well as MC expression of the cysteinyl protease dipeptidyl peptidase I (DPPI)302 and the transcription factor Smad3307, are also required for optimal survival during the CLP model.
MCs also can be activated by the endogenous peptide endothelin-1 (ET-1), primarily through the ET(A) receptor. Activation by ET-1 promotes MC degranulation and the release of proteases which in turn can degrade ET-1. MC protease-dependent degradation of ET-1 can contribute to optimal survival during CLP, which is associated with markedly elevated levels of ET-1301, and carboxypeptidase A3 (CPA3) is the critical protease which mediates degradation of ET-181, 83. Other MC-associated proteases also have been implicated in defense against bacteria. Studies in Mcpt4−/− mice indicate that the chymase MCPT4 has effects that can enhance survival in a moderately severe model of CLP, perhaps in part through degradation of TNF305. Orinska et al. reported evidence that intra-cellular IL-15 expression in MCs can transcriptionally limit the amount of the chymase MCPT2 in the cells, resulting in decreased MC antibacterial properties and reduced survival of the mice after CLP308.
MCs can mediate neutrophil recruitment after intraperitoneal injection of Klebsiella pneumoniae, probably via multiple mechanisms including the release of TNF133, IL-6304, and the tryptase MCPT6309, 310. There is evidence that MCs can enhance resistance to pulmonary infection with Mycoplasma pneumonia311. Histamine plays an important role in this model, but neutrophils, rather than MCs, were the major source of histamine in the lungs of the infected mice115. MCs also can contribute to Clostridium difficile toxin A-induced intestinal fluid secretion and neutrophil infiltration312. Malaviya et al. reported that, during infection with E. coli, neutrophil recruitment and bacterial clearance is controlled by JAK3 activation in MCs; this effect was attributed to the diminished ability of Jak3−/− MCs to produce TNF313. By contrast, there is evidence from work in MC knock-in mice that MC-derived TNF can enhance bacterial growth and hasten death after intraperitoneal inoculation of Salmonella typhimurium158.
Urinary tract infections (UTIs), mainly caused by uropathogenic E. coli, represent one of the most common bacterial infections in humans314. Using the MC knock-in approach in c-kit mutant mice, Shelburne and collaborators demonstrated that MCs and MC-derived TNF can amplify the protective adaptive immune response to infection with uropathogenic E. coli by promoting: 1) recruitment of DCs at the site of infection (in this case the footpad), 2) migration of DCs into the draining lymph nodes (DLNs), and 3) production of E. coli-specific IgG and IgM antibodies315. Increased numbers of surviving bacteria were found in the urinary bladder of c-kit mutant MC-deficient KitW/W-v mice as compared to Kit+/+ mice following experimental infection with E. coli316. Chan et al. compared the kinetics of E. coli clearance in the bladder and kidneys of infected mice and found that, while all bacteria were cleared within five days in the kidneys, significant numbers of bacteria were still found in the bladder as late as one month after infection317. They demonstrated that this prolonged bacterial survival was due to production of IL-10, and the absence of significant levels of E. coli specific antibodies, in the bladder317. There is evidence that mouse MCs can represent an important source of IL-10 during inflammation141, 317 and that MC-derived IL-10 can: 1) limit inflammation during contact hypersensitivity141 (although these findings have been recently challenged by Dudeck et al.168), as well as 2) diminish the severity of experimental graft-versus-host disease (GVHD)318. In line with these findings, Chan et al. demonstrated that MC-derived IL-10 contributed importantly to the suppression of E. coli-specific antibody production during experimental UTI in mice and accounted, at least in part, for the persistence of E. coli in the bladder317. Therefore, MCs appear to be able to play a dual role during E. coli infections in the bladder, first promoting the initial innate response to infection and later limiting the antibody response to E. coli by producing IL-10317.
Because c-kit mutant MC-deficient mice have many c-kit-related phenotypic abnormalities that may influence the responses of such animals to infection (including those which do or do not affect MC numbers or functions)152, 158, 175, it will be of great interest to continue to evaluate the roles of MCs in infection models using some of the newer, c-kit-independent, models of MC deficiency. For example, Rönnberg et al. recently reported that peritoneal MCs are activated by Staphylococcus aureus in vitro, however, the authors used c-kit-independent MC-deficient Mcpt5-Cre; DTA+ mice to demonstrate that MCs do not influence the in vivo manifestations of one model of intraperitoneal S. aureus infection319. Such work will help to clarify which roles of MCs are variably redundant with those of other cell types (including neutrophils or macrophages, among others) and which MC roles – whether to enhance and/or suppress aspects of these innate or acquired immune responses – may be non-redundant.
Viral infections
MCs have been implicated in the defense against certain viruses, although there have been relatively few studies in this area79. Sendai virus can induce histamine release from rat peritoneal MCs ex vivo320 and infection of rats with Sendai virus results in increased numbers of MCs in the lung321, 322. Kulka et al. showed that human peripheral blood-derived cultured MCs (HCMCs) and two lines of human MCs (LAD and HMC-1), as well as mouse BMCMCs, can respond to stimulation with dsRNA (poly[I:C]) by producing type I interferon (IFN-α) through TLR3. They also found that HCMCs can produce IFN-α when stimulated with live respiratory syncytial virus (RSV), reovirus type 1, or UV-inactivated influenza virus323. It has been reported, based on studies in MC knock-in KitW/W-v mice, that MCs can promote the recruitment of CD8+ T cells following i.p. injection of poly(I:C)324.
Several reports suggest that MCs can contribute to the pathology induced by some viruses in vivo. Both MC-deficient KitW/W-v mice and KitlSl/Sl-d mice exhibited reduced myocardial inflammation and necrosis as well as increased survival as compared to the respective littermate WT mice after i.p. infection with encephalomyocarditis virus325. Furthermore, adoptive transfer of BMCMCs into KitW/W-v mice or repeated subcutaneous treatment of KitlSl/Sl-d mice with recombinant SCF (which can induce the appearance of both CTMCs and MMCs in these mice63, 326) significantly increased the histopathological severity of the myocardial lesions induced by the virus (albeit not to levels observed in WT mice)325. By contrast, studies in KitW-sh/W-sh mice, including engraftment of these mice with BMCMCs, showed that MCs can participate in host defense against vaccinia virus, and MC production of the antimicrobial peptide cathelicidin was implicated as a key defense mechanism against this virus327, 328.
In humans, infection with Dengue Virus leads to increased levels of MC chymase in the serum329, and chymase levels are significantly higher in patients with severe dengue fever (also known as dengue hemorrhagic fever) as compared to patients with dengue fever329, 330. Using the MC knock-in model in KitW-sh/W-sh mice, St John and collaborators demonstrated that MCs can promote the recruitment of natural killer (NK) and NK T cells during Dengue Virus infection in mice329–331. Ebert et al. recently used a similar approach to demonstrate that MCs can contribute to clearance of Pulmonary Murine Cytomegalovirus in the lung by enhancing the recruitment of CD8+ T cells to the infection site332.
There is evidence that MCs may have roles in HIV infection. In vitro experiments show that the HIV gp120 envelope protein can promote production of Th2 cytokines (IL-4 and IL-5) in human MCs323. MCs and their progenitors might also serve as a reservoir for latent virus, a role which would be detrimental to the host333–336.
In line with the potential of MCs to help to orchestrate protective adaptive responses at mucosal sites, McLachlan et al. demonstrated that certain small molecules (‘MC activators’) are potent mucosal adjuvants, and provided evidence that these agents mediate such functions in a largely MC-dependent manner337. So-called ‘MC activators’ comprise a family of structurally diverse cationic peptides and polymeric compounds that can induce strong MC degranulation338, 339; such agents include compound 48/80 (c48/80)340–342, and a variety of peptide toxins, such as MC-degranulating peptide (MCD), which is found in honeybee and bumblebee venoms343. Using the MC knock-in system in c-kit mutant mice, McLachlan et al. demonstrated that compound 48/80 (which promotes MC degranulation, but also has other effects) can act as a potent mucosal adjuvant when co-administered in the footpad with recombinant anthrax protective antigen, and that this adjuvant effect largely depends on MCs and MC-derived TNF. Importantly, vaccination with c48/80 co-administered with the vaccinia virus antigen B5R intranasally conferred protection against intranasal challenge with a normally lethal dose of vaccinia virus337.
Conclusions
We are in the midst of an interesting period in MC research. For many years, an increasing understanding of the diversity of MC products, signaling mechanisms, and interactions with other cell types has led to the generation of many attractive hypotheses about the diverse potential effector and immunoregulatory roles of MCs in the biology and pathology of mucosal tissues (and in other settings). Increasingly, these hypotheses are being tested in ways that permit us to accrue definitive evidence regarding the nature, and the importance, of such proposed MC roles. In addition to long-established mouse model systems, including “MC knock-in c-kit mutant mice” and various MC protease-deficient mice, there are now many promising new models of constitutive or inducible MC deficiency, as well as many new models for achieving the targeted deletion of individual products in MCs. Based on the results obtained so far with both the older and newer models for MC research, we think that the most robust conclusions about the nature and importance of the roles of MCs in various biological responses in vivo, in mucosal tissues and other sites, probably will be derived from studies employing multiple informative model systems152. Taken together, such approaches offer many opportunities to obtain increasingly solid evidence to support (or discard) notions about how MCs might influence the development, physiology, homeostasis, immunology and pathology of mucosal tissues.
It hardly needs mentioning that findings in mice do not prove that the same processes occur in humans, and there are likely to be multiple differences in the details of immune responses and disease pathogenesis in the two species, not just differences in the roles of MCs in such settings. However, pre-clinical studies using models in which individual cells or products can be manipulated offer the promise of revealing pathways that, with luck, might be exploited to provide benefit to those suffering from any of the diverse mucosal pathologies in which MCs have been implicated. Time will tell to what extent this hope will be realized.
Acknowledgments
We thank the members of the Galli lab and our collaborators and colleagues for their contributions to some of the work reviewed herein and we apologize to the many contributors to this field whose work was not cited because of space limitations. L.L.R. is the recipient of fellowships from the French “Fondation pour la Recherche Médicale FRM” and the Stanford Pediatric Research Fund and grants from the Arthritis National Research Foundation and National Institutes of Health (K99AI110645); R.S. is supported by the Lucile Packard Foundation for Children’s Health and the Stanford NIH/NCRR CTSA award number UL1 RR025744; K.M. was supported in part by a Postdoctoral Fellowship for Research Abroad of the Japan Society for the Promotion of Science; S.J.G. acknowledges support from National Institutes of Health grants U19 AI104209, NS 080062 and from Tobacco-Related Disease Research Program at University of California.
References
- 1.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev. 2000;173:131–140. doi: 10.1034/j.1600-065x.2000.917305.x. [DOI] [PubMed] [Google Scholar]
- 2.Metz M, Grimbaldeston MA, Nakae S, Piliponsky AM, Tsai M, Galli SJ. Mast cells in the promotion and limitation of chronic inflammation. Immunol Rev. 2007;217:304–328. doi: 10.1111/j.1600-065X.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8(6):478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010;3(2):111–128. doi: 10.1038/mi.2009.136. [DOI] [PubMed] [Google Scholar]
- 5.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37(1):25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9(11):1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 8.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura Y, Shimada M, Hatanaka K, Miyano Y. Development of mast cells from grafted bone marrow cells in irradiated mice. Nature. 1977;268(5619):442–443. doi: 10.1038/268442a0. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 11.Douaiher J, Succar J, Lancerotto L, Gurish MF, Orgill DP, Hamilton MJ, et al. Development of mast cells and importance of their tryptase and chymase serine proteases in inflammation and wound healing. Adv Immunol. 2014;122:211–252. doi: 10.1016/B978-0-12-800267-4.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira SH, Lukacs NW. Stem cell factor: a hemopoietic cytokine with important targets in asthma. Curr Drug Targets Inflamm Allergy. 2003;2(4):313–318. doi: 10.2174/1568010033483990. [DOI] [PubMed] [Google Scholar]
- 13.Reber L, Da Silva CA, Frossard N. Stem cell factor and its receptor c-Kit as targets for inflammatory diseases. Eur J Pharmacol. 2006;533(1–3):327–340. doi: 10.1016/j.ejphar.2005.12.067. [DOI] [PubMed] [Google Scholar]
- 14.Galli SJ, Zsebo KM, Geissler EN. The kit ligand, stem cell factor. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 15.Razin E, Ihle JN, Seldin D, Mencia-Huerta JM, Katz HR, LeBlanc PA, et al. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984;132(3):1479–1486. [PubMed] [Google Scholar]
- 16.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Induction of the high-affinity IgE receptor (FcεRI) on human mast cells by IL-4. Int Immunol. 1996;8(9):1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 17.Valent P, Bevec D, Maurer D, Besemer J, Di Padova F, Butterfield JH, et al. Interleukin 4 promotes expression of mast cell ICAM-1 antigen. Proc Natl Acad Sci U S A. 1991;88(8):3339–3342. doi: 10.1073/pnas.88.8.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sillaber C, Strobl H, Bevec D, Ashman LK, Butterfield JH, Lechner K, et al. IL-4 regulates c-kit proto-oncogene product expression in human mast and myeloid progenitor cells. J Immunol. 1991;147(12):4224–4228. [PubMed] [Google Scholar]
- 19.Toru H, Eguchi M, Matsumoto R, Yanagida M, Yata J, Nakahata T. Interleukin-4 promotes the development of tryptase and chymase double-positive human mast cells accompanied by cell maturation. Blood. 1998;91(1):187–195. [PubMed] [Google Scholar]
- 20.Matsuzawa S, Sakashita K, Kinoshita T, Ito S, Yamashita T, Koike K. IL-9 enhances the growth of human mast cell progenitors under stimulation with stem cell factor. J Immunol. 2003;170(7):3461–3467. doi: 10.4049/jimmunol.170.7.3461. [DOI] [PubMed] [Google Scholar]
- 21.Mwamtemi HH, Koike K, Kinoshita T, Ito S, Ishida S, Nakazawa Y, et al. An increase in circulating mast cell colony-forming cells in asthma. J Immunol. 2001;166(7):4672–4677. doi: 10.4049/jimmunol.166.7.4672. [DOI] [PubMed] [Google Scholar]
- 22.Bailey DP, Kashyap M, Bouton LA, Murray PJ, Ryan JJ. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80(3):581–589. doi: 10.1189/jlb.0405201. [DOI] [PubMed] [Google Scholar]
- 23.Royer B, Varadaradjalou S, Saas P, Guillosson JJ, Kantelip JP, Arock M. Inhibition of IgE-induced activation of human mast cells by IL-10. Clin Exp Allergy. 2001;31(5):694–704. doi: 10.1046/j.1365-2222.2001.01069.x. [DOI] [PubMed] [Google Scholar]
- 24.Yeatman CF, 2nd, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, et al. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192(8):1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcεRI signals. J Leukoc Biol. 2007;82(6):1481–1490. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 26.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87(10):971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 27.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179(4):2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 28.Lin TJ, Issekutz TB, Marshall JS. Human mast cells transmigrate through human umbilical vein endothelial monolayers and selectively produce IL-8 in response to stromal cell-derived factor-1α. J Immunol. 2000;165(1):211–220. doi: 10.4049/jimmunol.165.1.211. [DOI] [PubMed] [Google Scholar]
- 29.Godot V, Arock M, Garcia G, Capel F, Flys C, Dy M, et al. H4 histamine receptor mediates optimal migration of mast cell precursors to CXCL12. J Allergy Clin Immunol. 2007;120(4):827–834. doi: 10.1016/j.jaci.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 30.Macey MR, Sturgill JL, Morales JK, Falanga YT, Morales J, Norton SK, et al. IL-4 and TGF-beta 1 counterbalance one another while regulating mast cell homeostasis. J Immunol. 2010;184(9):4688–4695. doi: 10.4049/jimmunol.0903477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda H, Kannan Y, Ushio H, Kiso Y, Kanemoto T, Suzuki H, et al. Nerve growth factor induces development of connective tissue-type mast cells in vitro from murine bone marrow cells. J Exp Med. 1991;174(1):7–14. doi: 10.1084/jem.174.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruitenberg EJ, Elgersma A. Absence of intestinal mast cell response in congenitally athymic mice during Trichinella spiralis infection. Nature. 1976;264(5583):258–260. doi: 10.1038/264258a0. [DOI] [PubMed] [Google Scholar]
- 33.Friend DS, Ghildyal N, Austen KF, Gurish MF, Matsumoto R, Stevens RL. Mast cells that reside at different locations in the jejunum of mice infected with Trichinella spiralis exhibit sequential changes in their granule ultrastructure and chymase phenotype. J Cell Biol. 1996;135(1):279–290. doi: 10.1083/jcb.135.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friend DS, Ghildyal N, Gurish MF, Hunt J, Hu X, Austen KF, et al. Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J Immunol. 1998;160(11):5537–5545. [PubMed] [Google Scholar]
- 35.Hunt JE, Stevens RL, Austen KF, Zhang J, Xia Z, Ghildyal N. Natural disruption of the mouse mast cell protease 7 gene in the C57BL/6 mouse. J Biol Chem. 1996;271(5):2851–2855. doi: 10.1074/jbc.271.5.2851. [DOI] [PubMed] [Google Scholar]
- 36.Jippo T, Lee YM, Ge Y, Kim DK, Okabe M, Kitamura Y. Tissue-dependent alteration of protease expression phenotype in murine peritoneal mast cells that were genetically labeled with green fluorescent protein. Am J Pathol. 2001;158(5):1695–1701. doi: 10.1016/S0002-9440(10)64125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12(11):1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irani AM, Bradford TR, Kepley CL, Schechter NM, Schwartz LB. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37(10):1509–1515. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Meng XW, Krilis SA. Mast cells expressing chymase but not tryptase can be derived by culturing human progenitors in conditioned medium obtained from a human mastocytosis cell strain with c-kit ligand. J Immunol. 1996;156(12):4839–4844. [PubMed] [Google Scholar]
- 40.Weidner N, Austen KF. Heterogeneity of mast cells at multiple body sites. Fluorescent determination of avidin binding and immunofluorescent determination of chymase, tryptase, and carboxypeptidase content. Pathol Res Pract. 1993;189(2):156–162. doi: 10.1016/S0344-0338(11)80086-5. [DOI] [PubMed] [Google Scholar]
- 41.Irani AM, Craig SS, DeBlois G, Elson CO, Schechter NM, Schwartz LB. Deficiency of the tryptase-positive, chymase-negative mast cell type in gastrointestinal mucosa of patients with defective T lymphocyte function. J Immunol. 1987;138(12):4381–4386. [PubMed] [Google Scholar]
- 42.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matin R, Tam EK, Nadel JA, Caughey GH. Distribution of chymase-containing mast cells in human bronchi. J Histochem Cytochem. 1992;40(6):781–786. doi: 10.1177/40.6.1588024. [DOI] [PubMed] [Google Scholar]
- 44.Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. 2009;31(2):185–205. doi: 10.1007/s00281-009-0165-4. [DOI] [PubMed] [Google Scholar]
- 45.Bischoff SC, Wedemeyer J, Herrmann A, Meier PN, Trautwein C, Cetin Y, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28(1):1–13. doi: 10.1046/j.1365-2559.1996.262309.x. [DOI] [PubMed] [Google Scholar]
- 46.Raithel M, Winterkamp S, Pacurar A, Ulrich P, Hochberger J, Hahn EG. Release of mast cell tryptase from human colorectal mucosa in inflammatory bowel disease. Scand J Gastroenterol. 2001;36(2):174–179. doi: 10.1080/003655201750065933. [DOI] [PubMed] [Google Scholar]
- 47.Cho EY, Choi SC, Lee SH, Ahn JY, Im LR, Kim JH, et al. Nafamostat mesilate attenuates colonic inflammation and mast cell infiltration in the experimental colitis. Int Immunopharmacol. 2011;11(4):412–417. doi: 10.1016/j.intimp.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, et al. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112(11):1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagel AF, deRossi T, Zopf Y, Konturek P, Dauth W, Kressel J, et al. Mast cell tryptase levels in gut mucosa in patients with gastrointestinal symptoms caused by food allergy. Int Arch Allergy Immunol. 2013;160(4):350–355. doi: 10.1159/000341634. [DOI] [PubMed] [Google Scholar]
- 50.Betts CJ, Else KJ. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21(1):45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 51.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392(6671):90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 52.Marone G, Triggiani M, de Paulis A. Mast cells and basophils: friends as well as foes in bronchial asthma? Trends Immunol. 2005;26(1):25–31. doi: 10.1016/j.it.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 54.Brightling CE, Symon FA, Birring SS, Bradding P, Wardlaw AJ, Pavord ID. Comparison of airway immunopathology of eosinophilic bronchitis and asthma. Thorax. 2003;58(6):528–532. doi: 10.1136/thorax.58.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu M, Tsai M, Tam SY, Jones C, Zehnder J, Galli SJ. Mast cells can promote the development of multiple features of chronic asthma in mice. J Clin Invest. 2006;116(6):1633–1641. doi: 10.1172/JCI25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu M, Eckart MR, Morgan AA, Mukai K, Butte AJ, Tsai M, et al. Identification of an IFN-γ/mast cell axis in a mouse model of chronic asthma. J Clin Invest. 2011;121(8):3133–3143. doi: 10.1172/JCI43598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawanami O, Ferrans VJ, Fulmer JD, Crystal RG. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest. 1979;40(6):717–734. [PubMed] [Google Scholar]
- 58.Edwards ST, Cruz AC, Donnelly S, Dazin PF, Schulman ES, Jones KD, et al. c-Kit immunophenotyping and metalloproteinase expression profiles of mast cells in interstitial lung diseases. J Pathol. 2005;206(3):279–290. doi: 10.1002/path.1780. [DOI] [PubMed] [Google Scholar]
- 59.Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, et al. CD19 regulates skin and lung fibrosis via Toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am J Pathol. 2008;172(6):1650–1663. doi: 10.2353/ajpath.2008.071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reber LL, Daubeuf F, Pejler G, Abrink M, Frossard N. Mast cells contribute to bleomycin-induced lung inflammation and injury in mice through a chymase/mast cell protease 4-dependent mechanism. J Immunol. 2014;192(4):1847–1854. doi: 10.4049/jimmunol.1300875. [DOI] [PubMed] [Google Scholar]
- 61.Sonoda T, Kanayama Y, Hara H, Hayashi C, Tadokoro M, Yonezawa T, et al. Proliferation of peritoneal mast cells in the skin of W/Wv mice that genetically lack mast cells. J Exp Med. 1984;160(1):138–151. doi: 10.1084/jem.160.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanakura Y, Thompson H, Nakano T, Yamamura T, Asai H, Kitamura Y, et al. Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood. 1988;72(3):877–885. [PubMed] [Google Scholar]
- 63.Tsai M, Shih LS, Newlands GF, Takeishi T, Langley KE, Zsebo KM, et al. The rat c-kit ligand, stem cell factor, induces the development of connective tissue-type and mucosal mast cells in vivo. Analysis by anatomical distribution, histochemistry, and protease phenotype. J Exp Med. 1991;174(1):125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dvorak AM, Mihm MC, Jr, Dvorak HF. Morphology of delayed-type hypersensitivity reactions in man. II. Ultrastructural alterations affecting the microvasculature and the tissue mast cells. Lab Invest. 1976;34(2):179–191. [PubMed] [Google Scholar]
- 65.Dahlin JS, Hallgren J. Mast cell progenitors: Origin, development and migration to tissues. Mol Immunol. 2014 doi: 10.1016/j.molimm.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 66.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102(50):18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, et al. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d-restricted NKT cells. J Immunol. 2009;183(8):5251–5260. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abramson J, Pecht I. Regulation of the mast cell response to the type 1 Fcε receptor. Immunol Rev. 2007;217:231–254. doi: 10.1111/j.1600-065X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 69.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reber LL, Frossard N. Targeting mast cells in inflammatory diseases. Pharmacol Ther. 2014;142(3):416–435. doi: 10.1016/j.pharmthera.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 71.Turner H, Kinet JP. Signalling through the high-affinity IgE receptor FcεRI. Nature. 1999;402(6760 Suppl):B24–30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 72.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng LE, Hartmann K, Roers A, Krummel MF, Locksley RM. Perivascular mast cells dynamically probe cutaneous blood vessels to capture immunoglobulin E. Immunity. 2013;38(1):166–175. doi: 10.1016/j.immuni.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suzuki R, Leach S, Liu W, Ralston E, Scheffel J, Zhang W, et al. Molecular editing of cellular responses by the high-affinity receptor for IgE. Science. 2014;343(6174):1021–1025. doi: 10.1126/science.1246976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, et al. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39(5):963–975. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39(5):976–985. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484(7395):465–472. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66(1):23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 79.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10(6):440–452. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, et al. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109(10):1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, et al. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313(5786):526–530. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 82.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, et al. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121(10):4180–4191. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204(11):2629–2639. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128(1):36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schafer B, Piliponsky AM, Oka T, Song CH, Gerard NP, Gerard C, et al. Mast cell anaphylatoxin receptor expression can enhance IgE-dependent skin inflammation in mice. J Allergy Clin Immunol. 2012 doi: 10.1016/j.jaci.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, et al. Activation of human mast cells through the platelet-activating factor receptor. J Allergy Clin Immunol. 2010;125(5):1137–1145. e1136. doi: 10.1016/j.jaci.2010.01.056. [DOI] [PubMed] [Google Scholar]
- 87.Metz M, Gilles S, Geldmacher A, Behrendt H, Traidl-Hoffmann C, Maurer M. Evidence for non-allergic mast cell activation in pollen-associated inflammation. J Invest Dermatol. 2011;131(4):987–990. doi: 10.1038/jid.2010.419. [DOI] [PubMed] [Google Scholar]
- 88.Cirino G, Cicala C, Bucci M, Sorrentino L, Ambrosini G, DeDominicis G, et al. Factor Xa as an interface between coagulation and inflammation. Molecular mimicry of factor Xa association with effector cell protease receptor-1 induces acute inflammation in vivo. J Clin Invest. 1997;99(10):2446–2451. doi: 10.1172/JCI119428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183(3):821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186(4):2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- 91.Lunderius-Andersson C, Enoksson M, Nilsson G. Mast cells respond to cell injury through the recognition of IL-33. Front Immunol. 2012;3:82. doi: 10.3389/fimmu.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaur D, Doe C, Woodman L, Wan H, Sutcliffe A, Hollins F, et al. Mast cell-airway smooth muscle crosstalk: the role of thymic stromal lymphopoietin. Chest. 2011 doi: 10.1378/chest.11-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Y, Tung H-Y, Tsai Y-M, Hsu S-C, Chang H-W, Kawasaki H, et al. Aryl hydrocarbon receptor controls murine mast cell homeostasis. Blood. 2013;121(16):3195–3204. doi: 10.1182/blood-2012-08-453597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sibilano R, Frossi B, Calvaruso M, Danelli L, Betto E, Dall’agnese A, et al. The aryl hydrocarbon receptor modulates acute and late mast cell responses. J Immunol. 2012 doi: 10.4049/jimmunol.1200009. [DOI] [PubMed] [Google Scholar]
- 95.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the FcεRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99(7):1492–1499. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakae S, Suto H, Iikura M, Kakurai M, Sedgwick JD, Tsai M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol. 2006;176(4):2238–2248. doi: 10.4049/jimmunol.176.4.2238. [DOI] [PubMed] [Google Scholar]
- 97.Kashiwakura J, Yokoi H, Saito H, Okayama Y. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol. 2004;173(8):5247–5257. doi: 10.4049/jimmunol.173.8.5247. [DOI] [PubMed] [Google Scholar]
- 98.Sibilano R, Frossi B, Suzuki R, D’Inca F, Gri G, Piconese S, et al. Modulation of FcεRI-dependent mast cell response by OX40L via Fyn, PI3K, and RhoA. J Allergy Clin Immunol. 2012;130(3):751–760. e752. doi: 10.1016/j.jaci.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 99.Lee H, Kashiwakura J, Matsuda A, Watanabe Y, Sakamoto-Sasaki T, Matsumoto K, et al. Activation of human synovial mast cells from rheumatoid arthritis or osteoarthritis patients in response to aggregated IgG through Fcγ receptor I and Fcγ receptor II. Arthritis Rheum. 2013;65(1):109–119. doi: 10.1002/art.37741. [DOI] [PubMed] [Google Scholar]
- 100.Malbec O, Daeron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev. 2007;217:206–221. doi: 10.1111/j.1600-065X.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 101.Fantozzi R, Brunelleschi S, Giuliattini L, Blandina P, Masini E, Cavallo G, et al. Mast cell and neutrophil interactions: a role for superoxide anion and histamine. Agents Actions. 1985;16(3–4):260–264. doi: 10.1007/BF01983155. [DOI] [PubMed] [Google Scholar]
- 102.Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. 2008;29(5):771–781. doi: 10.1016/j.immuni.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114(24):4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 104.Karra L, Levi-Schaffer F. Down-regulation of mast cell responses through ITIM containing inhibitory receptors. Adv Exp Med Biol. 2011;716:143–159. doi: 10.1007/978-1-4419-9533-9_9. [DOI] [PubMed] [Google Scholar]
- 105.Katz HR. Inhibitory receptors and allergy. Curr Opin Immunol. 2002;14(6):698–704. doi: 10.1016/s0952-7915(02)00400-4. [DOI] [PubMed] [Google Scholar]
- 106.Ott VL, Cambier JC. Activating and inhibitory signaling in mast cells: new opportunities for therapeutic intervention? J Allergy Clin Immunol. 2000;106(3):429–440. doi: 10.1067/mai.2000.109428. [DOI] [PubMed] [Google Scholar]
- 107.Hill PB, MacDonald AJ, Thornton EM, Newlands GF, Galli SJ, Miller HR. Stem cell factor enhances immunoglobulin E-dependent mediator release from cultured rat bone marrow-derived mast cells: activation of previously unresponsive cells demonstrated by a novel ELISPOT assay. Immunology. 1996;87(2):326–333. doi: 10.1046/j.1365-2567.1996.455545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, et al. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188(11):5428–5437. doi: 10.4049/jimmunol.1103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy. 2012;67(9):1118–1126. doi: 10.1111/j.1398-9995.2012.02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okayama Y, Kirshenbaum AS, Metcalfe DD. Expression of a functional high-affinity IgG receptor, FcγRI, on human mast cells: Up-regulation by IFN-γ. J Immunol. 2000;164(8):4332–4339. doi: 10.4049/jimmunol.164.8.4332. [DOI] [PubMed] [Google Scholar]
- 111.Riley JF. Histamine in tissue mast cells. Science. 1953;118(3064):332. doi: 10.1126/science.118.3064.332. [DOI] [PubMed] [Google Scholar]
- 112.Razin E, Mencia-Huerta JM, Stevens RL, Lewis RA, Liu FT, Corey E, et al. IgE-mediated release of leukotriene C4, chondroitin sulfate E proteoglycan, β-hexosaminidase, and histamine from cultured bone marrow-derived mouse mast cells. J Exp Med. 1983;157(1):189–201. doi: 10.1084/jem.157.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Windelborg Nielsen B, Engberg TM, Herlin T, Bjerke T, Schiotz PO. Histamine release from cord blood basophils. Int Arch Allergy Appl Immunol. 1990;93(4):314–322. doi: 10.1159/000235260. [DOI] [PubMed] [Google Scholar]
- 114.Ghosh AK, Hirasawa N, Ohtsu H, Watanabe T, Ohuchi K. Defective angiogenesis in the inflammatory granulation tissue in histidine decarboxylase-deficient mice but not in mast cell-deficient mice. The Journal of experimental medicine. 2002;195(8):973–982. doi: 10.1084/jem.20011782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, et al. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. The Journal of experimental medicine. 2006;203(13):2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caughey GH. Mast cell proteases as protective and inflammatory mediators. Adv Exp Med Biol. 2011;716:212–234. doi: 10.1007/978-1-4419-9533-9_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pejler G, Ronnberg E, Waern I, Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115(24):4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 118.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast cell proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 119.Huang C, Friend DS, Qiu WT, Wong GW, Morales G, Hunt J, et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J Immunol. 1998;160(4):1910–1919. [PubMed] [Google Scholar]
- 120.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1β and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161(4):1939–1946. [PubMed] [Google Scholar]
- 121.Algermissen B, Laubscher JC, Bauer F, Henz BM. Purification of mast cell proteases from murine skin. Exp Dermatol. 1999;8(5):413–418. doi: 10.1111/j.1600-0625.1999.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 122.Compton SJ, Cairns JA, Holgate ST, Walls AF. Human mast cell tryptase stimulates the release of an IL-8-dependent neutrophil chemotactic activity from human umbilical vein endothelial cells (HUVEC) Clin Exp Immunol. 2000;121(1):31–36. doi: 10.1046/j.1365-2249.2000.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gallwitz M, Reimer JM, Hellman L. Expansion of the mast cell chymase locus over the past 200 million years of mammalian evolution. Immunogenetics. 2006;58(8):655–669. doi: 10.1007/s00251-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 124.Tchougounova E, Pejler G, Abrink M. The chymase, mouse mast cell protease 4, constitutes the major chymotrypsin-like activity in peritoneum and ear tissue. A role for mouse mast cell protease 4 in thrombin regulation and fibronectin turnover. The Journal of experimental medicine. 2003;198(3):423–431. doi: 10.1084/jem.20030671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Andersson MK, Karlson U, Hellman L. The extended cleavage specificity of the rodent β-chymases rMCP-1 and mMCP-4 reveal major functional similarities to the human mast cell chymase. Mol Immunol. 2008;45(3):766–775. doi: 10.1016/j.molimm.2007.06.360. [DOI] [PubMed] [Google Scholar]
- 126.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factorα, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci U S A. 1991;88(10):4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-α, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. 1998;98(7):699–710. doi: 10.1161/01.cir.98.7.699. [DOI] [PubMed] [Google Scholar]
- 128.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature. 1990;346(6281):274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 129.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, et al. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206(11):2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Austen KF. The mast cell and the cysteinyl leukotrienes. Novartis Found Symp. 2005;271:166–175. doi: 10.1002/9780470033449.ch13. discussion 176–168, 198–169. [DOI] [PubMed] [Google Scholar]
- 131.Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev. 2007;217:168–185. doi: 10.1111/j.1600-065X.2007.00512.x. [DOI] [PubMed] [Google Scholar]
- 132.Dreskin SC, Abraham SN. Production of TNF-α by murine bone marrow derived mast cells activated by the bacterial fimbrial protein, FimH. Clin Immunol. 1999;90(3):420–424. doi: 10.1006/clim.1998.4657. [DOI] [PubMed] [Google Scholar]
- 133.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381(6577):77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 134.Gordon JR, Galli SJ. Promotion of mouse fibroblast collagen gene expression by mast cells stimulated via the FcεRI. Role for mast cell-derived transforming growth factor β and tumor necrosis factor α. J Exp Med. 1994;180(6):2027–2037. doi: 10.1084/jem.180.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, et al. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J Exp Med. 1989;170(1):245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hagaman DD, Okayama Y, D’Ambrosio C, Prussin C, Gilfillan AM, Metcalfe DD. Secretion of interleukin-1 receptor antagonist from human mast cells after immunoglobulin E-mediated activation and after segmental antigen challenge. Am J Respir Cell Mol Biol. 2001;25(6):685–691. doi: 10.1165/ajrcmb.25.6.4541. [DOI] [PubMed] [Google Scholar]
- 137.Lin TJ, Garduno R, Boudreau RT, Issekutz AC. Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 α and β. J Immunol. 2002;169(8):4522–4530. doi: 10.4049/jimmunol.169.8.4522. [DOI] [PubMed] [Google Scholar]
- 138.Oldford SA, Haidl ID, Howatt MA, Leiva CA, Johnston B, Marshall JS. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol. 2010;185(11):7067–7076. doi: 10.4049/jimmunol.1001137. [DOI] [PubMed] [Google Scholar]
- 139.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15(8):940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishizuka T, Okayama Y, Kobayashi H, Mori M. Interleukin-10 is localized to and released by human lung mast cells. Clin Exp Allergy. 1999;29(10):1424–1432. doi: 10.1046/j.1365-2222.1999.00636.x. [DOI] [PubMed] [Google Scholar]
- 141.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8(10):1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 142.Song C, Zhang Q, Liu X, Shan Y. IL-12 and IL-10 production are differentially regulated by phosphatidylinositol 3-kinase in mast cells. Scand J Immunol. 2012;75(3):266–272. doi: 10.1111/j.1365-3083.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 143.Mrabet-Dahbi S, Metz M, Dudeck A, Zuberbier T, Maurer M. Murine mast cells secrete a unique profile of cytokines and prostaglandins in response to distinct TLR2 ligands. Exp Dermatol. 2009;18(5):437–444. doi: 10.1111/j.1600-0625.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 144.Buckland J. New role for mast cells as IL-17-expressing effector cells in established RA. Nat Rev Rheumatol. 2010;6(5):243. doi: 10.1038/nrrheum.2010.50. [DOI] [PubMed] [Google Scholar]
- 145.Hueber AJ, Asquith DL, Miller AM, Reilly J, Kerr S, Leipe J, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184(7):3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 146.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, et al. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of Fcε receptor I expression. J Exp Med. 1998;188(6):1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Grützkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9(4):875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123(5):1142–1149. 1149 e1141–1145. doi: 10.1016/j.jaci.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 149.Zhang S, Anderson DF, Bradding P, Coward WR, Baddeley SM, MacLeod JD, et al. Human mast cells express stem cell factor. J Pathol. 1998;186(1):59–66. doi: 10.1002/(SICI)1096-9896(199809)186:1<59::AID-PATH140>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 150.de Paulis A, Minopoli G, Arbustini E, de Crescenzo G, Dal Piaz F, Pucci P, et al. Stem cell factor is localized in, released from, and cleaved by human mast cells. J Immunol. 1999;163(5):2799–2808. [PubMed] [Google Scholar]
- 151.Sibilano R, Frossi B, Pucillo CE. Mast cell activation: A complex interplay of positive and negative signaling pathways. Eur J Immunol. 2014 doi: 10.1002/eji.201444546. [DOI] [PubMed] [Google Scholar]
- 152.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33(12):613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Oka T, Kalesnikoff J, Starkl P, Tsai M, Galli SJ. Evidence questioning cromolyn’s effectiveness and selectivity as a ‘mast cell stabilizer’ in mice. Lab Invest. 2012 doi: 10.1038/labinvest.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19(1):31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 155.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167(3):835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18(6):751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 157.Zhou JS, Xing W, Friend DS, Austen KF, Katz HR. Mast cell deficiency in Kit(W-sh mice does not impair antibody-mediated arthritis. The Journal of experimental medicine. 2007;204(12):2797–2802. doi: 10.1084/jem.20071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am J Pathol. 2010;176(2):926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, et al. Genetic inversion in mast cell-deficient Wsh mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173(6):1693–1701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/Wv. J Cell Physiol. 1969;73(1):25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- 161.Nagle DL, Kozak CA, Mano H, Chapman VM, Bucan M. Physical mapping of the Tec and Gabrb1 loci reveals that the Wsh mutation on mouse chromosome 5 is associated with an inversion. Hum Mol Genet. 1995;4(11):2073–2079. doi: 10.1093/hmg/4.11.2073. [DOI] [PubMed] [Google Scholar]
- 162.Lyon MF, Glenister PH. A new allele sash (Wsh) at the W-locus and a spontaneous recessive lethal in mice. Genet Res. 1982;39(3):315–322. doi: 10.1017/s001667230002098x. [DOI] [PubMed] [Google Scholar]
- 163.Wolters PJ, Mallen-St Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, et al. Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient KitW-sh/KitW-sh sash mice. Clin Exp Allergy. 2005;35(1):82–88. doi: 10.1111/j.1365-2222.2005.02136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373(6512):347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 165.Nakano T, Sonoda T, Hayashi C, Yamatodani A, Kanayama Y, Yamamura T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Galli SJ, Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld miceTheir value for the analysis of the roles of mast cells in biologic responsesin vivo. Am J Pathol. 1987;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- 167.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, et al. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118(26):6930–6938. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dudeck A, Dudeck J, Scholten J, Petzold A, Surianarayanan S, Kohler A, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34(6):973–984. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 169.Scholten J, Hartmann K, Gerbaulet A, Krieg T, Muller W, Testa G, et al. Mast cell-specific Cre/loxP-mediated recombination in vivo. Transgenic Res. 2008;17(2):307–315. doi: 10.1007/s11248-007-9153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Musch W, Wege AK, Mannel DN, Hehlgans T. Generation and characterization of alpha-chymase-Cre transgenic mice. Genesis. 2008;46(3):163–166. doi: 10.1002/dvg.20378. [DOI] [PubMed] [Google Scholar]
- 171.Otsuka A, Kubo M, Honda T, Egawa G, Nakajima S, Tanizaki H, et al. Requirement of interaction between mast cells and skin dendritic cells to establish contact hypersensitivity. PLoS One. 2011;6(9):e25538. doi: 10.1371/journal.pone.0025538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Feyerabend TB, Weiser A, Tietz A, Stassen M, Harris N, Kopf M, et al. Cre-Mediated Cell Ablation Contests Mast Cell Contribution in Models of Antibody- and T Cell-Mediated Autoimmunity. Immunity. 2011;35(5):832–844. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 173.Furumoto Y, Charles N, Olivera A, Leung WH, Dillahunt S, Sargent JL, et al. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood. 2011;118(20):5466–5475. doi: 10.1182/blood-2010-09-309955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37(1):13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 175.Galli SJ, Tsai M, Marichal T, Chugunova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. doi: 10.1016/bs.ai.2014.11.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014 doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 177.Groschwitz KR, Ahrens R, Osterfeld H, Gurish MF, Han X, Abrink M, et al. Mast cells regulate homeostatic intestinal epithelial migration and barrier function by a chymase/Mcpt4-dependent mechanism. Proc Natl Acad Sci U S A. 2009;106(52):22381–22386. doi: 10.1073/pnas.0906372106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52(3):439–451. doi: 10.1136/gut.52.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Perdue MH, Masson S, Wershil BK, Galli SJ. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991;87(2):687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. quiz 21–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100(13):7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- 183.Kapur R, Cooper R, Xiao X, Weiss MJ, Donovan P, Williams DA. The presence of novel amino acids in the cytoplasmic domain of stem cell factor results in hematopoietic defects in Steel17H mice. Blood. 1999;94(6):1915–1925. [PubMed] [Google Scholar]
- 184.Wang L, Stanisz AM, Wershil BK, Galli SJ, Perdue MH. Substance P induces ion secretion in mouse small intestine through effects on enteric nerves and mast cells. Am J Physiol. 1995;269(1 Pt 1):G85–92. doi: 10.1152/ajpgi.1995.269.1.G85. [DOI] [PubMed] [Google Scholar]
- 185.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma. From bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. doi: 10.1164/ajrccm.161.5.9903102. [DOI] [PubMed] [Google Scholar]
- 186.Fish JE, Peters SP. Airway remodeling and persistent airway obstruction in asthma. The Journal of allergy and clinical immunology. 1999;104(3 Pt 1):509–516. doi: 10.1016/s0091-6749(99)70315-5. [DOI] [PubMed] [Google Scholar]
- 187.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 188.Busse WW, Lemanske RF. Asthma. N Engl J Med. 2001;344(5):350–362. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 189.Holt PG, Sly PD. Viral infections and atopy in asthma pathogenesis: new rationales for asthma prevention and treatment. Nat Med. 2012;18(5):726–735. doi: 10.1038/nm.2768. [DOI] [PubMed] [Google Scholar]
- 190.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 191.Slager RE, Hawkins GA, Li X, Postma DS, Meyers DA, Bleecker ER. Genetics of asthma susceptibility and severity. Clin Chest Med. 2012;33(3):431–443. doi: 10.1016/j.ccm.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Meyers DA, Bleecker ER, Holloway JW, Holgate ST. Asthma genetics and personalised medicine. Lancet Respir Med. 2014;2(5):405–415. doi: 10.1016/S2213-2600(14)70012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, et al. Accumulation of intraepithelial mast cells with a unique protease phenotype in TH2-high asthma. The Journal of allergy and clinical immunology. 2010;125(5):1046–1053. e1048. doi: 10.1016/j.jaci.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Balzar S, Fajt ML, Comhair SAA, Erzurum SC, Bleecker E, Busse WW, et al. Mast cell phenotype, location, and activation in severe asthma. Data from the Severe Asthma Research Program. Am J Respir Crit Care Med. 2011;183(3):299–309. doi: 10.1164/rccm.201002-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Carroll NG, Mutavdzic S, James AL. Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur Respir J. 2002;19(5):879–885. doi: 10.1183/09031936.02.00275802. [DOI] [PubMed] [Google Scholar]
- 196.Bradley BL, Azzawi M, Jacobson M, Assoufi B, Collins JV, Irani AM, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. The Journal of allergy and clinical immunology. 1991;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 197.Liesker JJW, Ten Hacken NHT, Rutgers SR, Zeinstra-Smith M, Postma DS, Timens W. Mast cell numbers in airway smooth muscle and PC20AMP in asthma and COPD. Respir Med. 2007;101(5):882–887. doi: 10.1016/j.rmed.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 198.Lamb D, Lumsden A. Intra-epithelial mast cells in human airway epithelium: evidence for smoking-induced changes in their frequency. Thorax. 1982;37(5):334–342. doi: 10.1136/thx.37.5.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy. 1999;29(Suppl 2):90–95. doi: 10.1046/j.1365-2222.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 200.Wilson JW, Bamford TL. Assessing the evidence for remodelling of the airway in asthma. Pulm Pharmacol Ther. 2001;14(3):229–247. doi: 10.1006/pupt.2001.0294. [DOI] [PubMed] [Google Scholar]
- 201.Altin J, Shen C, Liston A. Understanding the genetic regulation of IgE production. Blood Rev. 2010;24(4–5):163–169. doi: 10.1016/j.blre.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 202.Takhar P, Corrigan CJ, Smurthwaite L, O’Connor BJ, Durham SR, Lee TH, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. The Journal of allergy and clinical immunology. 2007;119(1):213–218. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 203.Williams CM, Galli SJ. Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J Exp Med. 2000;192(3):455–462. doi: 10.1084/jem.192.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Taube C, Wei X, Swasey CH, Joetham A, Zarini S, Lively T, et al. Mast cells, FcεRI, and IL-13 are required for development of airway hyperresponsiveness after aerosolized allergen exposure in the absence of adjuvant. J Immunol. 2004;172(10):6398–6406. doi: 10.4049/jimmunol.172.10.6398. [DOI] [PubMed] [Google Scholar]
- 205.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120(1):48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 206.Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31(4):773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 207.Kung TT, Stelts D, Zurcher JA, Jones H, Umland SP, Kreutner W, et al. Mast cells modulate allergic pulmonary eosinophilia in mice. Am J Respir Cell Mol Biol. 1995;12(4):404–409. doi: 10.1165/ajrcmb.12.4.7695919. [DOI] [PubMed] [Google Scholar]
- 208.Kobayashi T, Miura T, Haba T, Sato M, Serizawa I, Nagai H, et al. An essential role of mast cells in the development of airway hyperresponsiveness in a murine asthma model. J Immunol. 2000;164(7):3855–3861. doi: 10.4049/jimmunol.164.7.3855. [DOI] [PubMed] [Google Scholar]
- 209.Nogami M, Suko M, Okudaira H, Miyamoto T, Shiga J, Ito M, et al. Experimental pulmonary eosinophilia in mice by Ascaris suum extract. Am Rev Respir Dis. 1990;141(5 Pt 1):1289–1295. doi: 10.1164/ajrccm/141.5_Pt_1.1289. [DOI] [PubMed] [Google Scholar]
- 210.Okudaira H, Nogami M, Matsuzaki G, Dohi M, Suko M, Kasuya S, et al. T-cell-dependent accumulation of eosinophils in the lung and its inhibition by monoclonal anti-interleukin-5. Int Arch Allergy Appl Immunol. 1991;94(1–4):171–173. doi: 10.1159/000235354. [DOI] [PubMed] [Google Scholar]
- 211.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, et al. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24(1):73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 212.Takeda K, Hamelmann E, Joetham A, Shultz LD, Larsen GL, Irvin CG, et al. Development of eosinophilic airway inflammation and airway hyperresponsiveness in mast cell-deficient mice. J Exp Med. 1997;186(3):449–454. doi: 10.1084/jem.186.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Becker M, Reuter S, Friedrich P, Doener F, Michel A, Bopp T, et al. Genetic variation determines mast cell functions in experimental asthma. J Immunol. 2011;186(12):7225–7231. doi: 10.4049/jimmunol.1100676. [DOI] [PubMed] [Google Scholar]
- 214.Yang M, Kumar RK, Foster PS. Pathogenesis of steroid-resistant airway hyperresponsiveness: interaction between IFN-γ and TLR4/MyD88 pathways. J Immunol. 2009;182(8):5107–5115. doi: 10.4049/jimmunol.0803468. [DOI] [PubMed] [Google Scholar]
- 215.Li JJ, Wang W, Baines KJ, Bowden NA, Hansbro PM, Gibson PG, et al. IL-27/IFN-γ induce MyD88-dependent steroid-resistant airway hyperresponsiveness by inhibiting glucocorticoid signaling in macrophages. J Immunol. 2010;185(7):4401–4409. doi: 10.4049/jimmunol.1001039. [DOI] [PubMed] [Google Scholar]
- 216.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5(7):521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 217.Bullens DMA, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 219.Tan BB, Weald D, Strickland I, Friedmann PS. Double-blind controlled trial of effect of housedust-mite allergen avoidance on atopic dermatitis. Lancet. 1996;347(8993):15–18. doi: 10.1016/s0140-6736(96)91556-1. [DOI] [PubMed] [Google Scholar]
- 220.He R, Oyoshi MK, Jin H, Geha RS. Epicutaneous antigen exposure induces a Th17 response that drives airway inflammation after inhalation challenge. Proc Natl Acad Sci USA. 2007;104(40):15817–15822. doi: 10.1073/pnas.0706942104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, et al. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol. 2003;28(1):42–50. doi: 10.1165/rcmb.4832. [DOI] [PubMed] [Google Scholar]
- 222.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suurmond J, Dorjee AL, Boon MR, Knol EF, Huizinga TW, Toes RE, et al. Mast cells are the main interleukin 17-positive cells in anticitrullinated protein antibody-positive and -negative rheumatoid arthritis and osteoarthritis synovium. Arthritis Res Ther. 2011;13(5):R150. doi: 10.1186/ar3466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, et al. Increased IL-33 expression by epithelial cells in bronchial asthma. The Journal of allergy and clinical immunology. 2010;125(3):752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 225.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183(8):5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 226.Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet. 2009;41(3):342–347. doi: 10.1038/ng.323. [DOI] [PubMed] [Google Scholar]
- 227.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113(7):1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185(10):5743–5750. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 231.Junttila IS, Watson C, Kummola L, Chen X, Hu-Li J, Guo L, et al. Efficient cytokine-induced IL-13 production by mast cells requires both IL-33 and IL-3. The Journal of allergy and clinical immunology. 2013;132(3):704–712. e710. doi: 10.1016/j.jaci.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10(2):103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 233.Jung M-Y, Smrž D, Desai A, Bandara G, Ito T, Iwaki S, et al. IL-33 induces a hyporesponsive phenotype in human and mouse mast cells. J Immunol. 2013;190(2):531–538. doi: 10.4049/jimmunol.1201576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Waern I, Jonasson S, Hjoberg J, Bucht A, Abrink M, Pejler G, et al. Mouse mast cell protease 4 is the major chymase in murine airways and has a protective role in allergic airway inflammation. J Immunol. 2009;183(10):6369–6376. doi: 10.4049/jimmunol.0900180. [DOI] [PubMed] [Google Scholar]
- 235.Waern I, Lundequist A, Pejler G, Wernersson S. Mast cell chymase modulates IL-33 levels and controls allergic sensitization in dust-mite induced airway inflammation. Mucosal Immunol. 2013;6(5):911–920. doi: 10.1038/mi.2012.129. [DOI] [PubMed] [Google Scholar]
- 236.Sicherer SH, Sampson HA. Food allergy: recent advances in pathophysiology and treatment. Annu Rev Med. 2009;60:261–277. doi: 10.1146/annurev.med.60.042407.205711. [DOI] [PubMed] [Google Scholar]
- 237.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 238.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 239.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120(3):506–515. doi: 10.1016/j.jaci.2007.07.033. quiz 516–507. [DOI] [PubMed] [Google Scholar]
- 240.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 241.Jonsson F, Mancardi DA, Kita Y, Karasuyama H, Iannascoli B, Van Rooijen N, et al. Mouse and human neutrophils induce anaphylaxis. J Clin Invest. 2011;121(4):1484–1496. doi: 10.1172/JCI45232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sun J, Arias K, Alvarez D, Fattouh R, Walker T, Goncharova S, et al. Impact of CD40 ligand, B cells, and mast cells in peanut-induced anaphylactic responses. J Immunol. 2007;179(10):6696–6703. doi: 10.4049/jimmunol.179.10.6696. [DOI] [PubMed] [Google Scholar]
- 243.Reber LL, Marichal T, Mukai K, Kita Y, Tokuoka SM, Roers A, et al. Selective ablation of mast cells or basophils reduces peanut-induced anaphylaxis in mice. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Arias K, Chu DK, Flader K, Botelho F, Walker T, Arias N, et al. Distinct immune effector pathways contribute to the full expression of peanut-induced anaphylactic reactions in mice. J Allergy Clin Immunol. 2011;127(6):1552–1561. e1551. doi: 10.1016/j.jaci.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 245.Smit JJ, Willemsen K, Hassing I, Fiechter D, Storm G, van Bloois L, et al. Contribution of classic and alternative effector pathways in peanut-induced anaphylactic responses. PLoS One. 2011;6(12):e28917. doi: 10.1371/journal.pone.0028917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Khodoun M, Strait R, Orekov T, Hogan S, Karasuyama H, Herbert DR, et al. Peanuts can contribute to anaphylactic shock by activating complement. J Allergy Clin Immunol. 2009;123(2):342–351. doi: 10.1016/j.jaci.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ghosh S, Hoselton SA, Schuh JM. μ-chain-deficient mice possess B-1 cells and produce IgG and IgE, but not IgA, following systemic sensitization and inhalational challenge in a fungal asthma model. J Immunol. 2012;189(3):1322–1329. doi: 10.4049/jimmunol.1200138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Orinska Z, Osiak A, Lohler J, Bulanova E, Budagian V, Horak I, et al. Novel B cell population producing functional IgG in the absence of membrane IgM expression. Eur J Immunol. 2002;32(12):3472–3480. doi: 10.1002/1521-4141(200212)32:12<3472::AID-IMMU3472>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 249.Perona-Wright G, Mohrs K, Taylor J, Zaph C, Artis D, Pearce EJ, et al. Cutting edge: Helminth infection induces IgE in the absence of mu- or delta-chain expression. J Immunol. 2008;181(10):6697–6701. doi: 10.4049/jimmunol.181.10.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Simons FE. Anaphylaxis: Recent advances in assessment and treatment. J Allergy Clin Immunol. 2009;124(4):625–636. doi: 10.1016/j.jaci.2009.08.025. quiz 637–628. [DOI] [PubMed] [Google Scholar]
- 251.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, et al. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358(1):28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 252.Vadas P, Perelman B, Liss G. Platelet-activating factor, histamine, and tryptase levels in human anaphylaxis. J Allergy Clin Immunol. 2013;131(1):144–149. doi: 10.1016/j.jaci.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 253.Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98(6):1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 254.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 255.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128(4):1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 256.Hogan SP, Wang YH, Strait R, Finkelman FD. Food-induced anaphylaxis: mast cells as modulators of anaphylactic severity. Semin Immunopathol. 2012;34(5):643–653. doi: 10.1007/s00281-012-0320-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Caffarelli C, Romanini E, Caruana P, Street ME, de’ Angelis G. Clinical food hypersensitivity: the relevance of duodenal immunoglobulin E-positive cells. Pediatr Res. 1998;44(4):485–490. doi: 10.1203/00006450-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 258.Nolte H, Schiotz PO, Kruse A, Stahl Skov P. Comparison of intestinal mast cell and basophil histamine release in children with food allergic reactions. Allergy. 1989;44(8):554–565. doi: 10.1111/j.1398-9995.1989.tb04200.x. [DOI] [PubMed] [Google Scholar]
- 259.Wang M, Takeda K, Shiraishi Y, Okamoto M, Dakhama A, Joetham A, et al. Peanut-induced intestinal allergy is mediated through a mast cell-IgE-FcεRI-IL-13 pathway. J Allergy Clin Immunol. 2010;126(2):306–316. 316 e301–312. doi: 10.1016/j.jaci.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205(4):897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. Nutr Res. 2011;31(1):61–75. doi: 10.1016/j.nutres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Rance F. Food allergy in children suffering from atopic eczema. Pediatr Allergy Immunol. 2008;19(3):279–284. doi: 10.1111/j.1399-3038.2008.00719.x. quiz 285. [DOI] [PubMed] [Google Scholar]
- 263.Noti M, Kim BS, Siracusa MC, Rak GD, Kubo M, Moghaddam AE, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immunol. 2014;133(5):1390–1399. 1399 e1391–1396. doi: 10.1016/j.jaci.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Burton OT, Noval Rivas M, Zhou JS, Logsdon SL, Darling AR, Koleoglou KJ, et al. Immunoglobulin E Signal Inhibition during Allergen Ingestion Leads to Reversal of Established Food Allergy and Induction of Regulatory T Cells. Immunity. 2014;41(1):141–151. doi: 10.1016/j.immuni.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Choi HW, Abraham SN. Mast cell mediator responses and their suppression by pathogenic and commensal microorganisms. Mol Immunol. 2014 doi: 10.1016/j.molimm.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Chan CY, St John AL, Abraham SN. Plasticity in mast cell responses during bacterial infections. Curr Opin Microbiol. 2012;15(1):78–84. doi: 10.1016/j.mib.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kumar V, Sharma A. Mast cells: emerging sentinel innate immune cells with diverse role in immunity. Mol Immunol. 2010;48(1–3):14–25. doi: 10.1016/j.molimm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 268.Galli SJ, Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol. 2010;40(7):1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Woodbury RG, Miller HR, Huntley JF, Newlands GF, Palliser AC, Wakelin D. Mucosal mast cells are functionally active during spontaneous expulsion of intestinal nematode infections in rat. Nature. 1984;312(5993):450–452. doi: 10.1038/312450a0. [DOI] [PubMed] [Google Scholar]
- 270.Askenase PW. Immune inflammatory responses to parasites: the role of basophils, mast cells and vasoactive amines. Am J Trop Med Hyg. 1977;26(6 Pt 2):96–103. doi: 10.4269/ajtmh.1977.26.96. [DOI] [PubMed] [Google Scholar]
- 271.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192(12):1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Barrett KE, Neva FA, Gam AA, Cicmanec J, London WT, Phillips JM, et al. The immune response to nematode parasites: modulation of mast cell numbers and function during Strongyloides stercoralis infections in nonhuman primates. Am J Trop Med Hyg. 1988;38(3):574–581. doi: 10.4269/ajtmh.1988.38.574. [DOI] [PubMed] [Google Scholar]
- 273.Gustowska L, Ruitenberg EJ, Elgersma A, Kociecka W. Increase of mucosal mast cells in the jejunum of patients infected with Trichinella spiralis. Int Arch Allergy Appl Immunol. 1983;71(4):304–308. doi: 10.1159/000233412. [DOI] [PubMed] [Google Scholar]
- 274.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11(6):375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 275.Grencis RK, Humphreys NE, Bancroft AJ. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev. 2014;260(1):183–205. doi: 10.1111/imr.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206(10):2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Taylor MD, van der Werf N, Maizels RM. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33(4):181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 278.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 279.Crowle PK. Mucosal mast cell reconstitution and Nippostrongylus brasiliensis rejection by W/Wv mice. J Parasitol. 1983;69(1):66–69. [PubMed] [Google Scholar]
- 280.Abe T, Nawa Y. Reconstitution of mucosal mast cells in W/WV mice by adoptive transfer of bone marrow-derived cultured mast cells and its effects on the protective capacity to Strongyloides ratti-infection. Parasite Immunol. 1987;9(1):31–38. doi: 10.1111/j.1365-3024.1987.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 281.Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, et al. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med. 2005;202(5):607–616. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Khan AI, Horii Y, Nawa Y. Defective mucosal immunity and normal systemic immunity of Mongolian gerbils, Meriones unguiculatus, to reinfection with Strongyloides venezuelensis. Parasite Immunol. 1993;15(10):565–571. doi: 10.1111/pim.1993.15.10.565. [DOI] [PubMed] [Google Scholar]
- 283.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41(1):445–447. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Oku Y, Itayama H, Kamiya M. Expulsion of Trichinella spiralis from the intestine of W/Wv mice reconstituted with haematopoietic and lymphopoietic cells and origin of mucosal mast cells. Immunology. 1984;53(2):337–344. [PMC free article] [PubMed] [Google Scholar]
- 285.Koyama K, Ito Y. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunol. 2000;22(1):13–20. doi: 10.1046/j.1365-3024.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- 286.Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, et al. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A. 2012;109(17):6644–6649. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides ratti infection. Parasite Immunol. 1987;9(4):477–485. doi: 10.1111/j.1365-3024.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 288.Wedemeyer J, Galli SJ. Decreased susceptibility of mast cell-deficient KitW/KitW-v mice to the development of 1, 2-dimethylhydrazine-induced intestinal tumors. Lab Invest. 2005;85(3):388–396. doi: 10.1038/labinvest.3700232. [DOI] [PubMed] [Google Scholar]
- 289.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, et al. Mouse Mast Cell Tryptase mMCP-6 Is a Critical Link between Adaptive and Innate Immunity in the Chronic Phase of Trichinella spiralis Infection. J Immunol. 2008;180(7):4885–4891. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Blankenhaus B, Reitz M, Brenz Y, Eschbach ML, Hartmann W, Haben I, et al. Foxp3+ regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathog. 2014;10(2):e1003913. doi: 10.1371/journal.ppat.1003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86(5):1968–1976. [PubMed] [Google Scholar]
- 292.Arizono N, Kasugai T, Yamada M, Okada M, Morimoto M, Tei H, et al. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81(10):2572–2578. [PubMed] [Google Scholar]
- 293.Townsend JM, Fallon GP, Matthews JD, Smith P, Jolin EH, McKenzie NA. IL-9-deficient mice establish fundamental roles for IL-9 in pulmonary mastocytosis and goblet cell hyperplasia but not T cell development. Immunity. 2000;13(4):573–583. doi: 10.1016/s1074-7613(00)00056-x. [DOI] [PubMed] [Google Scholar]
- 294.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32(2):80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pritchard DI. Immunity to helminths: is too much IgE parasite--rather than host-protective? Parasite Immunol. 1993;15(1):5–9. doi: 10.1111/j.1365-3024.1993.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 296.Wakelin D. Immunity to intestinal parasites. Nature. 1978;273(5664):617–620. doi: 10.1038/273617a0. [DOI] [PubMed] [Google Scholar]
- 297.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8(5):518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11(4):446–449. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 299.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381(6577):75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 300.Maurer M, Echtenacher B, Hultner L, Kollias G, Mannel DN, Langley KE, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. 1998;188(12):2343–2348. doi: 10.1084/jem.188.12.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Maurer M, Wedemeyer J, Metz M, Piliponsky AM, Weller K, Chatterjea D, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature. 2004;432(7016):512–516. doi: 10.1038/nature03085. [DOI] [PubMed] [Google Scholar]
- 302.Mallen-St Clair J, Pham CT, Villalta SA, Caughey GH, Wolters PJ. Mast cell dipeptidyl peptidase I mediates survival from sepsis. J Clin Invest. 2004;113(4):628–634. doi: 10.1172/JCI19062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Piliponsky AM, Chen CC, Nishimura T, Metz M, Rios EJ, Dobner PR, et al. Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat Med. 2008;14(4):392–398. doi: 10.1038/nm1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181(8):5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Piliponsky AM, Chen CC, Rios EJ, Treuting PM, Lahiri A, Abrink M, et al. The chymase mouse mast cell protease 4 degrades TNF, limits inflammation, and promotes survival in a model of sepsis. Am J Pathol. 2012;181(3):875–886. doi: 10.1016/j.ajpath.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, et al. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109(11):4846–4855. doi: 10.1182/blood-2006-09-045641. [DOI] [PubMed] [Google Scholar]
- 307.Kanamaru Y, Sumiyoshi K, Ushio H, Ogawa H, Okumura K, Nakao A. Smad3 deficiency in mast cells provides efficient host protection against acute septic peritonitis. J Immunol. 2005;174(7):4193–4197. doi: 10.4049/jimmunol.174.7.4193. [DOI] [PubMed] [Google Scholar]
- 308.Orinska Z, Maurer M, Mirghomizadeh F, Bulanova E, Metz M, Nashkevich N, et al. IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat Med. 2007;13(8):927–934. doi: 10.1038/nm1615. [DOI] [PubMed] [Google Scholar]
- 309.Huang C, De Sanctis GT, O’Brien PJ, Mizgerd JP, Friend DS, Drazen JM, et al. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. J Biol Chem. 2001;276(28):26276–26284. doi: 10.1074/jbc.M102356200. [DOI] [PubMed] [Google Scholar]
- 310.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, et al. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. The Journal of biological chemistry. 2007;282(29):20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 311.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173(2):219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Wershil BK, Castagliuolo I, Pothoulakis C. Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology. 1998;114(5):956–964. doi: 10.1016/s0016-5085(98)70315-4. [DOI] [PubMed] [Google Scholar]
- 313.Malaviya R, Navara C, Uckun FM. Role of Janus kinase 3 in mast cell-mediated innate immunity against gram-negative bacteria. Immunity. 2001;15(2):313–321. doi: 10.1016/s1074-7613(01)00184-4. [DOI] [PubMed] [Google Scholar]
- 314.Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004;12(9):424–430. doi: 10.1016/j.tim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 315.Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, et al. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6(4):331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Malaviya R, Ikeda T, Abraham SN, Malaviya R. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett. 2004;91(2–3):103–111. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 317.Chan CY, St John AL, Abraham SN. Mast cell interleukin-10 drives localized tolerance in chronic bladder infection. Immunity. 2013;38(2):349–359. doi: 10.1016/j.immuni.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Leveson-Gower DB, Sega EI, Kalesnikoff J, Florek M, Pan Y, Pierini A, et al. Mast cells suppress murine GVHD in a mechanism independent of CD4+CD25+ regulatory T cells. Blood. 2013;122(22):3659–3665. doi: 10.1182/blood-2013-08-519157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ronnberg E, Johnzon CF, Calounova G, Faroldi GG, Grujic M, Hartmann K, et al. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology. 2014 doi: 10.1111/imm.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sugiyama K. Histamine release from rat mast cells induced by Sendai virus. Nature. 1977;270(5638):614–615. doi: 10.1038/270614a0. [DOI] [PubMed] [Google Scholar]
- 321.Castleman WL, Sorkness RL, Lemanske RF, Jr, McAllister PK. Viral bronchiolitis during early life induces increased numbers of bronchiolar mast cells and airway hyperresponsiveness. Am J Pathol. 1990;137(4):821–831. [PMC free article] [PubMed] [Google Scholar]
- 322.Sorden SD, Castleman WL. Virus-induced increases in bronchiolar mast cells in Brown Norway rats are associated with both local mast cell proliferation and increases in blood mast cell precursors. Lab Invest. 1995;73(2):197–204. [PubMed] [Google Scholar]
- 323.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114(1):174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 324.Orinska Z, Bulanova E, Budagian V, Metz M, Maurer M, Bulfone-Paus S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood. 2005;106(3):978–987. doi: 10.1182/blood-2004-07-2656. [DOI] [PubMed] [Google Scholar]
- 325.Higuchi H, Hara M, Yamamoto K, Miyamoto T, Kinoshita M, Yamada T, et al. Mast cells play a critical role in the pathogenesis of viral myocarditis. Circulation. 2008;118(4):363–372. doi: 10.1161/CIRCULATIONAHA.107.741595. [DOI] [PubMed] [Google Scholar]
- 326.Zsebo KM, Williams DA, Geissler EN, Broudy VC, Martin FH, Atkins HL, et al. Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell. 1990;63(1):213–224. doi: 10.1016/0092-8674(90)90302-u. [DOI] [PubMed] [Google Scholar]
- 327.Wang Z, Lai Y, Bernard JJ, Macleod DT, Cogen AL, Moss B, et al. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J Immunol. 2012;188(1):345–357. doi: 10.4049/jimmunol.1101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wang Z, MacLeod DT, Di Nardo A. Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses. J Immunol. 2012;189(4):1551–1558. doi: 10.4049/jimmunol.1200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.St John AL, Rathore AP, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife. 2013;2:e00481. doi: 10.7554/eLife.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.St John AL. Influence of mast cells on dengue protective immunity and immune pathology. PLoS Pathog. 2013;9(12):e1003783. doi: 10.1371/journal.ppat.1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.St John AL, Rathore AP, Yap H, Ng ML, Metcalfe DD, Vasudevan SG, et al. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc Natl Acad Sci U S A. 2011;108(22):9190–9195. doi: 10.1073/pnas.1105079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Ebert S, Becker M, Lemmermann NA, Buttner JK, Michel A, Taube C, et al. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog. 2014;10(4):e1004100. doi: 10.1371/journal.ppat.1004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Sundstrom JB, Ellis JE, Hair GA, Kirshenbaum AS, Metcalfe DD, Yi H, et al. Human tissue mast cells are an inducible reservoir of persistent HIV infection. Blood. 2007;109(12):5293–5300. doi: 10.1182/blood-2006-11-058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Bannert N, Farzan M, Friend DS, Ochi H, Price KS, Sodroski J, et al. Human Mast cell progenitors can be infected by macrophagetropic human immunodeficiency virus type 1 and retain virus with maturation in vitro. J Virol. 2001;75(22):10808–10814. doi: 10.1128/JVI.75.22.10808-10814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Sundstrom JB, Little DM, Villinger F, Ellis JE, Ansari AA. Signaling through Toll-like receptors triggers HIV-1 replication in latently infected mast cells. J Immunol. 2004;172(7):4391–4401. doi: 10.4049/jimmunol.172.7.4391. [DOI] [PubMed] [Google Scholar]
- 336.Li Y, Li L, Wadley R, Reddel SW, Qi JC, Archis C, et al. Mast cells/basophils in the peripheral blood of allergic individuals who are HIV-1 susceptible due to their surface expression of CD4 and the chemokine receptors CCR3, CCR5, and CXCR4. Blood. 2001;97(11):3484–3490. doi: 10.1182/blood.v97.11.3484. [DOI] [PubMed] [Google Scholar]
- 337.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14(5):536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 338.Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23(8):1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 339.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262(5139):1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- 340.Paton WD. Compound 48/80: a potent histamine liberator. Br J Pharmacol Chemother. 1951;6(3):499–508. doi: 10.1111/j.1476-5381.1951.tb00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Fawcett DW. Cytological and pharmacological observations on the release of histamine by mast cells. J Exp Med. 1954;100(2):217–224. doi: 10.1084/jem.100.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Rothschild AM. Mechanisms of histamine release by compound 48–80. Br J Pharmacol. 1970;38(1):253–262. doi: 10.1111/j.1476-5381.1970.tb10354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Gushchin IS, Miroshnikov AI, Martynov VI, Sviridov VV. Histamine releasing and anti-inflammatory activities of MCD-peptide and its modified forms. Agents Actions. 1981;11(1–2):69–71. doi: 10.1007/BF01991459. [DOI] [PubMed] [Google Scholar]
- 344.Galli SJ, Wershil BK, Bose R, Walker PA, Szabo S. Ethanol-induced acute gastric injury in mast cell-deficient and congenic normal mice. Evidence that mast cells can augment the area of damage. Am J Pathol. 1987;128(1):131–140. [PMC free article] [PubMed] [Google Scholar]
- 345.Pae S, Cho JY, Dayan S, Miller M, Pemberton AD, Broide DH. Chronic allergen challenge induces bronchial mast cell accumulation in BALB/c but not C57BL/6 mice and is independent of IL-9. Immunogenetics. 2010;62(8):499–506. doi: 10.1007/s00251-010-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xing W, Austen KF, Gurish MF, Jones TG. Protease phenotype of constitutive connective tissue and of induced mucosal mast cells in mice is regulated by the tissue. Proc Natl Acad Sci U S A. 2011;108(34):14210–14215. doi: 10.1073/pnas.1111048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400(6746):773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 348.Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400(6746):769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 349.Rönnberg E, Melo FR, Pejler G. Mast cell proteoglycans. J Histochem Cytochem. 2012;60(12):950–962. doi: 10.1369/0022155412458927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Schwartz LB. Mast cells: function and contents. Curr Opin Immunol. 1994;6(1):91–97. doi: 10.1016/0952-7915(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 351.Craig SS, Irani AM, Metcalfe DD, Schwartz LB. Ultrastructural localization of heparin to human mast cells of the MCTC and MCT types by labeling with antithrombin III-gold. Lab Invest. 1993;69(5):552–561. [PubMed] [Google Scholar]
- 352.Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–494. doi: 10.1038/nri3690. [DOI] [PubMed] [Google Scholar]
- 353.Wong GW, Tang Y, Feyfant E, Sali A, Li L, Li Y, et al. Identification of a new member of the tryptase family of mouse and human mast cell proteases which possesses a novel COOH-terminal hydrophobic extension. J Biol Chem. 1999;274(43):30784–30793. doi: 10.1074/jbc.274.43.30784. [DOI] [PubMed] [Google Scholar]
- 354.Xia HZ, Kepley CL, Sakai K, Chelliah J, Irani AM, Schwartz LB. Quantitation of tryptase, chymase, FcεRIα, and FcεRIγ mRNAs in human mast cells and basophils by competitive reverse transcription-polymerase chain reaction. J Immunol. 1995;154(10):5472–5480. [PubMed] [Google Scholar]
- 355.Caughey GH, Raymond WW, Blount JL, Hau LW, Pallaoro M, Wolters PJ, et al. Characterization of human γ-tryptases, novel members of the chromosome 16p mast cell tryptase and prostasin gene families. J Immunol. 2000;164(12):6566–6575. doi: 10.4049/jimmunol.164.12.6566. [DOI] [PubMed] [Google Scholar]
- 356.Schechter NM, Irani AM, Sprows JL, Abernethy J, Wintroub B, Schwartz LB. Identification of a cathepsin G-like proteinase in the MCTC type of human mast cell. J Immunol. 1990;145(8):2652–2661. [PubMed] [Google Scholar]
- 357.Irani AM, Goldstein SM, Wintroub BU, Bradford T, Schwartz LB. Human mast cell carboxypeptidase. Selective localization to MCTC cells. J Immunol. 1991;147(1):247–253. [PubMed] [Google Scholar]
- 358.Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci U S A. 1990;87(10):3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Katz HR, Stevens RL, Austen KF. Heterogeneity of mammalian mast cells differentiated in vivo and in vitro. J Allergy Clin Immunol. 1985;76(2 Pt 2):250–259. doi: 10.1016/0091-6749(85)90638-4. [DOI] [PubMed] [Google Scholar]
- 360.Li HJ, Johnston B, Aiello D, Caffrey DR, Giel-Moloney M, Rindi G, et al. Distinct cellular origins for serotonin-expressing and enterochromaffin-like cells in the gastric corpus. Gastroenterology. 2014;146(3):754–764. e753. doi: 10.1053/j.gastro.2013.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.von Stebut E, Metz M, Milon G, Knop J, Maurer M. Early macrophage influx to sites of cutaneous granuloma formation is dependent on MIP-1α /β released from neutrophils recruited by mast cell-derived TNFα. Blood. 2003;101(1):210–215. doi: 10.1182/blood-2002-03-0921. [DOI] [PubMed] [Google Scholar]
- 362.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119(2):498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 363.Lowman MA, Rees PH, Benyon RC, Church MK. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung, adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. 1988;81(3):590–597. [PubMed] [Google Scholar]
- 364.Rudich N, Ravid K, Sagi-Eisenberg R. Mast cell adenosine receptors function: a focus on the A3 adenosine receptor and inflammation. Front Immunol. 2012;3:134. doi: 10.3389/fimmu.2012.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171(1):338–345. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 366.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A3 receptors on mast cells. J Clin Invest. 2000;105(3):361–367. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sereda MJ, Bradding P, Vial C. Adenosine potentiates human lung mast cell tissue plasminogen activator activity. J Immunol. 2011;186(2):1209–1217. doi: 10.4049/jimmunol.1001563. [DOI] [PubMed] [Google Scholar]
- 368.Gomez G, Zhao W, Schwartz LB. Disparity in FcεRI-induced degranulation of primary human lung and skin mast cells exposed to adenosine. J Clin Immunol. 2011;31(3):479–487. doi: 10.1007/s10875-011-9517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, et al. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J Exp Med. 1999;189(10):1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in FcγRII-deficient mice. Nature. 1996;379(6563):346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 371.Hazenbos WL, Gessner JE, Hofhuis FM, Kuipers H, Meyer D, Heijnen IA, et al. Impaired IgG-dependent anaphylaxis and Arthus reaction in FcγRIII (CD16) deficient mice. Immunity. 1996;5(2):181–188. doi: 10.1016/s1074-7613(00)80494-x. [DOI] [PubMed] [Google Scholar]
- 372.Dombrowicz D, Flamand V, Miyajima I, Ravetch JV, Galli SJ, Kinet JP. Absence of FcεRI α chain results in upregulation of FcγRIII-dependent mast cell degranulation and anaphylaxis. Evidence of competition between FcεRI and FcγRIII for limiting amounts of FcR β and γ chains. J Clin Invest. 1997;99(5):915–925. doi: 10.1172/JCI119256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Coleman JW, Godfrey RC. The number and affinity of IgE receptors on dispersed human lung mast cells. Immunology. 1981;44(4):859–863. [PMC free article] [PubMed] [Google Scholar]
- 374.Sellge G, Barkowsky M, Kramer S, Gebhardt T, Sander LE, Lorentz A, et al. Interferon-γ regulates growth and controls Fcγ receptor expression and activation in human intestinal mast cells. BMC Immunol. 2014;15:27. doi: 10.1186/1471-2172-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Helm B, Marsh P, Vercelli D, Padlan E, Gould H, Geha R. The mast cell binding site on human immunoglobulin E. Nature. 1988;331(6152):180–183. doi: 10.1038/331180a0. [DOI] [PubMed] [Google Scholar]
- 376.Tkaczyk C, Okayama Y, Woolhiser MR, Hagaman DD, Gilfillan AM, Metcalfe DD. Activation of human mast cells through the high affinity IgG receptor. Mol Immunol. 2002;38(16–18):1289–1293. doi: 10.1016/s0161-5890(02)00077-9. [DOI] [PubMed] [Google Scholar]
- 377.Zhao W, Kepley CL, Morel PA, Okumoto LM, Fukuoka Y, Schwartz LB. FcγRIIa, not FcγRIIb, is constitutively and functionally expressed on skin-derived human mast cells. J Immunol. 2006;177(1):694–701. doi: 10.4049/jimmunol.177.1.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Trivedi NN, Tamraz B, Chu C, Kwok PY, Caughey GH. Human subjects are protected from mast cell tryptase deficiency despite frequent inheritance of loss-of-function mutations. J Allergy Clin Immunol. 2009;124(5):1099–1105. e1091–1094. doi: 10.1016/j.jaci.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Befus AD, Pearce FL, Gauldie J, Horsewood P, Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982;128(6):2475–2480. [PubMed] [Google Scholar]
- 380.Katz HR, Lobell RB. Expression and function of FcγR in mouse mast cells. Int Arch Allergy Immunol. 1995;107(1–3):76–78. doi: 10.1159/000236936. [DOI] [PubMed] [Google Scholar]