Abstract
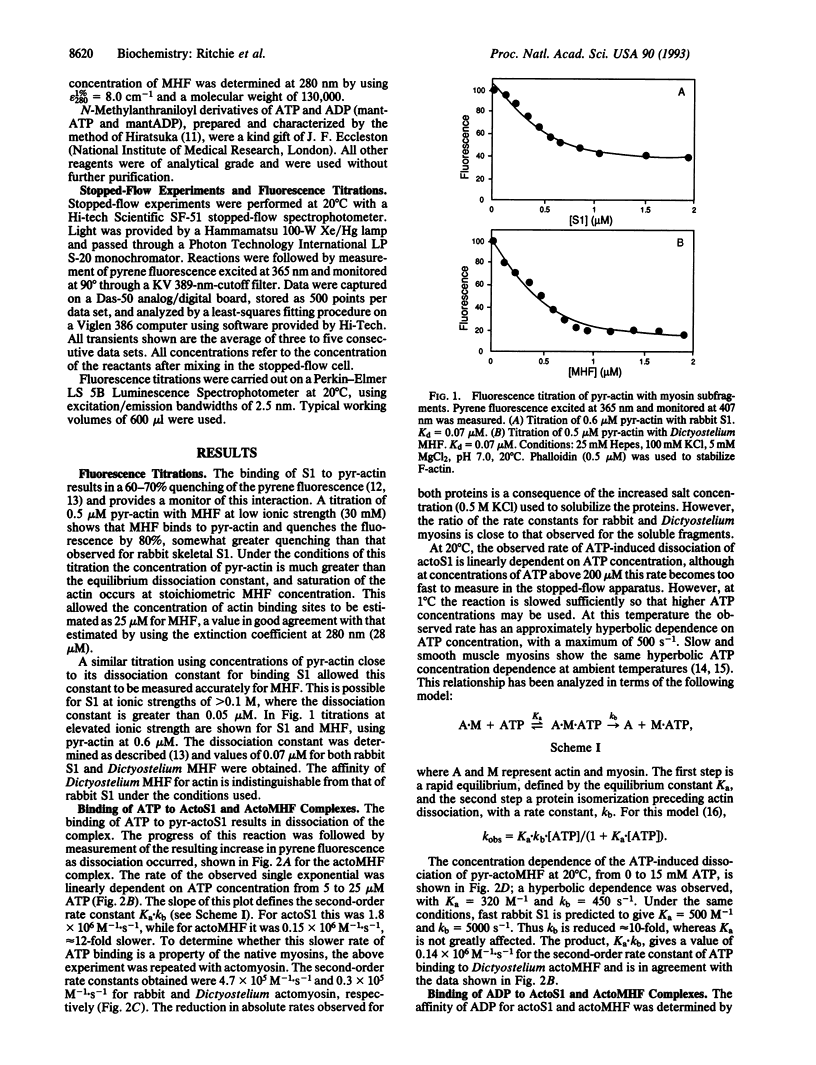
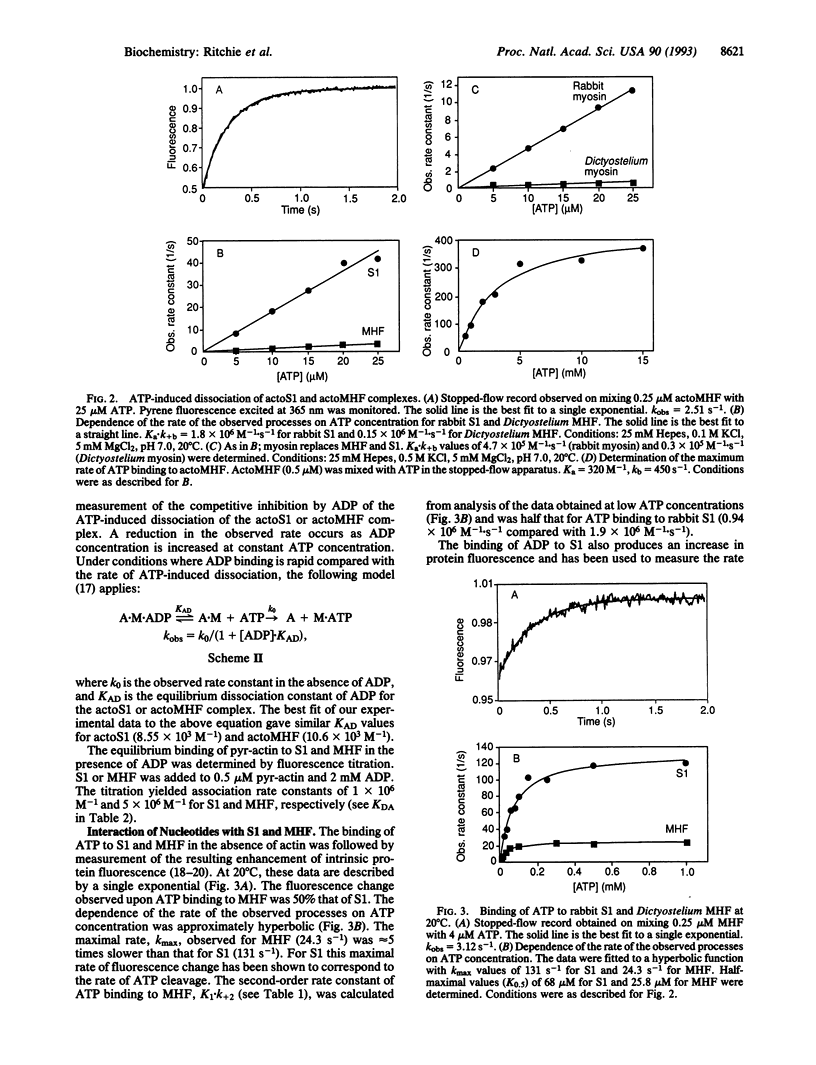
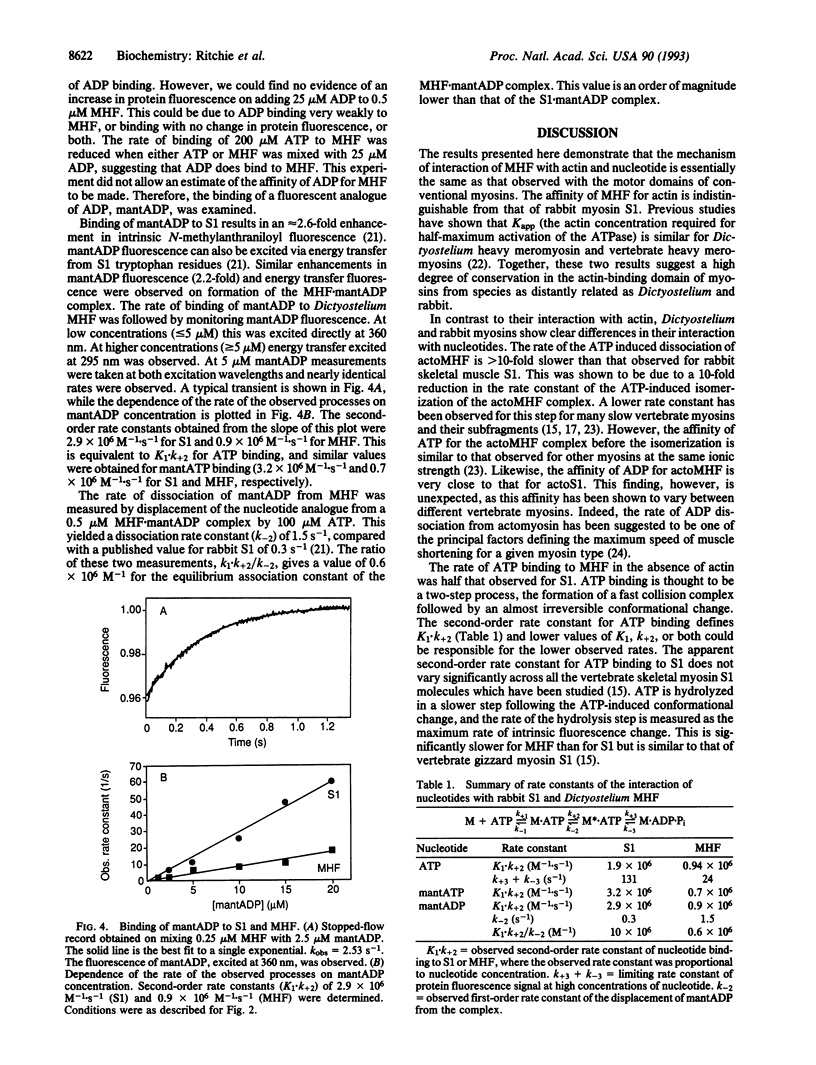
A detailed kinetic study of the interaction of a recombinant myosin head fragment (MHF) of Dictyostelium discoideum with actin and adenine nucleotides has been made by using a combination of rapid-reaction, equilibrium, and fluorescence methods. MHF is equivalent in size to a proteolytic fragment of skeletal muscle myosin, subfragment 1 (S1), the simplest unit of myosin to retain enzymatic and functional activity. The results show that qualitatively the interactions of MHF with nucleotides and actin are the same as those of S1. Both bind to rabbit actin with the same affinity, although differences in the rate constants of their interactions with nucleotides in the presence and absence of actin occur. The rate of ATP binding to MHF and the subsequent cleavage step are significantly slower than the corresponding rates with S1. The dissociation of a fluorescent analog of ADP from MHF was 5-fold faster than from S1, while its rate of binding MHF was 3-fold slower, resulting in a weaker association equilibrium constant. The ATP-induced isomerization of the actoMHF complex was 10-fold slower than for actoS1, but the binding affinities of ADP for actoMHF and actoS1 were indistinguishable. The results suggest a different degree of coupling between the nucleotide and actin binding sites of MHF and S1 which may be a common feature of nonmuscle myosins. They also provide the basis for a study of specifically modified myosins with which one can probe the sites of interaction with nucleotides or actin, as well as functional motility.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criddle A. H., Geeves M. A., Jeffries T. The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem J. 1985 Dec 1;232(2):343–349. doi: 10.1042/bj2320343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeves M. A., Goody R. S., Gutfreund H. Kinetics of acto-S1 interaction as a guide to a model for the crossbridge cycle. J Muscle Res Cell Motil. 1984 Aug;5(4):351–361. doi: 10.1007/BF00818255. [DOI] [PubMed] [Google Scholar]
- Geeves M. A., Jeffries T. E. The effect of nucleotide upon a specific isomerization of actomyosin subfragment 1. Biochem J. 1988 Nov 15;256(1):41–46. doi: 10.1042/bj2560041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka T. New ribose-modified fluorescent analogs of adenine and guanine nucleotides available as substrates for various enzymes. Biochim Biophys Acta. 1983 Feb 15;742(3):496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Taylor E. W. Intermediate states of subfragment 1 and actosubfragment 1 ATPase: reevaluation of the mechanism. Biochemistry. 1978 Aug 22;17(17):3432–3442. doi: 10.1021/bi00610a002. [DOI] [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Kubalek E. W., Uyeda T. Q., Spudich J. A. A Dictyostelium myosin II lacking a proximal 58-kDa portion of the tail is functional in vitro and in vivo. Mol Biol Cell. 1992 Dec;3(12):1455–1462. doi: 10.1091/mbc.3.12.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S. S., Kerwar G. Intrinsic fluorescence of actin. Biochemistry. 1972 Mar 28;11(7):1211–1217. doi: 10.1021/bi00757a015. [DOI] [PubMed] [Google Scholar]
- Manstein D. J., Ruppel K. M., Spudich J. A. Expression and characterization of a functional myosin head fragment in Dictyostelium discoideum. Science. 1989 Nov 3;246(4930):656–658. doi: 10.1126/science.2530629. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Taylor E. W. Comparison of the myosin and actomyosin ATPase mechanisms of the four types of vertebrate muscles. J Mol Biol. 1980 Jun 5;139(4):573–600. doi: 10.1016/0022-2836(80)90050-9. [DOI] [PubMed] [Google Scholar]
- Millar N. C., Geeves M. A. Protein fluorescence changes associated with ATP and adenosine 5'-[gamma-thio]triphosphate binding to skeletal muscle myosin subfragment 1 and actomyosin subfragment 1. Biochem J. 1988 Feb 1;249(3):735–743. doi: 10.1042/bj2490735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar N. C., Geeves M. A. The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal muscle myosin subfragment 1. FEBS Lett. 1983 Aug 22;160(1-2):141–148. doi: 10.1016/0014-5793(83)80954-5. [DOI] [PubMed] [Google Scholar]
- Siemankowski R. F., White H. D. Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J Biol Chem. 1984 Apr 25;259(8):5045–5053. [PubMed] [Google Scholar]
- Siemankowski R. F., Wiseman M. O., White H. D. ADP dissociation from actomyosin subfragment 1 is sufficiently slow to limit the unloaded shortening velocity in vertebrate muscle. Proc Natl Acad Sci U S A. 1985 Feb;82(3):658–662. doi: 10.1073/pnas.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- Truong T., Medley Q. G., Côté G. P. Actin-activated Mg-ATPase activity of Dictyostelium myosin II. Effects of filament formation and heavy chain phosphorylation. J Biol Chem. 1992 May 15;267(14):9767–9772. [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. The effect of EDTA on spectral properties of ATP-, ADP-, and ITP-G-actin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):611–616. doi: 10.1016/0006-291x(67)90530-x. [DOI] [PubMed] [Google Scholar]
- Woodward S. K., Eccleston J. F., Geeves M. A. Kinetics of the interaction of 2'(3')-O-(N-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragment 1: characterization of two acto-S1-ADP complexes. Biochemistry. 1991 Jan 15;30(2):422–430. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]


