Abstract
Plasmodium falciparum exports proteins into erythrocytes using the Plasmodium export element (PEXEL) motif, which is cleaved in the endoplasmic reticulum (ER) by plasmepsin V (PMV). A recent study reported that phosphatidylinositol-3-phosphate (PI(3)P) concentrated in the ER binds to PEXEL motifs and is required for export independent of PMV, and that PEXEL motifs are functionally interchangeable with RxLR motifs of oomycete effectors. Here we show that the PEXEL does not bind PI(3)P, and that this lipid is not concentrated in the ER. We find that RxLR motifs cannot mediate export in P. falciparum. Parasites expressing a mutated version of KAHRP, with the PEXEL motif repositioned near the signal sequence, prevented PMV cleavage. This mutant possessed the putative PI(3)P-binding residues but is not exported. Reinstatement of PEXEL to its original location restores processing by PMV and export. These results challenge the PI(3)P hypothesis and provide evidence that PEXEL position is conserved for co-translational processing and export.
 Export of Plasmodium falciparum proteins into infected erythrocytes relies upon the PEXEL motif in target proteins. Here Boddey et al. challenge the hypothesis that the PEXEL motif mediates export by binding PI(3)P and instead suggest it acts via cleavage by plasmepsin V.
Export of Plasmodium falciparum proteins into infected erythrocytes relies upon the PEXEL motif in target proteins. Here Boddey et al. challenge the hypothesis that the PEXEL motif mediates export by binding PI(3)P and instead suggest it acts via cleavage by plasmepsin V.
P. falciparum is a virulent parasite of humans. Following invasion of erythrocytes, the merozoite forms initiate remodelling, converting the terminally differentiated host cell into one in which the parasite can evade host responses and replicate (reviewed in ref. 1). Remodelling results in profound structural and morphological changes of the host cell central to parasite survival. This includes alterations of the cells physical properties, making it more rigid and gaining adhesive properties that can block flow in the microvasculature2,3. These parasite-induced changes of the erythrocyte play an important role in pathogenesis of malaria4.
The properties of P. falciparum-infected erythrocytes are conferred by export of hundreds of parasite proteins. To reach their destination, they traverse membranes of the parasite and parasitophorous vacuole (PV) and, in some cases, the erythrocyte membrane to reach the cell surface5. Consequently, the intracellular parasite exports many proteins establishing a trafficking network in the host cell. Most exported proteins contain a signal sequence for entry into the endoplasmic reticulum (ER) followed by 15–30 residues and a motif Plasmodium export element (PEXEL)/vacuolar transport signal6,7. The PEXEL is cleaved by the aspartyl protease plasmepsin V (PMV) in the ER, to uncover the export signal for trafficking to erythrocytes1,8,9,10,11. The PMV active site appears to be on the ER luminal side12. There are hundreds of proteins with a PEXEL predicted to be exported to the host erythrocyte6,7,13 via the Plasmodium translocon of exported proteins (PTEX) parasite translocon14,15.
Another group of exported proteins lack a PEXEL, the PEXEL-negative exported proteins16,17. This includes skeleton-binding protein 118, membrane-associated histidine-rich proteins 1 and 2 (refs 19, 20), ring-exported proteins 1 and 2 (REX1 (ref. 21) and REX2 (ref. 22)), and the MSP-7-related proteins17, as well as P. falciparum erythrocyte membrane protein 1 (PfEMP1)1,17. PfEMP1 is trafficked to the surface of the erythrocyte where it adheres to host receptors on the microvasculature (reviewed in ref. 23). PEXEL-negative exported proteins are not cleaved by PMV and are selected for export through an unknown pathway but translocated via PTEX14,15.
Recently, a study reported phosphatidylinositol-3-phosphate (PI(3)P) binds PEXEL with high affinity and this phospholipid is concentrated on the ER lumenal membrane in P. falciparum24. In addition, PI(3)P binding in the ER, via the PEXEL Arg, is required for export independent of cleavage by PMV24. It was also reported that PEXEL is functionally interchangeable with RxLR of effector proteins from oomycetes that are translocated into host cells24,25. Owing to the implications of these findings, our six laboratories have examined key findings of Bhattacharjee et al.24,25. In contrast, we find PEXEL does not bind PI(3)P, this phospholipid is not concentrated in the ER of P. falciparum but on the food vacuole and apicoplast membranes, that the PEXEL is not functionally interchangeable with the RxLR motif of oomycetes and the PEXEL is located at a conserved location to ensure co-translational processing by PMV for export. Therefore, PI(3)P binding of PEXEL in the ER is not the selective mechanism for export of malarial effector proteins to the P. falciparum-infected erythrocyte.
Results
P. falciparum exported proteins do not bind PI(3)P
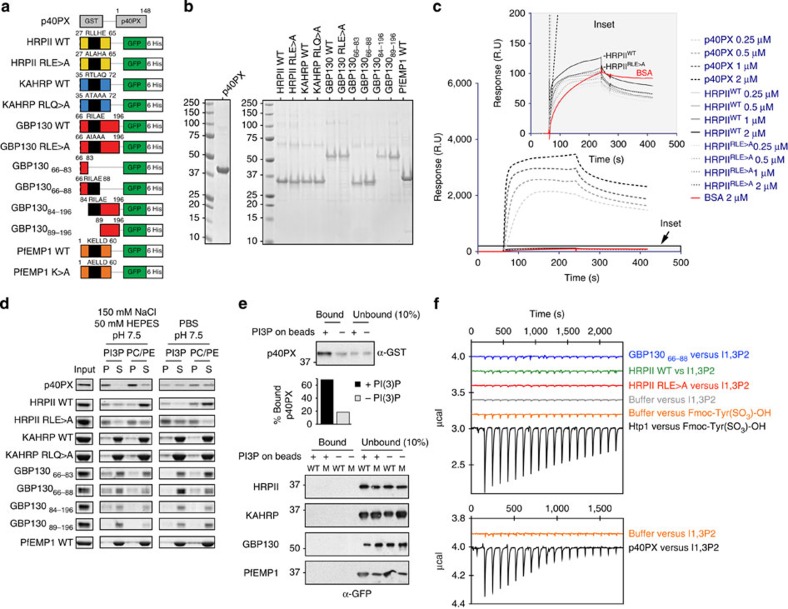
It has been suggested that PI(3)P specifically binds PEXEL of exported proteins24. This hypothesis was tested using proteins containing a PEXEL, or with this motif mutated. The amino terminus of exported proteins, histidine-rich protein II (HRPII)7,26, knob-associated histidine-rich protein (KAHRP)6 and glycophorin binding protein 130 (GBP130)7,26, which all contain a wild-type or mutant PEXEL motif, were expressed as green fluorescent protein (GFP)/6His fusions (Fig. 1a,b). The N terminus of PfEMP1, which does not have a PEXEL, has also been shown to bind PI(3)P24 and it was expressed fused to GFP/6His (Fig. 1a,b). The p40PX protein, which contains a PX domain that specifically binds PI(3)P, was used as a positive control24.
Figure 1. Exported P. falciparum proteins do not bind PI(3)P.
(a) Recombinant proteins expressed in this study as fusions to GST or GFP/6His. (b) Recombinant proteins stained with Coomassie blue stain. Molecular weights (kDa) are shown. (c) Binding of p40PX and HRPII with native PEXEL (WT) or mutant PEXEL (RLE>A) to PI(3)P liposomes measured by SPR. Binding to control PC/PE liposomes was subtracted to give the sensorgrams24. BSA was used to measure nonspecific binding to the liposome-coated chip (red). Inset: a similar level of low binding of HRPIIWT and HRPIIRLE>A to PI(3)P liposomes. Experiments were performed in triplicate. (d) Binding of proteins to PI(3)P liposomes or control PC/PE liposomes, determined by ultracentrifugation. Pellet and supernatant fractions were resolved by SDS–PAGE and Coomassie blue stain. Proteins were ultracentrifuged in buffer to remove potential aggregates before incubation with liposomes39, explaining why input and sum of pellet and supernatant sometimes differ. Experiments were performed in triplicate. Full-length gels shown in Supplementary Fig. 6. (e) Binding of p40PX and P. falciparum proteins with native PEXEL (WT) or mutant PEXEL (M) to PI(3)P-coated beads (+) or control beads lacking lipid (−). Bound protein was eluted in SDS–PAGE sample buffer and detected by immunoblot with anti-GST (p40PX) or anti-GFP (P. falciparum proteins). Ten per cent of unbound fraction volume was loaded to visualize protein inputs. Densitometry (histogram shown) of the p40PX bands shows that 68% of p40PX input bound to PI(3)P-coated beads (PI3P+) compared with 18% of input to control beads (PI3P−). No binding was detected for P. falciparum exported proteins. Experiments were performed in triplicate. Full-length gels shown in Supplementary Fig. 7. (f) Binding of recombinant proteins to phospholipid head group of PI(3)P (inositol 1,3 bisphosphate; I1,3P2) in solution, measured by isothermal calorimetry. The titration did not differ between I1,3P2 (2.4 mM) into dialysis buffer (150 mM NaCl, 20 mM HEPES pH 7.4) (negative control) and the same buffer containing either GBP13066–88, HRPIIWT or HRPIIRLE>A. As controls, binding of Htp1 from S. parasitica to Fmoc-Tyr(SO3)-OH and also p40PX binding to I1,3P2 are shown. Experiments were performed in duplicate.
To determine whether p40PX bound PI(3)P, surface plasmon resonance (SPR) was performed following methods of Battacharjee et al.24. Proteins were flowed over a chip coated with phosphatidylcholine (PC):phosphatidylethanolamine (PE):C16PI(3)P (75:20:5) liposomes (Fig. 1c). As expected, p40PX bound quantitatively to liposomes (Kd: 195 nM). HRPIIWT showed very little binding and the control protein HRPIIRLE>A, in which the PEXEL had been mutated, showed similarly low binding, demonstrating PEXEL of HRPII does not mediate specific binding. Both proteins bound with a similar response to background binding of BSA (Fig. 1c). HRPII–GFP proteins were fluorescent, indicating they were folded, and gel filtration analysis confirmed they were monomers (Supplementary Fig. 1a). Similarly, KAHRPWT, GBP130WT, KAHRPRLQ>A and GBP130RLE>A binding was too weak to quantify (Supplementary Fig. 1b). Although the purchased p40PX bound quantitatively to PI(3)P (Kd: 196 μM), the Req was less than p40PX expressed and purified by ourselves (Fig. 1c). This demonstrated that proteins expressed and purified by our laboratories could bind PI(3)P strongly; however, neither the PEXEL motif nor the surrounding sequences of exported P. falciparum proteins produced specific binding to PI(3)P above background.
As we could not detect specific PEXEL binding to PI(3)P by SPR, we tested other formats. Liposomes containing either PI(3)P or control (PC/PE) were tested for binding in solution24 and proteins sampled by ultracentrifugation. p40PX protein bound strongly to PI(3)P liposomes in HEPES buffer and could be blocked using PBS (phosphate competes with phosphorylated lipids for lipid binding). Some p40PX could be detected in the pellet of control liposomes, suggesting PI(3)P-independent binding (Fig. 1d). Nevertheless, KAHRPWT, KAHRPRLQ>A, GBP13066–83, GBP13066–88, GBP13084–196, GBP13089–196 and PfEMP1WT showed no PEXEL-specific binding. HRPIIWT showed binding to PI(3)P and PC/PE liposomes but the HRPII protein, in which the PEXEL was mutated (HRPIIRLE>A), bound at a similar level and PBS had no effect, providing strong evidence this motif was not responsible for binding. Our results demonstrated no correlation between the presence of PEXEL and specific binding to PI(3)P liposomes, suggesting they do not specifically interact.
To further test whether HRPIIWT, HRPIIRLE>A, KAHRPWT, KAHRPRLQ>A, GBP130WT, GBP130RLE>A and PfEMP1WT bound PI(3)P, they were incubated with PI(3)P-coated beads or controls. The p40PX protein bound strongly to PI(3)P (68% bound) but the P. falciparum proteins showed no binding (Fig. 1e). Similar results were obtained with GBP13066–83, GBP13066–88, GBP13084–196 and GBP13089–196, confirming these proteins did not bind PI(3)P (Supplementary Fig. 2a).
To analyse whether PEXEL could bind PI(3)P in solution, isothermal titration calorimetry (ITC) was performed with HRPIIWT, HRPIIRLE>A, GBP13066–88 and GBP13066–196 using the head group of PI(3)P, inositol 1,3 diphosphate (I1,3P2). The proteins did not show any thermal interaction signature with I1,3P2 that was indicative of binding (Fig. 1f and Supplementary Fig. 2c). This was not due to instrument sensitivity, as titrations were performed between Htp1 from Saprolegnia parasitica (SpHtp124-198(His)6) and the ligand Fmoc-Tyr(SO3)-OH and between p40PX and I1,3P2, and binding interactions were observed for these proteins (Fig. 1f)24,27.
Finally, lipid binding was assessed using spot membranes as described previously28. GBP13066–196 and GBP13066–196RLE>A containing a mutated PEXEL showed binding to PI(3)P, PI(4)P and PI(5)P on membranes; however, the PEXEL mutant also bound, suggesting it was not responsible for the interaction (Supplementary Fig. 2d). Binding to lipid spot membranes can occur by a small proportion of misfolded proteins and the interaction blocked by sub-stoichiometric amounts of DnaK, a chaperone that specifically binds misfolded proteins28. To test whether GBP130 proteins were binding lipids in this way, DnaK was incubated with GBP13066–196 and this blocked binding (Supplementary Fig. 2e), confirming PEXEL of GBP130 does not specifically interact with PI(3)P, and that misfolded PEXEL proteins interact with lipids.
In summary, we did not detect specific binding of HRPII, KAHRP, GBP130 or PfEMP1 to PI(3)P using methods published previously24, as well as alternative approaches.
PEXEL and RxLR proteins do not bind PI(3)P
It was reported that PEXEL protein HRPII binds PI(3)P in the P. falciparum ER before cleavage by PMV, as determined by immunoprecipitation and measuring bound lipid by enzyme-linked immunosorbent assay (ELISA)24. We followed the same protocol using proteins, to measure bound PI(3)P. We immunoprecipitated (IP) proteins that either had a PEXEL and were cleaved and exported or contained mutations that blocked cleavage and export. We expected PEXEL-cleaved proteins would not co-IP PI(3)P, owing to rapid removal of lipid-binding residues as reported (that is, conserved PEXEL Arg)24; however, if the PI(3)P-binding hypothesis was correct, that proteins containing such N-terminal lipid-binding residues such as KAHRPΔ38–48 (containing RTLAQ), HRPIIss-AVR3a (containing RLLR) and KAHRP R>K (containing KTLAQ; the PEXEL-like lysine residue in PfEMP1 previously reported to bind PI(3)P24), would co-IP PI(3)P. However, although PI(3)P ELISA controls were detected, allowing the generation of a standard curve, the quantity of PI(3)P on purified chimeras was minimal and no pattern was evident between PEXEL/RxLR-containing and PEXEL mutant proteins (Supplementary Fig. 3a). One possibility for this discrepancy is Bhattacharjee et al.24 used HIV protease inhibitor lopinavir to inhibit PMV, to retain more uncleaved HRPII precursor before IP. Lopinavir is a poor PMV inhibitor and lethal to P. falciparum, probably acting on other aspartyl proteases10. To test whether lopinavir affects viability under the conditions described24, we treated parasites and assessed translation. This showed lopinavir had a more potent effect on inhibiting translation than antimalarials, strongly suggesting the parasites were dying (Supplementary Fig. 3b). Under these conditions, it is possible that Bhatachaerjee et al.24 detected anomalous lipid binding to their reporters.
PI(3)P is present on the food vacuole membrane
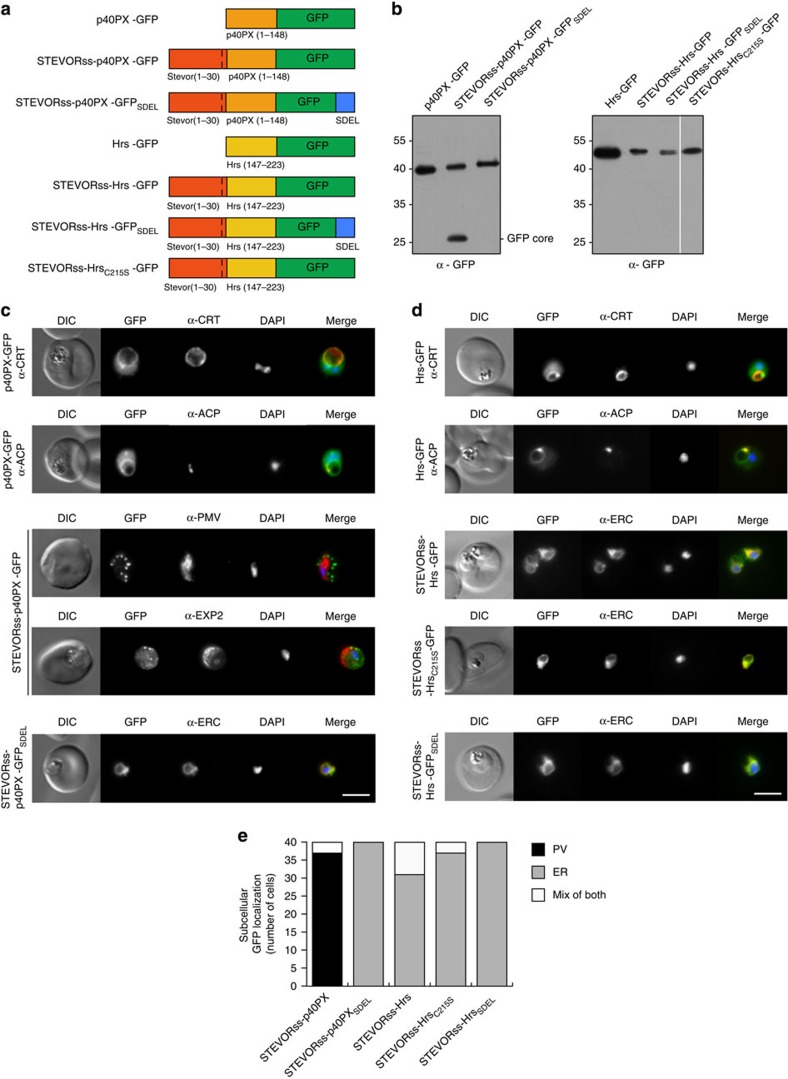
Bhattacharjee et al.24 localized PI(3)P to the inner leaflet of ER membranes in P. falciparum by expressing fluorescent reporters fused to PI(3)P-binding domains of two endosomal proteins, p40-phox-PX and early endosomal antigen 1 (EEA1)24. To localize PI(3)P, we made transgenic parasites expressing p40PX fused to GFP (p40PX-GFP) in different parasite subcellular compartments (Fig. 2a,b). The p40PX-GFP protein, lacking a signal sequence, was expressed in the cytoplasm and the fluorescence was concentrated around the food vacuole, as determined by co-localization with P. falciparum chloroquine resistance transporter29, and the apicoplast, as determined by co-localization with acyl carrier protein (ACP) (Fig. 2c). This was consistent with previous localization of PI(3)P at the food vacuole and apicoplast30, and the lysosomal membrane and endocytic pathway in other organisms31. To determine whether PI(3)P was present on the luminal side of ER membranes, we fused p40PX-GFP to a signal sequence from STEVOR (STEVORss-p40PX-GFP) (Fig. 2c)32. This targeted the protein into the ER, where it would be retained bound to PI(3)P as reported24. However, a circumferential localization around the parasite was observed, indicating it was secreted to the parasite periphery and PV. This was confirmed by the presence of a ‘GFP core' species by immunoblotting (Fig. 2b), which results from endocytosis from the PV to the food vacuole during haemoglobin uptake; this confirms the chimera was secreted and not ER retained. Experiments using antibodies to the ER resident protease PMV33 failed to show co-localization with GFP, indicating the reporter was not retained in the ER (Fig. 2c,e)24. The GFP localization was similar to EXP2 (ref. 34), a marker of the PV confirming secretion. To exclude the possibility that the STEVOR signal sequence influenced this localization, we fused p40PX-GFP to the ACP signal sequence and it trafficked to the PV and was not retained in the ER (Supplementary Fig. 4). This PV localization was also observed when the PI(3)P-binding residue of p40PX was mutated (R58Q, Supplementary Fig. 4). This suggests p40PX did not bind to the inner leaflet of the ER, consistent with a lack of PI(3)P. Only when an SDEL retention signal35 was fused to the carboxy terminus of STEVORss-p40PX-GFP did it accumulate in the ER, as shown by co-localization with the ER calcium-binding protein (ERC) (Fig. 2c,e). Therefore, PI(3)P is present on the food vacuole and apicoplast membranes of P. falciparum but not within the ER24.
Figure 2. PI(3)P is not localized in the ER of P. falciparum.
(a) Chimeric proteins expressed in P. falciparum. p40PX-GFP, no signal sequence; STEVORss-p40PX-GFP, STEVOR signal sequence; STEVORss-p40PX-GFPSDEL, STEVOR signal sequence and ER retention signal; Hrs-GFP, no signal sequence; STEVORss-Hrs-GFP, STEVOR signal sequence; STEVORss-HrsSDEL-GFP, signal sequence and ER retention signal; STEVORss-HrsC215S-GFP, STEVOR signal sequence and mutation of lipid-binding residue C215S in both FYVE fingers. (b) p40PX and Hrs reporters are expressed in P. falciparum-infected erythrocytes. Blots probed with anti-GFP antibodies. First panel: p40PX-GFP, STEVORss-p40PX-GFP and STEVORss-p40PX-GFPSDEL. A ‘GFP core' band was observed for STEVORss-p40PX-GFP. This is observed for all GFP chimeras secreted to the PV and does not indicate degradation specific to this protein. Note the absence of ‘GFP core' when SDEL ER-retention signal was attached, which prevented secretion to PV (lane 3). Second panel: Hrs-GFP, STEVORss-Hrs-GFP, STEVORss-Hrs-GFPSDEL and STEVORss-HrsC215S-GFP. Note the lack of ‘GFP core' for STEVORss-Hrs fusions, confirming they were not secreted. (c) p40PX-GFP localizes to the cytoplasm and to membranes of the food vacuole (labelled with anti-CRT) and apicoplast (labelled with anti-ACP). STEVORss-p40PX-GFP localizes to the PV in P. falciparum-infected erythrocytes, as shown by co-localization with anti-EXP2, and not in the ER, which was labelled with anti-PMV. If PI(3)P was located within the ER, this chimera would be expected to remain within the ER via p40PX binding. Fusion to an ER-retention signal (STEVORss-p40PX-GFPSDEL) retains the protein in ER, co-localization with anti-ERC. Scale bar, 5 μm. (d) Localization of Hrs-GFP to the cytoplasm and membranes of food vacuole (labelled anti-CRT) and apicoplast (labelled anti-ACP). STEVORss-Hrs-GFP localizes to the ER as shown by co-localization with anti-ERC; however, mutation of residues necessary for lipid binding in both Hrs FYVE fingers (STEVORss-HrsC215S-GFP) does not abrogate ER localization, demonstrating PI(3)P-independent retention in ER. Addition of ER retention signal (STEVORss-Hrs-GFPSDEL) also causes ER localization. Scale bar, 5 μm. (e) Quantification of GFP localization in the PV (black), ER (grey), or mix of both (white) determined using 40 P. falciparum-infected erythrocytes per construct (20 cells per experiment, performed twice).
To further assess the location of PI(3)P in P. falciparum, we expressed Hrs with two FYVE fingers36, in different subcellular compartments of the parasite, using a STEVOR signal peptide and GFP (Fig. 2a,b). When Hrs2xFYVE was expressed in the cytoplasm, Hrs-GFP was concentrated around the food vacuole and apicoplast, consistent with PI(3)P at these membranes (Fig. 2d). However, when it was directed through the ER (STEVORss-Hrs-GFP), the protein co-localized with the ER marker, ERC, similar to when an ER retention signal was added (Fig. 2d,e), and was not secreted to the PV (note the absence of ‘GFP core' Fig. 2b). However, when residues needed for PI(3)P binding were mutated, the protein remained in the ER. Therefore, ER retention observed for STEVORss-Hrs-GFP and STEVORss-HRSC215S-GFP was not due to PI(3)P-specific binding, suggesting another interaction(s) was retaining reporters in the ER, possibly involving unfolded proteins and ER chaperones. These results are consistent with the lack of PI(3)P in the parasite ER.
The RxLR motif cannot mediate export in P. falciparum
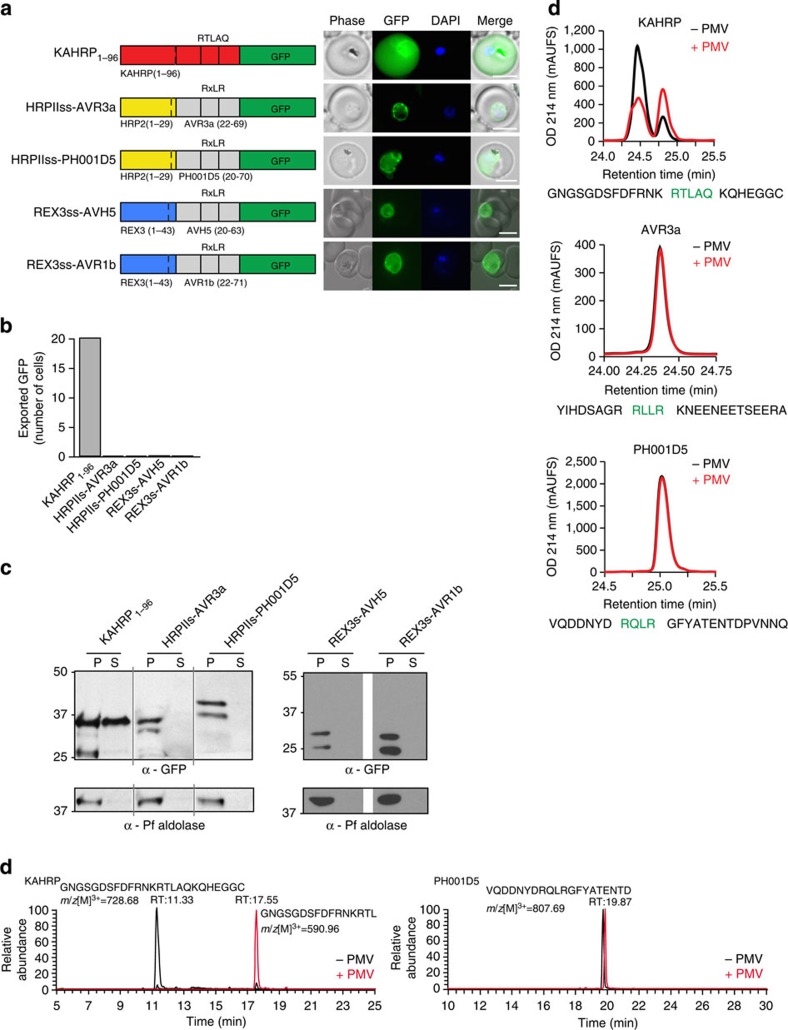
The RxLR amino acid motif in effectors of oomycetes has been implicated in export to host cells37. The RxLR motif from Phytophthora infestans also reportedly allows export from P. falciparum and Plasmodium berghei into erythrocytes24,25. Expression of PEXEL in P. infestans proteins may also aid in translocation of chimeric effector proteins38,39. This suggested RxLR was functionally transferable with PEXEL in Plasmodium spp. We therefore tested whether RxLR could mediate export in P. falciparum and if it was a substrate for PMV, as is the PEXEL. To examine this, we used the same constructs reported by Bhattacharjee et al.25, where the signal sequence of P. falciparum HRPII was fused to 47 or 49 amino acids of the P. infestans proteins, Avirulence protein 3a (AVR3a) or PH001D5, respectively, which contained RxLR in an equivalent position as PEXEL in P. falciparum6,7,40. These were fused to GFP (HRPIIss-AVR3a and HRPIIss-PH001D5) and expressed in P. falciparum. Both proteins were retained in the parasite and PV, and not exported, in contrast to KAHRP1–96, which contains a PEXEL and was exported (Fig. 3a,b). To further examine RxLR, we made two constructs where the signal sequence of REX3, a P. falciparum exported protein, was fused to the RxLR region of Phytophthora sojae AVH5 (REX3ss-AVH5)41 or AVR1b (REX3ss-AVR1b)42 followed by GFP. When expressed in P. falciparum, REX3ss-AVH5 and REX3ss-AVR1b entered the ER and were trafficked to the PV, but not exported (Fig. 3a,b). These data show RxLR from different Phytophthora spp. cannot mediate export to the host cell by P. falciparum.
Figure 3. Oomycete RxLR does not mediate export in P. falciparum.
(a) KAHRP1–96 is exported to the P. falciparum-infected erythrocyte; however, HRPIIss-AVR3a, HRPIIss-PH001D5, REX3ss-AVH5 and REX3ss-AVR1b are not exported but accumulate in the parasite and PV. Scale bar, 5 μm. (b) Quantification of cells with exported GFP determined using 20 P. falciparum-infected erythrocytes per construct (10 cells per experiment, performed twice). (c) Immunoblot with anti-GFP antibodies of each chimera from the tetanolysin pellet (P) and supernatant (S) shows that KAHRP1–96 is exported but RxLR chimeras are not. Tetanolysin inserts pores in the erythrocyte membrane, allowing sampling of the host cell cytosol. Aldolase was included as a control, as described previously71. Full-length gels shown in Supplementary Fig. 8. (d) RP-HPLC shows the KAHRP peptide is cleaved when incubated with PMV (red). AVR3a and PH001D5 peptides are not cleaved by PMV. PEXEL/RxLR sequences are labelled green. (e) MS/MS-extracted ion chromatograms of KAHRP and PH001D5 peptides after incubation with PMV shows that KAHRP peptides were cleaved after the PEXEL Leu (GNGSGDSFDFRNKRTL−) but PH001D5 was not cleaved.
Export of PEXEL proteins requires cleavage by PMV10,11,43. This was confirmed using KAHRP1–96, which was exported into the erythrocyte (Fig. 3a) and the cleaved protein detected in the tetanolysin supernatant (Fig. 3c). Tetanolysin inserts pores in the erythrocyte membrane, allowing sampling of proteins in the host cell cytosol44. Cleavage of KAHRP by PMV in P. falciparum occurs after the PEXEL Leu9. In contrast, HRPIIss-AVR3a, HRPIIss-PH001D5, REX3ss-AVH5 and REX3ss-AVR1b showed no signal in the tetanolysin supernatant, despite evidence of some N-terminal processing, demonstrating these proteins are not exported nor cleaved in a manner competent for export (Fig. 3c). To understand the mechanism, we examined whether RxLR is a substrate for PMV (Fig. 3d,e). Peptides containing RxLR from AVR3a or PH001D5 were incubated with PMV and analysed by mass spectrometry10. In contrast to KAHRP peptides that were cleaved after the PEXEL Leu by PMV, neither AVR3a nor PH001D5 peptides were affected. To confirm this, we affinity purified HRPIIss-PH001D5 from P. falciparum-infected erythrocytes and examined the proteins by mass spectrometry (MS). We detected peptides N-terminal to RxLR and the intact peptide as well (Supplementary Fig. 5). Thus, RxLR is not a substrate for PMV, consistent with it not mediating export in P. falciparum.
Export of PEXEL proteins is not independent of PMV
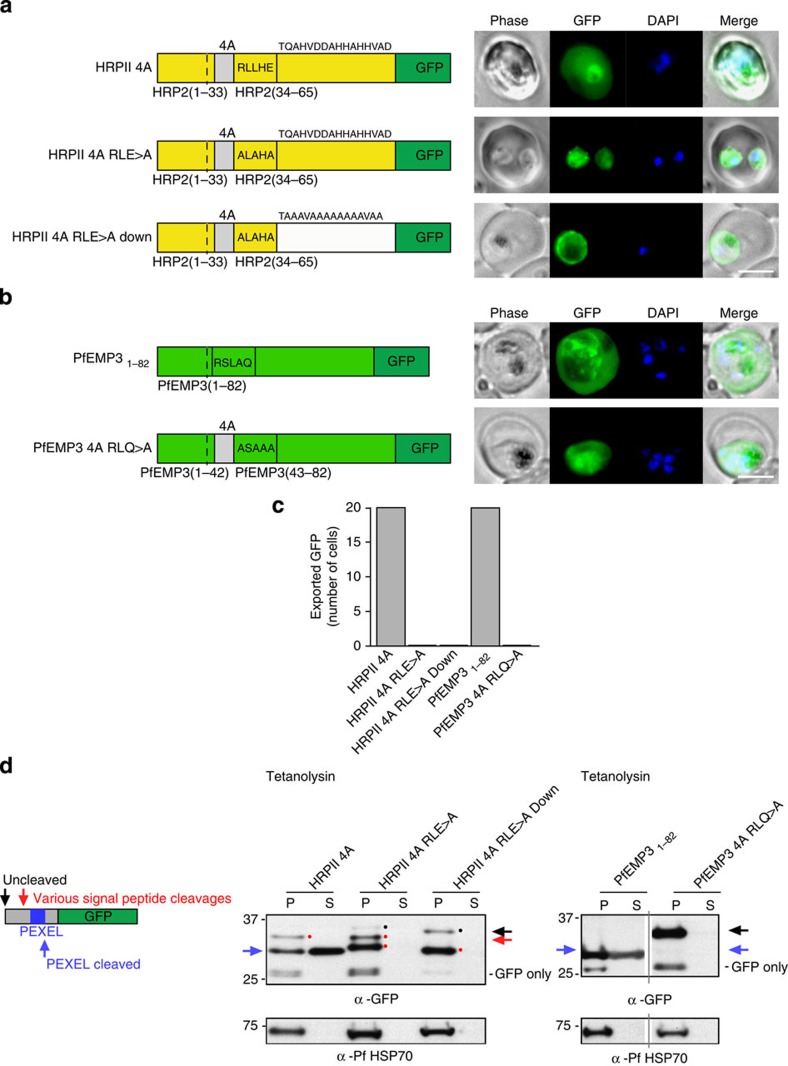
It was reported that PEXEL proteins HRPII and PfEMP3 can be exported via an alternative mechanism if PEXEL is mutated when four alanines are inserted immediately after the signal sequence24,45. To test this, we generated similar constructs24,45 of HRPII with a signal sequence and PEXEL (RLLHE) fused to GFP having four alanines inserted immediately after the signal sequence (Supplementary Table 1). HRPII 4A had a PEXEL and was exported (Fig. 4a). However, when the PEXEL was mutated (HRPII 4A RLE>A), no export was observed and it accumulated in the parasite and PV. Mutation of residues C-terminal to the mutant PEXEL (HRPII 4A RLE>A Down)24,45 did not allow export (Fig. 4a,b). The HRPII proteins were analysed by tetanolysin fractionation and immunoblotting. The HRPII 4A protein with a PEXEL was present in the supernatant fraction and processed by PMV, confirming it was exported (Fig. 4d). However, both HRPII 4A RLE>A and HRPII 4A RLE>A proteins were absent from supernatant fractions and not exported, despite evidence of some processing N-terminal to the mutant PEXEL sequence (Fig. 4d). The lack of export of HRPII 4A RLE>A was in contrast to previous work with this construct and shows cleavage by PMV is required24.
Figure 4. PEXEL-independent export of PEXEL proteins does not occur in P. falciparum.
(a) HRPII 4A is exported to the P. falciparum-infected erythrocyte. HRPII 4A RLE>A and HRPII 4A RLE>A Down are not exported. Scale bar, 5 μm. (b) PfEMP31–82 is exported by P. falciparum to the erythrocyte, but PfEMP3 4A RLQ>A is not. Scale bar, 5 μm. (c) Quantification of cells with exported GFP determined using 20 P. falciparum-infected erythrocytes per construct (10 cells per experiment, performed twice). (d) Immunoblot with anti-GFP antibodies of chimeras from the tetanolysin pellet (P) and supernatant (S) shows that HRPII 4A and PfEMP31–82 are cleaved at the PEXEL (blue arrow) and exported. Signal peptide-cleaved HRPII 4A was also visible (red spot). HRPII 4A RLE>A, HRPII 4A RLE>A Down and PfEMP31–82 RLQ>A were not exported and bands corresponding to signal peptide-cleaved (various positions possibly due to insertion of four alanines; red spots/arrow) and uncleaved protein (black spots/arrow) are present. Full-length gels shown in Supplementary Fig. 9.
Similarly, PfEMP31–82 with a PEXEL was exported (Fig. 4b,c) and processed by PMV (Fig. 4d). Cleavage of PfEMP3 by PMV in P. falciparum occurs after the PEXEL Leu10. However, mutation of PEXEL RLQ>A blocked cleavage by PMV and resulted in no export, including when four alanines were fused immediately after the signal sequence (Fig. 4b–d). In contrast to earlier work24,45, these results with HRPII and PfEMP3 demonstrate export occurs only when PEXEL was present and processed by PMV.
Spatial positioning of the PEXEL is important for export
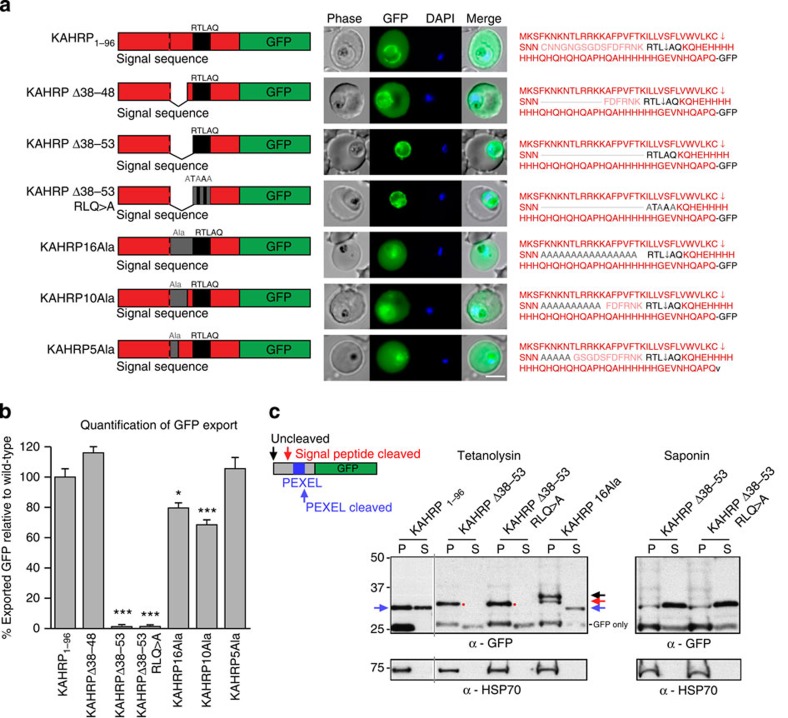
It has been reported that PEXEL proteins can be exported independent of PMV activity by binding PI(3)P. We sought to generate a protein containing a PEXEL not cleaved by PMV, to assess whether it could be exported by an alternative mechanism such as PI(3)P. The PEXEL is located at a conserved position in exported proteins (15–30 amino acids C-terminal to the signal sequence)13 and we investigated the effect of shifting this position towards the N-terminal signal sequence. KAHRP1–96 containing a PEXEL in the native position was efficiently exported (Fig. 5a,b). Deletion of 11 amino acids N-terminal to the PEXEL (KAHRPΔ38–48) had no negative effect on export, consistent with these amino acids having no essential role (Fig. 5a,b). However, deletion of 16 amino acids abutting the PEXEL (KAHRPΔ38–53), which placed the motif 3 residues beyond the signal sequence cleavage site (LKC—SNN), determined previously for KAHRP9, blocked export and the protein was secreted to the PV (Fig. 5a,b). The same PV localization was observed when PEXEL was mutated (KAHRPΔ38–53RLQ>A). Reinstatement of PEXEL to its native position using a 16 alanine spacer (16Ala) restored export, showing that motif positioning, and not primary amino acid sequence N-terminal to the PEXEL, is critical for export. Incorporation of six residues of KAHRP immediately abutting PEXEL (10Ala) did not improve export above 80% of controls; however, inclusion of 11 KAHRP residues abutting PEXEL (5Ala) fully restored export. It is possible that residues in this area assist export by improving the affinity of the substrate for PMV, although not being critical for processing.
Figure 5. Correct PEXEL positioning is essential for export.
(a) KAHRP1–96 and KAHRPΔ38–48 are exported to the P. falciparum-infected erythrocyte but KAHRPΔ38–53 and KAHRPΔ38–53 RLQ>A accumulate in the PV and are not exported. KAHRP16Ala, KAHRP10Ala and KAHRP5Ala are exported to the red blood cell. Scale bar, 5 μm. (b) Quantification of export in a. Data are the mean (±s.e.m.) GFP fluorescence intensity in the host cell from 20 cells per construct (20 replicates), shown as a percentage of KAHRP1–96. Data were analysed by t-test (***P<0.0001 and *P<0.003, compared with KAHRP1–96). (c) Immunoblot with anti-GFP antibodies of chimeras from the tetanolysin and saponin pellet (P) and supernatant (S). This shows KAHRP1–96 at a size corresponding to cleavage at the PEXEL and it was exported (32 kDa; blue arrows), KAHRPΔ38–53 and KAHRPΔ38–53 RLQ>E were cleaved to a size corresponding to cleavage by signal peptidase (32.5 kDa; red spots) and were not exported, but were present in the saponin supernatant, confirming they were secreted to the PV, and that KAHRP16Ala was of a size corresponding to cleavage at the PEXEL and it was exported (32 kDa; blue arrow); however, cleavage was inefficient as bands consistent with signal sequence cleaved (red arrow) and uncleaved (black) species were present in the pellet fraction. HSP70 was used as a control. Full-length gels shown in Supplementary Fig. 10.
To validate export of KHARP proteins, tetanolysin and saponin fractionation was performed (Fig. 5c). KHARP1–96 was present in the tetanolysin supernatant and efficiently processed by PMV. In comparison, KAHRPΔ38–53 and KAHRPΔ38–53RLQ>A were absent from the tetanolysin supernatant but present in the saponin supernatant, confirming their localization in the PV but not the erythrocyte. Their identical localization and indistinguishable molecular weight was consistent with cleavage by signal peptidase (Fig. 5a,c). Conversely, KAHRP16Ala was present as a PMV-cleaved protein in the tetanolysin supernatant, confirming it was exported; however, the presence of higher bands in pellet fractions, which correspond to uncleaved and signal peptidase-cleaved forms, determined previously9 indicate the 16Ala mutant form was cleaved inefficiently by PMV, which may explain why export was slightly less than the control (Fig. 5a,b). Collectively, these results show spatial position of PEXEL at the N terminus of KAHRP is important for export and linked to efficiency of cleavage by PMV. Further studies are needed to confirm this with other exported PEXEL proteins.
Co-translational PEXEL processing occurs before export
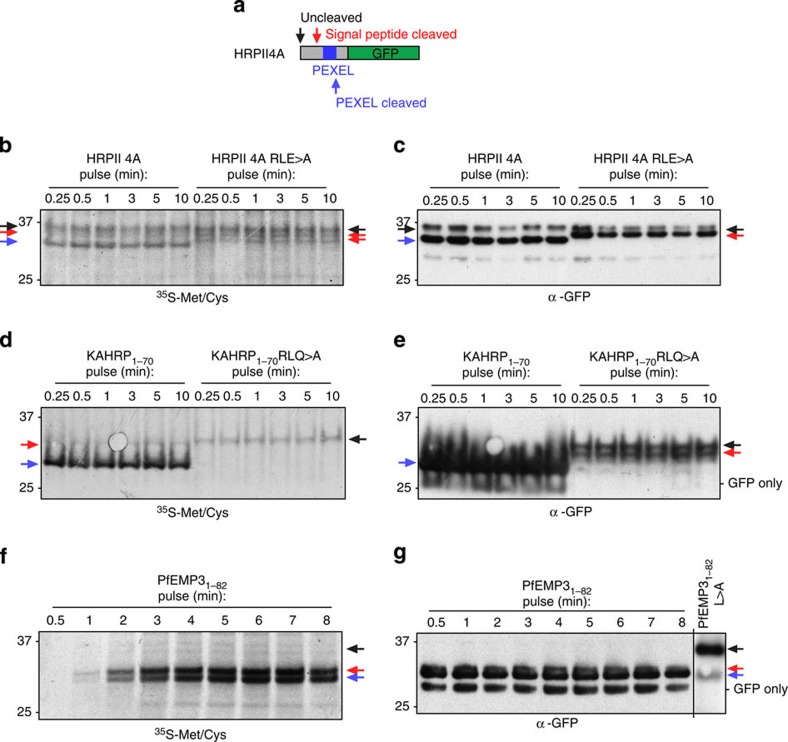
A paradox of the PI(3)P hypothesis is that the reported lipid-binding residues of PEXEL24 are removed by PMV in the ER. We investigated the dynamics of PEXEL cleavage to measure the rate of processing by labelling with 35S-methionine/cysteine. HRPII 4A (Fig. 6a) showed three bands of a size corresponding to uncleaved protein and the signal peptidase and PMV cleavage products just 0.25 min after addition of labelled amino acids (Fig. 6b,c). Mutation of the PEXEL RLE>A blocked its cleavage, while the uncleaved and the signal peptidase-cleaved bands were still observed, as well as an additional band that probably corresponds to a second signal peptidase-cleaved species derived from cleavage at the four alanines after the signal peptide. Similarly, for KAHRP1–70 there was rapid appearance of the PEXEL-cleaved protein, which was blocked by mutation of the motif RLQ>A (Fig. 6d,e). The KAHRP1–70RLQ>A construct was inefficiently cleaved by signal peptidase (cleavage of KAHRP by signal peptidase occurs at 32LKC↓SNN37 and this has been confirmed by MS9) and did not accumulate, raising the possibility that this protein may not efficiently enter the ER, resulting in reduced protein levels either by stalled translation or proteosomal degradation. This was supported by the absence of signal peptidase-cleaved protein in the 35S blot, which was however detected by immunoblot as this methodology detects protein produced over hours. KAHRP RLQ>A has been shown previously to be cleaved by signal peptidase inefficiently; however, a species that had the signal peptide removed was secreted to the PV9. Pulse-chase experiments with PfEMP31–82 also demonstrated that cleavage by signal peptidase and PMV occurred concomitantly with translation, although the product of PMV cleavage was consistently less abundant, raising the possibility this protein may be less co-translationally cleaved by PMV than HRPII and KAHRP (Fig. 6f,g)43. The rapid appearance of PEXEL-cleaved and signal sequence-cleaved species of HRPII, KAHRP and PfEMP3 suggest they are co-translationally processed by PMV and signal peptidase at the ER membrane. Thus, if the PEXEL was available for binding to PI(3)P for export, the Arg residue involved in binding24 would be very rapidly removed by PMV.
Figure 6. The PEXEL is cleaved co-translationally.
(a) Top: schematic of PEXEL proteins and their cleavage positions. (b) 35S-methionine/cysteine-labelling (35S-Met/Cys) of HRPII 4A and HRPII 4A RLE>A in P. falciparum for the indicated times shows bands corresponding to uncleaved (black arrow), signal peptide-cleaved (red arrow) and PEXEL-cleaved (blue arrow) species. Two signal peptide-cleaved species are present for RLE>A (red arrows), possibly due to insertion of four alanines following the signal sequence. (c) Immunoblot with anti-GFP antibodies of the same membrane shows the bands are GFP specific. (d) 35S-Met/Cys-labelling of KAHRP1–70 and KAHRP1–70-RLQ>A in P. falciparum. Bands corresponding to uncleaved (black arrow), signal peptide-cleaved (red arrow) and PEXEL-cleaved (blue arrow) species are indicated. (e) Immunoblot with anti-GFP antibodies of the same membrane. Note the absence of a signal peptidase cleaved product for KAHRP1–70 RLQ>A in the autoradiography that is present in the immunoblot as the latter reveals protein produced over hours. This shows signal peptidase activity was inefficient. (f) 35S-Met/Cys-labelling of PfEMP31–82 in P. falciparum. Bands corresponding to cleavage of the indented signal peptide (red arrow) and PEXEL (blue arrow) are indicated. (g) Immunoblot with anti-GFP antibodies of the same membrane. A PEXEL mutant (PfEMP31–82 L>A) was included as a size control. Most protein detected with anti-GFP was present before radiolabelling; thus, quantity is not comparable to 35S-Met/Cys. Incorporation of 35S-Met/Cys into HRPII and KAHRP constructs did not increase appreciably over time, in contrast to PfEMP3. The reason for this is unknown but may be due to saturation of anti-GFP agarose in the former experiments, which was used to enrich the GFP proteins. Full-length gels for all panels shown in Supplementary Fig. 11.
Discussion
Bhattacharjee et al.24,25,45 have suggested the PEXEL directs export by binding to PI(3)P on the inner ER leaflet, and that the RxLR motif of P. infestans can functionally replace PEXEL. They also showed RxLR was not cleaved by PMV and concluded export of PEXEL proteins occurs independent of this enzyme. In this study, we show PEXEL proteins and PfEMP1 do not bind PI(3)P, that this lipid is not present inside the ER, and that RxLR cannot functionally complement the PEXEL in P. falciparum. Thus, we find no evidence for PI(3)P in recognition of proteins in the ER for export. Rather, it is evident that P. falciparum has evolved PEXEL at a conserved spatial position ensuring co-translational cleavage by PMV at the ER for export.
Consistent with our findings, PMV activity previously has been shown to be essential for export and parasite survival. Expression of catalytic PMV mutants in independent laboratories gave a dominant-negative phenotype that reduced export and slowed parasite growth11,43. A similar but stronger effect on export and parasite lethality was observed using transition-state PEXEL mimetics that specifically inhibit PMV43,46. The function of PMV has also been determined showing it cleaves PEXEL proteins in the ER at the Leu residue, to expose an export signal necessary for the next step10,11. PEXEL appears to be cleaved rapidly, as we could not label uncleaved products even using short time points. Cleavage by signal peptidase was also observed at the earliest time points, suggesting both enzymes act co-translationally at the ER membrane. Although this model is based on the likelihood that the PMV catalytic domain is in the ER lumen, we cannot completely exclude it associates with the cytoplasmic face and PEXEL cleavage occurs on this side47,48. This would require that proteins are assembled into secretory vesicles on the outside of the ER and trafficked to the PV where the PTEX translocon is located14,15.
In the context of KAHRP, it is clear that the sequence around PEXEL is not critical but the distance from the signal sequence is important for export, and this was directly linked to the efficiency of PEXEL processing. The steps in export after PMV are not yet clear; however, the Ac-xE/Q/D revealed by cleavage may be a recognition signal for receptors that link cargo inside the ER to coat proteins on forming vesicles outside the ER membrane1,9.
A previous study reported P. falciparum recombinant exported proteins bind PI(3)P with high affinity and the conserved PEXEL Arg, or Lys in PfEMP1, was responsible24. When we tested equivalent PEXEL and PfEMP1 proteins using the methods published by Bhattacharjee et al.24, we did not detect reproducible binding to PI(3)P. Indeed, consistent binding to PI(3)P by p40PX was dependent on the conditions used. Under such conditions, if binding for a recombinant exported protein was detected, it was neither PEXEL dependent nor PI(3)P specific. If the PEXEL did bind PI(3)P as a conserved mechanism for export in P. falciparum, it would be expected to occur for multiple proteins and irrespective of the signal peptide chosen or insertion of four unnatural alanine amino acids beyond the signal sequence; however, this was not observed. Interestingly, binding of GBP130 with either a native or mutant PEXEL to PI(3)P, PI(4)P and PI(5)P was seen using spot assays but our data indicate that this was mediated by a minor population of unfolded GBP130, as 1/20 amount of DnaK could block the interaction. Similar nonspecific binding to PI(3)P was reported for a P. infestans RxLR protein28. This raises the possibility that partially unfolded proteins may have contributed to the PI(3)P binding reported previously24. We conclude that some experimental conditions may permit some lipid binding but it is not PEXEL dependent, reproducible across all exported proteins or physiologically relevant.
PI(3)P is a product of PI3 kinase and is found on endosomal and lysosomal membranes in mammalian cells and yeast36,49. We and others30 have localized PI(3)P to the food vacuole and apicoplast membranes in P. falciparum. Bhattacharjee et al.24 reported this lipid is localized on the inner ER leaflet in a steady state for the first time in a eukaryote. However, there is no obvious gene that encodes an ER-resident PI(3) kinase in P. falciparum and PI(3)P recruitment to the ER in eukaryotes is rare, occurring only transiently during autophagy50. Autophagosome formation requires a protein complex but orthologues to some proteins appear to be absent from Plasmodium spp.51,52,53. Bhattacharjee et al.24 used fluorescent proteins fused to EEA1 and p40PX to infer PI(3)P is located in the ER. However, the FYVE finger of EEA1 is a poor PI(3)P sensor, because its binding to this lipid is very weak and occurs via strong co-binding to Rab5:GTP36,54,55. As Rab5:GTP is located on endosomal membranes, EEA1 provides little information about PI(3)P on non-endosomal membranes, and several publications have advised against using this protein for such purposes36,55. We used p40PX and Hrs, and their expression in P. falciparum showed accumulation at the membrane of lysosome-like food vacuoles and the apicoplast, suggesting PI(3)P is located at these membranes, consistent with its widely conserved role in endosomal trafficking. When p40PX-GFP was directed into the P. falciparum ER, it was not retained but secreted to the PV. In contrast, when Hrs-GFP was directed into the ER, it remained there but mutation of resides required for PI(3)P binding did not alter this localization. This suggests PI(3)P-independent retention of exogenous PI(3)P-binding biosensors can occur in the P. falciparum ER and may explain discordant results and conclusions of Bhattacharjee et al.24 compared with our work. These results highlight the need for caution when using such PI(3)P reporters in P. falciparum.
The PI(3)P hypothesis originated from research on oomycetes, where virulence effectors containing an RxLR motif are directed into plant host cells by binding to PI(3)P on the cell surface followed by endocytosis39,56, although this has now been challenged (reviewed in ref. 57). The mechanism for translocation of RxLR proteins into the plant host is unclear and is an area of debate. Although effector binding to PI(3)P on the plant cell surface has been described39,56, other studies have found that when PI(3)P binding is observed it occurs through regions other than the RxLR motif. In examples where the motif has been found to be important for effector entry, it is not via PI(3)P binding, because the RxLR motif does not bind phospholipids in these studies28,58,59,60,61.
The PEXEL of Plasmodium spp. has obvious similarities to the RxLR motif of oomycetes and it was therefore possible they share a common mechanism. Indeed, it was reported that RxLR directs export to P. falciparum-infected erythrocytes24,25, whereas insertion of the PEXEL into a Phytophthora protein permitted its endocytosis by plant cells38. We tested this for P. falciparum, in independent laboratories, by making identical as well as different constructs as those described24,25. None of the chimeric RxLR proteins from P. infestans and P. sojae were exported. These results are important, because a primary conclusion by Bhattacharjee et al.24 was that RxLR mediates export in P. falciparum in the absence of PMV cleavage23. In agreement with Bhattacharjee et al.24, we showed the RxLR motif is not a substrate for PMV cleavage and this provides a clear mechanistic explanation for why this sequence cannot mediate export in P. falciparum.
In conclusion, we find no reproducible evidence that PI(3)P binding in the ER is the mechanism for export of PEXEL proteins. Rather, the PEXEL is present at a conserved distance from the signal peptide that permits co-translational processing by PMV, to reveal a signal for the next step in export to the human erythrocyte10,11.
Methods
Plasmids for recombinant protein expression
P. falciparum exported proteins GBP130 (PF3D7_1016300), KAHRP (PF3D7_0202000), HRPII (PF3D7_0831800) and PfEMP1 (PF3D7_1240600) were expressed in this study. GBP13066–196 and GBP13066–196RLE>A were described previously10. Briefly, DNA encoding the proteins was codon optimized (Genscript) and cloned into pET11a using NdeI/BamHI (generating pGBP1303Cmyc18His and pGBP130-3A3Cmyc18His). SacII followed by DNA encoding RGS18His was used in the constructs to improve Ni2+ binding, allowing stringent washing62. For the current study, GFP was incorporated into pGBP1303Cmyc18His by PCR from pGlux.1 using oligos JB176/JB177, with JB177 encoding 6His, and cloned via SacII/BamHI, generating pGBP1303CmycGFP6His. GBP130 fragments 66–83, 66–88, 84–196 and 89–196 were amplified from pGBP1303Cmyc18His using JB170/JB171, JB170/JB174, JB172/JB173 and JB175/JB173, respectively, and cloned into pGBP1303CmycGFP6His by NdeI/SacII, excising 3Cmyc. DNA encoding KAHRP35–72, KAHRP35–72 RLQ>A, HRPII22–65 WT, HRPII22–65 RLE>A, PfEMP11–60 and PfEMP11–60 K>A (PFL1960w), was codon optimized (Genscript) and cloned into pGBP1303CmycGFP6His by NdeI/SacII. pGEX-p40PX(aa1–148) was purchased from Addgene (19008), deposited by Michael Yaffe. See Supplementary Table 1 for more information.
Recombinant protein expression and purification
Escherichia coli BL21.DE3 was grown in Luria broth with 100 μg ml−1 carbenicillin at 37 °C, 200 r.p.m., to optical density (600 nm) 0.5, induced with 1 mM isopropyl-1-thio-β-D-galactopyranoside (Astral), grown for 5 h at 30 °C, 200 r.p.m., collected by centrifugation (8,000g, 4 °C) and lysed in 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole (Sigma-Aldrich) pH 8.0, containing 1 mg ml−1 lysozyme (Sigma), 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich), 1 × Complete cocktail (Roche). DNAse (0.01 mg ml−1; Roche), RNAse (0.01 mg ml−1; Life Technologies) and MgCl2 (5 mM) was added on ice for 30 min. Debris was collected (11,000g) and supernatants filtered through 5.0- and 0.8-μM filters (Millipore), and proteins were purified using Ni-NTA sepharose (Roche) in 10 ml chromatography columns (Bio-Rad), washed in 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole pH 8.0 and eluted in 100 mM followed by 200 mM imidazole. Elutions were viewed by SDS–PAGE and Coomassie blue staining. All GFP-tagged proteins were fluorescent. Fractions were pooled, dialysed against 150 mM NaCl, 20 mM HEPES pH 7.5, using Spectra/Por Dialysis tubing (MWCO 12–14,000; SpectrumLabs) at 4 °C overnight and concentrated to 2 mg ml−1 in Centricon YM-10 (MWCO 10,000) concentrators by centrifugation at 4,000g for 30 min at 4 °C. Size-exclusion chromatography was performed using Superdex 200 10/300 columns.
Recombinant p40PX(1–148) was expressed in E. coli using similar methodology, except the cells were expressed for 16 h at 18 °C post isopropyl-1-thio-β-D-galactopyranoside induction. p40PX was purified using glutathione sepaharose and eluted with 20 mM reduced glutathione, followed by size-exclusion chromatography using a Superdex 200 10/300 column. Recombinant GST-p40PX was also purchased (Echelon Biosciences; G-0302).
Preparation of liposomes
Liposomes were prepared as described by Bhattacharjee et al.24. PI(3)P-containing liposomes: solutions of 1.95 μmol PC and 0.52 μmol PE in CHCl3 (Avanti Polar Lipids; 850457C and 850757C), and 0.13 μmol D-myo-phosphatidylinositol 3-phosphate di-C16 (PI(3)P-diC16; Echelon Biosciences; P-3016) dissolved in CHCl3:MeOH:H20 (1:2:0.8) were mixed and dried under nitrogen to give 2.6 μmol total lipid at a ratio of 75:20:5 (PC:PE:PI(3)P-diC16). Control liposomes: solutions of 2.08 μmol PC and 0.52 μmol PE in CHCl3 were mixed and dried under nitrogen to give 2.6 μmol total lipid at a ratio of 80:20 (PC:PE). Lipid films were resuspended in 2.6 ml, 20 mM Tris Cl pH 7.4, containing 0.16 M KCl and incubated at 65 °C for 1 h with regular vortexing, to create a lipid emulsion. Four hundred aliquots were transferred to cryovials (Nunc) and were freeze–thawed three times in liquid nitrogen followed by 37 °C water bath and vortexing. Emulsions in glass tubes were sonicated in a Branson 2200 water bath for 30–60 min, ensuring the temperature remained below 40 °C. Liposomes were at stored at 4 °C and used within 5 days.
Binding to liposomes by SPR
SPR experiments were performed as described by Bhattacharjee et al.24 and others63,64 using a Biacore 3000 at 25 °C. Liposomes were coated onto an L1 sensor chip by injecting 100–300 μl of a 0.1-mM total lipid solution at 5 μl min−1 using 150 mM NaCl, 50 mM HEPES pH 7.5 as buffer. Control liposomes were injected over lane 1 and PI(3)P liposomes over lane 2. The amount injected was varied so that the total amount bound (typical equilibrium response of 3,000–6,000 response units24) was approximately equal between lanes. Following immobilization, lanes were washed with 3 × 10-μl 50 mM NaOH at 100 μl min−1. Before proceeding, 100 μl of 0.1 mg ml−1 BSA was injected over both lanes, to determine the level of residual nonspecific binding to the chip surface.
Recombinant proteins were passed over the liposome-coated L1 chip at a flow rate of 30 μl min−1 in 150 mM NaCl, 50 mM HEPES pH 7.5 buffer for 3 min at concentrations between 62.5 and 2,000 nM, and allowed to dissociate for 3 min. Binding to control liposomes (lane 1) was subtracted to yield the final sensorgrams, as described24. A pulse of 50 mM NaOH was applied at the end of each run to dissociate any bound protein. The chip surface was regenerated periodically by injecting 40 μl of 20 mM CHAPS at a flow rate of 30 μl min−1 and then re-coating with liposomes as described above. Req values were plotted against protein concentration and Kd values determined by nonlinear least-squares analysis of the binding isotherm using concentrations that spanned the Kd.
Binding to liposomes by ultracentrifugation
Liposomes and recombinant proteins were subjected to ultracentrifugation at 75,000g for 20 min at 25 °C separately. Liposomes and soluble recombinant proteins were resuspended to a final concentration of 1 mM total lipids and 5 μM recombinant proteins in either PBS pH 7.5 or 150 mM NaCl, 50 mM HEPES pH 7.5 as described24,64. Reactions were incubated for 20 min at 25 °C before ultracentrifugation again. Supernatants were collected and mixed with reducing sample buffer, pellets were resuspended in 100 μl of reducing sample buffer, and equal proportions were resolved by SDS–PAGE and visualized with Coomassie blue staining. Bands were quantified using a GS-800 Calibrated Densitometer (Bio-Rad).
Binding to PI(3)P-coated beads by pull down
PI(3)P-coated beads and control beads were purchased from Echelon Biosciences (P-B003a and P-B000) and protein binding was performed according to the manufacturer's instructions. Briefly, 1 μg protein was incubated in binding buffer (150 mM NaCl, 0.2% NP40, 10 mM HEPES pH7.4) with 50 μl beads in 100 μl total volumes for 1 h at 25 °C. Beads were collected in Micro Bio-Spin columns (Bio-Rad), washed with 5 × 1-ml binding buffer and bound proteins eluted in 25 μl reducing sample buffer for 15 min at 25 °C. Eluates were resolved by SDS–PAGE and detected by immunoblotting with anti-GST (p40PX) or anti-GFP (exported proteins) antibodies. Ten per cent of the unbound fraction volume was loaded to visualize the proteins.
Binding to lipid by ITC
Titrations were performed with a MicroCal iTC200 at 20 °C using proteins dialysed against 150 mM NaCl, 20 mM HEPES pH 7.5 as baits (200 μl) at 109 μM for HRPII WT, 58 μM for HRPII RLE>A, 49 μM for GBP13066–88 and 140 μM for Htp1 from S. parasitica27. Inositol 1,3 bisphosphate titrant was prepared at 2.4 mM and Fmoc-Tyr(SO3)-OH titrant at 5.12 mM. Titrations used 0.4 μl followed by 18 × 2 μl titration steps. The ITC stirring speed was 1,000 r.p.m. and the feedback gain mode was set to high.
Binding to lipid spot membranes
Lipid spot membranes (Echelon Biosciences, S-6000, P-6001 and P6002) equilibrated in PBS containing 0.1% Tween 20 and 5% lipid-free BSA (PBS/Tween/BSA) were incubated with 20 μM recombinant proteins for 20 min at 25 °C. Membranes were washed thrice in PBS/Tween/BSA and bound proteins detected with mouse anti-Penta-His antibodies (1:10,000; Qiagen) followed by horseradish peroxidase-conjugated secondary antibodies (1:2,000; Silenius) and visualized by enhanced chemiluminescence (Amersham).
Plasmids for transgenic parasites
P. falciparum expressing PfEMP31–82 and KAHRP1–96 were generated previously10, as were parasites expressing KAHRP1–70 (ref. 65). DNA encoding PfEMP3 L>A, PfEMP3 4A RLQ>A, HRPIIs-AVR3a, HRPIIs-PH001D5, HRPII 4A, HRPII 4A RLE>A, HRPII 4A RLE>A Down, KAHRP Δ38–48, KAHRP Δ38–53, KAHRP Δ38–53 RLQ>A, KAHRP16Ala, KAHRP 10Ala and KAHRP 5Ala was synthesized by Epoch Biolabs or Genscript, and cloned in-frame with GFP in pGlux.1 using XhoI/XmaI. p40PX-GFP: DNA encoding the PX domain from human p40phox was amplified from p40PX-EGFP (Addgene 19010) using primers p40_A_F/p40_K_R and cloned into pARL2-GFP66 using AvrII/KpnI (p40-GFP). STEVORss-p40PX: DNA encoding the first 30 amino acids (signal sequence) of STEVOR was released from pARL-STEVOR(1–30) (ref. 66) with XhoI and AvrII, and cloned into p40-GFP (pSTEV-40-GFP). STEVORss-p40PX-GFPSDEL: DNA encoding GFP-SDEL was amplified from pARL2-GFP using GFP_K_F/GFP_SDEL_X_R and cloned into pSTEV-40-GFP using KpnI/XmaI (pSTEV-40-GFPSDEL). ACPss-p40PX: human p40PX (residues 1–148) was PCR amplified from human complementary DNA using oligo 5′ACPsp-PX40p, which also encodes the first 20 amino acids (signal sequence) of ACP, and oligo 3′KpnI-PX40-rev (blunt-KpnI). The amplicon was cloned into pGFP_glmS67 digested with BglI, blunted using Klenow and re-digested with KpnI. Hrs-GFP: DNA encoding 2xFYVE from mouse Hrs separated by QGQGS was amplified from pGFP-2xFYVE30 using primers HRS_FYVE_AvrII_F/HRS_FYVE_KpnI_R and cloned into pARL2-GFP using AvrII/KpnI (pHRS-GFP). STEVORss-HRS: DNA encoding the first 30 amino acids (signal sequence) of STEVOR was released from pARL-STEVOR(1–30) with XhoI and AvrII, and cloned into pHRS-GFP (pSTEV-HRS-GFP). STEVORss-HRS-GFPSDEL: DNA encoding STEVORss-Hrs was released from pSTEV-HRS-GFP using AvrII/KpnI and cloned into similarly restricted pSTEV-40-GFPSDEL. STEVORss-HRSC2158-GFP: a fragment encoding 2xFYVEMUT (containing the double C215S mutation) was amplified from p-ddFYVEm30 using primers HRS_FYVE_AvrII_F and HRS_FYVE_KpnI_R, and cloned into pSTEV-HRS-GFP using AvrII/KpnI (pSTEV-HRSmutGFP).
To make plasmids encoding REX3s-Avh5 and REX3ss-Avr1b, DNA fragments Rex3(1–43)-Avr1b(22–71) and Rex3(1–43)-Avh5(20–63) were PCR amplified from truncated REX3-GFP68 using primers REX3fwK22, and reverse primers Rex3(1–43)-Avr1b(22–71)-KpnI-rev and Rex3Avh5(20–63)-AvrII-rev, respectively. These inserts were cloned KpnI/AvrII into pARL1GFP69. See Supplementary Table 1 for more information.
Oligonucleotides
Oligonucleotide sequences are shown in Supplementary Table 2. Oligonucleotides were made by Geneworks, Integrated DNA Technologies, Life Technologies or Sigma.
Parasites
P. falciparum 3D7 parasites were used for this study as was the case for Bhattacharjee et al.24 Parasites were transfected and grown in O+ human erythrocytes with selection under 2–5 nM WR99210 (a gift from Jacobus Pharmaceuticals), as described70.
For MS, 900 ml of trophozoite-infected erythrocytes grown to 10% parasitaemia in 5 nM WR99210 were purified by Varia Macs magnet separation through D columns (Miltenyi Biotech), fractionated with 0.15% saponin containing 1 × Complete protease inhibitors (Roche) and stored at −80 °C.
Microscopy and quantification of export and statistics
For immunofluorescence microscopy, cells were fixed in 4% paraformaldehyde, 0.01% glutaraldehyde (ProSciTech) for 30 min, to preserve GFP fluorescence, and permeabilized in 0.1% Triton X-100 in PBS for 10 min. Cells were probed with rabbit anti-CRT (1:600, MR4, MRA-308), rabbit anti-ACP (1:600, a kind gift from Geoff McFadden), rabbit anti-ERC (1:500, a kind gift from Leanne Tilley), rabbit anti-EXP2 (1:200, a kind gift from Jana McBride), rabbit anti-PMV (1:750)43, or mouse anti-PMV (1:200, MR4, MRA-815A, contributed by Dan Goldberg) diluted in PBS containing 3% BSA for 1 h followed by Alex Fluor 594-conjugated secondary immunoglobulin G antibodies (1:1,000; ThermoFisher Scientific) (catalogue number A-11032 and A-11037) for 1 h and nuclei were labelled with 0.2 μg ml−1 4′-6-diamidino-2-phenylindole (Roche) diluted in Vectorshield (Vector Labs). Samples were imaged on several platforms as follows: (1) a Deltavision Elite microscope with micrographs collected on a Coolsnap HQ2 charge-coupled device camera through a Olympus × 100 UPlanSApo numerical aperture 1.4 objective with SoftWorx software; (2) a Zeiss Axio Scope M1 microscope equipped with a × 100/numerical aperture 1.4 oil-immersion lens using a Hamamatsu Orca C4742–95 camera and Zeiss AxioVision software; and (3) a Zeiss Cell Observer epifluorescence imaging system. Images were assembled with ImageJ Fiji (versions 1.42q and 1.47d), Adobe Photoshop and Illustrator CS5 or CS6, or Corel PHOTO-PAINT X4.
For quantification of exported GFP, the number of infected erythrocytes with exported protein, or with protein localized in the ER/PV/mix of both, was counted using 20 cells per construct represented as a percentage. Live GFP images were blinded and the localization determined by two independent observers. For quantification of exported GFP in Fig. 5, Z-stacks of live-GFP images of synchronous parasites were captured on a Deltavision Elite microscope using a × 100 objective. At least 20 cells per condition were imaged using the same exposure settings, to allow quantitative analysis of GFP intensity in the host cell (signal outside the parasite and PV) between chimeras. All statistical analyses were performed using Prism 6 and mean differences were compared using the Mann–Whitney t-test.
Peptide cleavage and RP-HPLC
PMV-agarose was prepared by mixing goat anti-HA agarose (Sapphire Bioscience) with parasite lysates containing PMV-HA10. For peptide cleavage assays, 2 nmol synthetic peptides (Genscript, LifeTein) were mixed with 5 μl PMV-agarose (or PBS control) in 25 mM Tris HCl, 25 mM MOPS pH 6.4 and incubated at 37 °C for 24 h. Reactions were passed through Micro Bio-Spin columns (Bio-Rad), to remove agarose, and analysed with an Agilent 1100 modular HPLC10 and MS (liquid chromatography–tandem MS (LC–MS/MS), see below).
Subcellular fractionation and immunoblotting
Parasite-infected erythrocytes were magnet purified on CS columns (Miltenyi Biotech) and solubilized in 4 × Laemmli buffer as whole infected cells, or permeabilized in 100 units per ml tetanolysin (Sigma) or 0.15% saponin containing 1 × Complete protease inhibitors as described previously9, before pellet and supernatant fractions were resuspended in 4 × Laemmli buffer. Samples were boiled, separated by SDS–PAGE and transferred to nitrocellulose membrane. Membranes were blocked in PBS-T containing 10% skim milk and proteins detected using mouse anti-GFP (Roche; 1:1,000, catalogue number 11814460001), rabbit anti-Aldolase (1:1,000)46, rabbit anti-PfHSP70 (1:4,000)46, mouse anti-Penta-His (1:4,000, Qiagen, catalogue number 34460) or rabbit anti-GST (1:1,000; WEHI Antibody Facility) antibodies followed by horseradish peroxidase-conjugated secondary antibodies (1:2,000; Cell Signalling Technology, catalogue numbers 7074, 7076 and 7077) and visualized by enhanced chemiluminescence (Amersham). Images were scanned using a GS-800 Calibrated Densitometer (Bio-Rad) at 400 dpi using Quantity One v4.6.9 software (Bio-Rad). Uncleaved, signal peptide-cleaved and PEXEL-cleaved proteins were deduced based on predicted molecular weight or by MS9,10,11,43. Full-length versions of all gels and blots are provided in Supplementary Information.
Protein labelling IPs and densitometry
Synchronous early trophozoites were magnet purified on CS columns (Miltenyi Biotech) and incubated in Met/Cys-free medium for 30 min at 37 °C before addition of 300 μCi ml−1 35S-Met/Cys (Perkin/Elmer) to the medium. Parasites were incubated at 37 °C, pelleted and snap frozen in ethanol/dry ice bath at the indicated times and stored at −80 °C. Samples were solubilized in 25 mM Tris-HCl pH 8.0, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100 and Complete inhibitor cocktail (Roche), and GFP species were immunopurified with α-GFP agarose (MBL; catalogue number D153-8) at 4 °C for 1 h. Proteins were resolved by SDS–PAGE, visualized by autoradiography and quantified with a GS-800 Calibrated Densitometer (Bio-Rad).
Lopinavir experiment
Synchronous early trophozoites were magnet purified on CS columns (Miltenyi Biotech) and incubated with dimethyl sulfoxide, 50 μM lopinavir, 150 ng ml−1 chloroquine or 100 ng ml−1 artemisinin for 7 h before parasite proteins were radiolabelled as described above, but using 800 μCi ml−1 35S-Met/Cys for 15 min. Pellets were saponin treated (0.15%), snap frozen and stored at −80 °C. Samples were solubilized in Laemmli buffer, resolved by SDS–PAGE, visualized by autoradiography and quantified as above.
PI(3)P ELISA assays
ELISA assays were performed using a PI(3)P Mass ELISA kit (Echelon Biosciences, K-2500s) according to manufacturer's instructions and Bhattacharjee et al.24. Synchronous early trophozoites at 10% parasitaemia were magnet purified on CS columns (Miltenyi Biotech) and solubilized in lipid-binding buffer (20 mM Tris HCl pH 7.5, 150 mM NaCl, 0.25% Igepal, 1 mM EDTA) with Complete inhibitor cocktail (Roche) by rotating at 4 °C for 1 h. Insoluble debris was removed by centrifugation (20,800g) for 10 min at 4 °C. GFP species were immunopurified as above, beads were washed with lipid-binding buffer and proteins eluted in 100 mM Glycine pH 2.6, 500 mM NaCl. Elutions were immediately neutralized in 1 M Tris HCl pH 8.0. A total of five IPs was performed per sample. As conducted previously24, proteins were quantified by immunoblotting using mouse α-GFP (Roche; 1:1,000, catalogue number 11814460001) followed by densitometry of different exposures within the linear range. Equal protein concentrations were obtained by dilution and PI(3)P was then extracted from each protein and detected using the mass ELISA kit. Absorbance was read at 450 nm on an Envision fluorescence plate reader (Perkin-Elmer).
Mass spectrometry
HRPII-PH001D5 protein bands were in-gel digested with AspN (Promega) and eluted peptides, or synthetic peptides incubated with PMV-agarose, were fractionated by reversed-phase liquid chromatography on a nanoflow HPLC system (1200 series, Agilent, USA) using a nanoAcquity C18 150 mm × 0.15 mm I.D. column (Waters, USA) developed with a linear 60- min gradient with a flow rate of 0.5 μl min−1 at 45 °C from 100% solvent A (0.1% formic acid in Milli-Q water) to 60% solvent B (0.1% formic acid, 60% acetonitrile and 40% Milli-Q water). The nano HPLC was coupled on-line to an LTQ-Orbitrap mass spectrometer equipped with a nanoelectrospray ion source (ThermoFisher Scientific) for automated MS/MS. Up to five most intense ions per cycle were fragmented and analysed in the linear trap, with target ions already selected for MS/MS being dynamically excluded for 3 min.
Mass spectra database searching
LC–MS/MS data were searched against a custom decoy database comprising Plasmodium sequences from the LudwigNR non-redundant protein database (version Q211, 249275 entries), common contaminants (239 entries) and chimeric P. falciparum PEXEL constructs (44 entries), as well as their reverse sequences as described previously9. Peak lists for each nano LC–MS/MS run were used to search MASCOT v2.2.04 search algorithm (Matrix Science, UK) provided by the Australian Proteomics Computational Facility ( www.apcf.edu.au). Scaffold_3.6.2 (Proteome Software Inc.) was employed to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at >95.0% probability as specified by the Peptide Prophet algorithm. Extracted ion chromatograms of manually validated peptide sequences were generated using Excalibur v2.0 (ThermoFisher Scientific).
Additional information
How to cite this article: Boddey, J. A. et al. Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat. Commun. 7:10470 doi: 10.1038/ncomms10470 (2016).
Supplementary Material
Supplementary Figures 1-11 and Supplementary Tables 1-2 and Supplementary References
Acknowledgments
We thank the Red Cross blood bank in Melbourne for human erythrocytes. We thank Svenja Günther for expression of GBP130 66–196 proteins; Michelle Gazdik and Chris Burns for help in preparing lipids; Lachlan Whitehead (Centre for Dynamic Imaging, Walter and Eliza Hall Institute) for assistance with quantification of export; and David Böcher for help with generation of STEVOR constructs. This work was supported by the National Health and Medical Research Council of Australia (grants 637406, 1010326, 1049811 and 1057960), a Ramaciotti Foundation Establishment Grant (3197/2010), a Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS, and the Canadian Institutes for Health Research (MOP#130359). J.A.B is an Australian Research Council QEII Fellow, SF was supported by the Research Training Group GRK1459 of the German Research Foundation, and AFC is a Howard Hughes International Scholar.
Footnotes
Author contributions J.A.B., S.W., P.v.W., D.R., T.S., J.P., J.J.B. and A.F.C. designed the experiments and wrote the paper. M.T.O. and J.J.B. expressed recombinant proteins. J.J.B. performed SPR and gel filtration. J.A.B. performed liposome centrifugation, PI(3)P bead, lipid spot and proteomic experiments. S.W. and J.J.B. performed ITC. S.W. and J.A.B. performed the lipid spot experiments. J.P., S.F., T.S., Z.E., D.R., S.L. and J.A.B. localized PI(3)P in P. falciparum. J.A.B., T.G.C., A.N.H., S.F. and T.S. obtained RxLR, and HRPII 4A and PfEMP3 4A export data. S.L. and J.A.B. performed radiolabelling experiments. M.T.O. and J.A.B. performed spatial mutagenesis of the PEXEL in KAHRP. J.P., S.L. and J.A.B. performed PI3P ELISA assays.
References
- Boddey J. A. & Cowman A. F. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 67, 243–269 (2013). [DOI] [PubMed] [Google Scholar]
- Raventos S. C., Kaul D. K., Macaluso F. & Nagel R. L. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc. Natl Acad. Sci. USA 82, 3829–3833 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash G. B., O'Brien E., Gordon-Smith E. C. & Dormandy J. A. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood 74, 855–861 (1989). [PubMed] [Google Scholar]
- Leech J. H., Aley S. B., Miller L. H. & Howard R. J. Plasmodium falciparum malaria: cytoadherence of infected erythrocytes to endothelial cells and associated changes in the erythrocyte membrane. Prog. Clin. Biol. Res. 155, 63–77 (1984). [PubMed] [Google Scholar]
- Maier A. G. et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134, 48–61 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti M., Good R. T., Rug M., Knuepfer E. & Cowman A. F. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306, 1930–1933 (2004). [DOI] [PubMed] [Google Scholar]
- Hiller N. L. et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306, 1934–1937 (2004). [DOI] [PubMed] [Google Scholar]
- Chang H. H. et al. N-terminal processing of proteins exported by malaria parasites. Mol. Biochem. Parasitol. 160, 107–115 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey J. A., Moritz R. L., Simpson R. J. & Cowman A. F. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic 10, 285–299 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey J. A. et al. An aspartyl protease directs malaria effector proteins to the host cell. Nature 463, 627–631 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I. et al. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 463, 632–636 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr S. J. & Osborne A. R. Experimental determination of the membrane topology of the Plasmodium protease Plasmepsin V. PLoS One 10, e0121786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant T. J. et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 7, R12 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J. R., Muralidharan V., Oksman A. & Goldberg D. E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 511, 592–595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsworth B. et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 511, 587–591 (2014). [DOI] [PubMed] [Google Scholar]
- Gruring C. et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe 12, 717–729 (2012). [DOI] [PubMed] [Google Scholar]
- Heiber A. et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 9, e1003546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blisnick T. et al. Pfsbp1, a Maurer's cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol. Biochem. Parasitol. 111, 107–121 (2000). [DOI] [PubMed] [Google Scholar]
- Spycher C. et al. Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes. Mol. Cell. Biol. 26, 4074–4085 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachlatko E. et al. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol. Microbiol. 77, 1136–1152 (2010). [DOI] [PubMed] [Google Scholar]
- Dixon M. W. et al. Targeting of the ring exported protein 1 to the Maurer's clefts is mediated by a two-phase process. Traffic 9, 1316–1326 (2008). [DOI] [PubMed] [Google Scholar]
- Haase S. et al. Sequence requirements for the export of the Plasmodium falciparum Maurer's clefts protein REX2. Mol. Microbiol. 71, 1003–1017 (2009). [DOI] [PubMed] [Google Scholar]
- Kraemer S. M. & Smith J. D. A family affair: var genes, PfEMP1 binding, and malaria disease. Curr. Opin. Microbiol. 9, 374–380 (2006). [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Stahelin R. V., Speicher K. D., Speicher D. W. & Haldar K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell 148, 201–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S. et al. The malarial host-targeting signal is conserved in the Irish potato famine pathogen. PLoS Pathog. 2, e50 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Estrano C., Bhattacharjee S., Harrison T. & Haldar K. Cooperative domains define a unique host cell-targeting signal in Plasmodium falciparum-infected erythrocytes. Proc. Natl Acad. Sci. USA 100, 12402–12407 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawra S. et al. Host-targeting protein 1 (SpHtp1) from the oomycete Saprolegnia parasitica translocates specifically into fish cells in a tyrosine-O-sulphate-dependent manner. Proc. Natl Acad. Sci. USA 109, 2096–2101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawra S. et al. Avirulence protein 3a (AVR3a) from the potato pathogen Phytophthora infestans forms homodimers through its predicted translocation region and does not specifically bind phospholipids. J. Biol. Chem. 287, 38101–38109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidock D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawk L. et al. Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast. Eukaryot. Cell 9, 1519–1530 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. G. & Emr S. D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2, 157–162 (1998). [DOI] [PubMed] [Google Scholar]
- Gardner M. J. et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419, 498–511 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemba M. & Goldberg D. E. Characterization of plasmepsin V, a membrane-bound aspartic protease homolog in the endoplasmic reticulum of Plasmodium falciparum. Mol. Biochem. Parasitol. 143, 183–191 (2005). [DOI] [PubMed] [Google Scholar]
- de Koning-Ward T. F. et al. A newly discovered protein export machine in malaria parasites. Nature 459, 945–949 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. & Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell 48, 899–907 (1987). [DOI] [PubMed] [Google Scholar]
- Gillooly D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisson S. C. et al. A translocation signal for delivery of oomycete effector proteins into host plant cells. Nature 450, 115–118 (2007). [DOI] [PubMed] [Google Scholar]
- Grouffaud S., van West P., Avrova A. O., Birch P. R. & Whisson S. C. Plasmodium falciparum and Hyaloperonospora parasitica effector translocation motifs are functional in Phytophthora infestans. Microbiology 154, 3743–3751 (2008). [DOI] [PubMed] [Google Scholar]
- Kale S. D. et al. External lipid PI3P mediates entry of eukaryotic pathogen effectors into plant and animal host cells. Cell 142, 284–295 (2010). [DOI] [PubMed] [Google Scholar]
- Armstrong M. R. et al. An ancestral oomycete locus contains late blight avirulence gene Avr3a, encoding a protein that is recognized in the host cytoplasm. Proc. Natl Acad. Sci. USA 102, 7766–7771 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F. et al. Structural basis for interactions of the Phytophthora sojae RxLR effector Avh5 with phosphatidylinositol 3-phosphate and for host cell entry. Mol. Plant Microbe Interact. 26, 330–344 (2013). [DOI] [PubMed] [Google Scholar]
- Whisson S. C., Drenth A., Maclean D. J. & Irwin J. A. Phytophthora sojae avirulence genes, RAPD, and RFLP markers used to construct a detailed genetic linkage map. Mol. Plant Microbe Interact. 8, 988–995 (1995). [DOI] [PubMed] [Google Scholar]
- Sleebs B. E. et al. Inhibition of Plasmepsin V activity demonstrates its essential role in protein export, PfEMP1 display, and survival of malaria parasites. PLoS Biol. 12, e1001897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller N. L., Akompong T., Morrow J. S., Holder A. A. & Haldar K. Identification of a stomatin orthologue in vacuoles induced in human erythrocytes by malaria parasites. A role for microbial raft proteins in apicomplexan vacuole biogenesis. J. Biol. Chem. 278, 48413–48421 (2003). [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S., Speicher K. D., Stahelin R. V., Speicher D. W. & Haldar K. PI(3)P-independent and -dependent pathways function together in a vacuolar translocation sequence to target malarial proteins to the host erythrocyte. Mol. Biochem. Parasitol. 185, 106–113 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder A. N. et al. Structural basis for plasmepsin V inhibition that blocks export of malaria proteins to human erythrocytes. Nat. Struct. Mol. Biol. 22, 590–596 (2015). [DOI] [PubMed] [Google Scholar]
- Romisch K. Protein targeting from malaria parasites to host erythrocytes. Traffic 6, 706–709 (2005). [DOI] [PubMed] [Google Scholar]
- Romisch K. Diversion at the ER: How Plasmodium falciparum exports proteins into host erythrocytes. F1000 Res. 1, 12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly D. J., Simonsen A. & Stenmark H. Cellular functions of phosphatidylinositol 3-phosphate and FYVE domain proteins. Biochem. J. 355, 249–258 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman C. & Ktistakis N. T. Regulation of autophagy by phosphatidylinositol 3-phosphate. FEBS Lett. 584, 1302–1312 (2010). [DOI] [PubMed] [Google Scholar]
- Kitamura K. et al. Autophagy-related Atg8 localizes to the apicoplast of the human malaria parasite Plasmodium falciparum. PLoS ONE 7, e42977 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickel N. et al. Features of autophagic cell death in Plasmodium liver-stage parasites. Autophagy 9, 568–580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes S. et al. The multifunctional autophagy pathway in the human malaria parasite, Plasmodium falciparum. Autophagy 10, 80–92 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen A. et al. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494–498 (1998). [DOI] [PubMed] [Google Scholar]
- Lawe D. C., Patki V., Heller-Harrison R., Lambright D. & Corvera S. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and Rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 275, 3699–3705 (2000). [DOI] [PubMed] [Google Scholar]
- Tyler B. M. et al. Microbe-independent entry of oomycete RxLR effectors and fungal RxLR-like effectors into plant and animal cells is specific and reproducible. Mol. Plant Microbe Interact. 26, 611–616 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petre B. & Kamoun S. How do filamentous pathogens deliver effector proteins into plant cells? PLoS Biol. 12, e1001801 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan P. H. et al. Lipid binding activities of flax rust AvrM and AvrL567 effectors. Plant Signal. Behav. 5, 1272–1275 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeno T. et al. Phosphatidylinositol monophosphate-binding interface in the oomycete RXLR effector AVR3a is required for its stability in host cells to modulate plant immunity. Proc. Natl Acad. Sci. USA 108, 14682–14687 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaeno T. & Shirasu K. The RXLR motif of oomycete effectors is not a sufficient element for binding to phosphatidylinositol monophosphates. Plant Signal. Behav. 8, e23865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawra S. et al. In vitro translocation experiments with RxLR-reporter fusion proteins of Avr1b from Phytophthora sojae and AVR3a from Phytophthora infestans fail to demonstrate specific autonomous uptake in plant and animal cells. Mol. Plant Microbe Interact. 26, 528–536 (2013). [DOI] [PubMed] [Google Scholar]
- Tagwerker C. et al. A tandem affinity tag for two-step purification under fully denaturing conditions: application in ubiquitin profiling and protein complex identification combined with in vivocross-linking. Mol. Cell. Proteomics 5, 737–748 (2006). [DOI] [PubMed] [Google Scholar]
- Stahelin R. V. & Cho W. Differential roles of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2. Biochemistry 40, 4672–4678 (2001). [DOI] [PubMed] [Google Scholar]
- He J. et al. Membrane insertion of the FYVE domain is modulated by pH. Proteins 76, 852–860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddey J. A. et al. Role of Plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 14, 532–550 (2013). [DOI] [PubMed] [Google Scholar]
- Przyborski J. M. et al. Trafficking of STEVOR to the Maurer's clefts in Plasmodium falciparum-infected erythrocytes. EMBO J. 24, 2306–2317 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prommana P. et al. Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PloS One 8, e73783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruring C. et al. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2, 165 (2011). [DOI] [PubMed] [Google Scholar]
- Struck N. S. et al. Re-defining the Golgi complex in Plasmodium falciparum using the novel Golgi marker PfGRASP. J. Cell Sci. 118, 5603–5613 (2005). [DOI] [PubMed] [Google Scholar]
- Tonkin C. J. et al. Localization of organellar proteins in Plasmodium falciparum using a novel set of transfection vectors and a new immunofluorescence fixation method. Mol. Biochem. Parasitol. 137, 13–21 (2004). [DOI] [PubMed] [Google Scholar]
- Ansorge I., Benting J., Bhakdi S. & Lingelbach K. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem. J. 315, 307–314 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-11 and Supplementary Tables 1-2 and Supplementary References