Abstract
Background
Currently, targeted therapy has shown encouraging treatment benefits in selected patients with advanced non-small cell lung cancer (NSCLC). However, the comparative benefits of targeted drugs and chemotherapy (CT) treatments in unselected patients are not clear. We therefore conduct a network meta-analysis to assess the relative efficacy and safety of these regimens.
Methods
PubMed, EMBASE, Cochrane Library and abstracts from major scientific meetings were searched for eligible literatures. The odds ratio (OR) for objective response rate (ORR) and safety was used for pooling effect sizes. Bayesian network meta-analysis was conducted to calculate the efficacy and safety of all included treatments. All tests of statistical significance were two sided.
Results
A total of 13,060 patients from 24 randomized controlled trials (RCT) were assessed. The targeted agents included bevacizumab (Bev), gefitinib (Gef), erlotinib (Erl) and cetuximab (Cet). Network meta-analysis showed that Bev + CT had a statistically significantly higher incidence of ORR relative to the other six different treatments, including placebo (OR =6.47; 95% CI, 3.85–10.29), Erl (OR =2.81; 95% CI, 2.08–3.70), CT (OR =1.92; 95% CI, 1.61–2.28), Gef (OR =1.40; 95% CI, 1.10–1.75), Erl + CT (OR =1.46; 95% CI, 1.17–1.80) and Gef + CT (OR =1.75; 95% CI, 1.36–2.22), whereas placebo and Erl were associated with statistically significantly lower incidence of ORR. Trend analyses of rank probability revealed that Bev + CT had the highest probability of being the best treatment arm in term of ORR, followed by Cet + CT. Meanwhile, Cet + CT showed significant severer rash and thrombocytopenia compared with Bev + CT. Gef was probable to be the rank 3 for ORR but was associated with relatively low risk for grade ≥3 toxicities.
Conclusions
Our study suggested that Bev + CT may offer better ORR in the treatment of unselected patients with advanced NSCLC. Future studies will be needed to investigate whether the increase of ORR with targeted drugs would be translated into survival benefits.
Keywords: Targeted drugs, efficacy, non-small cell lung cancer (NSCLC), network meta-analysis
Introduction
Lung cancer is the leading cause of cancer-related death, with nearly 1.6 million deaths annually worldwide, as of 2012 (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancer and more than 40% of NSCLCs are diagnosed at advanced stage (III or IV), the 5-year survival rate is extremely low, ranging from 5% to 15% (2). Platinum-based double chemotherapy is recommended as standard first-line treatment, however, the objective response rate (ORR) is modest and recurrence eventually occurs for most patients (3).
Over the past decade, the NSCLC therapeutics landscape has been dominated by the increasing focus on identification and validation of molecular targets (4). Several drugs were designed to interfere with specific aberrant biological pathways in NSCLC, for example, epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) (such as gefitinib and erlotinib) (5), monoclonal antibodies targeting EGFR (such as cetuximab) (6,7) and angiogenesis inhibitors (such as, bevacizumab) (8-10). In addition, other targeted agents were at varying stages of clinical development, panitumumab (anti-EGFR monoclonal antibodies) (4), ALK inhibitor (crizotinib and Ceritinib) (11,12), selumetinib (MEK1/MEK2 inhibitor) (13) and so on.
Similar to many other cancers, NSCLC is not a singular entity but is in fact multiple pathologies, it is initiated by activation of oncogenes or inactivation of tumor suppressor genes. Thus, the optimal management of NSCLC is to identify the driver mutations that help to predict sensitivity to targeted therapy and estimate prognosis respectively. For example, large randomized controlled trials and meta-analysis showed that TKI treatment was superior to conventional chemotherapy drugs in terms of progression-free survival (PFS) and ORR for patients harboring EGFR-mutation (14-19). Unfortunately, there are no reliable clinical phenotypes or characteristics that allow for accurate prediction of driver mutation, all tumours must undergo specific mutational testing. As we know, in routine clinical practice, obtaining information on driver gene mutational status is not always feasible, due to insufficient testing facilities and low-quality tumor samples, especially, in some advanced patients or postoperative recurrence cases. Even if we can obtain the driver mutations from the peripheral blood circulating tumor DNA (ctDNA) (20) or circulating tumor cells (CTC) (21), the existing methods have insufficient sensitivity, and the testing cost is expensive. At the same time, the occurrence and development of tumors are a complicated process, and multiple signalling pathways have been identified in NSCLC that lead to malignant transformations, such as RAS-RAF-MEK-ERK or MAPK, PI3K-AKT-mTOR or JAK-STAT pathways (4). Single targeted therapy cannot obtain the expected effect and acquired resistance is frequently seen in clinical practice. So, the relative effects and safety of these targeted drugs compared with another in unselected patients with advanced NSCLC remains unclear.
Although many trials have been conducted to compare treatments, there is lack of integration information from head-to-head RCTs. Network meta-analysis provides a useful method for estimating the relative treatment effects of these agents (22). Unlike traditional meta-analysis, it enables us to synthesize data from both direct and indirect evidence of diverse regimens, and compare the results based on individual trial (23). Some previous researches reported that inferior response to EGFR-TKIs following treatment of chemotherapy (24,25). Therefore, we performed a systematic review and network meta-analysis of randomized controlled trials comparing the efficacy and safety of first-line chemotherapy and targeted therapy in unselected patients with advanced NSCLC and also estimated the rank probability of each treatment, expecting it will be helpful for making evidence-based clinical decision for physicians and patients.
Methods
Search strategy
We carried out a comprehensive systematic search for published articles from inception to 2015 using PubMed, EMBASE and Cochrane Library; the key words were as follows: NSCLC, bevacizumab, gefitinib, erlotinib, afatinib, cetuximab, and randomized controlled trial. No language limits were applied. At the same time, meeting abstracts and virtual presentations of American Society of Clinical Oncology (ASCO) annual meetings and European Society of Medical Oncology (ESMO) congresses were also searched to identify unpublished trials. Two authors (M.M.S and Y.G.Z) independently screened the selected eligible trials.
Selection criteria
Eligible studies should meet the following criteria: (I) randomized controlled trial; (II) patients with confirmed locally advanced or metastatic NSCLC were randomly assigned to first-line treatment; (III) at least two arms of different treatment regimens, chemotherapy, placebo or targeted therapy; (IV) studies with available data on patients’ EGFR unselected status; (V) outcomes of interest were ORR and safety. Studies failed to meet the inclusion criteria will be excluded. If overlap reports were identified, we included only the most recent and informative publication.
Data extraction and quality assessment
Two authors (M.M.S and Y.G.Z) independently extracted data according to a predefined information sheet, including first author, year of publication, number of patients, targeted treatment, chemotherapy regimens, patient characteristics (age, sex, ethnicity, histology and whether CT-native), and the outcomes of interest. The primary outcome in this study was ORR, it was defined as the proportion of complete response (CR) plus partial response (PR) among all evaluable patients, reflected the treatment by causing cancer cell death. For each trial, the OR with its 95% CI was directly extracted from research articles. Secondary outcome was the number of patients who had grade ≥3 adverse events, including rash, anemia, diarrhea, neutropenia and thrombocytopenia. Adverse events were graded according to National Cancer Institute Common Terminology Criteria (NCI-CTC) version 4.0.
Cochrane Collaboration’s tool was used to evaluate the quality of each eligible trials (26). Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to rate the overall quality of each outcome (27). Discrepancies were resolved by two reviewers (Y.L and W.R.T) to reach consensus.
Statistical methods
We first used random effects model to conduct direct meta-analysis, OR, 95% CI and P values were reported, two-side P<0.05 was considered statistically significant. If a direct comparison was based on two or more studies, I2 statistic was calculated to evaluate statistical heterogeneity. I2 values greater than 50% was considered high heterogeneity, 25–50% was indicative of modest heterogeneity, less than 25%, low heterogeneity (28).
Second, a Bayesian network meta-analysis was carried out to simultaneously compare the efficacy of all treatments which used in unselected patients with NSCLC. In the Bayesian framework, it incorporated both direct and indirect evidence to obtain estimate of the relative treatment effects between all the comparisons (23). The posterior distributions for each parameter of interest were estimated using Markov Chain Monte Carlo by placing suitable prior distributions (29). Both random-effects and fixed-effects models were used, then we evaluated the overall fit of the selected models base on deviance information criterion (DIC) statistics and the total residual deviance, DIC was an estimate of expected predictive error (lower deviance was better) (30). In addition, Bayesian framework for network meta-analysis provided a ranking probability curve of each treatment, we can rank treatments by counting the proportion of iterations of Markov Chain in which each drug had the highest OR (30).
Pairwise comparisons and node-splitting method were performed by STATA version 12.0 (STATA Corporation, College Station, TX, USA). Bayesian network meta-analysis was calculated using R2OpenBUGS version 3.2.3 (MRC, UK, and Imperial College, UK). Diagrams were made by R version 3.1.3 (R Project for Statistical Computing, Vienna, Austria). This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (31).
Results
Description of eligible trials
A total of 4,330 articles were identified according to the search strategy. Of these, 251 potentially eligible articles were evaluated in more detail, after review of full publications, 24 randomized clinical trials were finally selected for the study (Figure 1). Characteristics of the included trials were summarized in Table 1. Five trials applied bevacizumab (Bev) (9,10,32-34), seven trials applied gefitinib (Gef) (35-41), ten trials applied erlotinib (Erl) (24,42-50) and the other two trials applied cetuximab (Cet) (7,51). A total of 13,060 patients were enrolled, patients median age varied from 19-96; 38.2–100% of patients were adenocarcinoma; sixteen trials predominantly enrolled White patients (7,9,10,24,33,35-38,42,43,45,46,49-51) whereas other six had a majority of Asian patients (32,40,41,44,47,48) excluding the unreported data. For the outcomes of interest, eight different treatment arms were assessed: placebo, CT, Erl, Gef, Erl + CT, Gef + CT, Bev + CT, Cet + CT.
Figure 1.
Trial selection process.
Table 1. Characteristics of eligible studies included in the network meta-analysis.
| Study | No. of patients | Regimens arms 1 | Regimens arms 2 | Age, median [range], years | Female, N (%) | Ethnicity (%) | Smoking status: nonsmoker, N (%) | Adenocarcinoma, N (%) | CT-native |
|---|---|---|---|---|---|---|---|---|---|
| Sandler [2006] (10) | 850 | Paclitaxel + carboplatin + bevacizumab 15 mg/kg d1, iv, q3w | Paclitaxel 200 mg/m2 d1 + carboplatin (AUC =6) d1, iv, q3w ×6 cycles | NR | 387 (45.5) | White (85.8) | NR | 746 (87.7) | Yes |
| Reck [2010] (9) | 1,043 | Cisplatin + gemcitabine + bevacizumab 15 mg/kg d1, iv, q3w | Cisplatin 80 mg/m2 d1 + gemcitabine 1,250 mg/m2 d1,8, iv, q3w ×6 cycles | 57 [20–83] | 378 (36.2) | White (91.2) | NR | 876 (83.9) | Yes |
| Niho [2012] (32) | 180 | Paclitaxel + carboplatin + bevacizumab 15 mg/kg d1, iv, q3w | Paclitaxel 200mg/m2 d1 + carboplatin (AUC =6) d1, iv, q3w ×6 cycles | 60 [34–74] | 65 (36.1) | Asian (100.0) | 57 (31.6) | 166 (92.2) | Yes |
| Soria [2011] (33) | 213 | Paclitaxel + carboplatin + bevacizumab 15 mg/kg d1, iv, q3w | Paclitaxel 200 mg/m2 d1 + carboplatin (AUC=6) d1, iv, q3w ×6 cycles | 57 [23–79] | 78 (36.3) | White (98.6) | 36 (16.9) | 124 (58.2) | Yes |
| Boutsikou [2013] (34) | 229 | Docetaxel + carboplatin + bevacizumab 7.5 mg/kg d1, iv, q4w | Docetaxel 100 mg/m2 d1 + carboplatin (AUC =5.5) d1, iv, q4w ×6 cycles | NR | 38 (16.5) | NR | 27 (11.7) | 206 (89.9) | Yes |
| Docetaxel + carboplatin + erlotinib 150 mg/d d1, orally, 3 cycles | Docetaxel 100 mg/m2 d1 + carboplatin (AUC =5.5) d1, iv, q4w ×6 cycles | ||||||||
| Herbst [2004] (35) | 1,037 | Paclitaxel + carboplatin + gefitinib 250 mg/d orally, q3w | Paclitaxel 225 mg/m2 d1 + carboplatin (AUC =6) d1, iv, q3w ×6 cycles | 61 [26–86] | 418 (40.3) | White (90.2) | NR | 572 (55.1) | Yes |
| Crinò [2008] (36) | 196 | Gefitinib 250 mg/d, orally | Vinorelbine 30 mg/m2 d1,8, orally, q3w | 74 [70–89] | 48 (24.4) | White (82.6) | 28 (14.2) | 79 (40.3) | Yes |
| Goss [2009] (37) | 201 | Gefitinib 250 mg/d, orally | Placebo | 74 [42–90] | 79 (39.3) | White (96.0) | 19 (9.4) | 91 (45.2) | Yes |
| Giaccone [2004] (38) | 1,093 | Cisplatin + gemcitabine + gefitinib 250 mg/d orally | Cisplatin 80 mg/m2 d1 + gemcitabine 1,250 mg/m2 d1,8, iv, q3w ×6 cycles | 59 [31–85] | 863 (79.0) | White (90.4) | NR | 503 (46.1) | Yes |
| Morère [2010] (39) | 127 | Gefitinib 250 mg/d, orally | Docetaxel 75 mg/m2 d1, q3w | 70 [30–80] | 22 (17.3) | White (NR) | 8 (6.3) | 62 (48.8) | Yes |
| Fukuoka [2011] (40) | 1,217 | Gefitinib 250 mg/d, orally | Paclitaxel 200 mg/m2 d1 + carboplatin (AUC =5-6) d1, iv, q3w ×≤6 cycle | 57 [24–84] | 965 (79.2) | Asian (99.8) | 1140 (93.6) | 1172 (96.3) | Yes |
| Han [2012] (41) | 309 | Gefitinib 250 mg/d, orally | Cisplatin 75 mg/m2 d1 + gemcitabine 1,250 mg/m2 d1,8, iv, q3w ×9 cycles | 57 [19–74] | 274 (88.7) | Asian (100.0) | 309 (100.0) | 309 (100.0) | Yes |
| Herbst [2005] (42) | 1,078 | Paclitaxel + carboplatin + erlotinib 150 mg/d, orally | Paclitaxel 200 mg/m2 d1 + carboplatin (AUC =6) d1, iv, q3w ×6 cycles | 63 [24–84] | 424 (39.3) | White (86.6) | 116 (10.7) | 654 (60.6) | Yes |
| Lilenbaum [2008] (43) | 103 | Erlotinib 150 mg/d, orally | Paclitaxel 200 mg/m2 d1 + carboplatin (AUC =6) d1, iv, q3w ×4 cycles | NR | 52 (50.4) | White (66.0) | 10 (9.7) | 58 (56.3) | Yes |
| Mok [2009] (44) | 154 | Cisplatin/carboplatin + gemcitabine + erlotinib 150 mg/d, d15-28, orally | [Cisplatin 75 mg/m2 d1 or carboplatin (AUC =5) d1] + gemcitabine 1,250 mg/m2 d1,8, iv, q4w | 57 [27-79] | 46 (29.8) | Asian (94.1) | 52 (33.7) | 103 (66.8) | Yes |
| Gatzemeier [2007] (45) | 1,159 | Cisplatin + gemcitabine + erlotinib 150 mg/d, orally | Cisplatin 80 mg/m2 d1 + gemcitabine 1,250 mg/m2 d1,8, iv, q3w ×6 cycles | 60 [26–84] | 267 (23.0) | White (91.8) | NR | 445 (38.3) | Yes |
| Cappuzzo [2010] (46) | 889 | Erlotinib 150 mg/d, orally | Placebo | 60 [30–83] | 230 (25.8) | White (83.9) | 152 (17.0) | 403 (45.3) | No |
| Gridelli [2012] (24) | 760 | Erlotinib 150 mg/d, orally | Cisplatin 80 mg/m2 d1 + gemcitabine 1,200 mg/m2 d1,8, iv, q3w ×6 cycles | 62 [27–81] | 256 (33.7) | White (100.0) | 157 (20.7) | 422 (55.5) | No |
| Chen [2012] (47) | 113 | Erlotinib 150 mg/d, orally | Vinorelbine 60 mg/m2 d1,8, orally, q3w | 77 [70–90] | 21 (18.6) | Asian (100.0) | 24 (19.5) | 73 (64.6) | Yes |
| Wu [2013] (48) | 451 | Cisplatin/carboplatin + gemcitabine + erlotinib 150 mg/d, d15-28, orally | (Cisplatin 75 mg/m2 d1 or carboplatin (AUC =5) d1) +Gemcitabine 1,250 mg/m2 d1,8, iv, q4w×6 cycles | 57 [31–96] | 179 (39.7) | Asian (100.0) | 219 (52.8) | 342 (75.8) | Yes |
| Jänne [2012] (49) | 181 | Erlotinib 150 mg/d + paclitaxel 200 mg/m2 d1 carboplatin (AUC =6) d1, iv, q3w ×6 cycles | Erlotinib 150 mg/d, orally | 59 [32–81] | 107 (59.0) | White (80.0) | 143 (79.0) | 155 (86.0) | Yes |
| Lee [2012] (50) | 670 | Erlotinib 150 mg/d, orally | Placebo | 77 [72–82] | 261 (39.0) | White (97.0) | 37 (5.5) | 256 (38.2) | Yes |
| Butts [2007] (51) | 131 | Cisplatin/carboplatin + gemcitabine + cetuximab 400 mg/m2 on day 1, 250 mg/m2 weekly | Cisplatin 75 mg/m2d1 + gemcitabine 1,250 mg/m2 d1,8 or carboplatin (AUC =5) d1+ gemcitabine 1,000 mg/m2 d1,8, iv, q3w ×6 cycles | 64 [35–84] | 73 (55.7) | White (83.2) | 19 (14.5) | 61 (46.6) | Yes |
| Lynch [2010] (7) | 676 | Paclitaxel/docetaxel + carboplatin + cetuximab 400 mg/m2 on day 1, 250 mg/m2 weekly | (Paclitaxel 225 mg/m2 or docetaxel 75 mg/m2) + carboplatin (AUC =5) d1, iv, q3w ×≤6 cycle | 64 [34–87] | 280 (41.4) | White (88.1) | 53 (7.8) | 354 (52.4) | Yes |
d1, day 1; iv, intravenously; q3w, every 3 weeks; AUC, area under the curve. NR, not reported; CT, chemotherapy.
The quality of each eligible trial and other risks of bias were evaluated using Cochrane Collaboration’s tool, 14/24 studies were reported as high quality and the remaining 10 studies as acceptable quality (Table S1). Based on the GRADE criteria, the overall quality of the evidence about ORR, neutropenia, rash and diarrhea were rated as moderate, and the quality of the evidence about thrombocytopenia and anemia were rated as low (Table S2).
Direct comparisons
Pairwise comparisons were accomplished for the nine different comparisons. The number of patients who achieved ORR was reported in 24 studies. Grade ≥3 rash, anemia, diarrhea, neutropenia and thrombocytopenia were reported in 19 studies (7,10,24,34-46,48,49,51), 21 studies (7,9,10,24,33-45,48-51), 19 studies (7,24,34-46,48-51), 19 studies (7,9,10,24,32-36,38-42,44,45,48,49,51) and 16 studies (7,9,10,24,32-34,38,39,41,42,44,45,48,49,51) respectively. ORs and heterogeneity by I2 were listed in Table 2. For unselected patients, Bev + CT (OR =2.19; 95% CI, 1.55–3.11; P<0.001), Erl + CT (OR =1.64; 95% CI, 1.05–2.57; P=0.031) and Cet + CT (OR =1.68; 95% CI, 1.96–2.36; P=0.003) were associated with statistically significantly higher incidence of ORR than CT. The estimated OR for Gef + CT and Gef compared with CT showed a consistent trend for higher ORR, although they did not reach statistical significant. However, Erl was associated with inferior efficacy compared with CT (OR =0.81; 95% CI, 0.23–2.78; P=0.735).
Table 2. The odds ratios and heterogeneity for direct comparisons.
| Outcome | No. of studies | Events/total | OR (95% CI) | P | I2 (%) |
|---|---|---|---|---|---|
| CT vs. Bev + CT | |||||
| ORR | 5 | 181/896 vs. 355/949 | 2.19 (1.55–3.11) | <0.001 | 52.7 |
| Neutropenia | 5 | 235/927 vs. 349/973 | 1.53 (0.95–2.47) | 0.082 | 62.4 |
| Rash | 1 | 2/440 vs. 10/427 | 5.25 (1.14–24.11) | 0.033 | – |
| Thrombocytopenia | 5 | 82/927 vs. 96/973 | 1.15 (0.83–1.60) | 0.390 | 55.8 |
| Anemia | 4 | 52/869 vs. 40/854 | 0.67 (0.17–2.64) | 0.577 | 49.7 |
| CT vs. Erl + CT | |||||
| ORR | 5 | 355/1,478 vs. 446/1,469 | 1.64 (1.05–2.57) | 0.031 | 82.5 |
| Neutropenia | 5 | 234/1,149 vs. 229/1,141 | 0.98 (0.78–1.24) | 0.903 | 10.1 |
| Rash | 5 | 15/1,149 vs. 105/1,141 | 7.64 (2.64–22.08) | <0.001 | 56.2 |
| Diarrhea | 5 | 10/1,149 vs. 55/1,141 | 5.33 (2.78–10.21) | <0.001 | 26.4 |
| Thrombocytopenia | 4 | 126/1,088 vs. 142/1,089 | 1.43 (0.88–1.47) | 0.310 | 0.0 |
| Anemia | 5 | 116/1,149 vs. 150/1,141 | 1.35 (1.04–1.75) | 0.023 | 0.0 |
| CT vs. Gef + CT | |||||
| ORR | 2 | 252/669 vs. 277/681 | 1.13 (0.91–1.41) | 0.277 | 0.0 |
| Neutropenia | 2 | 37/696 vs. 44/704 | 1.19 (0.76–1.87) | 0.453 | 0.0 |
| Rash | 2 | 9/696 vs. 24/704 | 2.67 (1.23–5.81) | 0.013 | 0.0 |
| Diarrhea | 2 | 18/696 vs. 47/704 | 2.71 (1.56–4.71) | <0.001 | 48.6 |
| Thrombocytopenia | 1 | 20/355 vs. 21/362 | 1.03 (0.55–1.94) | 0.923 | – |
| Anemia | 2 | 8/696 vs. 9/704 | 1.11 (0.42–2.90) | 0.832 | 0.0 |
| CT vs. Gef | |||||
| ORR | 4 | 273/898 vs. 353/908 | 1.41 (0.99–2.01) | 0.055 | 31.5 |
| Neutropenia | 4 | 518/864 vs. 28/895 | 0.02 (0.01–0.05) | <0.001 | 38.6 |
| Rash | 4 | 10/876 vs. 69/903 | 4.56 (1.05–19.75) | 0.042 | 68.1 |
| Diarrhea | 4 | 15/876 vs. 33/903 | 2.19 (1.18–4.07) | 0.013 | 0.0 |
| Thrombocytopenia | 1 | 13/150 vs. 2/159 | 0.13 (0.03–0.61) | 0.009 | – |
| Anemia | 4 | 86/864 vs. 16/895 | 0.17 (0.10–0.29) | <0.001 | 0.0 |
| Placebo vs. Gef | |||||
| ORR | 1 | 1/101 vs. 6/100 | 6.38 (0.75–54.02) | 0.089 | – |
| Diarrhea | 1 | 3/101 vs. 3/100 | 1.01 (0.20–5.13) | 0.990 | – |
| Anemia | 1 | 0/101 vs. 3/100 | 7.28 (0.37–143.92) | 0.191 | – |
| Placebo vs. Erl | |||||
| ORR | 2 | 31/765 vs. 67/788 | 2.27 (1.46–3.53) | <0.001 | 0.0 |
| Rash | 1 | 0/445 vs. 37/433 | 84.27 (5.16–1,376.79) | 0.002 | – |
| Diarrhea | 2 | 4/758 vs. 35/767 | 8.04 (2.98–21.65) | <0.001 | 0.0 |
| Anemia | 1 | 3/313 vs. 6/334 | 1.89 (0.47–7.62) | 0.371 | – |
| CT vs. Erl | |||||
| ORR | 3 | 135/487 vs. 92/489 | 0.81 (0.23–2.78) | 0.735 | 78.9 |
| Neutropenia | 1 | 79/368 vs. 42/372 | 0.47 (0.31–0.70) | <0.001 | – |
| Rash | 2 | 26/419 vs. 44/424 | 2.13 (0.57–8.01) | 0.26 | 29.0 |
| Diarrhea | 2 | 2/419 vs. 23/424 | 9.78 (2.63–36.30) | 0.001 | 0.0 |
| Thrombocytopenia | 1 | 44/368 vs. 39/372 | 0.86 (0.55–1.36) | 0.526 | – |
| Anemia | 2 | 34/419 vs. 19/424 | 0.53 (0.29–0.95) | 0.032 | 0.0 |
| Erl vs. Erl + CT | |||||
| ORR | 1 | 28/81 vs. 46/100 | 1.61 (0.88–2.95) | 0.121 | – |
| Neutropenia | 1 | 0/81 vs. 41/100 | 113.7 (6.86–1,885.30) | 0.001 | – |
| Rash | 1 | 6/81 vs. 10/100 | 1.39 (0.48–4.00) | 0.543 | – |
| Diarrhea | 1 | 4/81 vs. 7/100 | 1.45 (0.41–5.13) | 0.566 | – |
| Thrombocytopenia | 1 | 0/81 vs. 5/100 | 9.38 (0.51–172.34) | 0.131 | – |
| Anemia | 1 | 1/81 vs. 7/100 | 6.02 (0.72–50.00) | 0.096 | – |
| CT vs. Cet + CT | |||||
| ORR | 2 | 70/404 vs. 105/403 | 1.68 (1.96–2.36) | 0.003 | 0.0 |
| Neutropenia | 2 | 209/386 vs. 229/389 | 1.21 (0.91–1.61) | 0.190 | 0.0 |
| Rash | 2 | 0/386 vs. 43/389 | 42.18 (5.70–312.13) | <0.001 | 0.0 |
| Diarrhea | 2 | 8/386 vs. 20/389 | 2.46 (1.09–5.55) | 0.029 | 0.0 |
| Thrombocytopenia | 2 | 58/386 vs. 70/389 | 1.32 (0.87–2.02) | 0.184 | 0.0 |
| Anemia | 2 | 28/386 vs. 34/389 | 1.26 (0.74–2.16) | 0.397 | 0.0 |
The reference of OR is treatment arm in the left column. ORR, objective response rate; Bev, bevacizumab; Gef, gefitinib; Erl, erlotinib; Cet, cetuximab; CT, chemotherapy.
In terms of rash and diarrhea, Erl + CT, Gef + CT, Cet + CT and Gef were associated with significantly greater odds compared with CT. While CT showed statistically significantly more incidence of neutropenia and anemia compared to Gef and Erl. The risk of thrombocytopenia did not show any statistically significant difference among all the treatment arms except CT vs. Gef (OR =0.13; 95% CI, 0.03–0.61; P=0.009) (Table 2).
An estimate consistent with large heterogeneity (I2>50%) was seen in three comparisons for ORR, two comparisons for rash, one comparison for neutropenia and one comparison for thrombocytopenia, while no large heterogeneity was seen in comparisons concerning anemia and diarrhea (Table 2).
Network meta-analysis for efficacy and toxicities
From the eligible studies, 28 indirect comparisons were made, and the network geometry of ORR was described in Figure 2. ORs and credibility interval for all possible comparisons were calculated by Bayesian network meta-analysis (Table 3). According to the results, Bev + CT had a statistically significantly higher incidence of ORR relative to the other six difference treatments, including placebo, Erl, CT, Gef, Erl + CT and Gef + CT, in contrast, placebo and Erl were associated with inferior ORR. Although no significant differences were observed among Bev + CT vs. Cet + CT and Erl + CT vs. Gef + CT, Bev + CT showed a trend of higher ORR than Cet + CT and Erl + CT showed a trend of higher ORR than Gef + CT.
Figure 2.
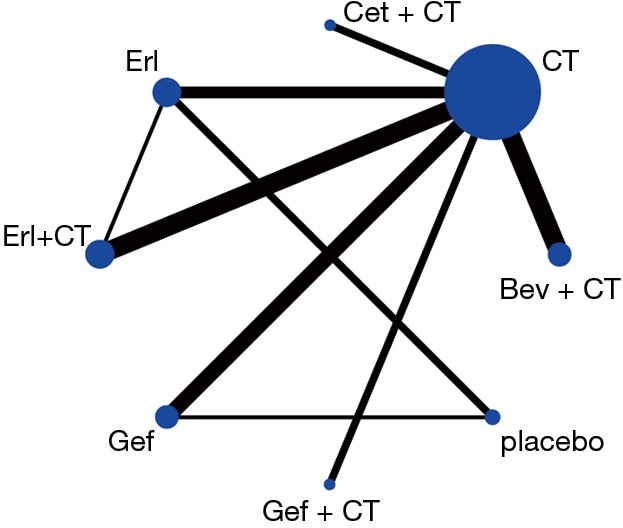
Network of studies comparing objective response rate of different agents for unselected patients with advanced non-small cell lung cancer. Each link represents at least one study, width of each link is number of trials per comparison, size of each node is proportional to the total sample size. CT, chemotherapy; Bev, bevacizumab; Gef, gefitinib; Erl, erlotinib; Cet, cetuximab.
Table 3. Multiple treatment comparison for efficacy based on network meta-analysis.
| Treatment | Comparator |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | CT | Erl | Gef | Erl + CT | Gef + CT | Bev + CT | Cet + CT | |
| ORR | ||||||||
| Placebo | 1 | 3.36 (2.07–5.25) | 2.30 (1.51–3.43) | 4.66 (2.80–7.38) | 4.46 (2.72–7.04) | 3.72 (2.22–5.99) | 6.47 (3.85–10.29) | 5.42 (3.01–9.13) |
| CT | 0.31 (0.19–0.48) | 1 | 0.69 (0.54–0.87) | 1.39 (1.18–1.62) | 1.33 (1.16–1.52) | 1.11 (0.93–1.31) | 1.92 (1.61–2.28) | 1.61 (1.18–2.16) |
| Erl | 0.45 (0.29–0.67) | 1.46 (1.15–1.84) | 1 | 2.03 (1.51–2.66) | 1.94 (1.49–2.48) | 1.62 (1.20–2.15) | 2.81 (2.08–3.70) | 2.35 (1.58–3.39) |
| Gef | 0.23 (0.13–0.36) | 0.73 (0.62–0.85) | 0.50 (0.38–0.66) | 1 | 0.96 (0.78–1.18) | 0.80 (0.63–1.01) | 1.40 (1.10–1.75) | 1.17 (0.82–1.63) |
| Erl + CT | 0.24 (0.14–0.37) | 0.76 (0.66–0.86) | 0.52 (0.40–0.67) | 1.05 (0.85–1.28) | 1 | 0.84 (0.67–1.04) | 1.46 (1.17–1.80) | 1.22 (0.86–1.68) |
| Gef + CT | 0.28 (0.16–0.45) | 0.91 (0.76–1.08) | 0.63 (0.46–0.83) | 1.26 (0.99–1.59) | 1.21 (0.96–1.50) | 1 | 1.75 (1.36–2.22) | 1.46 (1.01–2.05) |
| Bev + CT | 0.17 (0.10–0.26) | 0.53 (0.44–0.62) | 0.37 (0.27–0.48) | 0.73 (0.57–0.91) | 0.70 (0.56–0.86) | 0.58 (0.45–0.74) | 1 | 0.85 (0.59–1.18) |
| Cet + CT | 0.20 (0.11–0.33) | 0.64 (0.47–0.86) | 0.44 (0.30–0.64) | 0.89 (0.62–1.23) | 0.85 (0.60–1.17) | 0.71 (0.50–0.99) | 1.23 (0.86–1.72) | 1 |
| Rash | ||||||||
| Placebo | 1 | 1.3E7 (44.15–1.2E8) | 2.7E7 (94.78–2.3E8) | 1.1E8 (321.59–9.9E8) | 9.1E7 (292.3–7.6E8) | 3.8E7 (116.3–3.5E8) | 3.9E7 (127.2–3.7E8) | 1.9E28 (1.2E5–3.9E28) |
| CT | 3E–4 (0.00–1.4E–3) | 1 | 2..09 (1.29–3.26) | 8.15 (4.06–15.73) | 7.06 (4.29–11.43) | 3.00 (1.30–6.30) | 3.37 (1.02–8.96) | 2.2E33 (1.2E9–3.5E33) |
| Erl | 8E–4 (0.00–7.5E–3) | 0.50 (0.30–0.78) | 1 | 4.11 (1.69–8.94) | 3.54 (1.83–6.39) | 1.52 (0.56–3.48) | 1.69 (0.44–4.68) | 2.7E21 (37.43–3.4E21) |
| Gef | 4E–4 (0.00–3.6E–3) | 0.14 (0.06–0.25) | 0.29 (0.11–0.60) | 1 | 0.98 (0.36–2.05) | 0.41 (0.12–1.03) | 0.46 (0.10–1.39) | 3.4E32 (134–9.9E32) |
| Erl + CT | 3E–4 (0.00–3.0E–3) | 0.15 (0.09–0.24) | 0.32 (0.16–0.56) | 1.25 (0.51–2.69) | 1 | 0.46 (0.17–1.05) | 0.51 (0.14–1.41) | 2.5E22 (50.35–1.9E23) |
| Gef + CT | 1.8E–3 (0.00–0.02) | 0.39 (0.16–0.76) | 0.82 (0.29–1.82) | 3.10 (0.96–7.57) | 2.76 (0.97–6.12) | 1 | 1.30 (0.27–3.95) | 2.6E23 (24.16–1.8E24) |
| Bev + CT | 1.2E–3 (0.00–0.01) | 0.44 (0.12–1.11) | 0.93 (0.22–2.48) | 3.53 (0.76–10.52) | 3.04 (0.76–8.28) | 1.31(0.26–4.08) | 1 | 1.9E16 (63.44–1.3E17) |
| Cet + CT | 0.00 (0.00–0.00) | 0.00 (0.00–0.03) | 0.01 (0.00–0.07) | 0.03 (0.00–0.27) | 0.03 (0.00–0.23) | 0.01 (0.00–0.10) | 0.01 (0.00–0.11) | 1 |
| Anemia | ||||||||
| Placebo | 1 | 13.03 (2.44–46.19) | 5.83 (1.16–20.16) | 2.38 (0.42–8.52) | 17.49 (3.17–62.92) | 16.53 (1.96–67.28) | 10.17 (1.75–36.80) | 16.89 (2.80–63.12) |
| CT | 0.14 (0.02–0.43) | 1 | 0.47 (0.26–0.77) | 0.19 (0.11–0.30) | 1.34 (1.05–1.70) | 1.26 (0.42–2.98) | 0.78 (0.51–1.15) | 1.30 (0.76–2.09) |
| Erl | 0.30 (0.05–0.89) | 2.28 (1.29–3.79) | 1 | 0.42 (0.19–0.82) | 3.05 (1.65–5.28) | 2.88 (0.80–7.56) | 1.78 (0.86–3.34) | 2.95 (1.33–5.76) |
| Gef | 0.77 (0.11–2.39) | 5.74 (3.39–9.46) | 2.69 (1.20–5.27) | 1 | 7.71 (4.30–13.22) | 7.34 (2.08–19.23) | 4.50 (2.22–8.34) | 7.45 (3.44–14.48) |
| Erl + CT | 0.10 (0.02–0.32) | 0.76 (0.59–0.95) | 0.35 (0.19–0.60) | 0.14 (0.08–0.24) | 1 | 0.96 (0.31–2.33) | 0.59 (0.36–0.91) | 0.98 (0.54–1.65) |
| Gef + CT | 0.14 (0.01–0.53) | 1.03 (0.33–2.42) | 0.48 (0.13–1.25) | 0.19 (0.05–0.49) | 1.38 (0.43–3.31) | 1 | 0.80 (0.23–2.00) | 1.33 (0.37–3.38) |
| Bev + CT | 0.18 (0.03–0.59) | 1.34 (0.87–1.97) | 0.63 (0.30–1.15) | 0.25 (0.12–0.45) | 1.79 (1.08–2.79) | 1.70 (0.51–4.27) | 1 | 1.73 (0.86–3.14) |
| Cet + CT | 0.11 (0.02-0.37) | 0.83 (0.48-1.33) | 0.39 (0.17-0.76) | 0.15 (0.07-0.29) | 1.11 (0.61-1.87) | 1.05 (0.30-2.72) | 0.65 (0.32-1.17) | 1 |
| Diarrhea | ||||||||
| Placebo | 1 | 1.02 (0.30–2.70) | 8.35 (3.33–19.93) | 2.11 (0.59–5.68) | 7.47 (1.99–20.92) | 2.88 (0.70–8.22) | – | 2.93 (0.59–9.29) |
| CT | 1.35 (0.38–3.40) | 1 | 9.77 (3.88–21.52) | 2.14 (1.15–3.72) | 7.57 (3.76–14.34) | 2.81 (1.59–4.77) | – | 2.86 (1.17–6.25) |
| Erl | 0.15 (0.05–0.30) | 0.13 (0.05–0.26) | 1 | 0.26 (0.09–0.62) | 0.90 (0.33–1.97) | 0.36 (0.11–0.85) | – | 0.36 (0.09–1.00) |
| Gef | 0.67 (0.18–1.75) | 0.51 (0.27–0.88) | 4.92 (1.64–11.92) | 1 | 3.91 (1.50–8.51) | 1.45 (0.59–2.99) | – | 1.47 (0.47–3.61) |
| Erl + CT | 0.19 (0.05–0.50) | 0.15 (0.07–0.26) | 1.37 (0.52–3.01) | 0.32 (0.12–0.68) | 1 | 0.42 (0.16–0.89) | – | 0.43 (0.13–1.10) |
| Gef + CT | 0.52 (0.12–1.44) | 0.38 (0.21–0.63) | 3.71 (1.16–9.27) | 0.82 (0.33–1.70) | 2.92 (1.10–6.44) | 1 | – | 1.10 (0.35–2.70) |
| Cet + CT | 0.55 (0.11–1.63) | 0.42 (0.16–0.86) | 4.04 (0.97–10.96) | 0.90 (0.28–2.13) | 3.16 (0.92–7.73) | 1.19 (0.38–2.80) | – | 1 |
| Neutropenia | ||||||||
| CT | – | 1 | 0.34 (0.23–0.47) | 0.03 (0.02–0.05) | 1.07 (0.89–1.28) | 1.22 (0.76–1.85) | 1.35 (1.14–1.60) | 1.15 (0.94–1.38) |
| Erl | – | 3.05 (2.13–4.31) | 1 | 0.10 (0.06–0.16) | 3.26 (2.22–4.70) | 3.71 (2.02–6.29) | 4.13 (2.75–6.03) | 3.50 (2.30–5.16) |
| Gef | – | 32.20 (21.85–46.71) | 10.84 (6.22–17.66) | 1 | 34.51 (22.41–51.74) | 39.12 (21.18–67.37) | 43.61 (28.34–65.27) | 36.90 (23.70–55.70) |
| Erl + CT | – | 0.94 (0.78–1.12) | 0.32 (0.21–0.45) | 0.03 (0.02–0.04) | 1 | 1.14 (0.69–1.79) | 1.28 (0.99–1.62) | 1.08 (0.82–1.39) |
| Gef + CT | – | 0.86 (0.54–1.32) | 0.29 (0.16–0.49) | 0.03 (0.01–0.05) | 0.93 (0.56–1.45) | 1 | 1.17 (0.71–1.83) | 0.99 (0.59–1.57) |
| Bev + CT | – | 0.74 (0.62–0.88) | 0.25 (0.16–0.36) | 0.02 (0.01–0.04) | 0.80 (0.62–1.01) | 0.90 (0.55–1.41) | 1 | 0.85 (0.66–1.10) |
| Cet + CT | – | 0.88 (0.72–1.06) | 0.30 (0.19–0.43) | 0.03 (0.02–0.04) | 0.94 (0.72–1.22) | 1.07 (0.64–1.68) | 1.19 (0.91–1.53) | 1 |
| Thrombocytopenia | ||||||||
| CT | – | 1 | 0.81 (0.52–1.21) | 0.15 (0.02–0.46) | 1.16 (0.91–1.46) | 1.09 (0.55–1.92) | 1.16 (0.85–1.55) | 1.32 (0.91–1.86) |
| Erl | – | 1.29 (0.82–1.92) | 1 | 0.19 (0.02–0.63) | 1.50 (0.89–2.34) | 1.40 (0.62–2.74) | 1.49 (0.86–2.41) | 1.70 (0.94–2.84) |
| Gef | – | 21.20 (2.23–101.60) | 17.33 (1.65–81.56) | 1 | 24.59 (2.49–119.80) | 22.98 (2.01–114.30) | 24.73 (2.41–119.70) | 28.00(2.78–134.60) |
| Erl + CT | – | 0.88 (0.69–1.10) | 0.71 (0.43–1.11) | 0.13 (0.01–0.41) | 1 | 0.95 (0.46–1.74) | 1.02 (0.68–1.46) | 1.16 (0.74–1.73) |
| Gef + CT | – | 1.01 (0.52–1.77) | 0.82 (0.36–1.61) | 0.15 (0.01–0.52) | 1.17 (0.58–2.13) | 1 | 1.17 (0.56–2.19) | 1.33 (0.62–2.53) |
| Bev + CT | – | 0.88 (0.65–1.17) | 0.72 (0.41–1.16) | 0.13 (0.01–0.42) | 1.02 (0.69–1.46) | 0.96 (0.46–1.78) | 1 | 1.16 (0.72–1.79) |
| Cet + CT | – | 0.78 (0.54–1.09) | 0.63 (0.35–1.05) | 0.12 (0.01–0.37) | 0.90 (0.58–1.35) | 0.84 (0.39–1.60) | 0.90 (0.55–1.39) | 1 |
ORR, objective response rate; Bev, bevacizumab; Gef, gefitinib; Erl, erlotinib; Cet, cetuximab; CT, chemotherapy.
We selected rash, anemia, diarrhea, neutropenia and thrombocytopenia, which were the most common toxicities, as the representative of targeted drugs-related toxicities. The resluts showed that patients who received Cet + CT experienced more severe rash compared with the other seven treatments. Significantly increased odds for anemia observed in patients treated with Erl + CT compared to those treated with placebo, CT, Erl, Gef and Bev + CT. Erl had greater odds of diarrhea over four other agents: placebo, CT, Gef, Gef + CT. Bev + CT was associated with significantly greater odds for neutropenia compared to CT, Erl and Gef. In terms of thrombocytopenia, Cet + CT, Bev + CT, Gef + CT, Erl + CT, Erl and CT were significant severer than Gef while no other significant differences were observed among the rest comparisons (Table 3).
Ranking of treatment arms
Table 4 showed the rank probabilities among all the treatments, agents with greater value in the histogram were associated with greater probabilities for higher rank. This analysis indicated that Bev + CT had the highest probability of being the best treatment arm for ORR, followed by Cet + CT and Gef. In contrast, CT, Erl and placebo were associated with relatively inferior ORR rankings compared with other agents. Meanwhile, we could see that Bev + CT was associated with the highest risk for neutropenia and second risk for thrombocytopenia. Cet + CT was most probable to be the rank 1 for rash and thrombocytopenia, and the rank 2 for anemia. Gef was found to be associated with relatively low risk for grade ≥3 toxicities.
Table 4. Rank probabilities of each drug for different outcomes based on network meta-analysis.
| Treatment | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 |
|---|---|---|---|---|---|---|---|---|
| ORR | ||||||||
| Placebo | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
| CT | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | 0.86 | 0.00 | 0.00 |
| Erl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 |
| Gef | 0.00 | 0.17 | 0.51 | 0.29 | 0.02 | 0.00 | 0.00 | 0.00 |
| Erl + CT | 0.00 | 0.07 | 0.34 | 0.55 | 0.04 | 0.00 | 0.00 | 0.00 |
| Gef + CT | 0.00 | 0.00 | 0.01 | 0.07 | 0.78 | 0.13 | 0.00 | 0.00 |
| Bev + CT | 0.85 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Cet + CT | 0.15 | 0.61 | 0.14 | 0.09 | 0.02 | 0.00 | 0.00 | 0.00 |
| Rash | ||||||||
| Placebo | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 |
| CT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.96 | 0.00 |
| Erl | 0.00 | 0.00 | 0.00 | 0.11 | 0.36 | 0.53 | 0.00 | 0.00 |
| Gef | 0.00 | 0.58 | 0.35 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 |
| Erl + CT | 0.00 | 0.38 | 0.55 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gef + CT | 0.00 | 0.01 | 0.03 | 0.38 | 0.37 | 0.20 | 0.00 | 0.00 |
| Bev + CT | 0.00 | 0.03 | 0.06 | 0.38 | 0.26 | 0.22 | 0.03 | 0.00 |
| Cet + CT | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Anemia | ||||||||
| Placebo | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.23 | 0.76 |
| CT | 0.00 | 0.07 | 0.41 | 0.47 | 0.05 | 0.00 | 0.00 | 0.00 |
| Erl | 0.00 | 0.00 | 0.00 | 0.01 | 0.10 | 0.87 | 0.02 | 0.00 |
| Gef | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.75 | 0.24 |
| Erl + CT | 0.44 | 0.39 | 0.17 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 |
| Gef + CT | 0.15 | 0.14 | 0.31 | 0.17 | 0.18 | 0.05 | 0.00 | 0.00 |
| Bev + CT | 0.00 | 0.02 | 0.06 | 0.23 | 0.63 | 0.06 | 0.00 | 0.00 |
| Diarrhea | ||||||||
| Placebo | 0.00 | 0.00 | 0.04 | 0.06 | 0.12 | 0.39 | 0.40 | – |
| CT | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.40 | 0.59 | – |
| Erl | 0.67 | 0.30 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Gef | 0.00 | 0.00 | 0.12 | 0.28 | 0.49 | 0.11 | 0.00 | – |
| Erl + CT | 0.32 | 0.64 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | – |
| Gef + CT | 0.00 | 0.02 | 0.42 | 0.37 | 0.16 | 0.03 | 0.00 | – |
| Cet + CT | 0.01 | 0.04 | 0.36 | 0.29 | 0.22 | 0.08 | 0.01 | – |
| Neutropenia | ||||||||
| CT | 0.00 | 0.00 | 0.08 | 0.38 | 0.54 | 0.00 | 0.00 | – |
| Erl | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | 0.00 | – |
| Gef | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | – |
| Erl + CT | 0.01 | 0.13 | 0.32 | 0.34 | 0.19 | 0.00 | 0.00 | – |
| Gef + CT | 0.28 | 0.25 | 0.16 | 0.10 | 0.21 | 0.00 | 0.00 | – |
| Bev + CT | 0.64 | 0.30 | 0.05 | 0.01 | 0.00 | 0.00 | 0.00 | – |
| Cet + CT | 0.06 | 0.31 | 0.39 | 0.17 | 0.07 | 0.00 | 0.00 | – |
| Thrombocytopenia | ||||||||
| CT | 0.00 | 0.02 | 0.14 | 0.42 | 0.37 | 0.05 | 0.00 | – |
| Erl | 0.01 | 0.02 | 0.04 | 0.06 | 0.19 | 0.67 | 0.00 | – |
| Gef | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.00 | – |
| Erl + CT | 0.14 | 0.30 | 0.30 | 0.17 | 0.07 | 0.01 | 0.00 | – |
| Gef + CT | 0.19 | 0.14 | 0.12 | 0.11 | 0.22 | 0.22 | 0.00 | – |
| Bev + CT | 0.17 | 0.27 | 0.26 | 0.17 | 0.10 | 0.03 | 0.00 | – |
| Cet + CT | 0.49 | 0.25 | 0.14 | 0.07 | 0.04 | 0.01 | 0.00 | – |
ORR, objective response rate; Bev, bevacizumab; Gef, gefitinib; Erl, erlotinib; Cet, cetuximab; CT, chemotherapy.
Discussion
During the past few years, therapies for advanced NSCLC have significantly changed due to the development of molecular targeted drugs, either receptor monoclonal antibodies (mAb) or small molecule TKI (4). Through the identification of epigenetic mutations, tumour suppressor gene inactivation as well as oncogene driver mutations, they can provide more accurate therapeutic targets. Selection of driver genes is essential in targeted therapy, however, in routine clinical practice, a considerable number of patients are unable to provide adequate tissue samples for accurate genotyping. Although ctDNA or CTC would be a reliable method to detect mutations, its specificity, sensitivity and costs still need to be assessed (20,21). For the vast majority at present, no known drivers were detected and such patients were still empirically treated with standard cytotoxic chemotherapy. This network meta-analysis showed that Bev + CT offered superior ORR compared with other included regimens in treating patients with locally advanced or metastatic NSCLC without a known driver mutation.
Although other systematic reviews and meta-analysis have been conducted to evaluate the benefits of chemotherapy and targeted therapy in advanced NSCLC (52,53), direct head to head comparisons between these agents have not been well established, especially in unselected patients with advanced NSCLC. Unique to this analysis, multiple-treatments comparisons were used to accomplish a mixed-treatments analysis and obtained the information on the effectiveness of each agents. Our findings were similar to previous publications (44,53). A recent pooled analysis of available studies was performed to evaluate the efficacy of bevacizumab compared with other targeted drugs in patients with advanced NSCLC, they demonstrated that bevacizumab with chemotherapy significantly improved patients’ ORR among chemotherapy-native patients compared with other targeted drugs, which was consistent with our direct and indirect comparisons (52). However, it did not compare effect among other targeted drugs, nor did it explore the toxicity. In addition, treatment-line might affect the efficacy of TKIs, some previous studies found inferior response to EGFR-TKIs following chemotherapy exposure (24,25). Therefore, in order to minimize the crossover effects, we conducted a systematic review and network meta-analysis to assess the substantial differences among these first-line treatments in unselected patients with advanced NSCLC.
Moreover, Bayesian statistical model could also help us rank these regimens to determine which one is most likely to be the best or the worst, especially when the relative values fail to reach statistical significance (30). In this study, although no statistically significant differences between Bev + CT and Cet + CT in terms of ORR, Bev + CT arm had the greatest probability to rank the first, followed by Cet + CT. The formation of new blood vessels played an important role in the growth and invasiveness of primary tumors, vascular endothelial growth factor (VEGF) was a key potential target for the pharmacological inhibition of tumour angiogenesis, which may explain the relative good efficacy of bevacizumab (anti-VEGF monoclonal antibody) in the treatment of unselected patients with advanced NSCLC, in some ways. In regards to safety, although Bev + CT and Cet + CT presented potentially better efficacy, they were associated with severer rash, thrombocytopenia and neutropenia. Gef was probable to be the rank 3 for ORR and was associated with relatively low risk for grade ≥3 toxicities. Therefore, Gef therapy may remain as one of the options for patients with unknown driver mutation, particularly considering the rising cost of targeted drugs and limited medical resources.
The conclusion of this study will lead us to the argument about whether the targeted drugs should be used in clinical practice to have the best outcome as a whole. Several points needed to be considered. Firstly, cetuximab was not licensed in other countries except for the US. National Comprehensive Cancer Network (NCCN)-NSCLC guidelines showed that EGFR TKIs should be employed only in patients harboring EGFR-activating mutations. Bevacizumab was indicated as treatment for naïve patients. Secondly, in this study, we only analyzed ORR and toxicity as an efficacy, whether the increase of ORR with drugs would be translated into survival benefit was still not clear. A recent pooled analysis showed that a strong correlation between ORR and improved PFS and overall survival (OS) in chemotherapy-naïve patients treated with bevacizumab (52). In contrast, Boutsikou et al. (34) reported that administration of bevacizumab was associated with higher ORR compared with chemotherapy, but it did not translate into longer OS. This conflicting result indicated that data regarding ORR should be interpreted with caution, the surrogacy relation of ORR with survival data would be confirmed. We are currently planning to collect all relevant PFS and OS data to make up for our shortcomings.
Nevertheless, our network meta-analysis showed the different efficacy and safety of these included regimens from the available evidence. At the same time, several limitations needed to be considered. First, the number of studies included was relatively small. The indirect estimates were often very similar to the direct comparisons due to only single comparison was available. For example, the informative value of the direct comparison Cet + CT arms was limited by low number of events. Additionally, the established networks lacked sufficient direct comparisons between combination therapies. These resulted in trials’ heterogeneity. Second, given the retrospective nature of meta-analysis, publication bias and selection bias cannot be excluded. And many potentially important differences among these studies, including different dosage and administration schedules of targeted drugs and chemotherapy. Moreover, the treatment designs were not same in all arms. All of these would increase the clinical heterogeneity among included trials. Third, different baselines of trial populations, such as age, gender, ethnicity, interventions, comorbidities, and differences in other possible prognostic factors, these may introduce potential confounding and bias to the analysis. Fourth, due to a large proportion of trials were open label, an inherent risk of bias in the individual trial was introduced. Finally, this study only analyzed ORR and adverse events as an efficacy, progression free survival and OS data needed to be assessed in the future study.
In summary, our study suggested that the use of bevacizumab in combination with chemotherapy in the treatment of unselected patients with advanced NSCLC may offer a greater ORR and moderate toxicity. We hope this network meta-analysis may guide physicians in the therapeutic decision-making.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (grant number 81560451), Kunming University of Science and Technology Talent Introduction Fund (grant number KKSY201560001) and National Laboratory Special Fund (grant number 2060204).
Table S1. Quality assessment: risk of bias by Cochrane collaboration’s tool.
| Trial | Sequence generation | Allocation concealment | Blinding | Incomplete outcome data | Selective reporting | Other source of bias |
|---|---|---|---|---|---|---|
| Sandler [2006] (10) | Adequate | Inadequate | Unclear | Adequate | Adequate | – |
| Reck [2010] (9) | Adequate | Adequate | Adequatez | Adequate | Adequate | – |
| Niho [2012] (32) | Adequate | Adequate | Unclear | Adequate | Adequate | – |
| Soria [2011] (33) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Boutsikou [2013] (34) | Adequate | Inadequate | Unclear | Adequate | Unclear | – |
| Herbst [2004] (35) | Adequate | Inadequate | Unclear | Inadequate | Adequate | – |
| Crinò [2008] (36) | Adequate | Adequate | Inadequate | Adequate | Adequate | Vinorelbine is a less potent comparator; included only elderly patients |
| Goss [2009] (37) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Giaccone [2004] (38) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Morère [2010] (39) | Adequate | Inadequate | Unclear | Adequate | Adequate | Biased baseline characteristic: docetaxel arm had more never-smokers (14.3% vs. 4.7%) |
| Fukuoka [2011] (40) | Adequate | Adequate | Inadequate | Adequate | Adequate | – |
| Han [2012] (41) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Herbst [2005] (42) | Adequate | Inadequate | Unclear | Adequate | Adequate | – |
| Lilenbaum [2008] (43) | Adequate | Adequate | Unclear | Adequate | Adequate | – |
| Mok [2009] (44) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Gatzemeier [2007] (45) | Adequate | Adequate | Inadequate | Adequate | Adequate | – |
| Cappuzzo [2010] (46) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Gridelli [2012] (24) | Adequate | Adequate | Unclear | Adequate | Adequate | – |
| Chen [2012] (47) | Adequate | Adequate | Adequate | Adequate | Unclear | Vinorelbine is a less potent comparator; included only elderly patients |
| Wu [2013] (48) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Jänne [2012] (49) | Adequate | Adequate | Unclear | Adequate | Adequate | – |
| Lee [2012] (50) | Adequate | Adequate | Adequate | Adequate | Adequate | – |
| Butts [2007] (51) | Adequate | Inadequate | Unclear | Adequate | Adequate | – |
| Lynch [2010] (7) | Adequate | Inadequate | Unclear | Adequate | Adequate | – |
Table S2. GRADE summary of findings.
| Outcomes | No. of participants (studies) follow up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects |
|
|---|---|---|---|---|---|
| Risk with control | Risk difference with experiment (95% CI) | ||||
| Objective response rate | 11,666 (24 studies) | ⊕⊕⊕⊝; moderate (due to inconsistency) | RR 1.554 (1.259–1.918) | 229 per 1,000 | 127 more per 1,000 (from 59 more to 211 more) |
| Neutropenia | 9,045 (19 studies) | ⊕⊕⊕⊝; moderate (due to risk of bias, inconsistency, publication bias, plausible confounding would change the effect) | RR 0.644 (0.35–1.185) | 70 per 1,000 | 25 fewer per 1,000 (from 45 fewer to 13 more) |
| Rash | 9,331 (19 studies) | ⊕⊕⊕⊝; moderate (due to inconsistency, publication bias, plausible confounding would change the effect) | RR 5.292 (2.89–9.691) | 15 per 1,000 | 63 more per 1,000 (from 28 more to 127 more) |
| Diarrhea | 9,111 (19 studies) | ⊕⊕⊕⊝; moderate (due to risk of bias) | RR 3.453 (2.617–4.554) | 14 per 1,000 | 35 more per 1,000 (from 23 more to 50 more) |
| Thrombocytopenia | 6,996 (16 studies) | ⊕⊕⊝⊝; low (due to risk of bias, inconsistency) | RR 1.093 (0.931–1.283) | 99 per 1,000 | 9 more per 1,000 (from 7 fewer to 28 more) |
| Anemia | 9,819 (21 studies) | ⊕⊕⊝⊝; low (due to risk of bias, inconsistency) | RR 0.811 (0.542–1.212) | 67 per 1,000 | 13 fewer per 1,000 (from 31 fewer to 14 more) |
The basis for the assumed risk (e.g., the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). GRADE working group grades of evidence: high quality, further research is very unlikely to change our confidence in the estimate of effect; moderate quality, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; low quality, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; very low quality, we are very uncertain about the estimate. CI, confidence interval; RR, risk ratio.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [DOI] [PubMed] [Google Scholar]
- 2.Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13 Suppl 1:5-13. [DOI] [PubMed] [Google Scholar]
- 3.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10:1236-71. [DOI] [PubMed] [Google Scholar]
- 4.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cataldo VD, Gibbons DL, Pérez-Soler R, et al. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. N Engl J Med 2011;364:947-55. [DOI] [PubMed] [Google Scholar]
- 6.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [DOI] [PubMed] [Google Scholar]
- 7.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911-7. [DOI] [PubMed] [Google Scholar]
- 8.Sandler A. Bevacizumab in non small cell lung cancer. Clin Cancer Res 2007;13:s4613-6. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [DOI] [PubMed] [Google Scholar]
- 11.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Qi X, Huang Y, et al. Ceritinib (LDK378): a potent alternative to crizotinib for ALK-rearranged non-small-cell lung cancer. Clin Lung Cancer 2015;16:86-91. [DOI] [PubMed] [Google Scholar]
- 13.Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol 2013;14:38-47. [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [DOI] [PubMed] [Google Scholar]
- 15.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [DOI] [PubMed] [Google Scholar]
- 16.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [DOI] [PubMed] [Google Scholar]
- 17.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [DOI] [PubMed] [Google Scholar]
- 18.Bria E, Milella M, Cuppone F, et al. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol 2011;22:2277-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao G, Ren S, Li A, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: A meta-analysis from six phase III randomized controlled trials. Int J Cancer 2012;131:E822-9. [DOI] [PubMed] [Google Scholar]
- 20.Ma M, Zhu H, Zhang C, et al. "Liquid biopsy"-ctDNA detection with great potential and challenges. Ann Transl Med 2015;3:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ignatiadis M, Lee M, Jeffrey SS. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin Cancer Res 2015;21:4786-800. [DOI] [PubMed] [Google Scholar]
- 22.Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol 2012;30:3002-11. [DOI] [PubMed] [Google Scholar]
- 25.Chin TM, Quinlan MP, Singh A, et al. Reduced Erlotinib sensitivity of epidermal growth factor receptor-mutant non-small cell lung cancer following cisplatin exposure: a cell culture model of second-line erlotinib treatment. Clin Cancer Res 2008;14:6867-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mavridis D, Salanti G. A practical introduction to multivariate meta-analysis. Stat Methods Med Res 2013;22:133-58. [DOI] [PubMed] [Google Scholar]
- 30.Jansen JP, Crawford B, Bergman G, et al. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health 2008;11:956-64. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niho S, Kunitoh H, Nokihara H, et al. Randomized phase II study of first-line carboplatin-paclitaxel with or without bevacizumab in Japanese patients with advanced non-squamous non-small-cell lung cancer. Lung Cancer 2012;76:362-7. [DOI] [PubMed] [Google Scholar]
- 33.Soria JC, Márk Z, Zatloukal P, et al. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4442-51. [DOI] [PubMed] [Google Scholar]
- 34.Boutsikou E, Kontakiotis T, Zarogoulidis P, et al. Docetaxel-carboplatin in combination with erlotinib and/or bevacizumab in patients with non-small cell lung cancer. Onco Targets Ther 2013;6:125-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herbst RS, Giaccone G, Schiller JH, et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 2. J Clin Oncol 2004;22:785-94. [DOI] [PubMed] [Google Scholar]
- 36.Crinò L, Cappuzzo F, Zatloukal P, et al. Gefitinib versus vinorelbine in chemotherapy-naive elderly patients with advanced non-small-cell lung cancer (INVITE): a randomized, phase II study. J Clin Oncol 2008;26:4253-60. [DOI] [PubMed] [Google Scholar]
- 37.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaccone G, Herbst RS, Manegold C, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial--INTACT 1. J Clin Oncol 2004;22:777-84. [DOI] [PubMed] [Google Scholar]
- 39.Morère JF, Bréchot JM, Westeel V, et al. Randomized phase II trial of gefitinib or gemcitabine or docetaxel chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2 or 3 (IFCT-0301 study). Lung Cancer 2010;70:301-7. [DOI] [PubMed] [Google Scholar]
- 40.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [DOI] [PubMed] [Google Scholar]
- 41.Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8. [DOI] [PubMed] [Google Scholar]
- 42.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892-9. [DOI] [PubMed] [Google Scholar]
- 43.Lilenbaum R, Axelrod R, Thomas S, et al. Randomized phase II trial of erlotinib or standard chemotherapy in patients with advanced non-small-cell lung cancer and a performance status of 2. J Clin Oncol 2008;26:863-9. [DOI] [PubMed] [Google Scholar]
- 44.Mok TS, Wu YL, Yu CJ, et al. Randomized, placebo-controlled, phase II study of sequential erlotinib and chemotherapy as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2009;27:5080-7. [DOI] [PubMed] [Google Scholar]
- 45.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545-52. [DOI] [PubMed] [Google Scholar]
- 46.Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [DOI] [PubMed] [Google Scholar]
- 47.Chen YM, Tsai CM, Fan WC, et al. Phase II randomized trial of erlotinib or vinorelbine in chemonaive, advanced, non-small cell lung cancer patients aged 70 years or older. J Thorac Oncol 2012;7:412-8. [DOI] [PubMed] [Google Scholar]
- 48.Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86. [DOI] [PubMed] [Google Scholar]
- 49.Jänne PA, Wang X, Socinski MA, et al. Randomized phase II trial of erlotinib alone or with carboplatin and paclitaxel in patients who were never or light former smokers with advanced lung adenocarcinoma: CALGB 30406 trial. J Clin Oncol 2012;30:2063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SM, Khan I, Upadhyay S, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2012;13:1161-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin, with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol 2007;25:5777-84. [DOI] [PubMed] [Google Scholar]
- 52.Cui J, Cai X, Zhu M, et al. The efficacy of bevacizumab compared with other targeted drugs for patients with advanced NSCLC: a meta-analysis from 30 randomized controlled clinical trials. PLoS One 2013;8:e62038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang K, Wang YJ, Chen XR, et al. Effectiveness and safety of bevacizumab for unresectable non-small-cell lung cancer: a meta-analysis. Clin Drug Investig 2010;30:229-41. [DOI] [PubMed] [Google Scholar]