Abstract
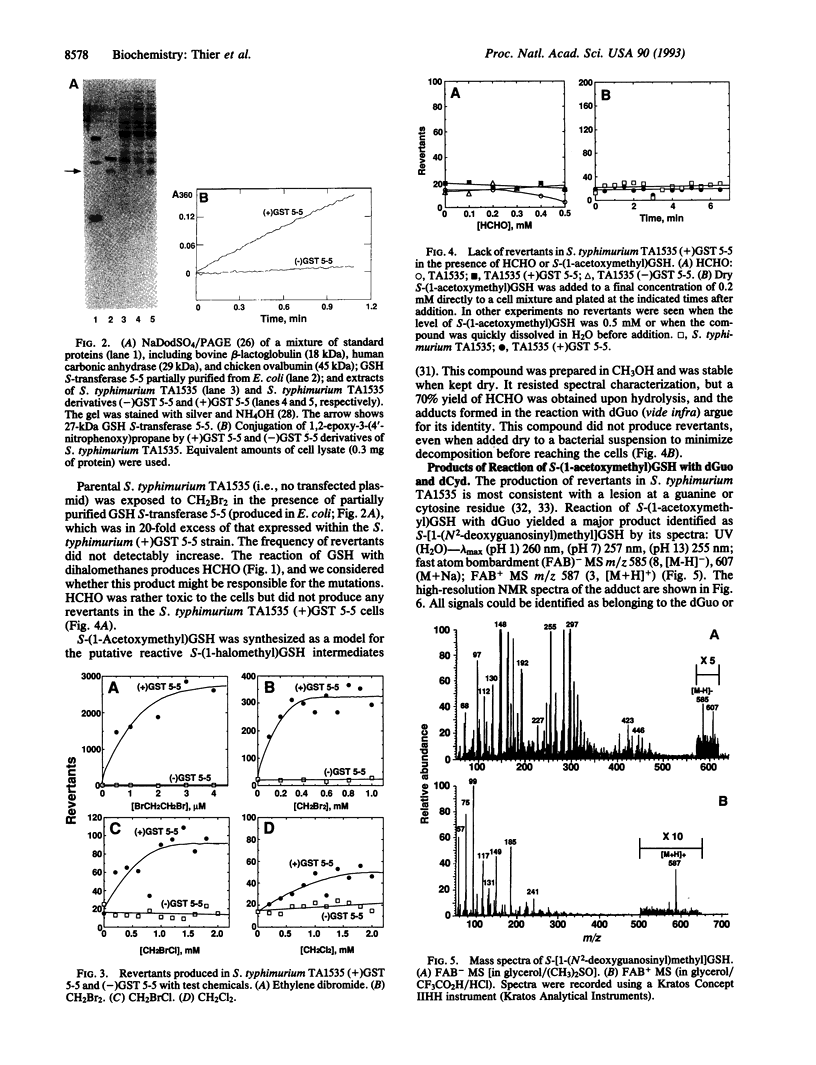
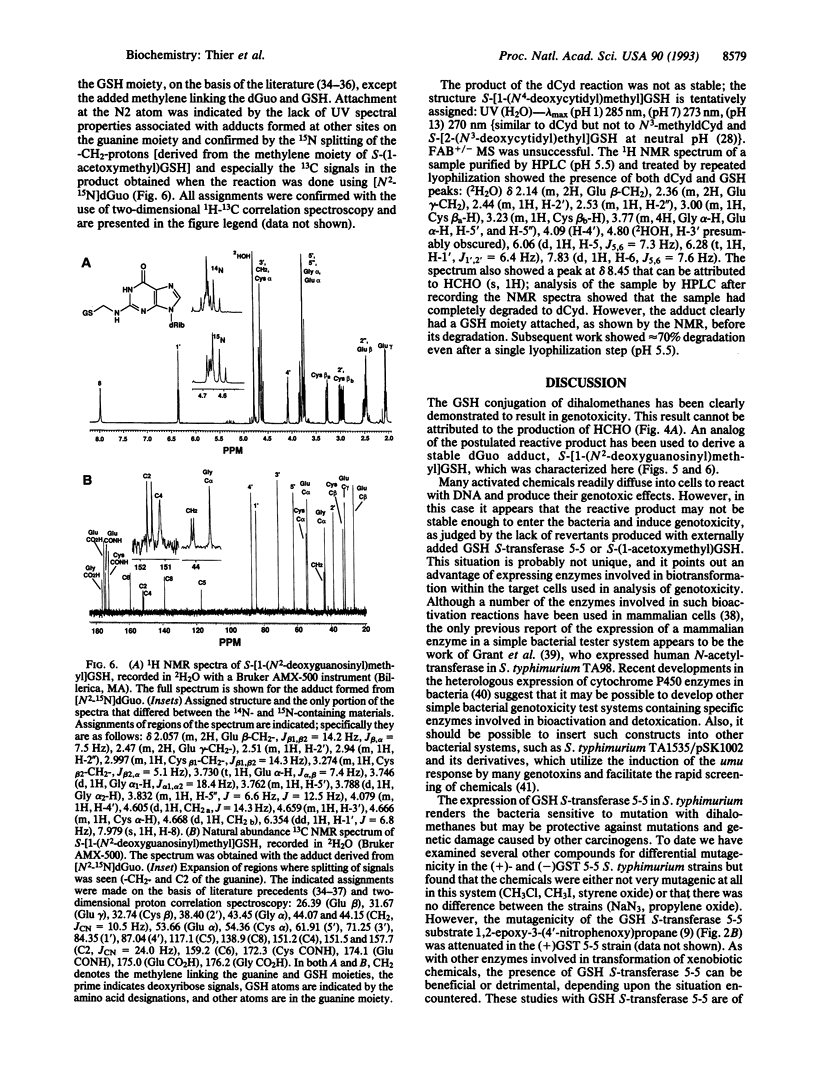
Dihalomethanes can produce liver tumors in mice but not in rats, and concern exists about the risk of these compounds to humans. Glutathione (GSH) conjugation of dihalomethanes has been considered to be a critical event in the bioactivation process, and risk assessment is based upon this premise; however, there is little experimental support for this view or information about the basis of genotoxicity. A plasmid vector containing rat GSH S-transferase 5-5 was transfected into the Salmonella typhimurium tester strain TA1535, which then produced active enzyme. The transfected bacteria produced base-pair revertants in the presence of ethylene dihalides or dihalomethanes, in the order CH2Br2 > CH2BrCl > CH2Cl2. However, revertants were not seen when cells were exposed to GSH, CH2Br2, and an amount of purified GSH S-transferase 5-5 (20-fold excess in amount of that expressed within the cells). HCHO, which is an end product of the reaction of GSH with dihalomethanes, also did not produce mutations. S-(1-Acetoxymethyl)GSH was prepared as an analog of the putative S-(1-halomethyl)GSH reactive intermediates. This analog did not produce revertants, consistent with the view that activation of dihalomethanes must occur within the bacteria to cause genetic damage, presenting a model to be considered in studies with mammalian cells. S-(1-Acetoxymethyl)GSH reacted with 2'-deoxyguanosine to yield a major adduct, identified as S-[1-(N2-deoxyguanosinyl)methyl]GSH. Demonstration of the activation of dihalomethanes by this mammalian GSH S-transferase theta class enzyme should be of use in evaluating the risk of these chemicals, particularly in light of reports of the polymorphic expression of a similar activity in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. E., Anders M. W. Metabolism of dihalomethanes to formaldehyde and inorganic halide. I. In vitro studies. Drug Metab Dispos. 1976 Jul-Aug;4(4):357–361. [PubMed] [Google Scholar]
- Andersen M. E., Clewell H. J., 3rd, Gargas M. L., Smith F. A., Reitz R. H. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol Appl Pharmacol. 1987 Feb;87(2):185–205. doi: 10.1016/0041-008x(87)90281-x. [DOI] [PubMed] [Google Scholar]
- Barnes H. J., Arlotto M. P., Waterman M. R. Expression and enzymatic activity of recombinant cytochrome P450 17 alpha-hydroxylase in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5597–5601. doi: 10.1073/pnas.88.13.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek J. D., Nitschke K. D., Bell T. J., Wackerle D. L., Childs R. C., Beyer J. E., Dittenber D. A., Rampy L. W., McKenna M. J. Methylene chloride: a two-year inhalation toxicity and oncogenicity study in rats and hamsters. Fundam Appl Toxicol. 1984 Feb;4(1):30–47. doi: 10.1016/0272-0590(84)90217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno M. S., Chiariotti L., Alifano P., Nappo A. G., Bruni C. B. Structure and function of the Salmonella typhimurium and Escherichia coli K-12 histidine operons. J Mol Biol. 1988 Oct 5;203(3):585–606. doi: 10.1016/0022-2836(88)90194-5. [DOI] [PubMed] [Google Scholar]
- Cmarik J. L., Humphreys W. G., Bruner K. L., Lloyd R. S., Tibbetts C., Guengerich F. P. Mutation spectrum and sequence alkylation selectivity resulting from modification of bacteriophage M13mp18 DNA with S-(2-chloroethyl)glutathione. Evidence for a role of S-(2-N7-guanyl)ethyl)glutathione as a mutagenic lesion formed from ethylene dibromide. J Biol Chem. 1992 Apr 5;267(10):6672–6679. [PubMed] [Google Scholar]
- Cmarik J. L., Inskeep P. B., Meredith M. J., Meyer D. J., Ketterer B., Guengerich F. P. Selectivity of rat and human glutathione S-transferases in activation of ethylene dibromide by glutathione conjugation and DNA binding and induction of unscheduled DNA synthesis in human hepatocytes. Cancer Res. 1990 May 1;50(9):2747–2752. [PubMed] [Google Scholar]
- Eisenstadt E., Miller J. K., Kahng L. S., Barnes W. M. Influence of uvrB and pKM101 on the spectrum of spontaneous, UV- and gamma-ray-induced base substitutions that revert hisG46 in Salmonella typhimurium. Mutat Res. 1989 Jan;210(1):113–125. doi: 10.1016/0027-5107(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Gerster J. F., Robins R. K. Purine nucleosides. 8. The synthesis of 2-fluoro- and 2-chloroinosine and certain derived purine nucleosides. J Org Chem. 1966 Oct;31(10):3258–3262. doi: 10.1021/jo01348a037. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Crespi C. L., Gelboin H. V. DNA-expressed human cytochrome P450s: a new age of molecular toxicology and human risk assessment. Mutat Res. 1991 Mar;247(1):113–127. doi: 10.1016/0027-5107(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Josephy P. D., Lord H. L., Morrison L. D. Salmonella typhimurium strains expressing human arylamine N-acetyltransferases: metabolism and mutagenic activation of aromatic amines. Cancer Res. 1992 Jul 15;52(14):3961–3964. [PubMed] [Google Scholar]
- Green T. The metabolic activation of dichloromethane and chlorofluoromethane in a bacterial mutation assay using Salmonella typhimurium. Mutat Res. 1983 Sep;118(4):277–288. doi: 10.1016/0165-1218(83)90211-2. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Humphreys W. G., Kim D. H., Cmarik J. L., Shimada T., Guengerich F. P. Comparison of the DNA-alkylating properties and mutagenic responses of a series of S-(2-haloethyl)-substituted cysteine and glutathione derivatives. Biochemistry. 1990 Nov 13;29(45):10342–10350. doi: 10.1021/bi00497a008. [DOI] [PubMed] [Google Scholar]
- Jones A. J., Grant D. M., Winkley M. W., Robins R. K. Carbon-13 magnetic resonance. XVII. Pyrimidine and purine nucleosides. J Am Chem Soc. 1970 Jul 1;92(13):4079–4087. doi: 10.1021/ja00716a042. [DOI] [PubMed] [Google Scholar]
- Jongen W. M., Alink G. M., Koeman J. H. Mutagenic effect of dichloromethane on Salmonella typhimurium. Mutat Res. 1978 Jan;56(3):245–248. doi: 10.1016/0027-5107(78)90191-4. [DOI] [PubMed] [Google Scholar]
- Jung G., Breitmaier E., Voelter W. Dissoziationsgleichgewichte von Glutathion. Eine Fourier-Transform- 13 C-NMR spektroskopische Untersuchung der PH-Abhängigkeit der Ladungsverteilung. Eur J Biochem. 1972 Jan 21;24(3):438–445. doi: 10.1111/j.1432-1033.1972.tb19704.x. [DOI] [PubMed] [Google Scholar]
- Koga N., Inskeep P. B., Harris T. M., Guengerich F. P. S-[2-(N7-guanyl)ethyl]glutathione, the major DNA adduct formed from 1,2-dibromoethane. Biochemistry. 1986 Apr 22;25(8):2192–2198. doi: 10.1021/bi00356a051. [DOI] [PubMed] [Google Scholar]
- Kubic V. L., Anders M. W. Metabolism of dihalomethanes to carbon monoxide--III. Studies on the mechanism of the reaction. Biochem Pharmacol. 1978;27(19):2349–2355. doi: 10.1016/0006-2952(78)90143-0. [DOI] [PubMed] [Google Scholar]
- La Roche S. D., Leisinger T. Sequence analysis and expression of the bacterial dichloromethane dehalogenase structural gene, a member of the glutathione S-transferase supergene family. J Bacteriol. 1990 Jan;172(1):164–171. doi: 10.1128/jb.172.1.164-171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D. M., Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983 May;113(3-4):173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Coles B., Pemble S. E., Gilmore K. S., Fraser G. M., Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochem J. 1991 Mar 1;274(Pt 2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S. I., Oda Y., Shimada T., Oki I., Sugimoto K. SOS-inducing activity of chemical carcinogens and mutagens in Salmonella typhimurium TA1535/pSK1002: examination with 151 chemicals. Mutat Res. 1987 Dec;192(4):239–246. doi: 10.1016/0165-7992(87)90063-7. [DOI] [PubMed] [Google Scholar]
- Nitschke K. D., Burek J. D., Bell T. J., Kociba R. J., Rampy L. W., McKenna M. J. Methylene chloride: a 2-year inhalation toxicity and oncogenicity study in rats. Fundam Appl Toxicol. 1988 Jul;11(1):48–59. doi: 10.1016/0272-0590(88)90269-2. [DOI] [PubMed] [Google Scholar]
- Osterman-Golkar S., Hussain S., Walles S., Anderstam B., Sigvardsson K. Chemical reactivity and mutagenicity of some dihalomethanes. Chem Biol Interact. 1983 Aug 15;46(1):121–130. doi: 10.1016/0009-2797(83)90011-x. [DOI] [PubMed] [Google Scholar]
- Pemble S. E., Taylor J. B. An evolutionary perspective on glutathione transferases inferred from class-theta glutathione transferase cDNA sequences. Biochem J. 1992 Nov 1;287(Pt 3):957–963. doi: 10.1042/bj2870957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz R. H., Mendrala A. L., Guengerich F. P. In vitro metabolism of methylene chloride in human and animal tissues: use in physiologically based pharmacokinetic models. Toxicol Appl Pharmacol. 1989 Feb;97(2):230–246. doi: 10.1016/0041-008x(89)90328-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder K. R., Hallier E., Peter H., Bolt H. M. Dissociation of a new glutathione S-transferase activity in human erythrocytes. Biochem Pharmacol. 1992 Apr 15;43(8):1671–1674. doi: 10.1016/0006-2952(92)90695-f. [DOI] [PubMed] [Google Scholar]
- Summer K. H., Göggelmann W., Greim H. Glutathione and glutathione S-transferases in the Salmonella mammalian-microsome mutagenicity test. Mutat Res. 1980 May;70(3):269–278. doi: 10.1016/0027-5107(80)90018-4. [DOI] [PubMed] [Google Scholar]
- Wiencke J. K., Christiani D. C., Kelsey K. T. Bimodal distribution of sensitivity to SCE induction by diepoxybutane in human lymphocytes. I. Correlation with chromosomal aberrations. Mutat Res. 1991 May;248(1):17–26. doi: 10.1016/0027-5107(91)90083-z. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van Bladeren P. J., Breimer D. D., Rotteveel-Smijs G. M., Mohn G. R. Mutagenic activation of dibromomethane and diiodomethane by mammalian microsomes and glutathione S-transferases. Mutat Res. 1980 Oct;74(5):341–346. doi: 10.1016/0165-1161(80)90192-2. [DOI] [PubMed] [Google Scholar]