Abstract
Fasting serum lipid levels and changes in plasma glucose, fatty acid non-esterified (Nefa), and blood pyruvate levels during intravenous glucose tolerance tests were measured in 13 normal subjects before and one, five, and 15 days after the administration of 1-triiodothyronine (T3) calculated as 6 μg/kg body weight.
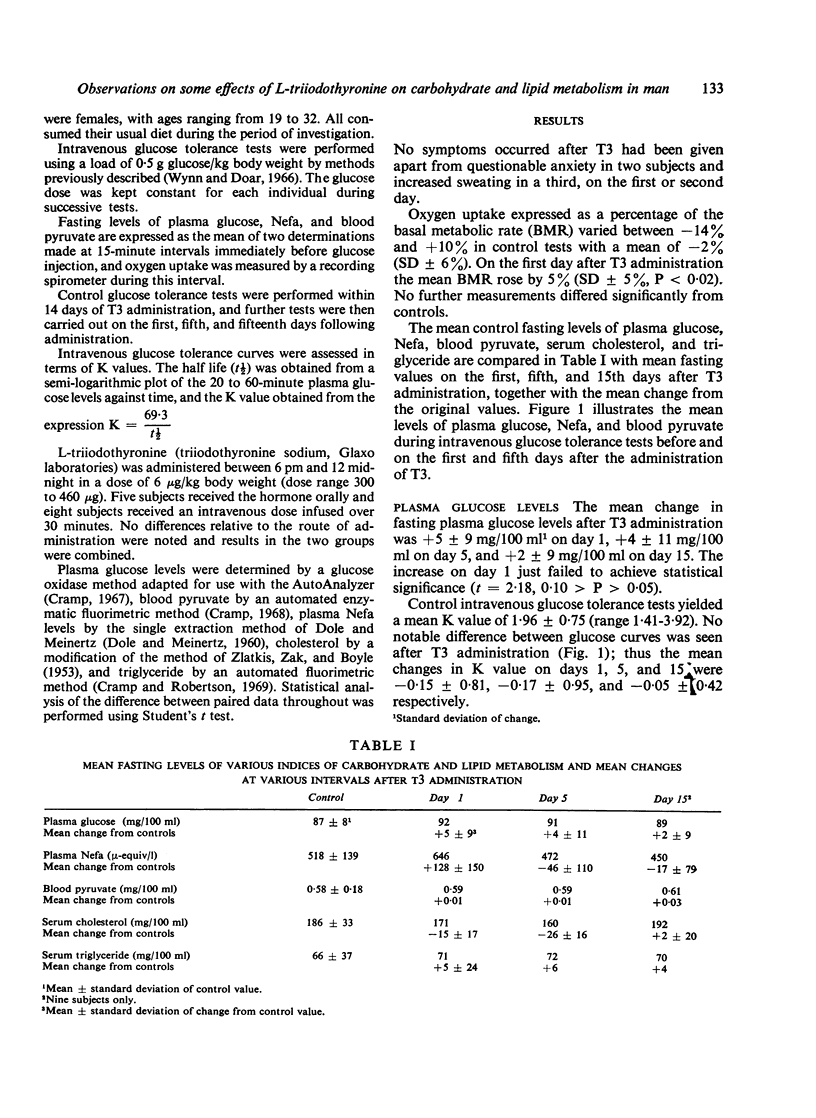
Significant increases in the mean basal metabolic rate and the mean fasting plasma Nefa level occurred within 10 to 17 hours of a single dose of T3, while a rise in the mean fasting plasma glucose concentration just failed to achieve significance. Fasting concentrations of blood pyruvate and serum triglyceride were unaffected. A significant fall in serum cholesterol levels was produced and lasted at least five days. All other indices returned to normal by five days.
During intravenous glucose tolerance tests performed at intervals after T3 administration no change in plasma glucose levels from control values was seen. Mean plasma Nefa and blood pyruvate levels, however, were significantly raised above control values during the early stages of the test 10 to 17 hours after T3. The relationship between these findings and those observed in clinical thyrotoxicosis is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMATUZIO D. S., SCHULTZ A. L., VANDERBILT M. J., RAMES E. D., NESBITT S. The effect of epinephrine, insulin, and hyperthyroidism on the rapid intravenous glucose tolerance test. J Clin Invest. 1954 Jan;33(1):97–102. doi: 10.1172/JCI102876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERFIELD W. J., WHICHELOW M. J. ARE THYROID HORMONES DIABETOGENIC?A STUDY OF PERIPHERAL GLUCOSE METABOLISM DURING GLUCOSE INFUSIONS IN NORMAL SUBJECTS AND HYPERTHYROID PATIENTS BEFORE AND AFTER TREATMENT. Metabolism. 1964 Jul;13:620–628. doi: 10.1016/0026-0495(64)90070-8. [DOI] [PubMed] [Google Scholar]
- Buckle R. M. Studies on blood keto acids in vitamin deficiency. Proc R Soc Med. 1967 Jan;60(1):48–52. [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPHE J., MAYER J. Effects of hormones on glucose utilization in fasted rats. Endocrinology. 1959 Sep;65:475–486. doi: 10.1210/endo-65-3-475. [DOI] [PubMed] [Google Scholar]
- Cramp D. G. New automated method for measuring glucose by glucose oxidase. J Clin Pathol. 1967 Nov;20(6):910–912. doi: 10.1136/jcp.20.6.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANOWSKI T. S., BONESSI J. V., SARVER M. E., MOSES C. HYDROCORTISONE AND/OR DESICCATED THYROID IN PHYSIOLOGIC DOSAGE. 13. CARBOHYDRATE METABOLISM DURING LARGE DOSAGE THYROID (PROLOID) THERAPY. Metabolism. 1964 Aug;13:739–746. doi: 10.1016/0026-0495(64)90019-8. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Doar J. W., Wynn V., Cramp D. G. Blood pyruvate and plasma glucose levels during oral and intravenous glucose tolerance tests in obese and non-obese women. Metabolism. 1968 Aug;17(8):690–701. doi: 10.1016/0026-0495(68)90053-x. [DOI] [PubMed] [Google Scholar]
- FRAWLEY T. F. The role of the adrenal cortex in glucose and pyruvic acid metabolism in man including the use of intravenous hydrocortisone in acute hypoglycemia. Ann N Y Acad Sci. 1955 May 27;61(2):464–493. doi: 10.1111/j.1749-6632.1955.tb42498.x. [DOI] [PubMed] [Google Scholar]
- FRY I. K., BUTTERFIELD W. J. Carbohydrate metabolism in diabetics. A possible intracellular block. Lancet. 1962 Jul 14;2(7246):66–68. [PubMed] [Google Scholar]
- Kreines K., Jett M., Knowles H. C., Jr Observations in hyperthyroidism of abnormal glucose tolerance and other traits related to diabetes mellitus. Diabetes. 1965 Nov;14(11):740–744. doi: 10.2337/diab.14.11.740. [DOI] [PubMed] [Google Scholar]
- Lamberg B. A. Glucose metabolism in thyroid disease. Acta Med Scand. 1965 Sep;178(3):351–362. doi: 10.1111/j.0954-6820.1965.tb04279.x. [DOI] [PubMed] [Google Scholar]
- MACHO L. The influence of endocrine glands on carbohydrate metabolism. II. The glucose tolerance and clearance of glucose in healthy subjects and in patients with hypo- and hyperthyroidism. Acta Med Scand. 1958 May 6;160(6):485–490. [PubMed] [Google Scholar]
- MARKS B. H., KIEM I., HILLS A. G. Endocrine influences on fat and carbohydrate metabolism in man. 1. Effect of hyperthyroidism on fasting serum nonesterified fatty acid concentration and on its response to glucose ingestion. Metabolism. 1960 Dec;9:1133–1138. [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- RICH C., BIERMAN E. L., SCHWARTZ I. L. Plasma nonesterified fatty acids in hyperthyroid states. J Clin Invest. 1959 Feb;38(2):275–278. doi: 10.1172/JCI103799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn V., Doar J. W. Some effects of oral contraceptives on carbohydrate metabolism. Lancet. 1966 Oct 1;2(7466):715–719. doi: 10.1016/s0140-6736(66)92978-3. [DOI] [PubMed] [Google Scholar]


