Abstract
The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide-1 (GLP-1) are secreted from enteroendocrine cells in the gut and regulate physiological and homeostatic functions related to glucose control, metabolism and food intake. This review provides a systematic summary of the molecular mechanisms underlying secretion from incretin cells, and an understanding of how they sense and interact with lumen and vascular factors and the enteric nervous system through transporters and G-protein coupled receptors (GPCRs) present on their surface to ultimately culminate in hormone release. Some of the molecules described below such as sodium coupled glucose transporter 1 (SGLT1), G-protein coupled receptor (GPR) 119 and GPR40 are targets of novel therapeutics designed to enhance endogenous gut hormone release. Synthetic ligands at these receptors aimed at treating obesity and type 2 diabetes are currently under investigation.
Keywords: Incretin, Glucagon-like peptide-1 (GLP-1), Glucose-dependent insulinotropic polypeptide (GIP)
The incretin effect
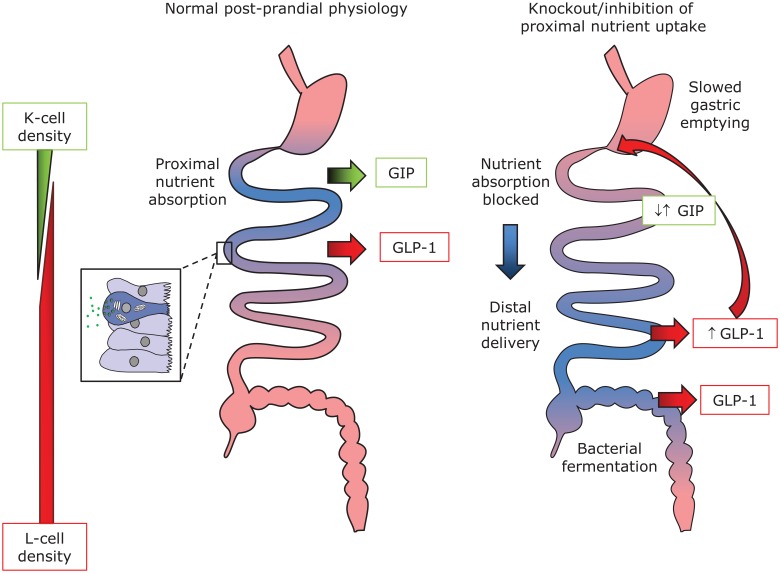
After the proposal of ‘secretin’ as a blood-borne factor coordinating the arrival of food in the upper small intestine with exocrine pancreatic secretion [Bayliss and Starling, 1902], a similar ‘hormonal’ axis promoting insulin release from the endocrine pancreas was proposed by La Barre in 1932, coining the term ‘incretin’. The incretin effect describes the fact that, even when the insulin-secreting and inherently glucose-sensitive pancreatic β cell is exposed to matching blood glucose levels, only approximately half as much insulin is secreted when glucose is provided by an intravenous infusion compared with oral ingestion/intestinal absorption of glucose [Perley and Kipnis, 1967]. We now know of a number of gut-derived hormones that boost glucose-dependent insulin release, including for example, sulfated cholecystokinin (CCK8S) [Zawalich et al. 1986], but the ‘true incretins’ known to date are glucose-dependent insulinotropic polypeptide (GIP) and glucagon like peptide-1 (GLP-1) [Dupre et al. 1973; Kreymann et al. 1987]. GIP and GLP-1 are produced and secreted from specialized endocrine cells of the gut, enteroendocrine cells (EECs), namely K and L cells, respectively (Figure 1).
Figure 1.
Incretin hormone secretion from K and L cells.
Glucose dependent insulinotropic polypeptide (GIP) is secreted from K cells, which are predominantly found in the duodenum, whereas glucagon-like peptide-1 (GLP-1) is secreted from L cells, which increase in numbers in the distal intestine. Both cell types are so-called open enteroendocrine cells with direct contact to the intestinal lumen, allowing sampling of the chyme. Nutrient transporters expressed in K and L cells play an important part in this chemosensation (see text for details). The difference in location, however, is likely to impact on the observed hormone responses to nutrient ingestion. It is now thought that sufficient L cells are present in the proximal intestine to account for early rises of GLP-1 under normal conditions (left hand side). However, when absorption is blocked (right hand side), the increased delivery to the distal intestine brings more L cells in contact with nutrients and/or their fermentation/putrification products, resulting in elevated GLP-1 levels. This in turn slows gastric emptying, potentially thereby prolonging exposure of proximal cells to chyme. While glucose stimulated GIP secretion is thus abolished in Sglt-1 knockout mice, emphasizing the importance of this transporter in K cells, GIP (as well as GLP-1) levels in B0at1 knockout animals are elevated in comparison with wildtype litter mates 60 min after food consumption.
Incretin secreting cells
K and L cells are subsets of enteroendocrine cells found scattered in the intestinal epithelium that, in their totality, contribute ~1% of intestinal epithelial cells. In recent years, transgenic technology has allowed the development of mice which express fluorescent protein reporters under the control of enteroendocrine hormone promoters [Reimann et al. 2008; Parker et al. 2009; Chandra et al. 2010; Wang et al. 2011; Suzuki et al. 2013]. This technique enabled the isolation, purification and systematic characterization of these otherwise elusive cells and led to rapid strides in our understanding of EEC biology. For one, the concept of these cells being uni/bi hormonal has been challenged and recent evidence points towards them being more plurihormonal than previously thought [Egerod et al. 2012; Habib et al. 2012; Sykaras et al. 2014]. Flow cytometric (FACS) analysis and immunostaining have revealed that, while in the colon, most L cells contained GLP-1 and PYY, the picture is different in the upper small intestine. Most small intestinal L cells contained CCK, ~10% were GIP positive and ~20% were PYY positive [Habib et al. 2012]. Recently, we identified insulin-like peptide-5 (INSL5) to be a product of colonic but not small intestinal L cells and showed that its levels were increased in calorie-restricted mice and reduced after feeding. INSL5 administration increased food intake in wildtype mice but not in mice lacking its receptor RXFP4, contrasting with the anorexic properties of other L-cell hormones [Grosse et al. 2014].
Like other EECs, K and L cells are polarized and exhibit an ‘open-type’ morphology with an apical pole consisting of microvilli in direct contact with the lumen and a broad basolateral side from which dense core secretory vesicles exocytose [Kieffer and Habener, 1999]. A unique feature of these EECs was revealed using laser scanning confocal microscopy of CCK-GFP and PYY-GFP cells. Pseudopod-like processes were observed at the base of the cells, extending towards adjacent cells and forming synapse-like structures, thereby presumably exerting a paracrine effect on neighbouring enterocytes [Chandra et al. 2010; Bohórquez et al. 2011]. However, the specific function of these pseudopod structures is yet to be established.
Another important aspect of the polarization of EECs is that their apical and basolateral surfaces differ in their accessibility to luminal and vascular factors; whether sensory receptors are located on the apical or basolateral membrane can be functionally critical, as for example the exclusive expression of the sodium coupled glucose transporter 1 (SGLT-1) on the apical membrane (see below), easily explains why incretin secreting cells should be ‘blind’ to elevation of vascular glucose concentrations. However, basolateral localization of receptors might be important to shield them from saturating ligand concentrations in the intestinal lumen, a scenario likely for the bile acid sensing receptor, G-protein coupled bile acid receptor 1 (GPBAR1) and the short chain fatty acid sensing receptors FFAR2/3 (see below).
Enteroendocrine cell sensing
For decades it has been known that the presence in the lumen of food or its macronutrient components (carbohydrates, fats and proteins) regulates incretin hormone secretion. Elevation of circulating GLP-1 levels can be detected within 10–15 minutes of eating and persists for several hours, depending on the nutritional composition of the meal. The molecular mechanisms behind this nutrient-dependent secretion have become clear from studies conducted by our group and others, aided by the engineering of transgenic mice wherein the EECs can be identified, isolated and studied. Several studies have shown that EECs directly sense nutrients in the lumen, and this has been attributed to the activity of nutrient transporters, receptors and metabolism. In addition, hormone secretion has been linked to non-nutrient stimulation by factors such as cytokines, bile acids and even gut microbial metabolites.
This review focuses on the nutrient and non-nutrient sensing mechanisms employed by GLP-1 and GIP-secreting L and K cells, respectively. While most of the mechanisms described here hold true for both cell types, some differences exist [Parker et al. 2009].
Nutrient regulation
Carbohydrate sensing
Glucose is a well-recognized stimulus of GLP-1 secretion and has consistently been demonstrated to cause GLP-1 release in in vitro enteroendocrine cell lines, primary murine intestinal culture systems, and in vivo mouse models and humans. The concentrations of glucose in the lumen required to trigger GLP-1 release in vivo are not clear and concentrations ranging from 5 to 1000 mM have been reported by various groups to trigger release from perfused ileum [Shima et al. 1990; Sugiyama et al. 1994; Ritzel et al. 1997]. The pathway underlying glucose-dependent secretion has been well characterized and requires the cotransport of sodium ions by the electrogenic sodium/glucose cotransporter 1 (SGLT1). The influx of Na+ ions depolarizes the plasma membrane, opening voltage sensitive calcium channels and exocytosis of GLP-1-containing secretory vesicles [Gribble et al. 2003].
Depletion of sodium chloride in the lumen impaired glucose-mediated GLP-1 secretion in isolated perfused rat small intestine [Kuhre et al. 2015], suggesting that secretion requires Na-coupled uptake by the L cells. Furthermore, support for the involvement of SGLT1-mediated glucose influx come from studies where co-administration of the SGLT inhibitor, phloridzin, with glucose in the upper small intestine blocked glucose-induced GLP-1 and GIP secretion in mice [Moriya et al. 2009] and rats [Kuhre et al. 2015]. Phloridzin also blocked hormone secretion triggered by nonmetabolizable substrates of SGLT: α-methyl-d-glucopyranoside (α-MDG) and 3-O-methyl-d-glucose (3-OMG) [Moriya et al. 2009].
Sglt1 deficient mice displayed impaired GIP and GLP-1 release early after oral glucose administration [Gorboulev et al. 2012], but surprisingly had higher GLP-1 levels at later time points following glucose gavage [Powell et al. 2013a]. One hypothesis to explain these divergent effects on early and late GLP-1 secretion would be that impaired glucose absorption in the small intestine causes increased glucose delivery and microbial fermentation in the distal intestine to produce short chain fatty acids which in turn stimulate secretion from L cells via alternative pathways [Powell et al. 2013a, 2013b]. This exemplifies an important difference between K and L cells – as K cells are mostly found in the duodenum, no late glucose stimulated GIP secretion was seen in Sglt1 knockout mice [Powell et al. 2013b] whereas, consistent with the increased L-cell number in the distal intestine, interventions that shift nutrients down the gut tend to increase GLP-1 secretion.
Whether cellular metabolism contributes to glucose-induced incretin secretion is debatable. In GLUTag cells, a murine enteroendocrine cell line that expresses the proglucagon gene and secretes GLP-1, glucose metabolism raises intracellular adenosine triphosphate (ATP) levels, causing closure of ATP-sensitive potassium (KATP) channels, membrane depolarization, intracellular Ca2+ elevation and GLP-1 secretion [Parker et al. 2012]. L and K cells express glucokinase and KATP channel subunits [Reimann et al. 2008; Parker et al. 2009] and, while this machinery was shown to be required for glucose-stimulated GLP-1 secretion in GLUTag cells, in humans treated with the KATP channel inhibitors glibenclamide and repaglinide, GLP-1 secretion after an oral glucose tolerance test was not affected [Stephens et al. 2011]. A recent study investigated the role of KATP channels in GIP secretion and concluded that, in healthy mice, the channels are mostly closed and are therefore unlikely to play a major role in glucose-stimulated hormone secretion [Ogata et al. 2014]. Other sugars like fructose, which is not a substrate for SGLT1, trigger GLP-1 release in GLUTag cells by closure of KATP channels in response to increased metabolism [Gribble et al. 2003]. Fructose also induced GLP-1, CCK, PYY and neutrotensin (NTS) secretion but not GIP secretion in healthy young humans and GLP-1 but not GIP secretion in mice and rats [Kuhre et al. 2014]. The authors speculated that K cells might be less excitable than L cells and so while, the weak fructose stimulus might activate L cells, it is not sufficient to activate K cells. However, reduced excitability of K cells has not been experimentally demonstrated and a recent report demonstrated fructose-stimulated GIP secretion in mice with streptozotocin-induced diabetes which seemed, however, also not dependent on KATP-channel closure [Seino et al. 2015].
Glucose transporter 2 (GLUT2), a facilitative glucose transporter expressed in K and L cells, has been proposed to play a role in incretin release upstream of metabolism. GLUT2 is thought to translocate to the apical membrane in response to elevated luminal glucose [Kellett and Helliwell, 2000], although the importance of this phenomenon is controversial [Gorboulev et al. 2012]. Phloretin, an inhibitor of facilitative glucose but not sodium coupled transport, abolished glucose uptake into L cells in primary murine intestinal cultures, although in contrast to phloridizin it had little effect on glucose stimulated secretion [Parker et al. 2012]. Nonetheless, phloretin impaired GLP-1 secretion in the GLUTag cell line model [Parker et al. 2012] and perfused rat small intestine [Kuhre et al. 2015], and reduced GIP and GLP-1 secretion in isolated rat small intestine loops [Mace et al. 2012]. GLUT2 knockout mice have been reported to have impaired GLP-1, but not GIP responses, possibly due to reduced GLP-1 content in the intestine [Cani et al. 2007a]. However, as a more recent study found no significant differences in GIP and GLP-1 secretion in GLUT2 knockout mice after an oral glucose gavage [Röder et al. 2014], it seems that GLUT2 and facilitative glucose transport in general is of less importance in K and L cells compared with its well established role in the stimulus secretion coupling of pancreatic β cells.
Sweet taste receptors have also been proposed as glucose sensors in incretin secreting cells. On the one hand, expression of members of the T1R sweet taste receptors and the α-subunit of the G-protein gustducin have been reported in the small intestine of mice and the enteroendocrine cell line, STC-1 [Dyer et al. 2005] and colocalization of α-gustducin with PYY and GLP-1 was demonstrated in L cells of the human colon [Rozengurt et al. 2006] and a subset of duodenal L and K cells [Young et al. 2013]. α-Gustducin null mice receiving glucose gavage showed deficient release of GLP-1 [Jang et al. 2007] in comparison with wildtype mice. Similar disruption of glucose-stimulated GLP-1 secretion was observed in T1r3 null mice [Kokrashvili et al. 2009] and a sweet taste receptor antagonist interfered with glucose-stimulated GLP-1 and PYY elevation in human volunteers [Steinert et al. 2011]. On the other hand, ingestion of artificial sweeteners did not trigger GLP-1 in mice [Fujita et al. 2009] or humans [Ma et al. 2009], and their intraluminal administration in the upper small intestine of mice did not trigger hormone release [Moriya et al. 2009].
The finding that SGLT-1 inhibition or knockout completely abolished GIP secretion in vivo [Powell et al. 2013b] argues against an apical location of a glucose sensing (‘taste’) receptor in K cells, as the resulting elevated (unabsorbed) luminal sugar should then result in increased secretion. It could be argued that SGLT-1 might transport glucose across the epithelium where it is then sensed by basolaterally located sweet taste receptor, a mechanism described later for bile acid detection by TGR5 (see below). However, this seems unlikely as incretin secretion from mixed primary epithelial cultures, which give unrestricted access to both sides of an enteroendocrine cell, was also abolished by SGLT-1 knockout/inhibition and was not responsive to saturating concentrations of artificial sweeteners [Parker et al. 2012; Reimann et al. 2008]. It remains, however, possible that sweet taste receptor expression is especially sensitive to culture conditions, although a recent study using a perfused intestinal preparation failed to observe effects of artificial sweeteners [Kuhre et al. 2014]. An alternative explanation for the impaired incretin secretion observed in mice with altered taste receptor pathway activity could be that the well documented taste receptor dependent increase in SGLT-1 expression and glucose absorption [Zietek and Daniel,2015] should be impaired in these mice, possibly reducing SGLT-1 expression in enteroendocrine cells. More work is needed to substantiate a role of sweet taste receptors in incretin hormone secretion.
Protein sensing
Dietary proteins are known to promote satiety and weight loss, and the satiating effects of proteins were found to be associated with the release of the anorectic hormone, PYY, and suppression of the orexigenic hormone, ghrelin. In age-matched normal weight and obese volunteers, a high protein diet caused a greater reduction in hunger and a larger increment in PYY compared with isocaloric high-carbohydrate or high-fat meals [Batterham et al. 2006]. Similar effects were seen in rodents, in which increasing the dietary protein content reduced ad libitum calorie intake and increased circulating PYY levels, and long-term high-protein feeding resulted in significantly less weight gain, less white adipose tissue, and lower plasma leptin levels compared with the corresponding isocaloric normal-protein diets. Additionally, Pyy null mice were found to be resistant to the satiating and weight-reducing effects of high protein diets [Batterham et al. 2006]. Oral whey protein in combination with glucose augmented GLP-1 responses in mice but had no effect on GIP secretion [Gunnarsson et al. 2006]. Interestingly, the same study showed that whereas total GIP secretion was unaffected, the active form of GIP, measured with N-terminally directed antibodies, was increased by whey protein. The authors suggested that ingested protein resulted in slower GIP inactivation, leading to an increase in the active form, but no change in circulating total GIP levels. Several other studies reported differential effects of oral protein ingestion on the two incretins, with greater effects observed on GLP-1 than GIP secretion [Elliott et al. 1993].
In the perfused rat intestine, GLP-1 release was triggered by luminal perfusion of a meat protein hydrolysate known as peptone, and the response magnitude was similar in the upper and lower half of the small intestine [Svendsen et al. 2015]. Meat peptones strongly stimulated GLP-1 secretion from primary murine colonic cultures [Diakogiannaki et al. 2013] and we recently demonstrated GLP-1 release from primary murine duodenal cultures after treatment with meat, vegetable and casein-derived peptones [Pais et al. 2015]. Mechanistic studies performed with meat peptones revealed the involvement of the calcium sensing receptor (CASR) and voltage gated calcium channels (VGCC) in mediating peptone-stimulated GLP-1 release [Pais et al. 2015]. CASR’s primary role as a calcium sensor regulating parathyroid hormone release [Brown and Herbert, 1995] is well recognized and established, but increasing evidence supports the idea that CASR also acts as an amino acid sensor in the gut, mediating macronutrient-dependent secretion of gut hormones such as gastrin [Feng et al. 2010], CCK [Liou et al. 2011; Wang et al. 2011], GIP and GLP-1 [Mace et al. 2012; Diakogiannaki et al. 2013; Pais et al. 2015]. Meat hydrolysate has been linked to the activation of mitogen-activated protein kinases (MAPK) and stimulation of GLP-1 secretion from murine-derived STC-1 and human-derived NCI-H716 cell lines [Reimer, 2006]. In GLUTag cells, albumin egg hydrolysate also increased transcription of the proglucagon gene [Cordier-Bussat et al. 1998]. The extent to which these effects are downstream of CASR activation remains to be established.
Among amino acids, l- glutamine (l-Gln) was consistently shown to trigger GLP-1 secretory responses in human volunteers and patients with type 2 diabetes [Greenfield et al. 2009; Samocha-Bonet et al. 2011] and also in in vitro studies performed in primary mouse cultures and GLUTag cells. The effect of l-Gln has been attributed to its ability both to trigger membrane depolarization via electrogenic Na+ dependent amino acid uptake leading to opening of L- and Q-type voltage gated calcium channels, and to elevate cytoplasmic cyclic adenosine monophosphate (cAMP) concentrations [Reimann et al. 2004; Tolhurst et al. 2011]. In primary small intestinal cultures, glutamine-triggered GLP-1 secretion was inhibited by antagonists of CASR [Pais et al. 2015]. Other amino acids, such as glycine and alanine, are strong stimulants of GLP-1 secretion from GLUTag cells through activation of the ionotropic glycine receptor [Gameiro et al. 2005], but this mechanism was not observed in colonic L cells in primary culture. l-Phenylalanine (l-Phe), however, was a highly effective stimulus of GLP-1 release in mouse small intestinal cultures [Pais et al. 2015], but was not an exceptional stimulus in colonic cultures, and was ineffective in GLUTag cells [Reimann et al. 2004; Tolhurst et al. 2011]. The identity of the L-cell sensor underlying l-Phe triggered secretion remains to be established, and although some studies have proposed the involvement of CASR [Mace et al. 2012], we were unable to block l-Phe triggered GLP-1 secretion from primary intestinal cultures using CASR antagonists [Pais et al. 2015]. Jiang and colleagues reported higher meal-dependent GLP-1 levels in mice lacking the neutral amino acid transporter B0AT1 than in controls and suggested that reduced neutral amino acid absorption in the null mice causes an overload of amino acids in the lower gut and stimulation of the large reservoir of distally located GLP-1 secreting cells [Jiang et al. 2015]. These results mirror the elevation of glucose-triggered GLP-1 release in mice lacking SGLT-1, which are similarly postulated to reflect increased glucose delivery to the distal gut, as discussed above.
Several other candidate sensory pathways for protein digestion products have been described. Activation of the G-protein coupled receptor GPRC6A in GLUTag cells is triggered by ornithine [Oya et al. 2013]. The proton-coupled electrogenic peptide transporter PEPT1 [Daniel, 2004] was shown to be critical for dipeptide triggered GLP-1 secretion in studies using agonists and antagonists of the transporter and pept1 deficient mice [Diakogiannaki et al. 2013]. Another receptor activated by polypeptides, lysophosphatidic acid receptor 5 (LPAR5, GPR92/93) was shown to mediate CCK [Choi et al. 2007] but not GLP-1 secretion [Diakogiannaki et al. 2013]. Further, the umami taste receptor dimer Tas1R1/Tas1R3 was shown to be involved in amino acid triggered CCK secretion [Daly et al. 2013].
Fat sensing
It was observed in the 1980s that infusion of lipid emulsion into the ileum of humans reduced food intake and promoted satiety, and it was later found that these effects were accompanied by an increase in plasma CCK levels [Welch et al. 1985; Drewe et al. 1992]. Indeed, fats also potently stimulate GLP-1 and PYY release [Feltrin et al. 2004; Little et al. 2005]. The main products of lipid digestion are monoglycerides (MGs) and fatty acids, and inhibition of fat digestion by lipase inhibitors attenuated the rise in plasma levels of CCK, PYY and GLP-1 after a fat-meal [Feinle et al. 2003; Feinle-Bisset et al. 2005; Ellrichmann et al. 2008; Beglinger et al. 2010]. CCK, GLP-1 and PYY release were found to be dependent on fatty acid chain length with only fatty acids greater than C10 being effective in stimulating hormone secretion [Feltrin et al. 2004; Feinle-Bisset et al. 2005; Little et al. 2005].
Several GPCRs have been implicated in the sensing of fatty acids and the concomitant release of gut hormones by EECs. Those identified until now include GPR40 (FFAR1), GPR120 (FFAR4) and GPR119. GPR40 is activated by medium to long chain free fatty acids (FFAs) [Briscoe et al. 2003] and found to be present in GIP and GLP-1 secreting cells in mice. Following an oral high-fat diet (HFD), Gpr40 deficient mice exhibited diminished secretion of GLP-1 and GIP with concomitant reduction in insulin levels [Edfalk et al. 2008]. GPR120 is also activated by unsaturated long chain FFAs and was found co-localized with GLP-1 in human colon. In HEK cells stably expressing GPR120, long chain FFAs evoked an increase in intracellular calcium [Ca2+]i in a dose-dependent fashion and, in STC-1 cells, α-linoleic acid strongly stimulated GLP-1 release [Hirasawa et al. 2005]. The increase in [Ca2+]i in STC-1 cells induced by α-linolenic acid was eliminated by siRNA specific for mouse Gpr120 confirming the role of this receptor in long chain FFA-mediated GLP-1 release [Hirasawa et al. 2005].
A recent report emphasized the greater importance of GPR120 for GIP compared with GLP-1 secretion [Iwasaki et al. 2015] and the same group reported specific enrichment of the fatty acid binding protein FABP5 in K cells, suggesting a role in fatty acid detection [Shibue et al. 2015]. Ligands of GPR119 include the fatty acid derivatives oleoylethanolamide (OEA) [Overton et al. 2006] and lysophophatidylcholine (LPC) [Soga et al. 2005], as well as monoacylglycerides [Hansen et al. 2011; Mandøe et al. 2015; Lauffer et al. 2009]. OEA administration in rats reduced food intake and weight gain [Rodríguez De Fonseca et al. 2001] and stimulated GLP-1 secretion from perfused rat ileum [Lauffer et al. 2009], GLUTag cells [Lauffer et al. 2009] and primary cultured murine L cells [Moss et al. 2015]. GPR119 is Gs-coupled and increases intracellular cAMP levels upon activation. GLUTag cells transfected with specific Gpr119 siRNA failed to increase cAMP levels in response to OEA [Lauffer et al. 2009]. Gpr119 knockout mice showed diminished nutrient-stimulated GLP-1 secretion [Lan et al. 2009]. Also, oral administration of the GPR119 agonist AR231453 increased plasma concentrations of GLP-1 and GIP in wildtype but not Gpr119 knockout animals [Chu et al. 2008]. 2-Oleoyl glycerol, a product of triglyceride digestion, also acts on GPR119 and its administration to humans significantly increased plasma GLP-1 and GIP levels [Hansen et al. 2011; Mandøe et al. 2015]. Pharmacological GPR119 agonists developed for human studies and tested in patients with type 2 diabetes have not, however, shown significant metabolic benefits [Katz et al. 2012]. The reason behind this is uncertain and the therapeutic potential of GPR119 is still under investigation.
A range of non-GPCR machinery has also been implicated in free-fatty-acid-triggered incretin secretion. Protein kinase Cζ was implicated in oleic acid triggered GLP-1 secretion in GLUTag cells [Iakoubov et al. 2007] and rodents [Iakoubov et al. 2011], and fatty acid transport protein was found to contribute to oleic acid induced GLP-1 secretion in vitro and in vivo [Poreba et al. 2012]. Triglycerides (TGs) are resynthesized in the enterocytes of small intestine from absorbed fatty acids and MGs by the sequential action of monoacylglycerol acyltransferase (MGAT) and diacylglycerol acyltransferase (DGAT). The newly formed TGs are then incorporated into chylomicrons (CM) by microsomal triglyceride transfer protein (MTP). MGAT2 and DGAT1 deficient mice displayed impaired GIP secretion after oral triglyceride gavage, but increased GLP-1 and PYY levels which remained elevated even 2 hours after gavage in comparison with wildtype controls [Okawa et al. 2009]. In addition, administration of a pharmacological inhibitor of DGAT1 prior to oil gavage reduced postprandial TG levels and led to prolonged elevated levels of GLP-1 in mice [Ables et al. 2012] Furthermore, an inhibitor of intestinal specific MTP was shown to elevate GLP-1 and PYY in rats fed a high fat diet [Hata et al. 2011]. CM formation and secretion were shown to be critical for GLP-1 [Lu et al. 2012] and GIP [Shimotoyodome et al. 2009] release, as revealed in rodent studies using surfactants that inhibited CM formation. Recently, Wang and colleagues showed that Apolipoprotein A-IV (ApoA-IV) knockout mice have increased lymphatic and plasma GLP-1 levels 30 minutes after intraduodenal administration of Ensure, a commercially available liquid meal, in comparison with wildtype controls. Interestingly, in another experiment conducted by the same group, acute administration of ApoA-IV prior to intraduodenal Ensure infusion did not alter GLP-1 levels in wildtype mice or the ApoA-IV-/- mice. They attributed the increased GLP-1 levels in the ApoA-IV knockout mice to increased expression of Gpr119 in the ileum [Wang et al. 2015].
Whereas fats acutely stimulate GLP-1 secretion as evidenced from above, chronic high-fat feeding in mice reduced numbers of GLP-1 and GIP positive cells and significantly decreased the expression of enteroendocrine hormones, nutrient sensing machinery and enteroendocrine-specific transcription factors [Richards et al. 2015]. Hayashi and colleagues demonstrated that endoplasmic reticulum stress, induced with excessive doses of palmitate, increased the expression of endoplasmic reticulum stress markers, Chop and BiP, reduced protein levels and function of prohormone convertase 1/3, and decreased GLP-1 secretion from GLUTag cells [Hayashi et al. 2014]. Aranias and colleagues, however, observed an increase in L-cell density in the jejunum of morbidly obese subjects and elevated GLP-1 secretion in HFD fed mice after an oral glucose challenge. They hypothesized that increased GLP-1 secretion would favour insulin secretion and counter balance diet-induced insulin resistance [Aranias et al. 2015]. The influence of diet on L cells and gut hormone secretion is clearly not completely understood, and more work needs to be done in this field.
Non nutrient regulation
Gut microbiota
Gut bacteria have a profound impact on the host and influence, in many ways, host physiology and metabolism [Tremaroli and Bäckhed, 2012]. EECs lie in close proximity to microbes and/or their metabolic products in the gut and the resulting crosstalk regulates hormone secretion. Bacterial fermentation of undigested prebiotics and complex carbohydrates produces short chain fatty acids (SCFAs) such as acetate, butyrate and propionate. SCFAs induced GLP-1 secretion from primary colonic cultures and this was shown to be mediated by GPR43 (FFAR2) and GPR41 (FFAR3). GPR43 is Gi- and Gq-coupled and triggered an increase in [Ca2+]i in colonic L cells following activation by SCFAs. Mice deficient in Ffar2 and Ffar3 displayed reduced SCFA-triggered GLP-1 secretion [Tolhurst et al. 2012]. Additionally, butyrate, acetate and propionate administration protected mice from developing diet-induced obesity and insulin resistance and stimulated gut hormone release [Lin et al. 2012]. A recent report, however, observed improved glucose tolerance in Ffar2/3 double knockout mice, which was explained by the direct inhibitory effect of these receptors in pancreatic β cells, and found no significant effect of Villin-Cre mediated conditional knockout of these receptors in the intestine [Tang et al. 2015]. In contrast, impaired glucose tolerance of Ffar3-/- mice was recapitulated, but a selective FFAR3 agonist also impaired glucose tolerance, presumably by direct action in the islet [Forbes et al. 2015], and it was concluded that the observed anorectic effect of this compound was mostly mediated through elevation of PYY.
It is now well established that obesity and type 2 diabetes affect gut microbiome diversity and composition [Ley et al. 2005; Qin et al. 2012]. Prebiotic feeding in ob/ob mice increased L-cell number, proglucagon mRNA levels and portal plasma GLP-1 levels, and this was mediated by the increased production of acetate and propionate [Everard et al. 2011]. Characterization of the microbiome revealed that the abundance of Akkermansia muciniphila was reduced in obese and diabetic mice [Everard et al. 2013] and feeding of this bacteria was positively associated with increased L-cell number and secretion [Everard et al. 2011]. Several other studies showed beneficial effects of prebiotics treatment on GLP-1, GLP-2 and PYY secretion in rodents and humans [Cani et al. 2007b, 2009a, 2009b].
Although a large body of evidence suggests that SCFAs produced by gut bacterial fermentation stimulate GLP-1 secretion, Wichmann and colleagues demonstrated that in germ-free (GF) mice, having severely reduced SCFAs, basal plasma GLP-1 levels were 3-fold higher than conventional raised mice (CONV-R). They attributed this observation to increased proglucagon (Gcg) expression in the caecum and colon. In addition, GF mice had more GLP-1 positive cells in the caecum and colon than the CONV-R mice. Colonization of GF mice with SCFA-producing bacteria increased acetate and propionate and correspondingly decreased Gcg expression and GLP-1 positive cells. Conversely, depletion of microbiota in CONV-R with antibiotics treatment over 3 days decreased SCFA levels and increased Gcg expression [Wichmann et al. 2013]. Other bacterial metabolites known to affect GLP-1 secretion include indole. Indole is produced from tryptophan by gut bacteria and, while acutely stimulated GLP-1 secretion by inhibiting voltage gated K+ channels, after prolonged exposure it slowed ATP production by blocking NADH dehydrogenase leading to a delayed reduction of GLP-1 secretion [Chimerel et al. 2014].
Bile acids
Bile acids play an important role in the digestion and absorption of dietary fats by facilitating the formation of micelles. Primary as well as secondary bile acids (formed as a result of bacterial modification) act as signalling molecules and strongly stimulate GLP-1 and PYY secretion [Ullmer et al. 2013]. They are known to act at two specific receptors: the nuclear farnesoid X receptor (FXR); and the transmembrane G-protein coupled receptor TGR5 (GPBAR1). Bile acid triggered GLP-1 secretion was found to be TGR5 dependent in STC-1 cells [Katsuma et al. 2005], GLUTag cells and primary murine intestinal cultures [Parker et al. 2012] and in vivo [Thomas et al. 2009]. Downstream signalling involved elevation of cAMP and [Ca2+]i [Parker et al. 2012]. Deoxycholic acid infusion in the colon increased enteroglucagon (GLP-1) and PYY secretion in humans undergoing colonoscopy [Adrian et al. 1993] and rectal taurocholate infusion reduced food intake, lowered glucose and increased GLP-1, PYY and insulin in obese type-2 diabetic volunteers [Adrian et al. 2012].
However, there has been debate about whether bile acids target their receptors on L cells from the apical or basolateral compartment. Brighton and colleagues measured GLP-1 release from murine ileum mounted in small volume Ussing chambers; they showed that basolateral taurodeoxycholate (TDCA) or a TGR5 agonist stimulated GLP-1 secretion but that the effects of apically-applied TDCA were abolished by inhibition of bile acid uptake across the epithelium. Preferential stimulation of GLP-1 release by basolateral versus apical bile acids was confirmed in the perfused rat gut, leading to the conclusion that bile acid triggered GLP-1 secretion is predominantly mediated by basolaterally located TGR5 on the L cell [Brighton et al. 2015]. These findings dampen the hope of developing TGR5 agonists as GLP-1 secretagogues, as TGR5 ligands entering the systemic circulation have been found to trigger gall bladder overfilling [Li et al. 2011]. It is interesting to note that a recent report suggests that FFAR1 (GPR40) is also located on the basolateral rather than the apical membrane of enteroendocrine cells [Christensen et al. 2015] and one might speculate that this will also extend to the related short-chain fatty acid receptors FFAR2 (GPR43) and FFAR3 (GPR41), as physiological observed luminal concentrations of short chain fatty acids are well above their reported potencies for these receptors.
A number of studies have shown that some of the beneficial effects of gastric bypass surgery on glucose metabolism in diabetic subjects can be attributed to increased bile acid delivery to the distal gut and enhanced release of gut hormones such as GLP-1 and PYY [Patti et al. 2009]. Bile acid sequestrants (BAS) are anion exchange resins that trap bile acids in the lumen and have cholesterol lowering and antidiabetic properties [Prawitt et al. 2014]. Additionally, they were shown to enhance GLP-1 secretion in diet-induced obese mice and increased proglucagon gene expression downstream of TGR5 [Harach et al. 2012]. Recently, Fxr was shown to be expressed in mouse ileal and colonic L cells and colocalized with GLP-1 in the human jejunum. Surprisingly, treatment with the FXR agonist GW406 decreased gcg expression and FXR activation was demonstrated to inhibit glycolysis, leading to reduced intracellular ATP levels and impaired glucose-dependent GLP-1 secretion [Trabelsi et al. 2015].
Hormones/cytokines
An emerging body of evidence shows that there is a crosstalk between the enteroendocrine system and other organs in the body and that gut hormone secretion is influenced by factors such as hormones and cytokines which act on the EECs to influence secretion either positively or negatively. Somatostatin (SST) is produced from D cells in the oxyntic and pyloric regions of the stomach and its secretion is stimulated by GIP, GLP-1 and CCK but not gastrin [Adriaenssens et al. 2015]. SST in turn exerts a suppressive tone over neighbouring EECs in the stomach as well as in the gut. Acting at least in part via the Gαi-coupled SSTR5 receptor, SST inhibited GIP and GLP-1 secretion in primary murine intestinal cultures and acts by suppressing cAMP levels in EECs [Moss et al. 2012].
Intestinal endocannabinoids exert orexigenic effects by signalling through vagal afferent neurons to satiety centres in the central nervous system [Izzo and Sharkey, 2010]. Levels of the endocannabinoid anandamide have been reported to increase in the small intestine of rats upon food deprivation and levels returned to baseline upon refeeding [Gómez et al. 2002]. The cannabinoid receptor CB1 was found to be highly expressed in GIP secreting K cells and, consistent with this finding, GIP but not GLP-1 secretion was reduced by the CB1 synthetic agonist methanandamide (mAEA) [Moss et al. 2012].
A number of studies have identified a stimulatory effect of GIP on GLP-1 secretion in rodents. Intravenous infusion of GIP, for example, elevated plasma levels of proglucagon-derived peptide (GLP-1) in rats [Roberge et al. 1993] and GIP treatment triggered GLP-1 release from primary canine L cells [Damholt et al. 1998]. It is not, however, currently believed that this plays an important role in humans, as even pharmacologically raised GIP levels had little effect on plasma GLP-1 [Nauck et al. 1993]. Ghrelin, an orexigenic hormone released from the stomach, enhanced glucose-induced GLP-1 release in vivo in mice and in vitro in rodent and human L cells through an extracellular-signal-regulated kinase ERK 1/2-dependent pathway [Gagnon et al. 2015a].
Obesity is a pro-inflammatory condition associated with elevated levels of circulating pro-inflammatory cytokines which are released from the adipose tissue and infiltrating immune cells. The endocrine and secretory behaviour of adipose tissue was revealed with the discovery that adipocytes synthesize and secrete the cytokine tumour necrosis factor α (TNFα) [Hotamisligil et al. 1993] and the hormone leptin [Zhang et al. 1994]. Since then, several adipose-derived proteins have been discovered that have profound physiological effects; they have collectively been given the term adipokines. Leptin has well established effects on food intake and elevated circulating levels in obesity are proportional to fat mass [Considine et al. 1996]. The long form of the leptin receptor, Ob-Rb, was found on human and rodent L cells and leptin was observed to stimulate GLP-1 secretion in vitro and in vivo in rats and mice [Anini and Brubaker, 2003]. Among other adipokines, interleukin (IL) 6 is regarded as a key player contributing to obesity linked insulin resistance [Senn et al. 2002; Eder et al. 2009]. IL-6 is also secreted from exercising skeletal muscle and was shown to stimulate GLP-1 secretion not only from intestinal L cells but also pancreatic α cells [Ellingsgaard et al. 2011]. Exercise has been shown to be associated with elevated levels of GLP-1, PYY and pancreatic polypeptide [Martins et al. 2007] and IL-6 is a candidate mediator of this effect.
Recently, TNFα was demonstrated to affect GLP-1 secretion. Whereas acute application of TNFα increased GLP-1 secretion from a human GLP-1 secreting cell line, TNFα pretreatment of both human and murine cell lines reduced gcg expression and impaired GIP-triggered GLP-1 secretion. Co-administration of a nuclear factor-κB (NFκβ) inhibitor abolished the inhibitory effect of TNFα pretreatment on GLP-1 responses and treatment with the TNFα inhibitor etanercept improved GLP-1 secretion in HFD-induced obese mice [Gagnon et al. 2015b]. Another adipokines, RANTES (CCL5) which is upregulated in obesity [Wu et al. 2007; Keophiphath et al. 2010] was shown to reduce glucose-stimulated GLP-1 release in vitro in NCI-H716 cells and plasma levels of GLP-1 and GLP-2 after an oral glucose load in mice [Pais et al. 2014].
Progesterone increased plasma levels of GIP and GLP-1 in mice, and was shown to be mediated by the G-protein coupled receptors PAQR5 and PAQR7 by receptor knockdown studies conducted in GLUTag cells [Flock et al. 2013]. Insulin receptor expression was also found on murine and human L cells, and insulin was found to stimulate GLP-1 secretion in vitro from murine L cells by activating the phosphatidylinositol 3 kinase-Akt and MAPK kinase (MEK)-ERK1/2 pathways. Pretreatment with high concentrations of insulin for 24 hours caused insulin resistance and reduced basal and insulin induced GLP-1 secretion [Lim et al. 2009]. Nesfatin-1, an anorexigenic peptide found in the hypothalamus [Oh-I et al. 2006], colocalized with GIP and GLP-1 positive cells in mouse small intestine and stimulated GLP-1 secretion from STC-1 cells [Ramesh et al. 2015].
Neural regulation
The enteric nervous system is sometimes referred to as a second brain because of its degree of autonomous function. It exerts several actions in the gastrointestinal tract such as control of gut motility, fluid exchange between the gut and the lumen, regulation of gastric and pancreatic secretion and gut defence, and it interacts with EECs in the gut epithelium to regulate hormone release. Previous immunohistochemistry studies performed on rat and human tissue detected GLP-1-positive cells predominantly in the lower small intestine and colon [Eissele et al. 1992] and led to the proposal that the initial rapid rise in GLP-1 following a meal was indirectly mediated by neural/endocrine pathways arising from nutrient detection in the proximal gut, rather than by direct nutrient stimulation of L cells.
More recent studies, however, have concluded that enough GLP-1 cells are present in the proximal small intestine of a number of species to account for the rapid postprandial rise of plasma GLP-1 concentrations [Theodorakis et al. 2006; Habib et al. 2012] and that they directly respond to nutrient stimulation [Pais et al. 2015]. L cells lie in close proximity to neurons and microvasculature in the gut and studies performed on isolated vascularly perfused rat colon and ileum demonstrated stimulatory roles for neurotransmitters, calcitonin-gene related peptide (CGRP) and gastrin releasing peptide (GRP) on GLP-1 secretion [Plaisancie et al. 1994; Dumoulin et al. 1995]. GRP is released from GRPergic neurons and its role in GLP-1 secretion was confirmed in studies using GRP agonists [Roberge and Brubaker, 1996] and GRP deficient mice [Persson et al. 2000]. Cholinergic regulation of GLP-1 secretion was shown in the murine STC-1 cell line, mediated by the muscarinic receptor M3 [Abello et al. 1994]. Other studies have shown roles for M1 and M2 in stimulating GLP-1 release from L cells in vitro, and M1 in mediating GLP-1 release from rat in vivo [Anini et al. 2002]. Pituitary adenylate cyclase-activating polypeptide (PACAP) increased GLP-1 secretion from GLUTag cells by increasing intracellular cAMP levels [Simpson et al. 2007]. γ-Aminobutyric acid (GABA) receptors were detected on the STC-1 and GLUTag cell lines: GABA triggered CCK and GLP-1 secretion mediated by the GABAA receptor subtype in STC-1 cells [Glassmeier et al. 1998] and by both GABAA and GABAC receptors in GLUTag cells [Gameiro et al. 2005].
Cell number and sensitivity
An alternative or supplementary approach to the acute stimulation of incretin secretion would be the promotion of increased incretin expression in the intestine. FXR-mediated effects of bile acids on pro-glucagon expression have already been mentioned. Alternatively, SCFA has been shown to increase the number of gcg-expressing cells in organotypic intestinal organoid cultures [Petersen et al. 2014], which might underlie the reported increase in L-cell density in response to prebiotic fibre supplementation in ob/ob mice [Everard et al. 2011] and the reduced gcg mRNA content of Ffar2-/- derived intestines [Tolhurst et al. 2012]. More recently Petersen and colleagues demonstrated that inhibition of NOTCH signalling pathways with the γ-secretase inhibitor dibenzazepine (DBZ), increased L-cell numbers in in vitro models of intestinal organoids cultures from mouse and human and augmented glucose-stimulated GLP-1 secretion. DBZ also increased L-cell numbers in the intestines of HFD fed mice and type 2 diabetes mouse models and improved glucose-stimulated insulin release and glucose tolerance [Petersen et al. 2015].
Another potentially interesting aspect of incretin secretion investigated only recently is its link to circadian rhythm and the possibility that it plays a role in entraining these rhythms to food intake. Gil-Lozano and colleagues demonstrated circadian rhythmicity in the secretion of GLP-1 in response to different secretagogues in vitro and well as in vivo in rats fasted for 4 hours before being administered a glucose load. They also showed rhythmic expression of clock genes in GLUTag cells and demonstrated that knockdown of two rhythmically expressed target genes (thyrotroph embryonic factor and protein tyrosine phosphatase 4a1) resulted in altered GLP-1 release [Gil-Lozano et al. 2014].
Physiological relevance
Therapies targeting the incretin axis have proved highly effective in treating type 2 diabetes and provide the additional benefits of weight loss and cardioprotection [Drucker and Nauck, 2006; Gejl et al. 2012]. Roux-en-Y gastric bypass (RYGB) is currently the most widely used bariatric procedure for treating severe obesity. Anastomosis of the jejunum to a small gastric pouch causes rapid postprandial delivery of nutrients to the distal gut with its large population of L cells. The consequent exaggerated release of GLP-1 is believed to underlie some of the remarkable improvements in glucose tolerance found in people undergoing surgery, in many cases resulting in remission of type 2 diabetes [Korner et al. 2007; Dirksen et al. 2010; Falkén et al. 2011; Jiménez et al. 2012]. High accompanying PYY levels after surgery likely contribute to the observed reductions in appetite [Korner et al. 2005; Morínigo et al. 2008]. Interestingly, nutrient malabsorption in mice deficient in Sglt1 [Powell et al. 2013a], B0at1 [Jiang et al. 2015] and Dgat1 [Okawa et al. 2009] seems to present a scenario that mimics some aspects of bariatric surgery. In each case elevated postprandial GLP-1 levels have been reported, probably reflecting the increased delivery of nonabsorbed nutrients to the hindgut (Figure 1), although not reproducing the rapid peaks found after surgery.
Conclusion
The enteroendocrine system secretes a myriad of hormones that are pivotal to glucose homeostasis and gut function. The incretins, in particular, have garnered a great deal of interest owing to their antidiabetic and satiating effects. GLP-1 based drugs (GLP-1 mimetics and inhibitors of GLP-1 degradation) and bariatric surgery have shown immense beneficial effects on glycaemia and weight loss in humans. Fuelled by our understanding of the intricate pathways by which EEC cells sense nutrients and other factors in their milieu, strategies to harness the tremendous potential of the enteroendocrine system by targeting endogenous gut hormone production offer great hope for the future treatment of diabetes and obesity.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research in the Reimann and Gribble laboratories is currently funded by the Wellcome Trust (grants 106262/Z/14/Z and 106263/Z/14/Z), Full4Health (grants FP7/2011-2015 no: 266408) and the Medical Research Council (MRC) (grant MRC_ MC_UU_12012/3).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Ramona Pais, The Wellcome Trust–MRC Institute of Metabolic Science, Metabolic Research Laboratories, University of Cambridge, Cambridge, UK.
Fiona M. Gribble, The Wellcome Trust–MRC Institute of Metabolic Science, Metabolic Research Laboratories, University of Cambridge, Addenbrookes’s Hospital, Box 289, Hills Road, Cambridge, CB2 0QQ, UK
Frank Reimann, The Wellcome Trust–MRC Institute of Metabolic Science, Metabolic Research Laboratories, University of Cambridge, Addenbrookes’s Hospital, Box 289, Hills Road, Cambridge, CB2 0QQ, UK.
References
- Abello J., Ye F., Bosshard A., Bernard C., Cuber J., Chayvialle J. (1994) Stimulation of glucagon-like peptide-1 secretion by muscarinic agonist in a murine intestinal endocrine cell line. Endocrinology 134: 2011–2017. [DOI] [PubMed] [Google Scholar]
- Ables G., Yang K., Vogel S., Hernandez-Ono A., Yu S., Yuen J., et al. (2012) Intestinal DGAT1 deficiency reduces postprandial triglyceride and retinyl ester excursions by inhibiting chylomicron secretion and delaying gastric emptying. J Lipid Res 53: 2364–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriaenssens A., Yee Hong Lam B., Billing L., Skeffington K., Sewing S., Reimann F., et al. (2015) A transcriptome-led exploration of molecular mechanisms regulating somatostatin-producing D-cells in the gastric epithelium. Endocrinology: en20151301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian T., Ballantyne G., Longo W., Bilchik A., Graham S., Basson M., et al. (1993) Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut 34: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian T., Gariballa S., Parekh K., Thomas S., Saadi H., Al Kaabi J., et al. (2012) Rectal Taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 55: 2343–2347. [DOI] [PubMed] [Google Scholar]
- Anini Y., Brubaker P. (2003) Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes 52: 252–259. [DOI] [PubMed] [Google Scholar]
- Anini Y., Hansotia T., Brubaker P. (2002) Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 143: 2420–2426. [DOI] [PubMed] [Google Scholar]
- Aranias T., Grosfeld A., Poitou C., Omar A., Le Gall M., Miquel S., et al. (2015) Lipid-rich diet enhances L-Cell density in obese subjects and in mice through improved L-cell differentiation. J Nutr Sci 4: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham R., Heffron H., Kapoor S., Chivers J., Chandarana K., Herzog H., et al. (2006) Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab 4: 223–233. [DOI] [PubMed] [Google Scholar]
- Bayliss W., Starling E. (1902) The mechanism of pancreatic secretion. J Physiol 28: 325–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beglinger S., Drewe J., Schirra J., Göke B., D’amato M., Beglinger C. (2010) Role of fat hydrolysis in regulating glucagon-like peptide-1 secretion. J Clin Endocrinol Metab 95: 879–886. [DOI] [PubMed] [Google Scholar]
- Bohórquez D., Chandra R., Samsa L., Vigna S., Liddle R. (2011) Characterization of basal pseudopod-like processes in ileal and colonic PYY cells. J Mol Histol 42: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brighton C., Rievaj J., Kuhre R., Glass L., Schoonjans K., Holst J., et al. (2015) Bile Acids trigger GLP-1 release predominantly by accessing basolaterally-located G-protein coupled bile acid receptors. Endocrinology: en20151321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe C., Tadayyon M., Andrews J., Benson W., Chambers J., Eilert M., et al. (2003) The orphan G protein-coupled receptor GPR40 Is activated by medium and long chain fatty acids. J Biol Chem 278: 11303–11311. [DOI] [PubMed] [Google Scholar]
- Brown E., Hebert S. (1995) Hot Papers. Signal transduction. ‘Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid’, Nature (1995), 366: 575–580 (by Brown E., et al. Comments. Scientist 9: 15–15. [DOI] [PubMed] [Google Scholar]
- Cani P., Holst J., Drucker D., Delzenne N., Thorens B., Burcelin R., et al. (2007a) GLUT2 and the incretin receptors are involved in glucose-induced incretin secretion. Mol Cell Endocrinol 276: 18–23. [DOI] [PubMed] [Google Scholar]
- Cani P., Hoste S., Guiot Y., Delzenne N. (2007b) Dietary Non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr 98: 32–37. [DOI] [PubMed] [Google Scholar]
- Cani P., Lecourt E., Dewulf E., Sohet F., Pachikian B., Naslain D., et al. (2009a) Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr 90: 1236–1243. [DOI] [PubMed] [Google Scholar]
- Cani P., Possemiers S., Van De Wiele T., Guiot Y., Everard A., Rottier O., et al. (2009b) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra R., Samsa L., Vigna S., Liddle R. (2010) Pseudopod-Like basal cell processes in intestinal cholecystokinin cells. Cell Tissue Res 341: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimerel C., Emery E., Summers D., Keyser U., Gribble F., Reimann F. (2014) Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep 9: 1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Lee M., Shiu A., Yo S., Halldén G., Aponte G. (2007) GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol 292: G1366–1375. [DOI] [PubMed] [Google Scholar]
- Christensen L., Kuhre R., Janus C., Svendsen B., Holst J. (2015) Vascular, but not luminal, activation of FFAR1 (GPR40) stimulates GLP-1 Secretion from isolated perfused rat small intestine. Physiol Rep 3: 312551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z., Carroll C., Alfonso J., Gutierrez V., He H., Lucman A., et al. (2008) A role for intestinal endocrine cell-expressed G Protein-coupled receptor 119 in glycemic control by enhancing glucagon-like peptide-1 and glucose-dependent insulinotropic peptide release. Endocrinology 149: 2038–2047. [DOI] [PubMed] [Google Scholar]
- Considine R., Sinha M., Heiman M., Kriauciunas A., Stephens T., Nyce M., et al. (1996) Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295. [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M., Bernard C., Levenez F., Klages N., Laser-Ritz B., Philippe J., et al. (1998) Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes 47: 1038–1045. [DOI] [PubMed] [Google Scholar]
- Daly K., Al-Rammahi M., Moran A., Marcello M., Ninomiya Y., Shirazi-Beechey S. (2013) Sensing of Amino acids by the gut-expressed taste receptor T1R1-T1R3 Stimulates cck secretion. Am J Physiol Gastrointest Liver Physiol 304: G271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damholt A., Buchan A., Kofod H. (1998) Glucagon-like-peptide-1 secretion from canine L-cells is increased by glucose-dependent-insulinotropic peptide but unaffected by glucose. Endocrinology 139: 2085–2091. [DOI] [PubMed] [Google Scholar]
- Daniel H. (2004) Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol 66: 361–384. [DOI] [PubMed] [Google Scholar]
- Diakogiannaki E., Pais R., Tolhurst G., Parker H., Horscroft J., Rauscher B., et al. (2013) Oligopeptides Stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia 56: 2688–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen C., Hansen D., Madsbad S., Hvolris L., Naver L., Holst J., et al. (2010) Postprandial diabetic glucose tolerance is normalized by gastric bypass feeding as opposed to gastric feeding and is associated with exaggerated GLP-1 secretion: a case report. Diabetes Care 33: 375–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe J., Gadient A., Rovati L., Beglinger C. (1992) Role of circulating cholecystokinin in control of fat-induced inhibition of food intake in humans. Gastroenterology 102: 1654–1659. [DOI] [PubMed] [Google Scholar]
- Drucker D., Nauck M. (2006) The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696–1705. [DOI] [PubMed] [Google Scholar]
- Dumoulin V., Dakka T., Plaisancie P., Chayvialle J., Cuber J. (1995) Regulation of glucagon-like peptide-1-(7–36) amide, peptide YY, and neurotensin secretion by neurotransmitters and gut hormones in the isolated vascularly perfused rat ileum. Endocrinology 136: 5182–5188. [DOI] [PubMed] [Google Scholar]
- Dupre J., Ross S., Watson D., Brown J. (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37: 826–828. [DOI] [PubMed] [Google Scholar]
- Dyer J., Salmon K., Zibrik L., Shirazi-Beechey S. (2005) Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans 33: 302–305. [DOI] [PubMed] [Google Scholar]
- Eder K., Baffy N., Falus A., Fulop A. (2009) The major inflammatory mediator interleukin-6 and obesity. Inflamm Res 58: 727–736. [DOI] [PubMed] [Google Scholar]
- Edfalk S., Steneberg P., Edlund H. (2008) GPR40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57: 2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod K., Engelstoft M., Grunddal K., Nøhr M., Secher A., Sakata I., et al. (2012) A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153: 5782–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissele R., Göke R., Willemer S., Harthus H., Vermeer H., Arnold R., et al. (1992) Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest 22: 283–291. [DOI] [PubMed] [Google Scholar]
- Ellingsgaard H., Hauselmann I., Schuler B., Habib A., Baggio L., Meier D., et al. (2011) Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17: 1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R., Morgan L., Tredger J., Deacon S., Wright J., Marks V. (1993) Glucagon-like peptide-1 (7–36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol 138: 159–166. [DOI] [PubMed] [Google Scholar]
- Ellrichmann M., Kapelle M., Ritter P., Holst J., Herzig K., Schmidt W., et al. (2008) Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7–36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab 93: 3995–3998. [DOI] [PubMed] [Google Scholar]
- Everard A., Belzer C., Geurts L., Ouwerkerk J., Druart C., Bindels L., et al. (2013) Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G., Muccioli G., et al. (2011) Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60: 2775–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkén Y., Hellström P., Holst J., Näslund E. (2011) Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab 96: 2227–2235. [DOI] [PubMed] [Google Scholar]
- Feinle-Bisset C., Patterson M., Ghatei M., Bloom S., Horowitz M. (2005) Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab 289: E948–E953. [DOI] [PubMed] [Google Scholar]
- Feinle C., O’Donovan D., Doran S., Andrews J., Wishart J., Chapman I., et al. (2003) Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol 284: G798–G807. [DOI] [PubMed] [Google Scholar]
- Feltrin K., Little T., Meyer J., Horowitz M., Smout A., Wishart J., et al. (2004) Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 287: R524–533. [DOI] [PubMed] [Google Scholar]
- Feng J., Petersen C., Coy D., Jiang J., Thomas C., Pollak M., et al. (2010) Calcium-Sensing receptor is a physiologic multimodal chemosensor regulating gastric G-cell growth and gastrin secretion. Proc Natl Acad Sci U S A 107: 17791–17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock G., Cao X., Maziarz M., Drucker D. (2013) Activation of enteroendocrine membrane progesterone receptors promotes incretin secretion and improves glucose tolerance in mice. Diabetes 62: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S., Stafford S., Coope G., Heffron H., Real K., Newman R., et al. (2015) Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake, but does not improve glucose tolerance in mouse models. Diabetes 64: 3763–3771. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Wideman R., Speck M., Asadi A., King D., Webber T., et al. (2009) Incretin release from gut is acutely enhanced by sugar but not by sweeteners in vivo. Am J Physiol Endocrinol Metab 296: E473–E479. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Baggio L., Drucker D., Brubaker P. (2015a) Ghrelin is a novel regulator of GLP-1 secretion. Diabetes 64: 1513–1521. [DOI] [PubMed] [Google Scholar]
- Gagnon J., Sauvé M., Zhao W., Stacey H., Wiber S., Bolz S., et al. (2015b) Chronic exposure to tumor necrosis factor Α impairs secretion of glucagon-like peptide-1. Endocrinology: en20151361. [DOI] [PubMed] [Google Scholar]
- Gameiro A., Reimann F., Habib A., O’Malley D., Williams L., Simpson A., et al. (2005) The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol 569: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl M., Søndergaard H., Stecher C., Bibby B., Møller N., Bøtker H., et al. (2012) Exenatide alters myocardial glucose transport and uptake depending on insulin resistance and increases myocardial blood flow in patients with type 2 diabetes. J Clin Endocrinol Metab 97: E1165–1169. [DOI] [PubMed] [Google Scholar]
- Gil-Lozano M., Mingomataj E., Wu W., Ridout S., Brubaker P. (2014) Circadian Secretion of the intestinal hormone GLP-1 by the rodent L cell. Diabetes 63: 3674–3685. [DOI] [PubMed] [Google Scholar]
- Glassmeier G., Herzig K., Höpfner M., Lemmer K., Jansen A., Scherubl H. (1998) Expression of functional GABAA receptors in cholecystokinin-secreting gut neuroendocrine murine STC-1 cells. J Physiol 510: 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez R., Navarro M., Ferrer B., Trigo J., Bilbao A., Del Arco I., et al. (2002) A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22: 9612–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorboulev V., Schürmann A., Vallon V., Kipp H., Jaschke A., Klessen D., et al. (2012) Na(+)-D-SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 61: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield J., Farooqi I., Keogh J., Henning E., Habib A., Blackwood A., et al. (2009) Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr 89: 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble F., Williams L., Simpson A., Reimann F. (2003) A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes 52: 1147–1154. [DOI] [PubMed] [Google Scholar]
- Grosse J., Heffron H., Burling K., Akhter Hossain M., Habib A., Rogers G., et al. (2014) Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci U S A 111: 11133–11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnarsson P., Winzell M., Deacon C., Larsen M., Jelic K., Carr R., et al. (2006) Glucose-Induced incretin hormone release and inactivation are differently modulated by oral fat and protein in mice. Endocrinology 147: 3173–3180. [DOI] [PubMed] [Google Scholar]
- Habib A., Richards P., Cairns L., Rogers G., Bannon C., Parker H., et al. (2012) Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153: 3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen K., Rosenkilde M., Knop F., Wellner N., Diep T., Rehfeld J., et al. (2011) 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 96: E1409–E1417. [DOI] [PubMed] [Google Scholar]
- Harach T., Pols T., Nomura M., Maida A., Watanabe M., Auwerx J., et al. (2012) TGR5 potentiates GLP-1 secretion in response to anionic exchange resins. Sci Rep 2: 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata T., Mera Y., Ishii Y., Tadaki H., Tomimoto D., Kuroki Y., et al. (2011) JTT-130, a novel intestine-specific inhibitor of microsomal triglyceride transfer protein, suppresses food intake and gastric emptying with the elevation of plasma peptide YY and glucagon-like peptide-1 in a dietary fat-dependent manner. J Pharmacol Exp Ther 336: 850–856. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Yamada R., Das S., Sato T., Takahashi A., Hiratsuka M., et al. (2014) Glucagon-Like peptide-1 production in the GLUTag cell line is impaired by free fatty acids via endoplasmic reticulum stress. Metabolism 63: 800–811. [DOI] [PubMed] [Google Scholar]
- Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., et al. (2005) Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G., Shargill N., Spiegelman B. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91. [DOI] [PubMed] [Google Scholar]
- Iakoubov R., Ahmed A., Lauffer L., Bazinet R., Brubaker P. (2011) Essential role for protein kinase Cζ in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology 152: 1244–1252. [DOI] [PubMed] [Google Scholar]
- Iakoubov R., Izzo A., Yeung A., Whiteside C., Brubaker P. (2007) Protein kinase Czeta is required for oleic acid-induced secretion of glucagon-like peptide-1 by intestinal endocrine L cells. Endocrinology 148: 1089–1098. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Harada N., Sasaki K., Yamane S., Iida K., Suzuki K., et al. (2015) Free fatty acid receptor GPR120 is highly expressed in enteroendocrine K cells of the upper small intestine and has a critical role in GIP secretion after fat ingestion. Endocrinology 156: 837–846. [DOI] [PubMed] [Google Scholar]
- Izzo A., Sharkey K. (2010) Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther 126: 21–38. [DOI] [PubMed] [Google Scholar]
- Jang H., Kokrashvili Z., Theodorakis M., Carlson O., Kim B., Zhou J., et al. (2007) Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A 104: 15069–15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Rose A., Sijmonsma T., Bröer A., Pfenninger A., Herzig S., et al. (2015) Mice lacking neutral amino acid transporter B(0)AT1 (SlC6A19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol Metab 4: 406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A., Casamitjana R., Flores L., Viaplana J., Corcelles R., Lacy A., et al. (2012) Long-term effects of sleeve gastrectomy and Roux-en-Y gastric bypass surgery on type 2 diabetes mellitus in morbidly obese subjects. Ann Surg 256: 1023–1029. [DOI] [PubMed] [Google Scholar]
- Katsuma S., Hirasawa A., Tsujimoto G. (2005) Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun 329: 386–390. [DOI] [PubMed] [Google Scholar]
- Katz L., Gambale J., Rothenberg P., Vanapalli S., Vaccaro N., Xi L., et al. (2012) Effects of JNJ-38431055, a novel GPR119 receptor agonist, in randomized, double-blind, placebo-controlled studies in subjects with type 2 diabetes. Diabetes Obes Metab 14: 709–716. [DOI] [PubMed] [Google Scholar]
- Kellett G., Helliwell P. (2000) The diffusive component of intestinal glucose absorption is mediated by the glucose-induced recruitment of GLUT2 to the brush-border membrane. Biochem J 350: 155–162. [PMC free article] [PubMed] [Google Scholar]
- Keophiphath M., Rouault C., Divoux A., Clément K., Lacasa D. (2010) CCl5 promotes macrophage recruitment and survival in human adipose tissue. Arterioscler Thromb Vasc Biol 30: 39–45. [DOI] [PubMed] [Google Scholar]
- Kieffer T., Habener J. (1999) The glucagon-like peptides. Endocrine Reviews 20: 876–913. [DOI] [PubMed] [Google Scholar]
- Kokrashvili Z., Mosinger B., Margolskee R. (2009) T1R3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci 1170: 91–94. [DOI] [PubMed] [Google Scholar]
- Korner J., Bessler M., Cirilo L., Conwell I., Daud A., Restuccia N., et al. (2005) Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365. [DOI] [PubMed] [Google Scholar]
- Korner J., Bessler M., Inabnet W., Taveras C., Holst J. (2007) Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M., Bloom S. (1987) Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 2: 1300–1304. [DOI] [PubMed] [Google Scholar]
- Kuhre R., Frost C., Svendsen B., Holst J. (2015) Molecular mechanisms of glucose-stimulated GLP-1 secretion from perfused rat small intestine. Diabetes 64: 370–382. [DOI] [PubMed] [Google Scholar]
- Kuhre R., Gribble F., Hartmann B., Reimann F., Windeløv J., Rehfeld J., et al. (2014) Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol 306: G622–G630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H., Vassileva G., Corona A., Liu L., Baker H., Golovko A., et al. (2009) GPR119 Is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol 201: 219–230. [DOI] [PubMed] [Google Scholar]
- Lauffer L., Iakoubov R., Brubaker P. (2009) GPR119 Is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 58: 1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R., Bäckhed F., Turnbaugh P., Lozupone C., Knight R., Gordon J. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Holmstrom S.R., Kir S., Umetani M., Schmidt D.R., Kliewer S.A., Mangelsdorf D.J. (2011) The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol 25: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim G., Huang G., Flora N., Leroith D., Rhodes C., Brubaker P. (2009) Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology 150: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Frassetto A., Kowalik E., Nawrocki A., Lu M., Kosinski J., et al. (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7: e35240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou A., Sei Y., Zhao X., Feng J., Lu X., Thomas C., et al. (2011) The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am J Physiol Gastrointest Liver Physiol 300: G538–G546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T., Feltrin K., Horowitz M., Smout A., Rades T., Meyer J., et al. (2005) Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am J Physiol Regul Integr Comp Physiol 289: R1090–R1098. [DOI] [PubMed] [Google Scholar]
- Lu W., Yang Q., Yang L., Lee D., D’alessio D., Tso P. (2012) Chylomicron formation and secretion is required for lipid-stimulated release of incretins GLP-1 and GIP. Lipids 47: 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Bellon M., Wishart J., Young R., Blackshaw L., Jones K., et al. (2009) Effect of the artificial sweetener, sucralose, on gastric emptying and incretin hormone release in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296: G735–G739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace O., Schindler M., Patel S. (2012) The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CASR in rat small intestine. J Physiol 590: 2917–2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandøe M., Hansen K., Hartmann B., Rehfeld J., Holst J., Hansen H. (2015) The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Am J Clin Nutr. DOI: 10.3945/ajcn.115.106799. [DOI] [PubMed] [Google Scholar]
- Martins C., Morgan L., Bloom S., Robertson M. (2007) Effects of exercise on gut peptides, energy intake and appetite. J Endocrinol 193: 251–258. [DOI] [PubMed] [Google Scholar]
- Morínigo R., Vidal J., Lacy A., Delgado S., Casamitjana R., Gomis R. (2008) Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg 247: 270–275. [DOI] [PubMed] [Google Scholar]
- Moriya R., Shirakura T., Ito J., Mashiko S., Seo T. (2009) Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab 297: E1358–E1365. [DOI] [PubMed] [Google Scholar]
- Moss C., Glass L., Diakogiannaki E., Pais R., Lenaghan C., Smith D., et al. (2015) Lipid derivatives activate GPR119 and trigger GLP-1 secretion in primary murine L-cells. Peptides. DOI: 10.1016/j.peptides.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C., Marsh W., Parker H., Ogunnowo-Bada E., Riches C., Habib A., et al. (2012) Somatostatin receptor 5 and cannabinoid receptor 1 activation inhibit secretion of glucose-dependent insulinotropic polypeptide from intestinal K cells in rodents. Diabetologia 55: 3094–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck M., Heimesaat M., Orskov C., Holst J., Ebert R., Creutzfeldt W. (1993) Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91: 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H., Seino Y., Harada N., Iida A., Suzuki K., Izumoto T., et al. (2014) KATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. J Endocrinol 222: 191–200. [DOI] [PubMed] [Google Scholar]
- Oh-I S., Shimizu H., Satoh T., Okada S., Adachi S., Inoue K., et al. (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712. [DOI] [PubMed] [Google Scholar]
- Okawa M., Fujii K., Ohbuchi K., Okumoto M., Aragane K., Sato H., et al. (2009) Role of MGAT2 and DGAT1 in the release of gut peptides after triglyceride ingestion. Biochem Biophys Res Commun 390: 377–381. [DOI] [PubMed] [Google Scholar]
- Overton H., Babbs A., Doel S., Fyfe M., Gardner L., Griffin G., et al. (2006) Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab 3: 167–175. [DOI] [PubMed] [Google Scholar]
- Oya M., Kitaguchi T., Pais R., Reimann F., Gribble F., Tsuboi T. (2013) The G Protein-coupled receptor family C group 6 subtype a (GPRC6a) receptor is involved in amino acid-induced glucagon-like peptide-1 secretion from GLUTag cells. J Biol Chem 288: 4513–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais R., Gribble F., Reimann F. (2015) Signalling pathways involved in the detection of peptones by murine small intestinal enteroendocrine L-cells. Peptides. DOI: 10.1016/j.peptides.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais R., Zietek T., Hauner H., Daniel H., Skurk T. (2014) Rantes (CCL5) reduces glucose-dependent secretion of glucagon-like peptides 1 and 2 and impairs glucose-induced insulin secretion in mice. Am J Physiol Gastrointest Liver Physiol 307: G330–G337. [DOI] [PubMed] [Google Scholar]
- Parker H., Adriaenssens A., Rogers G., Richards P., Koepsell H., Reimann F., et al. (2012) Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia 55: 2445–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H., Habib A., Rogers G., Gribble F., Reimann F. (2009) Nutrient-dependent secretion of glucose-dependent insulinotropic polypeptide from primary murine K cells. Diabetologia 52: 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H., Wallis K., Le Roux C., Wong K., Reimann F., Gribble F. (2012) Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol 165: 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patti M., Houten S., Bianco A., Bernier R., Larsen P., Holst J., et al. (2009) Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity 17: 1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perley M., Kipnis D. (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic subjects. J Clin Invest 46: 1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Gingerich R., Nayak S., Wada K., Wada E., Ahrén B. (2000) Reduced GLP-1 and insulin responses and glucose intolerance after gastric glucose in GRP receptor-deleted mice. Am J Physiol Endocrinol Metab 279: E956–E962. [DOI] [PubMed] [Google Scholar]
- Petersen N., Reimann F., Bartfeld S., Farin H., Ringnalda F., Vries R., et al. (2014) Generation of L cells in mouse and human small intestine organoids. Diabetes 63: 410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N., Reimann F., Van Es J., Van Den Berg B., Kroone C., Pais R., et al. (2015) Targeting development of incretin-producing cells increases insulin secretion. J Clin Invest 125: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisancie P., Bernard C., Chayvialle J., Cuber J. (1994) Regulation of Glucagon-like peptide-1-(7–36) amide secretion by intestinal neurotransmitters and hormones in the isolated vascularly perfused rat colon. Endocrinology 135: 2398–2403. [DOI] [PubMed] [Google Scholar]
- Poreba M., Dong C., Li S., Stahl A., Miner J., Brubaker P. (2012) Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am J Physiol Endocrinol Metab 303: E899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D., Dacosta C., Gay J., Ding Z., Smith M., Greer J., et al. (2013a) Improved glycemic control in mice lacking SGLT1 and SGLT2. Am J Physiol Endocrinol Metab 304: E117–E130. [DOI] [PubMed] [Google Scholar]
- Powell D., Smith M., Greer J., Harris A., Zhao S., Dacosta C., et al. (2013b) Lx4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther 345: 250–259. [DOI] [PubMed] [Google Scholar]
- Prawitt J., Caron S., Staels B. (2014) Glucose-Lowering effects of intestinal bile acid sequestration through enhancement of splanchnic glucose utilization. Trends Endocrinol Metab 25: 235–244. [DOI] [PubMed] [Google Scholar]
- Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., et al. (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490: 55–60. [DOI] [PubMed] [Google Scholar]
- Ramesh N., Mortazavi S., Unniappan S. (2015) Nesfatin-1 stimulates glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide secretion from STC-1 cells in vitro. Biochem Biophys Res Commun 462: 124–130. [DOI] [PubMed] [Google Scholar]
- Reimann F., Habib A., Tolhurst G., Parker H., Rogers G., Gribble F. (2008) Glucose sensing in L cells: a primary cell study. Cell Metabolism 8: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F., Williams L., Da Silva Xavier G., Rutter G., Gribble F. (2004) Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia 47: 1592–1601. [DOI] [PubMed] [Google Scholar]
- Reimer R. (2006) Meat hydrolysate and essential amino acid-induced glucagon-like peptide-1 secretion, in the human NCI-H716 enteroendocrine cell line, is regulated by extracellular signal-regulated kinase1/2 and P38 mitogen-activated protein kinases. J Endocrinol 191: 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards P., Pais R., Habib A., Brighton C., Yeo G., Reimann F., et al. (2015) High Fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides. DOI: 10.1016/j.peptides.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel U., Fromme A., Ottleben M., Leonhardt U., Ramadori G. (1997) Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol 34: 18–21. [DOI] [PubMed] [Google Scholar]
- Roberge J., Brubaker P. (1993) Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology 133: 233–240. [DOI] [PubMed] [Google Scholar]
- Roberge J., Gronau K., Brubaker P. (1996) Gastrin-releasing peptide is a novel mediator of proximal nutrient-induced proglucagon-derived peptide secretion from the distal gut. Endocrinology 137: 2383–2388. [DOI] [PubMed] [Google Scholar]
- Röder P., Geillinger K., Zietek T., Thorens B., Koepsell H., Daniel H. (2014) The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One 9: e89977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez De Fonseca F., Navarro M., Gómez R., Escuredo L., Nava F., Fu J., et al. (2001) An anorexic lipid mediator regulated by feeding. Nature 414: 209–212. [DOI] [PubMed] [Google Scholar]
- Rozengurt N., Wu S., Chen M., Huang C., Sternini C., Rozengurt E. (2006) Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol 291: G792–G802. [DOI] [PubMed] [Google Scholar]
- Samocha-Bonet D., Wong O., Synnott E., Piyaratna N., Douglas A., Gribble F., et al. (2011) Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr 141: 1233–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn J., Klover P., Nowak I., Mooney R. (2002) Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes 51: 3391–3399. [DOI] [PubMed] [Google Scholar]
- Seino Y., Ogata H., Maekawa R., Izumoto T., Iida A., Harada N., Miki T., Seino S., Inagaki N., Tsunekawa S., Oiso Y., Hamada Y. (2015) Fructose induces glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 and insulin secretion: Role of adenosine triphosphate-sensitive K(+) channels. J Diabetes Investig 6: 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue K., Yamane S., Harada N., Hamasaki A., Suzuki K., Joo E., et al. (2015) Fatty acid-binding protein 5 regulates diet-induced obesity via GIP secretion from enteroendocrine K cells in response to fat ingestion. Am J Physiol Endocrinol Metab 308: E583–E591. [DOI] [PubMed] [Google Scholar]
- Shima K., Suda T., Nishimoto K., Yoshimoto S. (1990) Relationship between molecular structures of sugars and their ability to stimulate the release of glucagon-like peptide-1 from canine ileal loops. Acta Endocrinol 123: 464–470. [DOI] [PubMed] [Google Scholar]
- Shimotoyodome A., Fukuoka D., Suzuki J., Fujii Y., Mizuno T., Meguro S., et al. (2009) Coingestion of acylglycerols differentially affects glucose-induced insulin secretion via glucose-dependent insulinotropic polypeptide in C57BL/6J mice. Endocrinology 150: 2118–2126. [DOI] [PubMed] [Google Scholar]
- Simpson A.K., Ward P.S., Wong K.Y., Collord G.J., Habib A.M., Reimann F., Gribble F.M. (2007) Cyclic AMP triggers glucagon-like peptide-1 secretion from the GLUTag enteroendocrine cell line. Diabetologia 50: 2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soga T., Ohishi T., Matsui T., Saito T., Matsumoto M., Takasaki J., et al. (2005) Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem Biophys Res Commun 326: 744–751. [DOI] [PubMed] [Google Scholar]
- Steinert R., Gerspach A., Gutmann H., Asarian L., Drewe J., Beglinger C. (2011) The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (Pyy). Clin Nutr 30: 524–532. [DOI] [PubMed] [Google Scholar]
- Stephens J., Bodvarsdottir T., Wareham K., Prior S., Bracken R., Lowe G., et al. (2011) Effects of short-term therapy with glibenclamide and repaglinide on incretin hormones and oxidative damage associated with postprandial hyperglycaemia in people with type 2 diabetes mellitus. Diabetes Res Clin Pract 94: 199–206. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Manaka H., Kato T., Yamatani K., Tominaga M., Sasaki H. (1994) Stimulation of truncated glucagon-like peptide-1 release from the isolated perfused canine ileum by glucose absorption. Digestion 55: 24–28. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Harada N., Yamane S., Nakamura Y., Sasaki K., Nasteska D., et al. (2013) Transcriptional regulatory factor X6 (RFX6) increases gastric inhibitory polypeptide (GIP) expression in enteroendocrine K-cells and is involved in GIP hypersecretion in high fat diet-induced obesity. J Biol Chem 288: 1929–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svendsen B., Pedersen J., Albrechtsen N., Hartmann B., Toräng S., Rehfeld J., et al. (2015) An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 156: 847–857. [DOI] [PubMed] [Google Scholar]
- Sykaras A., Demenis C., Cheng L., Pisitkun T., McLaughlin J., Fenton R., et al. (2014) Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155: 3339–3351. [DOI] [PubMed] [Google Scholar]
- Tang C., Ahmed K., Gille A., Lu S., Gröne H., Tunaru S., et al. (2015) Loss of FFA2 and FFA3 Increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 21: 173–177. [DOI] [PubMed] [Google Scholar]
- Theodorakis M., Carlson O., Michopoulos S., Doyle M., Juhaszova M., Petraki K., et al. (2006) Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am J Physiol Endocrinol Metab 290: E550–E559. [DOI] [PubMed] [Google Scholar]
- Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., et al. (2009) TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G., Heffron H., Lam Y., Parker H., Habib A., Diakogiannaki E., et al. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the g-protein-coupled receptor FFAR2. Diabetes 61: 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolhurst G., Zheng Y., Parker H., Habib A., Reimann F., Gribble F. (2011) Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and camp. Endocrinology 152: 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabelsi M., Daoudi M., Prawitt J., Ducastel S., Touche V., Sayin S., et al. (2015) Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat Commun 6: 7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremaroli V., Bäckhed F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489: 242–249. [DOI] [PubMed] [Google Scholar]
- Ullmer C., Alvarez Sanchez R., Sprecher U., Raab S., Mattei P., Dehmlow H., et al. (2013) Systemic bile acid sensing by G protein-coupled bile acid receptor 1 (GPBAR1) promotes PYY and GLP-1 release. Br J Pharmacol 169: 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Yang Q., Huesman S., Xu M., Li X., Lou D., et al. (2015) The role of apolipoprotein a-IV in regulating glucagon-like peptide-1 secretion. Am J Physiol Gastrointest Liver Physiol 309: G680–G687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chandra R., Samsa L., Gooch B., Fee B., Cook J., et al. (2011) Amino Acids stimulate cholecystokinin release through the Ca2+-sensing receptor. Am J Physiol Gastrointest Liver Physiol 300: G528–G537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch I., Saunders K., Read N. (1985) Effect of ileal and intravenous infusions of fat emulsions on feeding and satiety in human volunteers. Gastroenterology 89: 1293–1297. [DOI] [PubMed] [Google Scholar]
- Wichmann A., Allahyar A., Greiner T., Plovier H., Lundén G., Larsson T., et al. (2013) Microbial modulation of energy availability in the colon regulates intestinal transit. Cell Host Microbe 14: 582–590. [DOI] [PubMed] [Google Scholar]
- Wu H., Ghosh S., Perrard X., Feng L., Garcia G., Perrard J., et al. (2007) T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 115: 1029–1038. [DOI] [PubMed] [Google Scholar]
- Young R., Chia B., Isaacs N., Ma J., Khoo J., Wu T., et al. (2013) Disordered Control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes 62: 3532–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawalich W., Cote S., Diaz V. (1986) Influence of cholecystokinin on insulin output from isolated perifused pancreatic islets. Endocrinology 119: 616–621. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432. [DOI] [PubMed] [Google Scholar]
- Zietek T., Daniel H. (2015) Intestinal nutrient sensing and blood glucose control. Curr Opin Clin Nutr Metab Care 18: 381–388. [DOI] [PubMed] [Google Scholar]