Abstract
Background
The high relapse and mortality rate of small-cell lung cancer (SCLC) fuels the need for epidemiologic study to aid in its prevention.
Methods
We included 24 studies from the ILCCO collaboration. Random-effects panel logistic regression and cubic spline regression were used to estimate the effects of smoking behaviors on SCLC risk and explore their non-linearity. Further, we explored whether the risk of smoking on SCLC was mediated through COPD.
Findings
Significant dose–response relationships of SCLC risk were observed for all quantitative smoking variables. Smoking pack-years were associated with a sharper increase of SCLC risk for pack-years ranged 0 to approximately 50. The former smokers with longer cessation showed a 43%quit_for_5–9 years to 89%quit_for_≥ 20 years declined SCLC risk vs. subjects who had quit smoking < 5 years. Compared with non-COPD subjects, smoking behaviors showed a significantly higher effect on SCLC risk among COPD subjects, and further, COPD patients showed a 1.86-fold higher risk of SCLC. Furthermore, smoking behaviors on SCLC risk were significantly mediated through COPD which accounted for 0.70% to 7.55% of total effects.
Interpretation
This is the largest pooling study that provides improved understanding of smoking on SCLC, and further demonstrates a causal pathway through COPD that warrants further experimental study.
Highlights
-
•
Cumulative smoking of the first 50 pack-years is associated with a sharper increase in SCLC risk.
-
•
Smoking behaviors have a higher risk on SCLC among COPD subjects, and COPD patients have a 1.86-fold higher risk of SCLC.
-
•
Risks of smoking behaviors on SCLC are partially mediated through COPD.
A strong association between smoking and SCLC is noted whereas the dose–response relationships are less clear. We demonstrate that cumulative smoking of the first 50 pack-years is associated with a sharper increase in SCLC risk. Moreover, although the relationship between smoking and COPD or COPD and SCLC is well-established, no study has investigated the causal pathway among smoking, COPD, and SCLC. Here we reveal the risks of smoking behaviors on SCLC which are partially mediated (up to 7.6%) through COPD. The findings warrant further experimental study to elucidate the mechanisms in this causal pathway.
1. Introduction
Small cell lung cancer (SCLC) comprises approximately 15–18% of all lung cancers worldwide (Fruh et al., 2013). SCLC is the most aggressive subtype of lung cancer and is characterized by rapid doubling time, high growth fraction, and early widespread metastasis (Kalemkerian et al., 2013). Despite high response rates to initial treatment, SCLC usually relapses and becomes refractory to treatment within one year. The median survival is 14–20 months for limited SCLC and 9–11 months for extensive SCLC (Kalemkerian et al., 2013). These statistics highlight the need for new tools to aid in diagnosis and prevention.
Smoking is the major risk factor for SCLC (Pesch et al., 2012, Engeland et al., 1996, Freedman et al., 2008). However, previous studies were limited in sample size and statistical power to estimate more precise effect size of smoking on SCLC risk as well as the non-linear exposure–response relationships, which have been thoroughly explored in the previous non-small cell lung cancer (NSCLC) studies (Zhai et al., 2014a, b). Furthermore, smoking is also an independent risk factor for chronic obstructive pulmonary disease (COPD), which shares similar genetic and biological characteristics to lung cancer (Houghton, 2013, Roca et al., 2012, Schwartz and Ruckdeschel, 2006, Young and Hopkins, 2011), while concomitant COPD has not been fully examined with regard to SCLC risk (Purdue et al., 2007, Fan et al., 2011). Precise understanding of the association between smoking, COPD, and SCLC using a large sample size will shed light on its pathogenesis.
To address these knowledge gaps, we conducted a pooling analysis of 24 case–control studies in the International Lung Cancer Consortium (ILCCO) that in total included 4346 SCLC cases and 37,942 cancer-free controls. We examined: 1) exposure–response relationships between SCLC risk and cigarette smoking indicators, including cumulative smoking, age of initiation, and time since quitting smoking; 2) the association between physician diagnosis of COPD and SCLC risk; and 3) the interaction and mediation effects of COPD and cigarette smoking on SCLC risk.
2. Methods
2.1. Ethics
Individual studies were approved by their respective ethics committees.
2.2. Study Population
This pooled analysis comprised data from the ILCCO collaboration (http://ilcco.iarc.fr), which was established in 2004 to share data among ongoing lung cancer studies (Hung et al., 2008). We included 24 ILCCO studies that met the following criteria: 1) had histologically confirmed SCLC cases; 2) used a structured questionnaire to evaluate lifestyle; and 3) provided an intact study protocol. Among the 24 studies, two (Schottker et al., 2013, Goodman et al., 1998) were cohort studies. The remaining 22 had a case–control design, ten (Miller et al., 2002, Muscat et al., 1995, Loriot et al., 2001, Lopez-Cima et al., 2012, Kim and Hong, 2013, Ito et al., 2012, Lee et al., 2009, Ruano-Ravina et al., 2014, Zhang et al., 2010, Park et al., 2005) were hospital-based, ten studies (Kreienbrock et al., 2001, Landi et al., 2008, Luce and Stucker, 2011, Schwartz et al., 2009, Field et al., 2005, Heck et al., 2009, Sevilya et al., 2014, Cote et al., 2012, Hashibe et al., 2006, Wang et al., 2014) were population-based, and the other two (Yang et al., 2005, Brenner et al., 2010) were mixed case–control studies. The included studies were performed in North America (Goodman et al., 1998, Miller et al., 2002, Muscat et al., 1995, Park et al., 2005, Schwartz et al., 2009, Heck et al., 2009, Hashibe et al., 2006, Wang et al., 2014, Yang et al., 2005, Brenner et al., 2010), Europe (Schottker et al., 2013, Loriot et al., 2001, Lopez-Cima et al., 2012, Lee et al., 2009, Kreienbrock et al., 2001, Landi et al., 2008, Luce and Stucker, 2011, Field et al., 2005, Cote et al., 2012, Ruano-Ravina et al., 2004), and Asia and Oceania (Lopez-Cima et al., 2012, Kim and Hong, 2013, Ruano-Ravina et al., 2014, Heck et al., 2009). Each included study was approved by the institutional review boards of the respective institutions, and each participant provided informed consent.
2.3. Case Ascertainment
Incident lung cancer cases were diagnosed pathologically and verified through review of medical records (Schottker et al., 2013, Goodman et al., 1998, Miller et al., 2002, Muscat et al., 1995, Loriot et al., 2001, Kim and Hong, 2013, Ito et al., 2012, Lee et al., 2009, Ruano-Ravina et al., 2014, Park et al., 2005, Kreienbrock et al., 2001, Landi et al., 2008, Schwartz et al., 2009, Field et al., 2005, Wang et al., 2014, Yang et al., 2005, Brenner et al., 2010, Etzel et al., 2006), linkage to cancer registries (Lopez-Cima et al., 2012, Ito et al., 2012, Luce and Stucker, 2011, Schwartz et al., 2009, Field et al., 2005, Cote et al., 2012, Hashibe et al., 2006, Wang et al., 2014, Brenner et al., 2010), or linkage to mortality registries (Goodman et al., 1998, Field et al., 2005). Histology information was ascertained based on the ICD for Oncology morphology codes or by individual studies. The proportion of SCLC over total lung cancers ranged from 4 to 24%. The cases that were staged as either limited stage or extensive stage were combined in the analysis.
Among the ten studies that had COPD diagnostic information (Table S1), the baseline questionnaires listed chronic emphysema, bronchitis, and/or COPD, and other lung disorders such as asthma and tuberculosis. Each subject was asked to self-report whether he/she was ever diagnosed by a physician of chronic bronchitis, emphysema, or COPD, categorized as present or absent. Subjects who had physician diagnosed chronic emphysema, bronchitis, and/or COPD were defined as having COPD. One study also validated the COPD diagnosis with pulmonary function tests (Yang et al., 2005).
2.4. Smoking and Other Factors
All studies collected information on lifetime history of cigarette smoking, including age of initiation of smoking, duration, intensity, and time since quitting for former smokers. To explore the non-linear association between smoking and SCLC, we generated common categorical variables related to smoking status (never smoker defined as no cumulative smoking, current smoker defined as cumulative smoking of any amount plus time since quitting smoking less than or equal to 1 year, and former smoker defined as smokers who had quit more than 1 year before diagnosis or interview), daily smoking intensity (1–9, 10–19, 20–29, 30–39, and 40 or more cigarettes/day), smoking duration (1–19, 20–29, 30–39, 40–49, and 50 or more years), and lifetime cumulative smoking (1–19, 20–39, 40–59, 60–79, and 80 or more pack-years; one pack-year being equivalent to 20 cigarettes/day smoked during 1 year). Former smokers were further categorized according to age of smoking initiation (less than 15 years, 15–20, 20–25, 25–30, or more than 30 years) and time since quitting (< 5, 5–9, 10–19, or 20 or more years).
Other variables included in the pooled analysis were gender, age at diagnosis or interview, geographical region (North America, Europe, and Asia and Oceania), self-reported race (Asian, Black, White, Hawaiian, Hispanic, Other), family history of lung cancer (yes, no), and education level (non, elementary, vocational, postsecondary, university).
2.5. Statistical Analysis
2.5.1. Non-linear Exposure–Response Relationships of Smoking Behaviors on SCLC
The odds ratios (ORs) of SCLC and their 95% confidence intervals (95% CIs) for daily smoking intensity, duration of smoking, lifetime cumulative smoking, age of smoking initiation, and time since quitting were estimated using random-effects panel logistic regression (Conway, 1990). This multilevel model takes account of the variation among studies (panels), and study heterogeneity, during model fitting. The ρ, ranging from 0 to 1, represents the proportion of the total variance contributed by the panel (study)-level variance component. When ρ is zero, the panel-level variance component is negligible, and the estimators from the panel logistic regression are no different from that from the traditional logistic regression. Age at diagnosis and gender were adjusted for in all the regression models. Trends of SCLC risk across smoking categories were evaluated by fitting the categorical smoking variables into an ordinal regression model (Armstrong and Sloan, 1989). To better visualize the exposure–response relationship, we plotted cumulative smoking, age of smoking initiation, and time since quitting smoking with the SCLC risks using estimates from restricted cubic spline models (Hastie and Tibshirani, 1995, Campbell, 1996). The non-linear association was explored by applying the likelihood test to compare the spline model to its nested linear model. Subgroup analyses were performed stratified by COPD status, gender, study area (Caucasian-dominated areas vs. non-Caucasian dominated areas), source of controls (hospital-based controls vs. population-based controls), and 1st degree family history of lung cancer (yes vs. no), and the difference of risk effects between subgroups was evaluated by including the interaction term of smoking and stratifying variable into the model.
2.5.2. Interaction and Mediation Analyses
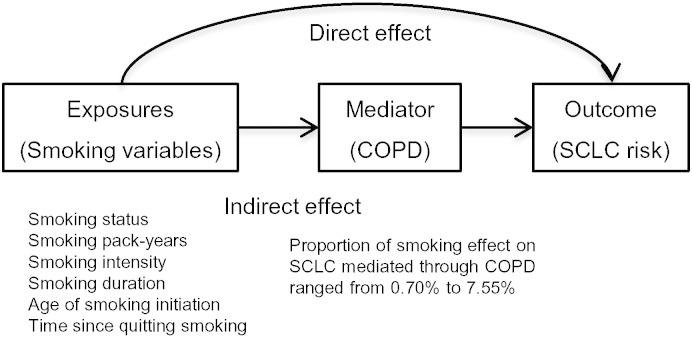
The associations between cumulative smoking and SCLC risk were further tested in subgroups with and without preexisting COPD. We conducted the Wald test for effect modification from COPD by adding an interaction term. It is well-established that smoking is the risk to both COPD and SCLC, and COPD is a risk factor to SCLC. To explore whether the effect of smoking on the risk of SCLC is mediated through COPD, the VanderWeele's mediation analysis was performed (VanderWeele and Vansteelandt, 2010). The smoking effect on SCLC was decomposed to two parts: the indirect effect which represents the effect of smoking is mediated through COPD and the direct effect which represents the effect of smoking on SCLC by pathways other than COPD. To obtain direct and indirect effects of smoking on SCLC risk, ORs for mediation analysis in the case–control setting were calculated by combining the regression of COPD and the regression of SCLC risk (Vanderweele and Vansteelandt, 2010, VanderWeele et al., 2012). The proportion mediated was obtained by ORd × (ORi − 1) / (ORd × ORi − 1), where ORd is the direct effect odds ratio and ORi is the indirect effect odds ratio (Campbell, 1996).
All tests were two-sided and evaluated using SAS software (version 9·4; SAS Institute, Cary, NC) or STATA statistical package (Version 14; Stata Corp. LP, College Station, TX, USA). A P-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Characteristics of Study Populations
In the 24 studies with recruitment initiated since 1969, 4346 SCLC patients and 37,942 non-SCLC controls were identified (Table S1). Among ten studies with available COPD status, 1543 COPD and 14,665 non-COPD subjects were further analyzed to explore stratified and mediation effects. Demographic characteristics are summarized in Table 1. SCLC patients were significantly older, male-predominant, less educated, and more commonly had a family history of lung cancer than their respective controls (P < 0.01). For smoking behaviors, the proportions of current smokers/former smokers, the amount of lifetime cumulative smoking pack-years, smoking duration, and smoking intensity (cigarettes per day) in SCLCs or COPDs were significantly higher than in their respective controls, while the time since quitting smoking was significantly lower than that in controls (P < 0.01). The frequency of COPD diagnosis was higher among SCLC cases (20.6%) than controls (7.6%) (P < 0.001).
Table 1.
Basic characteristics and smoking behaviors in the pooled dataset.
| Variable | SCLC (n = 4346) |
Non-SCLC (n = 37,942) |
||||
|---|---|---|---|---|---|---|
| COPDa (n = 503) |
Non-COPDa (n = 1940) |
COPDa (n = 1040) |
Non-COPDa (n = 12,725) |
|||
| Study area | ||||||
| Caucasian-dominated | 4153(95.6) | 503(100) | 1940 (100) | 35,944 (94.7) | 1040(100) | 12,725 (100) |
| Non-Caucasian-dominated | 193(4.4) | 1995 (5.3) | ||||
| Age, mean (SD) | 61.2(10.5) | 63.9(9.4) | 60.6 (10.7)b | 58.8 (12.7)c | 61.3(10.3) | 58.1 (12.5)d |
| Gender (female), n (%) | 1371(31.6) | 193(38.4) | 601 (31.0)b | 14,269 (37.6)c | 373(35.9) | 5262 (41.4)d |
| Ethnicity (Caucasian), n (%) | 2310(87.1) | 299(59.4) | 1000 (51.6)b | 23,507 (85.2)c | 427(41.1) | 7442 (58.5)d |
| Education (greater than university), n (%) | 487(15.9) | 82(20.0) | 260 (16.8) | 7047 (32.1)c | 228(27.6) | 3733 (43.9)d |
| First degree family history of lung cancer (≥ 1), n (%) | 173(15.2) | 25(19.5) | 83 (17.5) | 1435 (9.8)c | 67(17.3) | 764 (12.2)d |
| Smoking status | b | c | d | |||
| Never | 166(4.5) | 3(0.8) | 101 (7.1) | 13,613 (37.5) | 278(28.7) | 5075 (45.0) |
| Former | 987(27.0) | 114(31.2) | 332 (23.4) | 13,002 (35.8) | 400(41.3) | 3875 (34.4) |
| Current | 2510(68.5) | 249(68.0) | 987 (69.5) | 9696 (26.7) | 291(30.0) | 2332 (20.7) |
| Smoking pack-years, mean (SD) | 46.8(27.9) | 53.7(31.5) | 43.3 (27.1)b | 27.6 (24.2)c | 32.7(27.7) | 24.2 (23.4)d |
| Smoking intensity (cigarettes per day), mean (SD) | 24.1(12.4) | 25.6(13.0) | 23.0 (12.1)b | 18.7 (12.2)c | 19.3(12.5) | 18.0 (12.4)d |
| Smoking duration (years), mean (SD) | 39.1(10.6) | 42.0(10.4) | 38.9 (10.4)b | 31.2 (14.3)c | 39.7(11.7) | 32.7 (14.4)d |
| Age of initiation (years), mean (SD) | 18.1(4.9) | 18.0(4.5) | 18.4 (4.8)b | 18.8 (5.7)c | 18.6(5.2) | 18.8 (5.4)d |
| Age of cessation (years), mean (SD) | 55.9(10.9) | 58.9(9.6) | 53.9 (10.7)b | 46.0 (14.8)c | 47.5(13.3) | 42.8 (14.5)d |
| Time since quitting smoking among former smokers (years), mean (SD) | 3.9(7.7) | 4.0(8.0) | 3.5 (7.4)b | 13.8 (13.3)c | 11.6(13.0) | 12.5 (13.1)d |
Values are presented as n (%) for categorical data or mean(standard deviation [SD]) for continuous variables. The categorical variables were tested by Fisher's exact test and continuous variables were compared by Student t-test between groups/subgroups. The quantitative smoking variables were summarized among former or current smokers. SCLC: small cell lung cancer; COPD: chronic obstructive pulmonary disease.
A subset of 10 studies that have reported COPD status in both case and control including the ReSoLuCENT study, NELCS, FHS, CAPUA, MSH-PMH, SLRI, ICARE, Mayo Clinic, MGH, and HMGU.
P-value ≤ 0.01 of COPD vs. non-COPD among SCLC cases.
P-value < 0.01 of SCLC vs. non-SCLC among overall samples.
P-value < 0.01 of COPD vs. non-COPD among non-SCLC subjects.
3.2. Association of Smoking Behaviors With SCLC Risk
Former smokers had a significantly higher risk on SCLC vs. non-smokers (OR, 6.21, 95% CI 5.21–7.41, P < 0.001) while a much higher risk existed among current smokers vs non-smokers (OR, 26.72, 95% CI 22.54–31.68, P < 0.001) (Table 2). A statistically significant dose–response for SCLC risk was observed for all quantitative smoking variables (Table 2). Cumulative smoking intensity (smoking pack-years) was associated with increased risk of SCLC vs. non-smokers in a significant dose–response manner [ORs ranged from 4.33 for those who had pack-years < 20 to 69.03 for those who had pack-years ≥ 80, P for trend (Ptrend) < 0.001]. Smoking intensity had a similar dose–response model (ORs from 4.35 < 10_cigarettes-per-day to 34.49 ≥ 40_cigarettes-per-day, Ptrend < 0.001), as well as smoking duration (ORs ranged from 2.37 < 20_years to 48.80 ≥ 50_years, Ptrend < 0.001), and age of initiation (ORs from 7.09smoking_after_30 to 24.04smoking_before_15, Ptrend < 0.001). The former smokers with longer cessation showed a considerably decreased risk on SCLC risk in a dose–response trend vs. subjects who had quit smoking for less than 5 years [OR for those who had quit for 5–9 years (OR5–9), 0.57, 95% CI 0.45–0.73; OR10–19, 0.28, 95% CI 0.23–0.36; OR ≥ 20, 0.11, 95% CI 0.09–0.14; Ptrend < 0.001]. The sensitivity analysis yielded similar results with further adjustment for study areas (Caucasian-dominated areas vs. non-Caucasian-dominated areas), source of controls (hospital-based vs. population-based), and family history of lung cancer (yes vs. no) (Table 2).
Table 2.
Smoking behaviors and the risk of SCLC.
| Variable | NSCLC/Nnon-SCLC | Overall analysis |
Sensitivity analysisa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI)b | Z | P | ρc | OR (95% CI)a | Z | P | ρc | ||
| Smoking status | 0.16 | 0.19 | |||||||
| Never | 166/13,613 | 1.00 (ref) | — | — | 1.00 (ref) | — | — | ||
| Former | 987/13,002 | 6.21 (5.21,7.41) | 20.36 | 0.000 | 6.13 (5.10,7.37) | 19.32 | 0.000 | ||
| Current | 2510/9696 | 26.72 (22.54,31.68) | 37.83 | 0.000 | 26.96 (22.57,32.21) | 36.32 | 0.000 | ||
| Trend test | 48.06 | 0.000 | 46.24 | 0.000 | |||||
| Smoking pack-years | 0.16 | 0.20 | |||||||
| Never smokers | 166/13,613 | 1.00 (ref) | — | — | |||||
| < 20 | 462/9110 | 4.33 (3.61,5.19) | 15.80 | 0.000 | 4.28 (3.51,5.21) | 14.42 | 0.000 | ||
| 20–39 | 1365/6996 | 19.19 (16.23,22.69) | 34.54 | 0.000 | 18.61 (15.49,22.36) | 31.20 | 0.000 | ||
| 40–59 | 1063/3533 | 35.86 (30.12,42.71) | 40.17 | 0.000 | 33.88 (27.93,41.09) | 35.75 | 0.000 | ||
| 60–79 | 527/1275 | 55.04 (45.32,66.84) | 40.43 | 0.000 | 57.91 (46.51,72.10) | 36.29 | 0.000 | ||
| ≥ 80 | 474/889 | 69.03 (56.38,84.52) | 41.00 | 0.000 | 73.91 (58.69,93.08) | 36.58 | 0.000 | ||
| Trend test | 57.32 | 0.000 | 51.33 | 0.000 | |||||
| Smoking intensity (cigarettes per day) | 0.16 | 0.19 | |||||||
| Never smokers | 166/13,613 | 1.00 (ref) | — | — | 1.00 (ref) | — | — | ||
| < 10 | 246/4371 | 4.35 (3.55,5.34) | 14.17 | 0.000 | 4.06 (3.26,5.05) | 12.51 | 0.000 | ||
| 10–19 | 1142/6979 | 13.70 (11.56,16.24) | 30.15 | 0.000 | 13.08 (10.88,15.73) | 27.36 | 0.000 | ||
| 20–29 | 1307/6670 | 20.31 (17.14,24.08) | 34.68 | 0.000 | 20.12 (16.70,24.24) | 31.57 | 0.000 | ||
| 30–39 | 578/2154 | 29.93 (24.83,36.07) | 35.68 | 0.000 | 31.67 (25.76,38.92) | 32.83 | 0.000 | ||
| ≥ 40 | 609/1929 | 34.49 (28.56,41.65) | 36.78 | 0.000 | 35.99 (29.15,44.45) | 33.28 | 0.000 | ||
| Trend test | 48.01 | 0.000 | 43.96 | 0.000 | |||||
| Smoking duration (years) | 0.12 | 0.10 | |||||||
| Never smokers | 166/13,613 | 1.00 (ref) | — | — | 1.00 (ref) | — | — | ||
| < 20 | 100/4000 | 2.37 (1.83,3.07) | 6.53 | 0.000 | 2.52 (1.90,3.34) | 6.40 | 0.000 | ||
| 20–29 | 543/3635 | 14.67 (12.18,17.67) | 28.33 | 0.000 | 16.04 (13.05,19.72) | 26.36 | 0.000 | ||
| 30–39 | 1039/4645 | 25.54 (21.40,30.48) | 35.89 | 0.000 | 28.89 (23.65,35.28) | 32.97 | 0.000 | ||
| 40–49 | 1197/4259 | 37.43 (31.16,44.95) | 38.76 | 0.000 | 41.92 (34.07,51.59) | 35.30 | 0.000 | ||
| ≥ 50 | 561/1731 | 48.80 (39.60,60.12) | 36.50 | 0.000 | 52.69 (41.73,66.54) | 33.30 | 0.000 | ||
| Trend test | 46.09 | 0.000 | 42.50 | 0.000 | |||||
| Age of initiation (years) | 0.14 | 0.10 | |||||||
| Never smokers | 165/13,612 | 1.00 (ref) | — | — | 1.00 (ref) | — | — | ||
| ≥ 30 | 80/840 | 7.09 (5.32,9.46) | 13.32 | 0.000 | 6.70 (4.92,9.13) | 12.05 | 0.000 | ||
| 25–29 | 138/858 | 12.57 (9.79,16.15) | 19.82 | 0.000 | 12.44 (9.49,16.30) | 18.25 | 0.000 | ||
| 20–24 | 636/3592 | 14.17 (11.71,17.15) | 27.22 | 0.000 | 13.58 (11.01,16.75) | 24.36 | 0.000 | ||
| 15–19 | 1603/8826 | 15.74 (13.15,18.84) | 30.07 | 0.000 | 15.48 (12.71,18.87) | 27.16 | 0.000 | ||
| < 15 | 497/2049 | 24.04 (19.67,29.39) | 31.04 | 0.000 | 25.30 (20.27,31.58) | 28.56 | 0.000 | ||
| Trend test | 35.38 | 0.000 | 31.96 | 0.000 | |||||
| Time since quitting smoking among former smokers (years) | 0.05 | 0.04 | |||||||
| < 5 | 199/718 | 1.00 (ref) | — | — | 1.00 (ref) | — | — | ||
| 5–9 | 158/927 | 0.57 (0.45,0.73) | 4.61 | 0.000 | 0.57 (0.45,0.72) | 4.63 | 0.000 | ||
| 10–19 | 197/2092 | 0.28 (0.23,0.36) | 11.08 | 0.000 | 0.28 (0.23,0.35) | 11.16 | 0.000 | ||
| ≥ 20 | 149/3493 | 0.11 (0.09,0.14) | 18.15 | 0.000 | 0.11 (0.09,0.14) | 18.23 | 0.000 | ||
| Trend test | 19.40 | 0.000 | 19.49 | 0.000 | |||||
With further adjustment for family history of lung cancer (yes vs. no), study area (Caucasian-dominated areas vs. non-Caucasian-dominated areas), source of controls (hospital-based vs. population-based).
Odds ratio (OR), 95% confidence interval (95% CI), Z, and P-values were estimated using random-effects panel logistic regression by study center with adjustment for age and gender.
The proportion of the total variance contributed by the panel (study)-level variance component. It is ranged from 0 to 1. The closer of ρ to 0 represents the less heterogeneity among studies. When ρ is zero, the panel-level variance component is unimportant, and the panel estimator is no different from the pooled logistic estimator.
3.3. Stratified Analyses of Smoking Behaviors on SCLC Risk
Further, we performed the stratified analyses by COPD, gender, ethnicity, source of control, and 1st degree family history of lung cancer. All the smoking variables showed a higher effect on SCLC risk in COPD subgroup than those in non-COPD subjects with significance or borderline significance except for time since quitting smoking which was probably due to insufficient sample size (Table S2). Male smokers had a trend of stronger dose–response on SCLC risk than that in female but with a lack of statistical significance (Table S3). Smoking variables in Caucasian-dominated populations showed stronger effects on SCLC risk than those in non-Caucasian dominant populations (Table S4). No statistical significance was observed for time since quitting smoking probably due to insufficient sample size from non-Caucasian populations (Table S4). Further, stratified analyses by control type showed a trend of higher effects of smoking behaviors on SCLC risk in the studies with population-based controls than those in the studies with hospital-based controls (Table S5). Furthermore, in stratified analysis by family history of lung cancer, smoking behaviors showed a trend of, but non-significant, stronger effects in subjects with family history of lung cancer than the others (Table S6).
3.4. Non-linear Exposure–Response Relationships
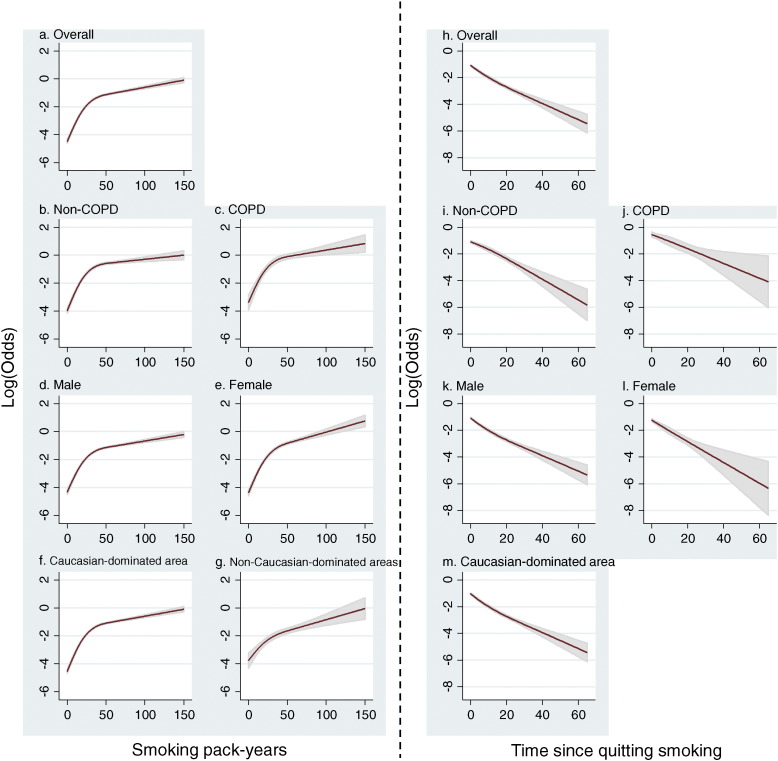
Further, non-linear exposure–response relationships of smoking pack-years and time since quitting smoking were explored using restricted cubic spline regression model (Fig. 1). The SCLC risk for cumulative smoking pack-years revealed an upward spline with a knot at approximately 50 pack-years (Pnon-linear < 0.001); the slope of the first segment was larger than that of the second segment (Fig. 1a). The results were consistent in the subgroup analyses stratified by COPD status (Fig. 1b for non-COPD, 1c for COPD), by gender (Fig. 1d for male, 1e for female), and study area (Fig. 1f for Caucasian-dominated areas, Fig. 1g for non-Caucasian-dominated areas). In contrast, there were significantly decreasing trends between time since quitting smoking and SCLC risk among former smokers (Fig. 1h) which obtained consistent results among the subgroup analyses (Fig. 1i–m). We were not able to perform the cubic spline analysis among the former smokers in studies from non-Caucasian-dominated areas due to insufficient cases recruited.
Fig. 1.
The dose–response relationship between smoking behaviors and the risk of SCLC. Smoking pack-years were explored on the non-linear dose–response relationship on SCLC risk among all samples (a), or stratified by COPD status (b, c), by gender (d, e), or by study areas (f, g). Time since quitting smoking was also explored by cubic spline regressions for non-linearity among all samples (h), or stratified by COPD status (i, j), by gender (k, l), or among Caucasian-dominated areas (m). Due to insufficient sample size, there was no subgroup analysis done among non-Caucasian-dominated areas. The x-axis represents the quantitative smoking information while the y-axis represents the odds in loge scale.
3.5. Smoking Effect on SCLC Mediated Through COPD
Smoking behaviors were positively associated with COPD risk (Table S7). COPD status was independently associated with SCLC risk (OR, 1.86, 95% CI 1.61–2.16, P < 0.001) with adjustment for age, gender, and smoking pack-years. Furthermore, to explore whether the association between smoking and SCLC risk was mediated through COPD, we performed a series of mediation analyses (Table 3). A statistically significant indirect effect on SCLC risk mediated through COPD was observed for former smokers [ORindirect, 1.03, P < 0.001; proportion mediated (%M), 3.57%] or current smokers (ORindirect, 1.06, P < 0.001; %M, 5.86%), smoking pack-years (ORindirect per SD, 1.03, P < 0.001; %M, 4.99%), smoking intensity (ORindirect per SD, 1.01, P < 0.001; %M, 2.52%), smoking duration (ORindirect per SD, 1.05, P < 0.001; %M, 7.55%), and time since quitting smoking (ORindirect per SD, 0.98, P = 0.005; %M, 0.70%). Overall, less than 10% of smoking's risk effect on SCLC was mediated through COPD (Fig. 2).
Table 3.
Causal mediation analysis of smoking behaviors, COPD, and the risk of SCLC.
| Variable | ORindirect (95% CI) | P | ORdirect (95% CI) | P | %M |
|---|---|---|---|---|---|
| Smoking status | |||||
| Never vs former | 1.03 (1.02, 1.05) | 0.000 | 5.30 (4.84, 5.81) | 0.000 | 3.57 |
| Never vs current | 1.06 (1.04, 1.09) | 0.000 | 28.13 (23.41, 33.80) | 0.000 | 5.86 |
| Smoking pack-years per SD (25.9) increment |
1.03 (1.02, 1.04) | 0.000 | 2.33 (2.22, 2.44) | 0.000 | 4.99 |
| Smoking intensity per SD (13.2) increment |
1.01 (1.01, 1.02) | 0.000 | 1.63 (1.56, 1.71) | 0.000 | 2.52 |
| Smoking duration (years) per SD (17.9) increment |
1.05 (1.03, 1.07) | 0.000 | 2.58 (2.35 2.84) | 0.000 | 7.55 |
| Age of initiation (years) per SD (5.6) increment |
0.99 (0.80, 1.01) | 0.158 | 0.89 (0.82, 0.95) | 0.001 | 7.49 |
| Time since quitting smoking among former smokers per SD (13.0) increment |
0.98 (0.97, 0.99) | 0.005 | 0.26 (0.22, 0.30) | 0.000 | 0.70 |
Odds ratios (OR) were in per standard deviation (SD, among all samples) increment. The SDs of smoking pack years, smoking intensity, smoking duration, age of initiation, time since quitting smoking among former smokers were 25.9 pack-years, 13.2 cigarettes per day, 17.9 years, 5.6 years, and 13.0 years, respectively.
Fig. 2.
A diagram of mediation model.
4. Discussion
To our knowledge, this is the largest study investigating the relationship among multiple quantitative smoking risk factors, COPD, and risk of SCLC (Pesch et al., 2012). The major strength of this study is its large sample size, which allowed us to have greater power to detect the exposure–response relationship of smoking behaviors, COPD, and SCLC risk in more homogeneous subgroups, and the risks of smoking mediated through COPD (Zhai et al., 2014a). Furthermore, the multi-ethnic design makes this study generalizable to the other populations (St Sauver et al., 2012). The pooling study also took advantage of the well-planned questionnaires that collected data on detailed smoking behaviors such as cumulative smoking, age since smoking initiation, and time since quitting smoking, as well as COPD status.
Our study addressed the information gap regarding the non-linear exposure-response relationships between the cigarette smoking behaviors, COPD, and risk of SCLC. SCLC risk rises sharply with the first 50 pack-years of cumulative smoking, and increases continuously with further smoking. A similar steep slope with a subsequent leveling-off of lung cancer risk for intensity of smoking and a plateauing of the SCLC risks by duration of smoking were seen in previous studies (Pesch et al., 2012, Zhai et al., 2014a, Vineis et al., 2000). Findings of very high relative risks for SCLC in smokers are in agreement with experimental findings that more extensive damage triggers the regeneration of quiescent subpopulations of cells (Li and Clevers, 2010). Those cells that are centrally located in the lungs are possibly the cellular precursors of SCLC that react to more extensive damage (Liu and Engelhardt, 2008, Liu et al., 2006). The leveling-off association among extremely heavy and long-term smokers might be explained by a potential saturation effect, or competing risks among heavy smokers (Vineis et al., 2000).
Juvenile initiated cigarette smoking would have over 15-fold higher risk of SCLC in the following years compared with non-smokers. The sensitivity analysis by further adjustment for smoking duration showed a reduced magnitude of risk, which implies that the effect of smoking initiation is, to some extent, dependent on the smoking duration, while still retaining significance.
SCLC risk steadily decreased as years since smoking cessation increased, which underscores the importance of quitting smoking as early as possible. The risk remains 3.59 fold higher (95% CI 2.71–7.46, P < 0.001) after 20 years' cessation compared with never smokers. A possible mechanism for this long-term carcinogenic effect of smoking is that cigarette smoke can exert a wide range of irreversible changes in lung tissue that affect its function (Thorley and Tetley, 2007).
The risk of lung cancer in patients with COPD has long been established (Zhai et al., 2014a, Purdue et al., 2007, Raviv et al., 2011). However, most of the studies focused on the risk of overall lung cancer or NSCLC, while the relationship with SCLC was rarely explored or underpowered (Kato et al., 2011). Our analysis offers insights suggesting that 86% of increased risk of SCLC occurs in persons with COPD independent from smoking. Further, our study suggests that smoking has a higher damaging effect on lungs among subjects diagnosed of COPD than non-COPD subjects, which indicates a synergetic mechanism in lung cancer pathophysiology. This finding agrees with that of our previous study of non-small cell lung cancer (Zhai et al., 2014a). One biological explanation for this association between COPD and SCLC is that long-term pulmonary inflammation from COPD damages lung tissue and produces free radicals that may induce mutagenesis during tissue regeneration (Ballaz and Mulshine, 2003). Another potential mechanistic explanation is that impaired mucociliary function in COPD patients could hamper the removal of harmful particles (Zhai et al., 2014b, Houtmeyers et al., 1999). Other mechanisms associated with the presence of COPD that might be associated with the inflammation and associated cytokines, development of lung cancer includes alterations to cell cycle regulation, shared genetic and epigenetic susceptibilities (Houghton, 2013). Furthermore, no study has previously investigated the role of COPD as a causal mediator between smoking and SCLC. Our study demonstrates that less than 10% of the smoking risk effect on SCLC is mediated through COPD. Wang et al. reported one-third of the effect of smoking behavior on lung cancer mediated through COPD. The findings indicate a histologically-different causal role of COPD among smoking and lung cancer, which warrants further validation and experimental study (Wang et al., 2010).
We acknowledge some limitations in our study. First, misclassification of SCLC or COPD has to be considered since our study included diverse countries in which different diagnostic criteria may apply. A pathology comparability analysis was performed by Stang et al. in a German case series; the agreement between pathologists was 94% for SCLC, and lower in never smokers (Stang et al., 2006). Second, studies included were lacking information on spirometry, and underdiagnostics of COPD was thus significant among non-COPD subjects, which is about 70% of the total population (Mannino et al., 2000, Lamprecht et al., 2015, Bednarek et al., 2008). Due to underdiagnosed COPD patients, risks of smoking behaviors among non-COPD subgroups were, to some extent, overestimated. However, physician-diagnosed COPD is compatible with spirometry-based COPD for epidemiological studies (Straus et al., 2002, Eisner et al., 2005, Murgia et al., 2014). A validation assessment also confirmed that self-reported physician-diagnosed COPD correlates with high rates of true COPD in medical records (Barr et al., 2002). Therefore, such underdiagnosis of COPD contributes to the more conservative results for the evaluation of the risk difference between COPD and non-COPD. On the other hand, a more accurate COPD diagnostic method will result in a higher stratified effect as well as stronger statistical power. Third, medication information of COPD patients was also important to this association study. Inhaled corticosteroids (ICS) are anti-inflammatory drugs that have proven benefits for worsening COPD patients (Kew and Seniukovich, 2014), as well as a decreased risk of lung cancer in a dose–response manner (Parimon et al., 2007, Lee et al., 2013). Statins are also recognized as powerful anti-inflammatory agents beyond low-density lipoprotein cholesterol reduction (Pruefer et al., 2002), which have a beneficial role in COPD treatment including reduced risk of lung cancer (Janda et al., 2009, van Gestel et al., 2009). Inclusion of the medication information in future study increases both statistical power and clinical interpretation. Besides, the source of controls, SCLC case ascertainment, COPD verification, geographical area, and recruitment period could explain partial heterogeneity. Though we detected a significant indirect effect of smoking on SCLC risk mediated by COPD, we were not able to determine the temporal relationship between COPD and SCLC in this study, and reverse causality of the pre-diagnosed stage of SCLC could thus possibly affect COPD development as well.
5. Conclusion
This study emphasizes the non-linear association of smoking with the relative risk of SCLC. The pattern is partially supported by prior SCLC studies (Pesch et al., 2012, Vineis et al., 2000) and hypothesis-generating experiments. Smoking also has a strong effect on COPD, and COPD is an independent risk factor on SCLC, and further, a part of smoking risk effect on SCLC is mediated through COPD. The mutually shared genetic predisposition or common mechanistic pathway among smoking behaviors, COPD and SCLC warrants investigation to facilitate early detection of SCLC.
Funding
This work was supported by the National Institutes of Health and National Cancer Institute (Grants CA092824, CA074386, CA090578, ES00002, CA80127, CA84354, CA68384, the Intramural Research Program, CA060691, HHSN261201000028C, P30CA022453, CA167462, DA11386, CA90833, CA77954, CA09142, CA96134, and ES011667); BfS (Bundesamt für Strahlenschutz, Germany) (Grant St. Sch 1066, 4074, 4074/1); Mayo Clinic Foundation; Canadian Cancer Society Research Institute (Grant 020214); FIS/Spain (Grant FIS-01/310, FIS-PI03-0365, and FIS-07-BI060604); FICYT/Asturias (Grants FICYT PB02-67 and FICYT IB09-133); Roy Castle Lung Cancer Foundation in UK; Ministry of Education, Science, Sports, Culture, and Technology of Japan (Grants-in-Aid for Scientific Research on Priority and Innovative Areas); Ministry of Health, Labor, and Welfare of Japan [Third-Term Comprehensive 10-Year Strategy for Cancer Control]; National Cancer Center Research and Development Fund (23-A-4); Health and Labor Sciences Research Grants for Research on Applying Health Technology from Ministry of Health, Labor and Welfare; the Bi-national Israel–US Science Foundation; the Baden-Württemberg Ministry of Science Research and Arts Foundation; the Ann Fitzpatrick Alper Research Program for Environmental Genomics of the University of California at Los Angeles Jonsson Comprehensive Cancer Center. Dr. Wei is partially supported by the National Natural Science Foundation of China (Grant 81402764) and the Natural Science Foundation of Jiangsu, China (Grant BK20140907). Dr. Zhang is partially supported by the National Natural Science Foundation of China (Grant No. 81402763).
Role of Sponsors
The sponsors of all the funding bodies had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Author Contributions
Dr. Ru-Yi Huang generated the conception, done the data analysis, interpreted the data, and wrote the manuscript. Dr. Yongyue Wei generated the conception together with analyzing the data in depth and revising the manuscript. Dr. Rayjean Hung was in charge of the data harmonization, monitor of the consortium work and offered statistical assistance for the manuscript. All authors from the ILCCO group contributed to the design and execution of the work and to the preparation and drafting critically of this report. Additionally, all had the opportunity to contribute to the interpretation of the results and to the redrafting of the report. Approval of the final report was obtained from all authors. Dr. David Christiani wrote and supervised the project concept and was responsible for the final report.
Conflicts of Interest
We declare that we have no conflicts of interest.
Acknowledgments
We thank Dr. June Carroll and the Mount Sinai Hospital Granovsky Gluskin Family Medicine Centre for helping with the recruitment of the MSH-PMH study. We thank the study participants and the Lung Cancer Study Team of the MGH Thoracic Oncology Center.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.09.031.
Appendix A Supplementary Data
Supplementary analyses on smoking habits, SCLC, and COPD.
References
- Armstrong B.G., Sloan M. Ordinal regression models for epidemiologic data. Am. J. Epidemiol. 1989;129(1):191–204. doi: 10.1093/oxfordjournals.aje.a115109. [DOI] [PubMed] [Google Scholar]
- Ballaz S., Mulshine J.L. The potential contributions of chronic inflammation to lung carcinogenesis. Clin. Lung Cancer. 2003;5(1):46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- Barr R.G., Herbstman J., Speizer F.E., Camargo C.A., Jr. Validation of self-reported chronic obstructive pulmonary disease in a cohort study of nurses. Am. J. Epidemiol. 2002;155(10):965–971. doi: 10.1093/aje/155.10.965. [DOI] [PubMed] [Google Scholar]
- Bednarek M., Maciejewski J., Wozniak M., Kuca P., Zielinski J. Prevalence, severity and underdiagnosis of COPD in the primary care setting. Thorax. 2008;63(5):402–407. doi: 10.1136/thx.2007.085456. [DOI] [PubMed] [Google Scholar]
- Brenner D.R., Hung R.J., Tsao M.S. Lung cancer risk in never-smokers: a population-based case–control study of epidemiologic risk factors. BMC Cancer. 2010;10:285. doi: 10.1186/1471-2407-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. Usefulness of generalized additive models. Stat. Methods Med. Res. 1996;5(3):331–332. doi: 10.1177/096228029600500307. [DOI] [PubMed] [Google Scholar]
- Conway M.R. A random effects model for binary data. Biometrics. 1990;46:317–328. [Google Scholar]
- Cote M.L., Liu M., Bonassi S. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur. J. Cancer. 2012;48(13):1957–1968. doi: 10.1016/j.ejca.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner M.D., Trupin L., Katz P.P. Development and validation of a survey-based COPD Severity Score. Chest. 2005;127(6):1890–1897. doi: 10.1378/chest.127.6.1890. [DOI] [PubMed] [Google Scholar]
- Engeland A., Haldorsen T., Andersen A., Tretli S. The impact of smoking habits on lung cancer risk: 28 years' observation of 26,000 Norwegian men and women. Cancer Causes Control. 1996;7(3):366–376. doi: 10.1007/BF00052943. [DOI] [PubMed] [Google Scholar]
- Etzel C.J., Lu M., Merriman K., Liu M., Vaporciyan A., Spitz M.R. An epidemiologic study of early onset lung cancer. Lung Cancer. 2006;52(2):129–134. doi: 10.1016/j.lungcan.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Fan Y.G., Jiang Y., Chang R.S. Prior lung disease and lung cancer risk in an occupational-based cohort in Yunnan, China. Lung Cancer. 2011;72(2):258–263. doi: 10.1016/j.lungcan.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Field J.K., Smith D.L., Duffy S., Cassidy A. The Liverpool Lung Project Research protocol. Int. J. Oncol. 2005;27(6):1633–1645. [PubMed] [Google Scholar]
- Freedman N.D., Leitzmann M.F., Hollenbeck A.R., Schatzkin A., Abnet C.C. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9(7):649–656. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruh M., De Ruysscher D., Popat S., Crino L., Peters S., Felip E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. 6):vi99–v105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- van Gestel Y.R., Hoeks S.E., Sin D.D. COPD and cancer mortality: the influence of statins. Thorax. 2009;64(11):963–967. doi: 10.1136/thx.2009.116731. [DOI] [PubMed] [Google Scholar]
- Goodman G.E., Valanis B., Meyskens F.L., Jr. Strategies for recruitment to a population-based lung cancer prevention trial: the CARET experience with heavy smokers. Beta-carotene and retinol efficacy trial. Cancer Epidemiol. Biomark. Prev. 1998;7(5):405–412. [PubMed] [Google Scholar]
- Hashibe M., Morgenstern H., Cui Y. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case–control study. Cancer Epidemiol. Biomark. Prev. 2006;15(10):1829–1834. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- Hastie T., Tibshirani R. Generalized additive models for medical research. Stat. Methods Med. Res. 1995;4(3):187–196. doi: 10.1177/096228029500400302. [DOI] [PubMed] [Google Scholar]
- Heck J.E., Andrew A.S., Onega T. Lung cancer in a U.S. population with low to moderate arsenic exposure. Environ. Health Perspect. 2009;117(11):1718–1723. doi: 10.1289/ehp.0900566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton A.M. Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer. 2013;13(4):233–245. doi: 10.1038/nrc3477. [DOI] [PubMed] [Google Scholar]
- Houtmeyers E., Gosselink R., Gayan-Ramirez G., Decramer M. Regulation of mucociliary clearance in health and disease. Eur. Respir. J. 1999;13(5):1177–1188. doi: 10.1034/j.1399-3003.1999.13e39.x. [DOI] [PubMed] [Google Scholar]
- Hung R.J., Christiani D.C., Risch A. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol. Biomark. Prev. 2008;17(11):3081–3089. doi: 10.1158/1055-9965.EPI-08-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., McKay J.D., Hosono S. Association between a genome-wide association study-identified locus and the risk of lung cancer in Japanese population. J. Thorac. Oncol. 2012;7(5):790–798. doi: 10.1097/JTO.0b013e3182475028. [DOI] [PubMed] [Google Scholar]
- Janda S., Park K., FitzGerald J.M., Etminan M., Swiston J. Statins in COPD: a systematic review. Chest. 2009;136(3):734–743. doi: 10.1378/chest.09-0194. [DOI] [PubMed] [Google Scholar]
- Kalemkerian G.P., Akerley W., Bogner P. Small cell lung cancer. J. Natl. Compr. Cancer Netw. 2013;11(1):78–98. doi: 10.6004/jnccn.2013.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K., Kiyokawa T., Mori K. Current state of treatment for advanced gastric cancer. Gan To Kagaku Ryoho. 2011;38(2):184–186. [PubMed] [Google Scholar]
- Kew K.M., Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014;3:CD010115. doi: 10.1002/14651858.CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Hong Y.C. No association between tumor necrosis factor-alpha gene polymorphisms and lung cancer risk. Environ. Health Toxicol. 2013;28 doi: 10.5620/eht.2013.28.e2013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreienbrock L., Kreuzer M., Gerken M. Case–control study on lung cancer and residential radon in Western Germany. Am. J. Epidemiol. 2001;153(1):42–52. doi: 10.1093/aje/153.1.42. [DOI] [PubMed] [Google Scholar]
- Lamprecht B., Soriano J.B., Studnicka M. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015 doi: 10.1378/chest.14-2535. [DOI] [PubMed] [Google Scholar]
- Landi M.T., Consonni D., Rotunno M. Environment and genetics in lung cancer etiology (EAGLE) study: an integrative population-based case–control study of lung cancer. BMC Public Health. 2008;8:203. doi: 10.1186/1471-2458-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Hyun M.K., Jang E.J., Lee N.R., Kim K., Yim J.J. Inhaled corticosteroid use and risks of lung cancer and laryngeal cancer. Respir. Med. 2013;107(8):1222–1233. doi: 10.1016/j.rmed.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Lee S.M., Woll P.J., Rudd R. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J. Natl. Cancer Inst. 2009;101(15):1049–1057. doi: 10.1093/jnci/djp200. [DOI] [PubMed] [Google Scholar]
- Li L., Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Engelhardt J.F. The glandular stem/progenitor cell niche in airway development and repair. Proc. Am. Thorac. Soc. 2008;5(6):682–688. doi: 10.1513/pats.200801-003AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kho A.T., Kohane I.S., Sun Y. Predicting survival within the lung cancer histopathological hierarchy using a multi-scale genomic model of development. PLoS Med. 2006;3(7) doi: 10.1371/journal.pmed.0030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Cima M.F., Alvarez-Avellon S.M., Pascual T., Fernandez-Somoano A., Tardon A. Genetic polymorphisms in CYP1A1, GSTM1, GSTP1 and GSTT1 metabolic genes and risk of lung cancer in Asturias. BMC Cancer. 2012;12:433. doi: 10.1186/1471-2407-12-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loriot M.A., Rebuissou S., Oscarson M. Genetic polymorphisms of cytochrome P450 2A6 in a case–control study on lung cancer in a French population. Pharmacogenetics. 2001;11(1):39–44. doi: 10.1097/00008571-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Luce D., Stucker I. Investigation of occupational and environmental causes of respiratory cancers (ICARE): a multicenter, population-based case–control study in France. BMC Public Health. 2011;11:928. doi: 10.1186/1471-2458-11-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino D.M., Gagnon R.C., Petty T.L., Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 2000;160(11):1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- Miller D.P., Liu G., De Vivo I. Combinations of the variant genotypes of GSTP1, GSTM1, and p53 are associated with an increased lung cancer risk. Cancer Res. 2002;62(10):2819–2823. [PubMed] [Google Scholar]
- Murgia N., Brisman J., Claesson A., Muzi G., Olin A.C., Toren K. Validity of a questionnaire-based diagnosis of chronic obstructive pulmonary disease in a general population-based study. BMC Pulm. Med. 2014;14:49. doi: 10.1186/1471-2466-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat J.E., Stellman S.D., Wynder E.L. Insulation, asbestos, smoking habits, and lung cancer cell types. Am. J. Ind. Med. 1995;27(2):257–269. doi: 10.1002/ajim.4700270210. [DOI] [PubMed] [Google Scholar]
- Parimon T., Chien J.W., Bryson C.L., McDonell M.B., Udris E.M., Au D.H. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007;175(7):712–719. doi: 10.1164/rccm.200608-1125OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y., Chen L., Elahi A., Lazarus P., Tockman M.S. Genetic analysis of microsomal epoxide hydrolase gene and its association with lung cancer risk. Eur. J. Cancer Prev. 2005;14(3):223–230. doi: 10.1097/00008469-200506000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch B., Kendzia B., Gustavsson P. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case–control studies. Int. J. Cancer. 2012;131(5):1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruefer D., Makowski J., Schnell M. Simvastatin inhibits inflammatory properties of Staphylococcus aureus alpha-toxin. Circulation. 2002;106(16):2104–2110. doi: 10.1161/01.cir.0000034048.38910.91. [DOI] [PubMed] [Google Scholar]
- Purdue M.P., Gold L., Jarvholm B., Alavanja M.C., Ward M.H., Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax. 2007;62(1):51–56. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv S., Hawkins K.A., DeCamp M.M., Jr., Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am. J. Respir. Crit. Care Med. 2011;183(9):1138–1146. doi: 10.1164/rccm.201008-1274CI. [DOI] [PubMed] [Google Scholar]
- Roca M., Roca I.C., Mihaescu T. Lung cancer — a comorbidity in chronic obstructive pulmonary disease. Rev. Med. Chir. Soc. Med. Nat. Iasi. 2012;116(4):1055–1062. [PubMed] [Google Scholar]
- Ruano-Ravina A., Figueiras A., Barros-Dios J.M. Type of wine and risk of lung cancer: a case–control study in Spain. Thorax. 2004;59(11):981–985. doi: 10.1136/thx.2003.018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano-Ravina A., Pereyra M.F., Castro M.T., Perez-Rios M., Abal-Arca J., Barros-Dios J.M. Genetic susceptibility, residential radon, and lung cancer in a radon prone area. J. Thorac. Oncol. 2014;9(8):1073–1080. doi: 10.1097/JTO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- Schottker B., Haug U., Schomburg L. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am. J. Clin. Nutr. 2013;97(4):782–793. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- Schwartz A.G., Ruckdeschel J.C. Familial lung cancer: genetic susceptibility and relationship to chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2006;173(1):16–22. doi: 10.1164/rccm.200502-235PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A.G., Cote M.L., Wenzlaff A.S., Land S., Amos C.I. Racial differences in the association between SNPs on 15q25.1, smoking behavior, and risk of non-small cell lung cancer. J. Thorac. Oncol. 2009;4(10):1195–1201. doi: 10.1097/JTO.0b013e3181b244ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilya Z., Leitner-Dagan Y., Pinchev M. Low integrated DNA repair score and lung cancer risk. Cancer Prev. Res. (Phila.) 2014;7(4):398–406. doi: 10.1158/1940-6207.CAPR-13-0318. [DOI] [PubMed] [Google Scholar]
- St Sauver J.L., Grossardt B.R., Leibson C.L., Yawn B.P., Melton L.J., 3rd, Rocca W.A. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin. Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang A., Pohlabeln H., Muller K.M., Jahn I., Giersiepen K., Jockel K.H. Diagnostic agreement in the histopathological evaluation of lung cancer tissue in a population-based case–control study. Lung Cancer. 2006;52(1):29–36. doi: 10.1016/j.lungcan.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Straus S.E., McAlister F.A., Sackett D.L., Deeks J.J. Accuracy of history, wheezing, and forced expiratory time in the diagnosis of chronic obstructive pulmonary disease. J. Gen. Intern. Med. 2002;17(9):684–688. doi: 10.1046/j.1525-1497.2002.20102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley A.J., Tetley T.D. Pulmonary epithelium, cigarette smoke, and chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulm. Dis. 2007;2(4):409–428. [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J., Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am. J. Epidemiol. 2010;172(12):1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele T.J., Asomaning K., Tchetgen Tchetgen E.J. Genetic variants on 15q25.1, smoking, and lung cancer: an assessment of mediation and interaction. Am. J. Epidemiol. 2012;175(10):1013–1020. doi: 10.1093/aje/kwr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P., Kogevinas M., Simonato L., Brennan P., Boffetta P. Levelling-off of the risk of lung and bladder cancer in heavy smokers: an analysis based on multicentric case–control studies and a metabolic interpretation. Mutat. Res. 2000;463(1):103–110. [PubMed] [Google Scholar]
- Wang Y., McKay J.D., Rafnar T. Rare variants of large effect in BRCA2 and CHEK2 affect risk of lung cancer. Nat. Genet. 2014;46(7):736–741. doi: 10.1038/ng.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Spitz M.R., Amos C.I., Wilkinson A.V., Wu X., Shete S. Mediating effects of smoking and chronic obstructive pulmonary disease on the relation between the CHRNA5-A3 genetic locus and lung cancer risk. Cancer. 2010;116(14):3458–3462. doi: 10.1002/cncr.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Allen M.S., Aubry M.C. Clinical features of 5628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- Young R.P., Hopkins R.J. How the genetics of lung cancer may overlap with COPD. Respirology. 2011;16(7):1047–1055. doi: 10.1111/j.1440-1843.2011.02019.x. [DOI] [PubMed] [Google Scholar]
- Zhai R., Yu X., Shafer A., Wain J.C., Christiani D.C. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145(2):346–353. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai R., Yu X., Wei Y., Su L., Christiani D.C. Smoking and smoking cessation in relation to the development of co-existing non-small cell lung cancer with chronic obstructive pulmonary disease. Int. J. Cancer. 2014;134(4):961–970. doi: 10.1002/ijc.28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Hu Z., Huang J. A 3'-untranslated region polymorphism in IGF1 predicts survival of non-small cell lung cancer in a Chinese population. Clin. Cancer Res. 2010;16(4):1236–1244. doi: 10.1158/1078-0432.CCR-09-2719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary analyses on smoking habits, SCLC, and COPD.