Isoprene emission in plants is correlated with structural organization and function of plastidic membranes.
Abstract
Isoprene is a small lipophilic molecule with important functions in plant protection against abiotic stresses. Here, we studied the lipid composition of thylakoid membranes and chloroplast ultrastructure in isoprene-emitting (IE) and nonisoprene-emitting (NE) poplar (Populus × canescens). We demonstrated that the total amount of monogalactosyldiacylglycerols, digalactosyldiacylglycerols, phospholipids, and fatty acids is reduced in chloroplasts when isoprene biosynthesis is blocked. A significantly lower amount of unsaturated fatty acids, particularly linolenic acid in NE chloroplasts, was associated with the reduced fluidity of thylakoid membranes, which in turn negatively affects photosystem II photochemical efficiency. The low photosystem II photochemical efficiency in NE plants was negatively correlated with nonphotochemical quenching and the energy-dependent component of nonphotochemical quenching. Transmission electron microscopy revealed alterations in the chloroplast ultrastructure in NE compared with IE plants. NE chloroplasts were more rounded and contained fewer grana stacks and longer stroma thylakoids, more plastoglobules, and larger associative zones between chloroplasts and mitochondria. These results strongly support the idea that in IE species, the function of this molecule is closely associated with the structural organization and functioning of plastidic membranes.
Isoprene is globally the most abundant biogenic hydrocarbon constitutively emitted from many plant species (Guenther et al., 2012). It has been proposed that leaf isoprene emission is an important adaptation for plants, conferring tolerance to different environmental constraints (Vickers et al., 2009; Loreto and Schnitzler, 2010; Loreto and Fineschi, 2014). However, biogenic isoprene emission represents a nontrivial carbon loss in plants, particularly under stress conditions (Fang et al., 1996; Brilli et al., 2007; Teuber et al., 2008; Ghirardo et al., 2014), and the reason(s) why plants emit isoprene are still ambiguous, and the true role of isoprene emission remains elusive. Different approaches and techniques have been used to determine whether and how the cost of this expensive carbon emission is matched by the accomplishment of the physiological function in planta. It has been shown that isoprene might quench and/or regulate reactive oxygen and nitrogen species formation (Behnke et al., 2010a; Velikova et al., 2012), thereby indirectly providing a general antioxidant action (for review, see Vickers et al., 2009; Loreto and Schnitzler, 2010) and stabilizing thylakoid membrane structures due to the lipophilic properties of this molecule (Sharkey et al., 2001; Velikova et al., 2011).
Protein and pigment-protein complexes are assembled and embedded in a lipid matrix, which has a unique lipid composition. The thylakoid lipid bilayer of chloroplasts is characterized by a high proportion of galactolipids with one (monogalactosyldiacylglycerol [MGDG]) or two (digalactosyldiacylglycerol [DGDG]) Gal molecules (Joyard et al., 2010). MGDGs are the primary constituents (approximately 50%) of thylakoid membrane glycerolipids, followed by DGDGs (approximately 30%), sulfoquinovosyldiacylglycerol (approximately 5%–12%), and phosphatidylglycerol (approximately 5%–12%; Kirchhoff et al., 2002). Galactolipids contain a large proportion of polyunsaturated fatty acids, and consequently, the thylakoid membrane is a relatively fluid system (Gounaris and Barber, 1983) compared with other biological membranes. The fluidity of the thylakoid membrane is essential for photosynthetic processes.
The thylakoid membranes are highly organized internal membrane chloroplast systems that conduct the light reactions of photosynthesis. These membranes comprise pigments and proteins organized in complexes. Thylakoid membranes are arranged into stacked and unstacked regions called grana and stroma thylakoids, respectively, differentially enriched in PSI and PSII complexes (Mustárdy et al., 2008). The spatial separation of the PSI and PSII complexes in the stacked and unstacked membrane regions and the macromolecular organization of PSII in stacked grana thylakoids are self-organizing processes and important features to maintain the functional integrity of the photosynthetic apparatus (Kirchhoff et al., 2007).
It is not known how changes in the lipid matrix affect lipid-protein interactions and vice versa, and how membrane macroorganization ensures the efficient diffusion of protein complexes associated with plant adaptation to the changing environment remains elusive. The isoprene impact on thylakoid intactness and functionality has been assessed using different biophysical techniques (Velikova et al., 2011). Thermoluminescence data demonstrated that the position of the main peak (Qb peak; Sane, 2004) was upshifted approximately 10° in isoprene-emitting (IE) plants, suggesting modifications in the lipid environment due to the presence of isoprene in heterologous Arabidopsis (Arabidopsis thaliana) plants expressing the isoprene synthase gene from poplar (Populus spp.). It was also shown that isoprene improves the stability of PSII light-harvesting complex II (LHCII) through the modification of pigment-protein complex organization in thylakoid membranes (Velikova et al., 2011). Moreover, we recently showed that knocking down isoprene emission in poplar remodels the chloroplast proteome (Velikova et al., 2014). The lack of isoprene resulted in the down-regulation of proteins associated with the light reactions of photosynthesis, redox regulation, and oxidative stress defenses and several proteins responsible for lipid metabolism (Velikova et al., 2014).
In this study, we focused on the lipid composition of thylakoid membranes in IE and nonisoprene-emitting (NE) poplar (Populus × canescens) leaves. Specifically, we determined whether the translational suppression of isoprene synthase in NE leaves influences the lipid matrix of thylakoids and how this phenomenon affects membrane structure and function. Here, we provide evidence that the suppression of isoprene biosynthesis in poplar (1) reduced total galactolipids, phospholipids (PLs), and linolenic fatty acid (18:3), (2) altered the chloroplast ultrastructure, and (3) stimulated photoprotective mechanisms.
RESULTS
Lipid, Fatty Acid, and Malondialdehyde Analyses
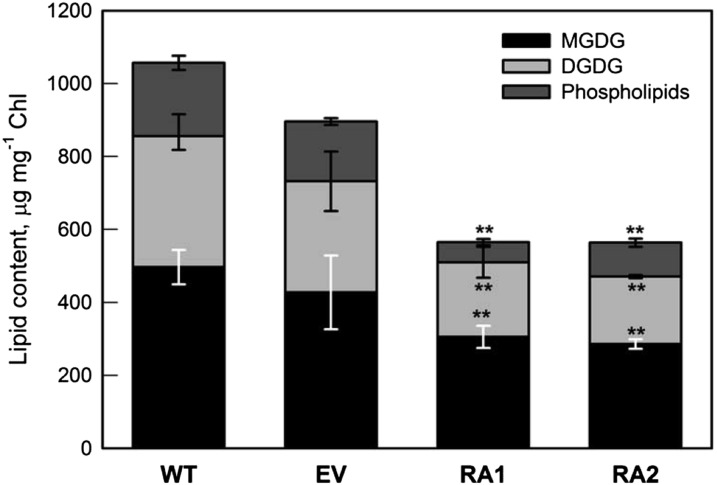
Chloroplast membranes isolated from NE poplar had significantly lower (−53%; P < 0.01) lipid contents than the membranes of IE plants (Fig. 1). In both NE and IE plants, the major molecular species of MGDG were 18:2-18:3 and 18:3 dimers. In DGDG, the major molecular species were n16:0-18:3 and 18:3 dimers. Linolenic acid (18:3) was the major fatty acid of both IE and NE chloroplast membranes, but the content of this fatty acid was consistently much lower in NE than in IE plants (Table I; Supplemental Fig. S1). The fatty acid analysis also revealed significantly lower palmitic (n16:0), linoleic (18:2), and stearic (n18:0) acid contents in NE plants compared with IE plants. In the fraction of the PLs, the phosphatidic acid (18:1) levels were lower in NE plants.
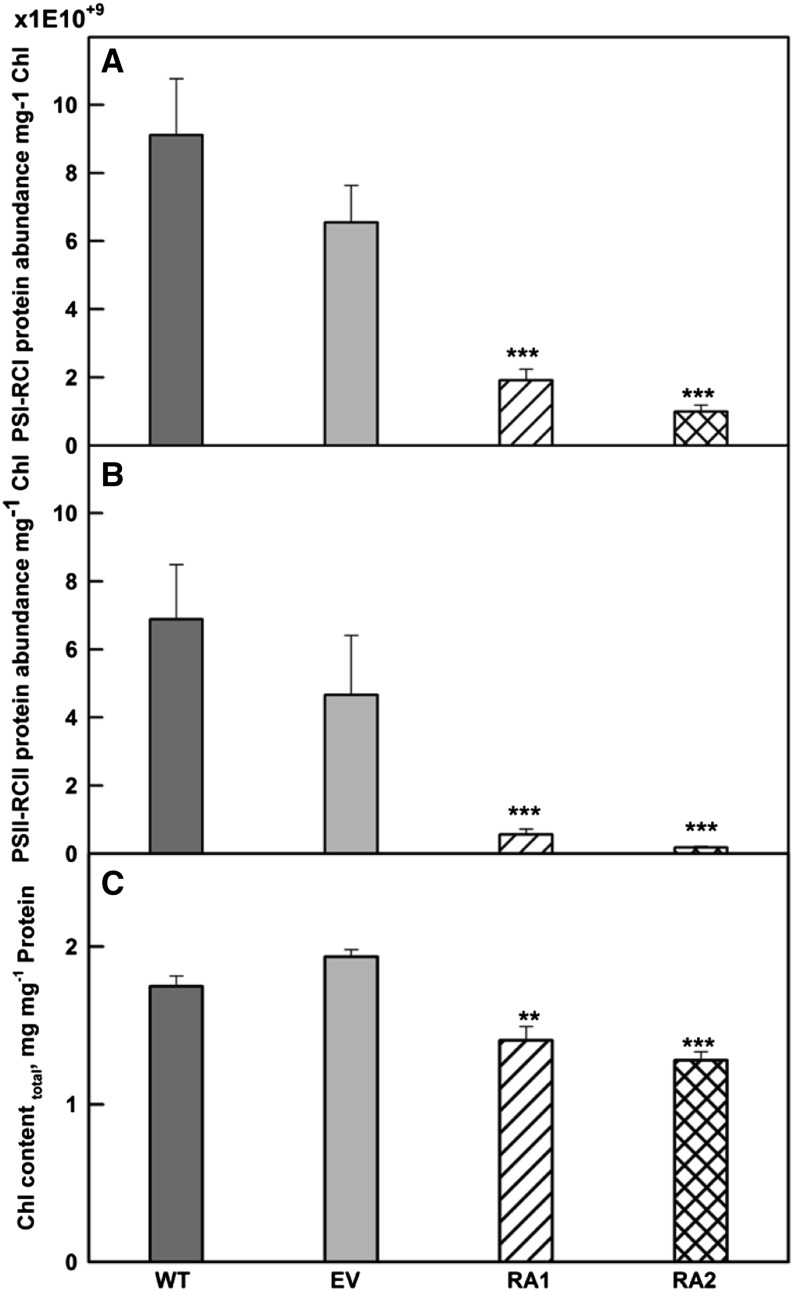
Figure 1.
Lipid contents in isolated chloroplasts of IE (wild type [WT] and empty vector [EV]) and NE (RNA interference transgenic lines, RA1 and RA2) poplar. Error bars display the se (n = 4). Asterisks indicate significant differences from the wild type: **, P < 0.01.
Table I. Fatty acid composition (μg mg−1 chlorophyll) of the main lipid classes in C16 to C23 saturated (:0) and unsaturated (:1, :2, and :3) compounds in chloroplasts of IE (wild type [WT] and empty vector [EV]) and NE (RA1 and RA2) poplar plant lines.
Fatty acids are designated as the total number of carbon atoms followed by the number of double bonds and their location (omega) after the colon: n16:0, palmitic acid; 16:1, palmitoleic acid; n18:0, stearic acid; 18:1, oleic acid; 18:2, linoleic acid; 18:3, linolenic acid; and n23:0, tricosanoic acid. Saturated straight-chain fatty acids are indicated with an n. Means ± se are shown; n = 4. Asterisks and boldface indicate significant differences from the wild type: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
| Line | Lipid Classes | n16:0 | 16:1 | n18:0 | 18:1 | 18:2 | 18:3 | 20:3 | n23:0 |
|---|---|---|---|---|---|---|---|---|---|
| WT | MGDG | 21.1 ± 3.9 | 2.8 ± 0.6 | 61.8 ± 13.6 | 411.1 ± 29.0 | ||||
| DGDG | 77.5 ± 22.1 | 13.2 ± 5.4 | 10.7 ± 3.3 | 265.1 ± 17.6 | 1.4 ± 0.6 | ||||
| PLs | 17.7 ± 3.6 | 3.5 ± 1.2 | 4.1 ± 0.5 | 12.9 ± 4.7 | 7.6 ± 1.3 | 151.9 ± 16.5 | 4.0 ± 1.0 | ||
| EV | MGDG | 19.5 ± 6.0 | 2.6 ± 0.9 | 54.9 ± 19.5 | 350.3 ± 75.2 | ||||
| DGDG | 56.0 ± 15.9 | 8.7 ± 2.7 | 8.8 ± 3.2 | 230.3 ± 59.4 | 1.4 ± 0.6 | ||||
| PLs | 17.8 ± 2.2 | 2.4 ± 0.4 | 4.3 ± 0.2 | 13.8 ± 6.4 | 9.1 ± 0.5 | 120.9 ± 3.0 | 4.1 ± 1.9 | ||
| RA1 | MGDG | 12.7 ± 1.7* | 1.7 ± 0.2 | 38.0 ± 8.9* | 253.2 ± 20.0* | ||||
| DGDG | 37.8 ± 9.0** | 5.7 ± 1.5* | 5.7 ± 1.6* | 154.2 ± 30.1*** | 0.5 ± 0.2* | ||||
| PLs | 9.1 ± 2.7 | 1.5 ± 0.5 | 3.3 ± 0.5 | 3.7 ± 0.7 | 5.7 ± 0.8 | 66.1 ± 9.0* | 0.7 ± 0.1* | ||
| RA2 | MGDG | 11.9 ± 1.1** | 1.9 ± 0.4 | 35.9 ± 4.9* | 236.0 ± 8.8* | ||||
| DGDG | 35.0 ± 1.8** | 5.4 ± 0.4* | 4.7 ± 0.7* | 139.5 ± 1.5*** | 0.5 ± 0.1* | ||||
| PLs | 11.5 ± 1.2* | 0.9 ± 0.3 | 2.7 ± 0.3** | 3.6 ± 1.3 | 3.2 ± 0.4 | 72.6 ± 6.6** | 2.5 ± 0.5 |
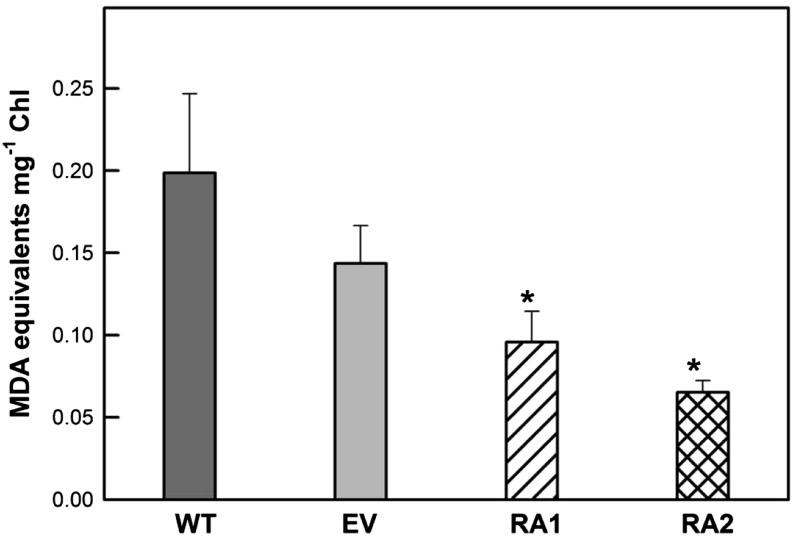
The concentration of malondialdehyde (MDA), the principal product of polyunsaturated fatty acid peroxidation, was lower in chloroplasts isolated from NE plants than in those isolated from IE poplar (Fig. 2), associated with a lower concentration of polyunsaturated fatty acids in NE chloroplasts (Table I; Supplemental Figure S1).
Figure 2.
MDA levels in isolated chloroplasts of IE (wild type [WT] and empty vector [EV]) and NE (RA1 and RA2) poplar. Error bars display the se (n = 4). Asterisks indicate significant differences from the wild type: *, P < 0.05.
Chloroplast Ultrastructure Observations and Protein Abundance in Photosynthetic Membranes
To determine whether the different lipid concentrations and changes in lipid composition affect the chloroplast structure, thin leaf segments obtained from the middle region of IE and NE leaves were subjected to transmission electron microscopy (TEM) analyses. Representative micrographs of chloroplasts from IE and NE specimens are shown in Figures 3 and 4.
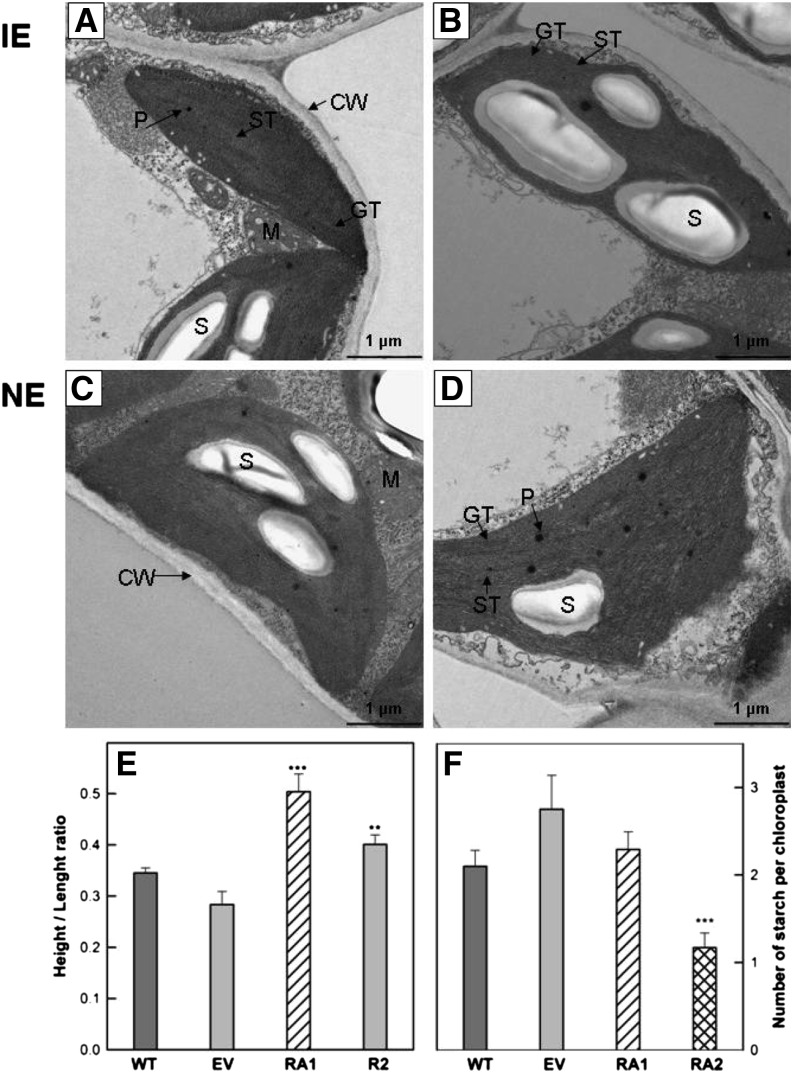
Figure 3.
A to D, Transmission electron micrographs of representative chloroplast cross sections taken from the intact leaves of IE (wild type [WT] and empty vector [EV]; A and B) and NE (RA1 and RA2; C and D) poplar. E and F, Height-length ratio (E) and average number of starch grains (F) in IE and NE chloroplasts. CW, Cell wall; GT, granal thylakoids; M, mitochondrion; P, plastoglobuli; S, stroma; SI, starch gain. Bars = 1 μm at 6,300× magnification.
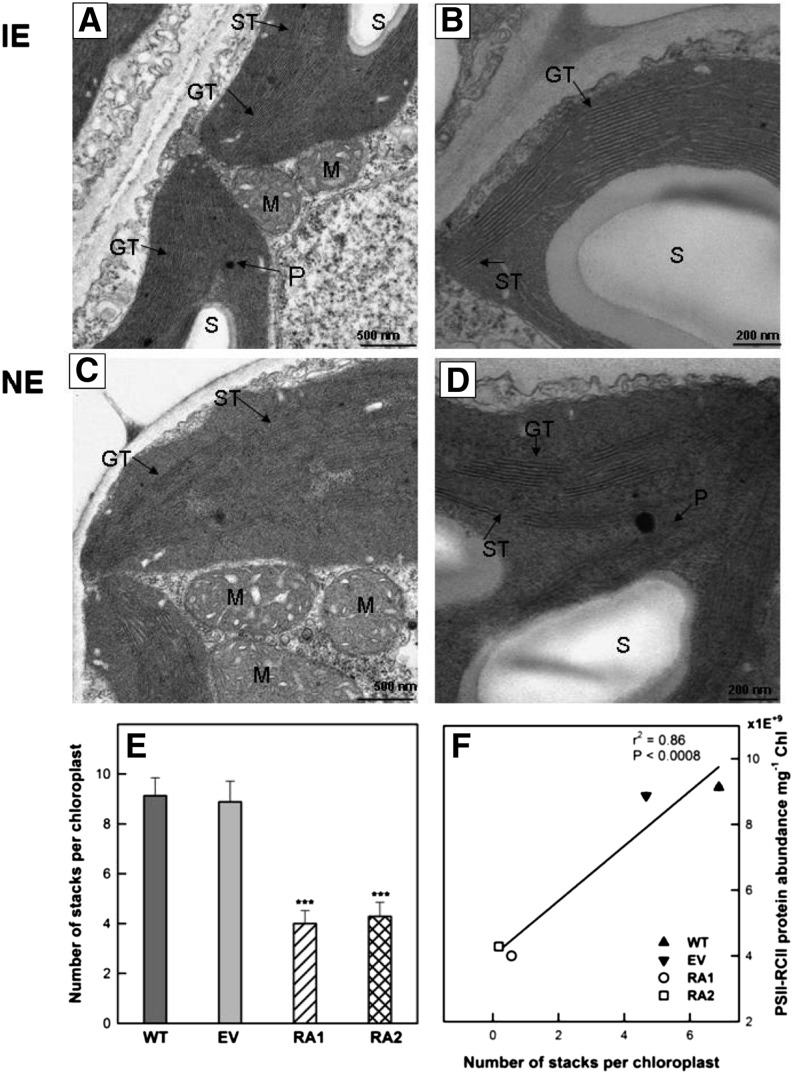
Figure 4.
A to D, Transmission electron micrographs of representative chloroplast cross sections taken from the intact leaves of IE (wild type [WT] and empty vector [EV]; A and B) and NE (RA1 and RA2; C and D) poplar. E and F, Average number of stacks per chloroplast (E) and correlation between PSII-RCII protein abundance (10 peptides; for protein accession nos., see “Materials and Methods”) and number of stacks (F). CW, Cell wall; GT, granal thylakoids; M, mitochondrion; P, plastoglobuli; S, stroma; SI, starch gain. Bars = 500 nm in A and C at 10,000× magnification; bars = 200 nm in B and D at 20,000× magnification.
The typical elliptic shape of mesophyll chloroplasts was more oval in NE than in IE specimens (Fig. 3). The mesophyll cells of IE leaves are characterized by a well-developed inner membrane system, comprising grana of different sizes and relatively long stromal thylakoids. IE chloroplasts contained single, midsize starch granules and less numerous peroxisomes, and these organelles were associated with relatively small-sized mitochondria (Figs. 3, A and B, and 4, A and B).
Conversely, the chloroplasts of NE plants were characterized by a less developed membrane system, with shorter and fewer grana stacks and longer stroma thylakoids (Fig. 4, C–E). NE chloroplasts contained more plastoglobules and smaller starch grains than IE chloroplasts (Fig. 3, C and D). NE chloroplasts were also in close structural contact with mitochondria through relatively large associative regions (Fig. 4C). A relatively large number of NE chloroplasts were undeveloped (data not shown).
To further understand how the structural changes were related to the protein enrichment in photosynthetic membranes, we extracted mass spectrometry (MS) data from our recent proteome study (Velikova et al., 2014). The concentrations of PSI-PSI reaction centers (RCI) and PSII-PSII reaction centers (RCII) were strongly decreased in NE chloroplasts compared with IE chloroplasts (Fig. 5, A and B). Lower protein abundance of PSII-RCII correlated with fewer stacks (Fig. 4F). Chlorophyll concentrations in the NE lines RA1 and RA2 were also significantly reduced (Fig. 5C).
Figure 5.
Protein abundance of PSI-RCI (four peptides; for protein accession nos., see “Materials and Methods”; A), PSII-RCII (10 peptides; for protein accession nos., see “Materials and Methods”; B), and chlorophyll content (C) in IE (wild type [WT] and empty vector [EV]) and NE (RA1 and RA2) poplar plants. Protein abundance represents the sum of MS data extracted from our proteome study (Velikova et al., 2014). Error bars display the se (n = 4). Asterisks indicate significant differences from the wild type: **, P < 0.01; and ***, P < 0.001.
Chlorophyll Fluorescence
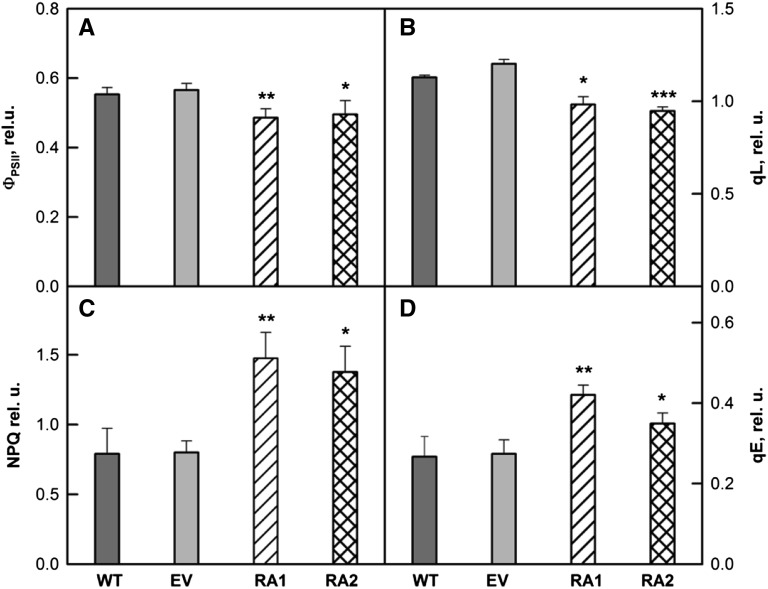
We measured light- and dark-adapted states of chlorophyll fluorescence in IE and NE poplar plants grown under ambient greenhouse conditions (Fig. 6). There was no significant difference in maximal PSII activity between IE and NE plants (data not shown), suggesting that the efficiency of PSII, when all reaction centers were open, was similarly high in both groups of plants. However, NE leaves exhibited significantly lower photosystem II photochemical efficiency (ΦPSII) and PSII redox state (qL) and higher nonphotochemical quenching (NPQ) and energy-dependent quenching (qE; Fig. 6). Importantly, the true efficiency of PSII (ΦPSII) was lower in NE compared with IE, indicating that a smaller fraction of the absorbed light energy was used for photochemistry. Indeed, the accurate indicator of the PSII redox state, qL (Baker, 2008), was significantly lower in NE, suggesting that the fraction of open PSII reaction centers was much lower in these mutants (Figs. 5 and 7; Supplemental Fig. S2).
Figure 6.
ΦPSII (A), redox state of PSII (qL; B), NPQ (C), and qE (D) of IE (wild type [WT] and empty vector [EV]) and NE (RA1 and RA2) poplar plants at growth conditions. Values represent means of five to seven different plants out of three independent experiments (n = 15–21; se is given). Photosynthetic parameters are described in “Materials and Methods.” Asterisks indicate significant differences from the wild type: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
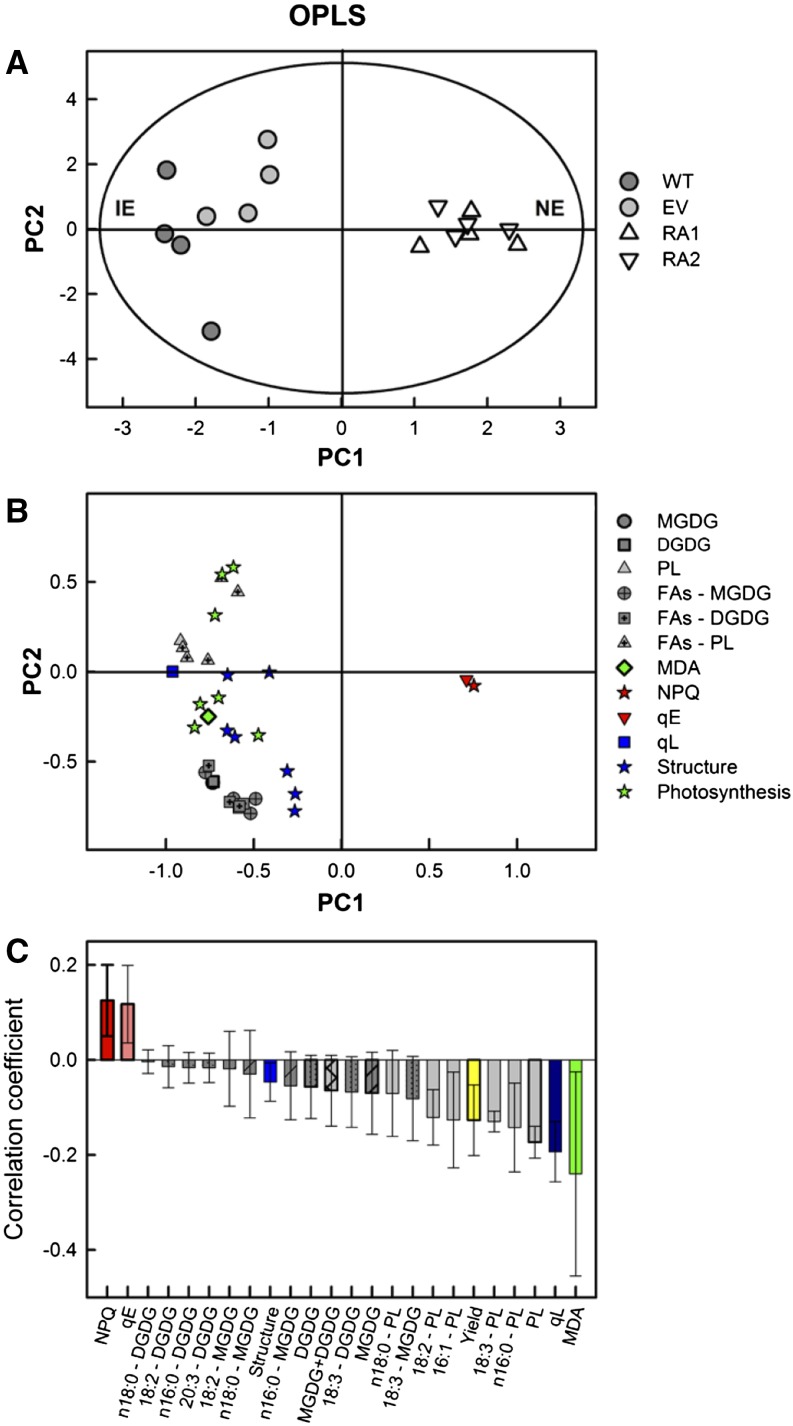
Figure 7.
Score (A), loading (B), and correlation coefficient plots (C) of orthogonal partial least squares (OPLS) of lipid classes, fatty acid composition, and MDA contents in isolated chloroplasts, chlorophyll fluorescence parameters measured in intact leaves (NPQ, ΦPSII, qE, and qL), and chloroplast proteins related to photosynthesis and proteins with structural activity. A, IE plants (wild type [WT] and empty vector [EV]), gray circles; NE plants (RA1 and RA2), white triangles. B, Each parameter is indicated with a different symbol: dark-gray circles, MGDG; dark-gray squares, DGDG; gray triangles, PL; dark-gray circles with a dot, MGDG fatty acids; dark-gray squares with a dot, DGDG fatty acids; gray triangles with a dot, PL fatty acids; green diamonds, MDA; red stars, NPQ; red down triangles, qE; blue squares, qL; blue stars, proteins with structural activity; and green stars, proteins related to photosynthesis. C, Parameter colors match those of the symbols in B. Only discriminant data with variable of importance for the projection (VIP) > 1 (for all except proteins) and VIP > 0.5 (proteins) are presented. Model fitness: Q2(Y) = 84%, R2(X) = 44%, r2 = 93%, and R2(Y) = 100% using one principal component; P = 0.00061, cross-validated ANOVA.
Multivariate Data Analyses
We examined the involvement of lipid content and fatty acid composition in NE and IE chloroplasts, compared with chlorophyll fluorescence measurements, MDA contents, and previously described proteomic differences (Velikova et al., 2014). Principal component analysis (PCA) showed that the isoprene emission traits reflected the largest variance of the measured data, indicated by the separation between NE and IE samples in the first two principal components (Supplemental Fig. S2; explained variance, PC1 = 51%, PC2 = 16%), where Q2 is the fraction of Y variation predicted by the X model and according to cross-validation, and R2(X) and R2(Y) are the fraction of X variation modeled, using the X and Y model, respectively. Additionally, there were no appreciable differences between the two groups, as both RA1 and RA2 lines and WT and EV lines clustered together in the NE and IE groups, respectively.
We performed a discriminant analysis to determine which of the analyzed parameters were significantly affected by the suppression of isoprene biosynthesis and to assess the relative importance of these parameters in distinguishing NE from IE plants (Fig. 7). The OPLS analysis indicated, overall, that differences between NE and IE chloroplasts reflect the lipid composition, fatty acid and MDA contents, chlorophyll fluorescence parameters, and chloroplastic proteins associated with photosynthesis or cell structure. Each singular factor had a different importance (Fig. 7C). Specifically, the most important (high VIP values) variables negatively correlated with NE (positive and high correlation coefficient values) were MDA, qL, PL, saturated and unsaturated fatty acids, MGDG and DGDG, and photosynthetic proteins (Fig. 7, B and C). Importantly, the unsaturated fatty acid 18:3 (linolenic acid) was strongly negatively correlated with NE in all lipids (MGDG, DGDG, and PL). Additionally, linolenic acid was well correlated with the lipid degradation product MDA, detected in both MGDG and DGDG. The PL content was highly correlated with photosynthetic proteins, namely PSI proteins, ATP synthase, cytochrome b6f, and an intrinsic protein of PSII oxygen-evolving complex, PsbP. Conversely, NPQ and qE were strongly and positively correlated with the NE genotype.
The computed OPLS model was reliable, resulting in a significant (P = 0.00061, cross-validated ANOVA) cross-validated predictive ability of Q2(Y) = 84% to distinguish NE from IE samples and a cross-validated goodness of R2(X) = 44%, r2 = 93%, R2(Y) = 100% using only the first principal component.
DISCUSSION
Suppression of Isoprene Biosynthesis Decreases the Chloroplastic Lipid Content and Alters Chloroplast Ultrastructure
One of the proposed biological functions of isoprene is the stabilization of thylakoid membrane structures through modification of the lipid environment and organization of the pigment-protein complexes in thylakoid membranes (Velikova et al., 2011). Indeed, several clear alterations were evident in thylakoid membrane lipids and fatty acid composition due to the translational suppression of isoprene synthase activity in poplar plants. The most important changes in NE chloroplasts were the absolute decrease in the contents of galactolipids (MGDG and DGDG) and PLs through the down-regulation of the unsaturated fatty linolenic acid (18:3). The functional role of MGDGs in the bioactivity of various membrane proteins is well known (Lee, 2003, 2004): a mutant with a defective MGDG SYNTHASE1 is unable to produce photosynthetically active membranes (Kobayashi et al., 2013). MGDGs are essential for the efficient activity of violaxanthin deepoxidase (Yamamoto and Higashi, 1978). The ability of the lipid mixture to segregate into bilayer and nonbilayer phases might regulate the protein content in chloroplast membranes (Garab et al., 2000). Indeed, we could demonstrate that the proteins related to photosynthesis were strongly down-regulated in NE compared with IE plants (Figs. 7 and 8; Velikova et al., 2014).
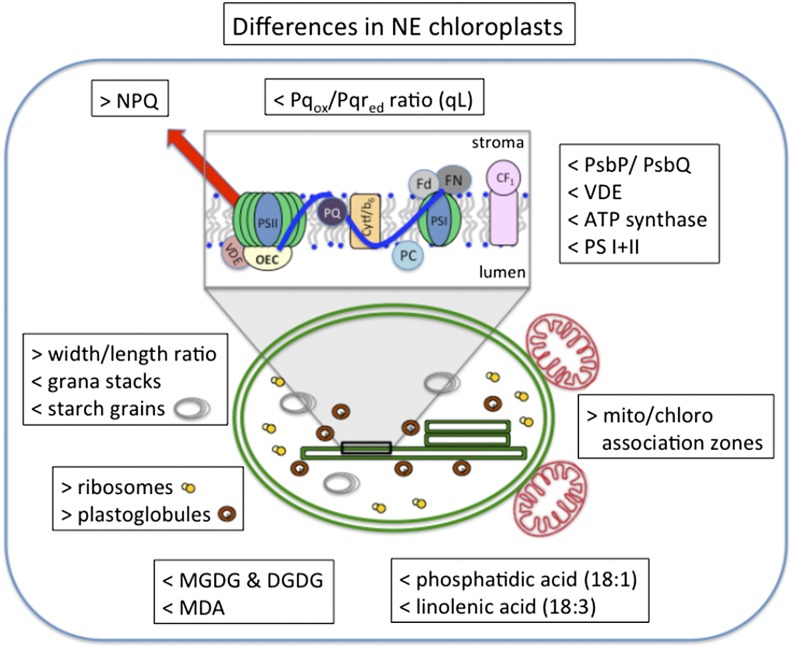
Figure 8.
Schematic overview of the changes in chloroplast ultrastructure, lipid composition, protein abundance, and PSII photochemical efficiency triggered by the suppression of isoprene biosynthesis and emission in poplar plants. VDE, Violaxanthin deepoxidase.
DGDGs are the predominant bilayer lipid species in thylakoid membranes of higher plants (Joyard et al., 2010). They exert structural functions and improve the thermal stability of membranes, particularly at high temperatures (Krumova et al., 2010). DGDGs bind to PSII (Loll et al., 2007) through the formation of hydrogen bonds with Tyr in PSII (Gabashvili et al., 1998), and DGDGs are also important for binding of extrinsic proteins required for the stabilization of the oxygen-evolving complex (Sakurai et al., 2007). Our data clearly indicate that the suppression of isoprene biosynthesis significantly diminished the level of DGDGs in poplar chloroplasts, which was accompanied by a reduction of the RCI and RCII concentrations (Fig. 5, A and B), PsbP and PsbQ protein subunits of PSII, and light-harvesting complex I and LHCII (Velikova et al., 2014). When the thylakoid membrane protein complexes were resolved by blue native-PAGE, the protein patterns of the two groups of poplar lines looked quite similar in content and intensity of the individual bands (supplemental figure S3 in Velikova et al., 2014). However, semiquantitative analysis of the individual protein bands showed that the levels of PSI, the PSII dimer, ATP synthase, the PSII monomer, and the cytochrome b6f complex were slightly reduced in NE compared with IE chloroplasts (supplemental figure S3 in Velikova et al., 2014).
Decreased lipid and protein levels were associated with changes in the ultrastructure of the chloroplasts from NE plants, suggesting a role for isoprene biosynthesis in the structural organization of plastidic membranes. These results are consistent with previous studies that indicated a role for MGDGs and DGDGs in the structure of thylakoid membranes (Dörmann et al., 1995; Jarvis et al., 2000). In this study, we observed a significant reduction of grana stacks per chloroplast in NE compared with IE poplar lines (Fig. 4, E and F), which was related to an important decrease in PSI and PSII proteins (Fig. 5, A and B). Alterations in protein stoichiometry could exert a direct influence on the thylakoid membrane ultrastructure (Pribil et al., 2014), in particular, the assembly of PSII and LHCII into supercomplexes and megacomplexes (Kouřil et al., 2012). It was demonstrated (Labate et al., 2004) that the constitutive expression of the pea (Pisum sativum) Lhcb1 gene in transgenic tobacco (Nicotiana tabacum) plants leads to increased grana stacking, indicating that increased concentrations of LHCII result in more stacking.
Typically, chloroplast membranes have a unique lipid composition characterized by a high proportion of galactolipids containing a large portion of triunsaturated fatty acids (C16 or C18; Joyard et al., 2010). The high content of triunsaturated fatty acids guarantees the high fluidity of the thylakoid membranes and the precise allocation of the photosynthetic machinery to efficiently acquire light energy (Gounaris and Barber, 1983). The level of membrane viscosity is an important factor for photosynthetic performance (e.g. providing optimal conditions for the diffusion of hydrophobic molecules, such as plastoquinol; Kirchhoff et al., 2000, 2002) or membrane intrinsic protein complexes (e.g. during state transitions; Allen and Forsberg, 2001; Tikkanen et al., 2008). The low linolenic acid (18:3) content in all lipid fractions from NE chloroplasts indicates that, in the absence of isoprene, the thylakoid membrane fluidity is reduced, which in turn negatively affects the efficiency of PSII photochemistry (Fig. 6). A low level of unsaturation in thylakoid membranes makes PSII extremely susceptible to photoinhibition and causes a significant reduction in the content of the D1 protein (the reaction center protein) at high irradiance (Kanervo et al., 1995), suggesting that membrane fluidity is a critical factor for PSII D1 protein turnover. Moreover, we detected in NE chloroplast lower amounts of phosphatidic acid (18:1), an important intermediate in lipid biosynthesis (Joyard et al., 2010) with functions as a signaling lipid (Testerink and Munnik, 2005, 2011; Horváth et al., 2012; McLoughlin and Testerink, 2013).
We observed that the lower level of linolenic acid (18:3) detected in NE chloroplasts was associated with significantly lower MDA chloroplast content in NE compared with IE poplar (Fig. 2; Table I). Previous studies have reported that MDA is primarily derived from triunsaturated fatty acids in chloroplasts (Yamauchi et al., 2008; Schmid-Siegert et al., 2012). MDA can be used as an oxidative stress marker when plants are exposed to unfavorable conditions (Esterbauer et al., 1991) but is also present in healthy plants (Weber et al., 2004; Mène-Saffrané et al., 2007, 2009). At the whole-leaf extract level, MDA levels were higher in NE poplar (Behnke et al., 2010b), which agrees with their higher concentrations of linolenic acid (Way et al., 2013). The production of MDA from triunsaturated fatty acids serves to adsorb a portion of the reactive oxygen species (Mène-Saffrané et al., 2009); therefore, MDA is a by-product in the mechanism of cell protection.
Another remarkable observation in this study was the increased abundance of plastoglobules in NE compared with IE chloroplasts. Plastoglobules are lipoprotein particles containing isoprenoid-driven metabolites (primarily prenylquinones, including plastoquinone and phylloquinone), tocopherols (Vidi et al., 2006), and structural proteins (plastoglobulins; Bréhélin et al., 2007). The increased number of plastoglobules in NE compared with IE chloroplasts might reflect the higher levels of α-tocopherol in leaves of these lines, as demonstrated previously (Behnke et al., 2010b).
Electron tomography revealed that plastoglobules are physically coupled to thylakoid membranes via a half-lipid bilayer, providing a direct lipid conduit for metabolite channeling between plastoglobules and thylakoid membranes (Austin et al., 2006). Moreover, plastoglobules are involved in different secondary metabolism pathways, stress responses, and the development of thylakoids (Bréhélin et al., 2007). In a previous study, we showed that the lipid-associated protein fibrillin content, comprising lipid-associated proteins and fibrillins, is negatively correlated with the NE plants (Velikova et al., 2014). This observation suggests the involvement of isoprene in the maintenance of thylakoid membranes.
Interestingly, we observed larger associative zones between chloroplasts and mitochondria in NE plants. Mitochondria are instrumental for the generation of metabolic energy in eukaryotic cells, and these organelles deliver intermediates to support different metabolic pathways, including photosynthesis (Jacoby et al., 2012). One of the important benefits of mitochondria-chloroplast interactions is the optimization of photosynthetic carbon assimilation through the coordinated production and utilization of ATP and NADPH, the induction of photosynthesis, the activation of enzymes, and the maintenance of metabolite levels (Raghavendra and Padmasree, 2003). We propose that the larger associative zones between chloroplasts and mitochondria in NE plants reflect a higher demand for assimilatory power (ATP and NADPH) compared with IE plants. Indeed, the down-regulation of the cytochrome b6f complex in NE chloroplasts indicates the inhibition of ATP production, associated with the down-regulation of extrinsic subunits of ATP synthase in isoprene-suppressed lines (Velikova et al., 2014). Because isoprene functions as a protective molecule against oxidative stress (Loreto and Schnitzler, 2010), the isoprene suppression in NE plants might be balanced by enhancing other compensatory protective mechanisms such as photorespiration and oxidative electron transport, which are both mediated by mitochondria.
Recent analyses demonstrated that the suppression of isoprene biosynthesis dramatically reduces carbon fluxes throughout the methylerythritol 4-phosphate pathway (Ghirardo et al., 2014), followed by the reallocation of carbon to other pathways, which in turn induces profound metabolic changes, particularly in lipid biosynthesis (Way et al., 2013; Kaling et al., 2015). Thus, at the cellular level, lipid metabolism is up-regulated in NE leaves, whereas at the subcellular level, as shown here, low levels of galactolipids and PLs comprise the structure of NE chloroplasts. These results suggest that the crucial needs of NE plants to maintain the correct fluidity of thylakoid membranes induces the up-regulation of lipid metabolism, including lipid intermediates, likely compensating for the low levels of galactolipids and PLs packed into chloroplast membranes. Thus, isoprene might (1) directly improve the fluidity of thylakoid membranes in synergy with galactolipids or (2) indirectly affect lipid biosynthesis or trafficking into the chloroplast. Whether the lack of isoprene function or the alteration of the plastidic isoprenoid pathway itself induces changes in the chloroplast lipid levels, thereby affecting membrane fluidity, should be examined in future studies.
Functional Changes Relate to Structural Alterations in NE Chloroplasts
We measured light- and dark-adapted states of chlorophyll fluorescence in IE and NE poplar plants grown under unstressed conditions in order to assess whether the structural changes have functional significance with regard to the differences in the ability to emit isoprene. Our results showed that ΦPSII was lower in NE plants than in IE plants, consistent with previous observations that the proteins involved in photosynthetic processes are down-regulated in NE plants, potentially decreasing the efficiency of the photochemistry of photosynthesis (Fig. 5; Velikova et al., 2014). The lower ΦPSII values in NE plants were negatively correlated to NPQ, a protective mechanism for the removal of excess excitation energy within pigment complexes and the inhibition of the formation of free radicals (Demmig-Adams and Adams, 2006).
Higher NPQ levels in concert with restricted electron transport rate between both photosystems and a reduced plastoquinone pool have been shown (Härtel et al., 1998) to be accompanied by DGDG modifications in the Arabidopsis mutant (dgd1). Moreover, in this mutant, PSI showed an increased capacity for cyclic electron transfer and a reduced capacity for state transitions (Ivanov et al., 2006). Similar to the dgd1 mutant (Dörmann et al., 1995), the NE poplar plants showed a lower DGDG content, modified chloroplastic ultrastructure, increased NPQ, restricted electron transport rate (Behnke et al., 2007), and decreased total chlorophyll content (Fig. 5C; Behnke et al., 2013; Way et al., 2013; Ghirardo et al., 2014).
The NPQ comprises qE (i.e. dependent on the energization of thylakoid membranes), state transitions (Minagawa, 2011), photoinhibition quenching (Müller et al., 2001), and zeaxanthin-dependent quenching (Nilkens et al., 2010). qE is the most important and well-characterized component of NPQ. This transition is triggered through the acidification of the thylakoid lumen (Ruban et al., 2012), which in turn leads to the protonation of violaxanthin deepoxidase, for the conversion of violaxanthin to zeaxanthin, and PsbS, a polypeptide of the PSII-associated light-harvesting complex (Kiss et al., 2008; Murchie and Niyogi, 2011).
Here, we showed higher values of qE in NE plants, which might reflect a particular conformation of the LHCII complex resulting from chlorophyll and/or xanthophyll-protein interactions (Horton et al., 2005). Indeed, we observed that many proteins associated with photosynthesis are less abundant in NE chloroplasts (Figs. 5 and 7; Velikova et al., 2014). This lack of photosynthetic proteins could lead to specific conformational changes, which in turn could determine the higher qE in NE poplar. However, the supramolecular organization of the PSII antenna involves numerous interactions between proteins, suggesting that the changes in these interactions (Garab and Mustardy, 1999; Horton et al., 2005) could be responsible for the increase in NPQ we observed in NE plants. Indeed, with circular dichroism spectroscopy, it has been shown that isoprene deficiency inhibits the formation of the chirally organized macrodomains. This effect in turn decreases the thermal stability of thylakoid membranes (Velikova et al., 2011). We also observed the significant down-regulation of the cytochrome b6f complex in NE lines (Velikova et al., 2014), which might inhibit the production of ATP in isoprene-suppressed plants. The increase of qE in NE lines might reflect the optimization of electron transport and ATP synthesis through the modulation of the cyclic electron transfer around PSI, the activation state of ATP synthase, and the partitioning of the proton-motive force between ΔpH and the membrane electrical potential (Horton et al., 2005).
CONCLUSION
The proposed biological functions of isoprene in plants have been associated with the ability of this molecule to affect thylakoid membrane organization and reduce the formation of reactive oxygen species, conferring tolerance to heat and oxidative stress. It has been hypothesized that isoprene improves the thermal stability of thylakoid membranes by affecting the membrane lipid composition (Velikova et al., 2011). Here, we provided direct evidence of the relationship between isoprene emission and the level of main lipid classes and their fatty acid composition, and we characterized the structural organization of the photosynthetic machinery in IE and NE poplar genotypes. The suppressed isoprene production in NE plastids was associated with the reduced amount of galactolipids and PLs, the lower level of the major fatty acid (18:3), and the altered chloroplast ultrastructure (Fig. 8). The suppression of isoprene biosynthesis causes considerable metabolic changes, particularly in lipid biosynthesis (Way et al., 2013; Kaling et al., 2015), and significant alterations in the chloroplast proteome (Velikova et al., 2014). The majority of the plastidic and mitochondrial proteome is encoded in the nuclear genome, and there is a continuous exchange of forward information from nucleus to organelle (anterograde) and of backward information from organelle to nucleus (retrograde; Pfannschmidt, 2010). According to the retrograde signaling concept, based on the available experimental data, signals originating in chloroplasts and/or mitochondria modulate nuclear gene expression (Leister, 2012). These signals originate from carotenoid biosynthesis, reactive oxygen species, photosynthetic redox processes, and changes in the pool of metabolites (Pfannschmidt, 2010; Leister, 2012). The plastidic signals identified so far have been associated with specific stress conditions. It is likely that the comprehensive changes in the metabolome (Way et al., 2013; Kaling et al., 2015), liposome, proteome (Velikova et al., 2014), and ultrastructure of the chloroplasts in NE poplar (Fig. 8), as well as the distinct physiological behavior of these plants, reflect finely tuned retrograde signaling. The precise mechanisms for the transmission of the changes in chloroplast to the nucleus in NE plant cells remain elusive.
MATERIALS AND METHODS
Plant Material
In this study, we used the same gray poplar (Populus × canescens; syn. Populus tremula × Populus alba) genotypes as utilized in previous chloroplast proteome research (Velikova et al., 2014), namely, two IE lines (WT and EV) and two NE lines (RA1 and RA2). The empty vector line was used to ensure that the differences between NE and IE plants reflected specific alterations in the isoprene synthase gene and not a more general genetic manipulation effect. The plants were grown in a greenhouse as described previously (Velikova et al., 2014). Briefly, the ambient temperature was 25°C/20°C with a relative humidity of 50%/60% and a photoperiod of 16 h of day/8 h of night. The plants were fertilized weekly with Triabon (Compo) and Osmocote (Scotts Miracle-Gro; 1:1 [v/v]; 10 g L−1 soil).
Four-month-old plants were used for the experiments. Fully expanded leaves (ninth node from the apical meristem) from six to seven different plants, considered as biological replicates, were used for physiological, biochemical, and structural studies. The chloroplasts were isolated as described previously (Velikova et al., 2014) and used for lipid and MDA analyses.
Lipid Extraction Procedure
The total lipids from chloroplasts were extracted according to Bligh and Dyer (1959). All procedures were performed in dim light using chilled solvents (containing 0.01% [w/v] butylated hydroxytoluene) and glassware. The chloroplast samples (0.5 mL) were mixed with chloroform:methanol (1:2 [v/v]; 1.9 mL) for approximately 2 min; subsequently, 0.625 mL of chloroform and 0.625 mL of distilled water were added. The lower chloroform phase, containing the lipids, was removed, and aliquots were transferred into vials and exsiccated under N2. The residues were weighed and calculated for total lipids.
Gas Chromatography-MS Analysis of PL Fatty Acid Composition
PL fatty acids were analyzed as described previously (Behnke et al., 2013; Way et al., 2013). Briefly, the PL fatty acids were separated from other lipids using a silica-bonded phase column (MEGA-BE-SI, 2 g 12 mL−1, 20/PK, Bond ELUT; Agilent Technologies). Fatty acid methyl esters were obtained after mild alkaline hydrolysis. Myristic acid was used as an internal standard for gas chromatography analysis. Unsubstituted fatty acid methyl esters were measured using the 5973MSD gas chromatograph-mass spectrometer (Agilent Technologies) coupled with a combustion unit to an isotope ratio mass spectrometer (DeltaPlus; Thermo Electron) and identified using the established fatty acid libraries and characteristic retention times of pure standards. The fatty acids were named according to the total number of carbon atoms and double bonds. Saturated straight-chain fatty acids are indicated with an n.
Ultraperformance Liquid Chromatography Electrospray Time-of-Flight MS of Galactolipids
Lipids were dissolved in 1 mL of liquid chromatography-MS-grade methanol (Fluka). MGDG and DGDG contents were analyzed using the ultra-performance liquid chromatography electrospray time-of-flight MS system (maXis; Bruker). Aliquots of 2.5 µL of each sample were analyzed in three technical replicates in randomized order.
The chromatographic separation was achieved on a C18 ACQUITYUPLC BEH column, 50 mm, 2.1 mm, and 1.7 µm (Waters), using a gradient elution. The composition was changed from 50% to 92% B for 10 min and maintained for an additional 10 min, then changed to 100% B for 1 min and maintained for 5 min. The flow was set to 0.4 mL h−1. Mobile phase A comprised water:isopropyl alcohol (95:5, v/v), and mobile phase B comprised acetonitrile:isopropyl alcohol (95:5, v/v). A format of 0.001 mm sodium (Sigma-Aldrich) was added to both mobile phases. This method has been published previously for profiling photosynthetic glycerol lipids (Xu et al., 2010).
MGDGs and DGDGs were detected as sodium adducts through positive electrospray ionization. The instrument was calibrated with ESI Tune Mix (Agilent Technologies). Acquired spectra were internally calibrated and exported to GENEDATA software for chromatographic alignment and peak picking. MGDGs and DGDGs were identified based on the retention times and detected exact masses (mass error < 0.01 D).
MGDG and DGDG standards (Larodan) were used to evaluate the analytical performance and determine the quality control, which was injected 10 times in the beginning for column conditioning and after every 10th sample to validate the measuring performance.
MDA Content
The lipid peroxidation level in extracted chloroplast samples was quantified after measuring the MDA content using the thiobarbituric acid-reactive substances assay according to Hodges et al. (1999). The chloroplast sample (0.100 mL) was mixed with 1.2 mL of 80% (w/w) ethanol (containing 0.01% [w/v] butylated hydroxytoluene) and sonicated in a water bath sonicator for 3 min, followed by centrifugation at 5,000g for 10 min at 4°C. An aliquot of the obtained supernatant (0.5 mL) was mixed with the same volume of 0.65% (w/v) thiobarbituric acid solution containing 20% (w/v) TCA. Another aliquot of the supernatant (0.5 mL) was mixed with 0.5 mL of 20% (w/v) TCA, representing the zero control. The mixture was heated at 95°C for 30 min. The reaction was terminated after incubation in an ice bath. The cooled mixture was centrifuged at 10,000g for 10 min at 4°C, and the absorbance of the supernatant was measured at 532, 600, and 440 nm (Perkin Elmer). MDA equivalents were calculated according to Hodges et al. (1999):
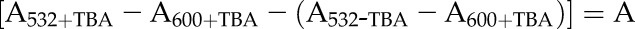 |
(1) |
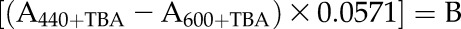 |
(2) |
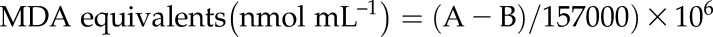 |
(3) |
Protein and Chlorophyll Analyses
For the calculation of the abundance of reaction center proteins in PSI and PSII, we used the chloroplast proteome data published by Velikova et al. (2014). Peak intensities of peptides identified as RCI (protein accession nos. POPTR_0008s15100.1, POPTR_0006s27030.1, POPTR_0003s14870.1, and POPTR_0002s25510.2) and RCII (protein accession nos. POPTR_0011s03390.1, POPTR_0004s03160.1, POPTR_0005s22780.1, POPTR_0002s05660.1, POPTR_0005s01430.1, POPTR_0005s27800.3, POPTR_0002s05720.1, POPTR_0002s25810.1, POPTR_0001s44210.1, and POPTR_0006s26270.1) were summed and expressed per mg of chlorophyll.
The chlorophyll content was measured in isolated chloroplast suspension after extraction with 80% (v/v) ice-cold acetone. Absorbance at 663 and 646 nm was detected to determine chlorophyll a and b concentrations, calculated according to Porra et al. (1989).
Chlorophyll Fluorescence Measurements
The chlorophyll fluorescence parameters were measured on intact leaves using a MINI-PAM Photosynthesis Yield Analyzer (Heinz-Walz). The leaves were dark adapted for 15 min prior to the determination of the minimal (Fo) and maximal (Fm) chlorophyll fluorescence, and subsequently, the leaves were exposed to actinic light (430 μmol m−2 s−1). After steady-state fluorescence was obtained, a saturating pulse was applied to determine the maximum fluorescence in the light (Fm′). The ΦPSII was calculated from (Fm′ – F′)/Fm′ (Genty et al., 1989). The redox state of PSII was assessed based on the parameter qL = (Fq′/Fv′)/(Fo′/F′), where F′ is the fluorescence emission from the light-adapted leaf, Fv′ is variable fluorescence from the light-adapted leaf, and Fq′ is the difference in fluorescence between Fm′ and F′ (Baker, 2008). Fo′ was estimated using the following equation: Fo′ = Fo/[(Fv/Fm) + Fo/Fm′] (Oxborough and Baker, 1997). The NPQ was calculated as NPQ = (Fm − Fm′)/Fm′ (Bilger and Björkman, 1991). The NPQ relaxation kinetics in the dark was used to calculate qE. qE was assigned as a fast-relaxing component (within the first 2 min of dark relaxation after switching off the actinic light), calculated as qE = (Fm′′ – Fm′)/F′′m, 2min dark (Zaks et al., 2013).
TEM
Leaf segments (1 mm2) were cut from the middle of the leaves for TEM analyses. The segments were fixed in 2.5% (v/v) glutaraldehyde (electron microscopy grade) in 0.1 m sodium cacodylate buffer, pH 7.4 (Science Services), postfixed in 2% (v/v) aqueous osmium tetraoxide (Dalton, 1955), dehydrated in an ethanol gradient (30%–70%), stained with uranyl acetate (2% in 70% ethanol), dehydrated in an ethanol gradient (70%–100%) and propylene oxide (100%), embedded in Epon (Merck), and cured for 24 h at 60°C. Semithin sections (300 nm) were cut and stained with Toluidine Blue. Ultrathin sections of 50 nm were collected onto 200-mesh copper grids before examination using TEM (Zeiss Libra 120 Plus; Carl Zeiss). The images were acquired using a Slow Scan CCD camera and iTEM software (Olympus Soft Imaging Solutions).
Statistical Analyses
Correlation analyses between different data sets of PL and galactolipid (MGDG and DGDG) contents, fatty acid compositions, chlorophyll fluorescence parameters, MDA content, the data groups, and IE or NE genotypes were performed using PCA and OPLS from the software package SIMCA-P (version 13.0.0.0; Umetrics). In addition, we included the chloroplastic protein contents associated with photosynthesis and structure (Velikova et al., 2014) to correlate lipids to proteins. Because the proteomic data originated from three samples for each plant genotype (containing six to seven different leaves each of the three samples), multivariate analyses were performed using only the data matching the same three samples used for both proteomic and PL analyses. Galactolipids, MDA, and chlorophyll fluorescence measurements were obtained from more and different extracts; therefore, only the data from three samples were taken randomly and used for these analyses. We added the means of all biological replicates to examine the correlations between genotypes using data originating from different leaf extracts. The resulting matrix size, therefore, was 78 × 16 (variables × observations). Thus, our analyses could correlate any data value with an isoprene emission trait (IE and NE plant genotype), but correlations between/within variables could be achieved only using data from the same leaf material (i.e. within PL and proteins, MGDG, DGDG, and MDA, and within chlorophyll fluorescence data).
The multivariate data analyses followed the established procedures to analyze MS data as described previously (Ghirardo et al., 2005, 2012; Kreuzwieser et al., 2014; Vanzo et al., 2014; Velikova et al., 2014). The isoprene emission trait was selected as the Y variable for the OPLS analysis by setting NE = 1 and IE = 0. The X variables were centered, and each type of data was block-wise scaled with 1 sd−1, considering the different number of X variables in each group of data. Each calculated significant principal component was validated using full cross validation, with 95% confidence level on parameters. The regression model OPLS was further tested for significance using cross-validated ANOVA (Eriksson et al., 2008). Variables showing VIP values greater than 1 and jack-knifing method uncertainty bars smaller than the respective VIP values were defined as discriminant variables to distinguish IE from NE samples. For the proteomics data, containing a much higher number of variables, the VIP threshold was set to 0.5. The statistical significance of the differences between the means of discriminant variables and the functional and structural parameters measured in NE and IE plants was additionally evaluated using Student’s t test and at an α level of 0.05, unless otherwise stated.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Lipid content and fatty acid composition in isolated chloroplasts of IE (WT and EV) and NE (RA1 and RA2) poplar.
Supplemental Figure S2. Score and loading plots of all PCA parameters analyzed (lipid and fatty acid composition, MDA, NPQ, ΦPSII, qE, qL), proteins related to photosynthesis, and proteins with structural activity.
Glossary
- MGDG
monogalactosyldiacylglycerol
- DGDG
digalactosyldiacylglycerol
- LHCII
light-harvesting complex II
- IE
isoprene-emitting
- NE
nonisoprene-emitting
- PL
phospholipid
- MDA
malondialdehyde
- TEM
transmission electron microscopy
- MS
mass spectrometry
- NPQ
nonphotochemical quenching
- qE
energy-dependent quenching
- ΦPSII
photosystem II photochemical efficiency
- PCA
principal component analysis
- OPLS
orthogonal partial least squares
- VIP
variable of importance for the projection
Footnotes
This work was supported by the Alexander von Humboldt Foundation (to V.V.).
References
- Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6: 317–326 [DOI] [PubMed] [Google Scholar]
- Austin JR II, Frost E, Vidi PA, Kessler F, Staehelin LA (2006) Plastoglobules are lipoprotein subcompartments of the chloroplast that are permanently coupled to thylakoid membranes and contain biosynthetic enzymes. Plant Cell 18: 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Behnke K, Ehlting B, Teuber M, Bauerfeind M, Louis S, Hänsch R, Polle A, Bohlmann J, Schnitzler JP (2007) Transgenic, non-isoprene emitting poplars don’t like it hot. Plant J 51: 485–499 [DOI] [PubMed] [Google Scholar]
- Behnke K, Ghirardo A, Janz D, Kanawati B, Esperschütz J, Zimmer I, Schmitt-Kopplin P, Niinemets Ü, Polle A, Schnitzler JP, et al. (2013) Isoprene function in two contrasting poplars under salt and sunflecks. Tree Physiol 33: 562–578 [DOI] [PubMed] [Google Scholar]
- Behnke K, Kaiser A, Zimmer I, Brüggemann N, Janz D, Polle A, Hampp R, Hänsch R, Popko J, Schmitt-Kopplin P, et al. (2010a) RNAi-mediated suppression of isoprene emission in poplar transiently impacts phenolic metabolism under high temperature and high light intensities: a transcriptomic and metabolomic analysis. Plant Mol Biol 74: 61–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke K, Loivamäki M, Zimmer I, Rennenberg H, Schnitzler JP, Louis S (2010b) Isoprene emission protects photosynthesis in sunfleck exposed Grey poplar. Photosynth Res 104: 5–17 [DOI] [PubMed] [Google Scholar]
- Bilger W, Björkman O (1991) Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva parviflora L. Planta 184: 226–234 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bréhélin C, Kessler F, van Wijk KJ (2007) Plastoglobules: versatile lipoprotein particles in plastids. Trends Plant Sci 12: 260–266 [DOI] [PubMed] [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M (2007) Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. New Phytol 175: 244–254 [DOI] [PubMed] [Google Scholar]
- Dalton AJ. (1955) A chrome-osmium fixative for electron microscopy. Anat Rec 121: 281 [Google Scholar]
- Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation. New Phytol 172: 11–21 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Trygg J, Wold S (2008) CV-ANOVA for significance testing of PLS and OPLS models. J Chemometr 22: 594–600 [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81–128 [DOI] [PubMed] [Google Scholar]
- Fang C, Monson RK, Cowling EB (1996) Isoprene emission, photosynthesis, and growth in sweetgum (Liquidambar styraciflua) seedlings exposed to short- and long-term drying cycles. Tree Physiol 16: 441–446 [DOI] [PubMed] [Google Scholar]
- Gabashvili IS, Menikh A, Segui J, Fragata M (1998) Protein structure of photosystem II studied by FTIR spectroscopy: effect of digalactosyldiacylglycerol on the tyrosine side chain residues. J Mol Struct 444: 123–133 [Google Scholar]
- Garab G, Lohner K, Laggner P, Farkas T (2000) Self-regulation of the lipid content of membranes by non-bilayer lipids: a hypothesis. Trends Plant Sci 5: 489–494 [DOI] [PubMed] [Google Scholar]
- Garab G, Mustardy L (1999) Role of LHCII-containing macrodomains in the structure, function and dynamics of grana. Aust J Plant Physiol 26: 649–658 [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Ghirardo A, Heller W, Fladung M, Schnitzler JP, Schroeder H (2012) Function of defensive volatiles in pedunculate oak (Quercus robur) is tricked by the moth Tortrix viridana. Plant Cell Environ 35: 2192–2207 [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Sørensen HA, Petersen M, Jacobsen S, Søndergaard I (2005) Early prediction of wheat quality: analysis during grain development using mass spectrometry and multivariate data analysis. Rapid Commun Mass Spectrom 19: 525–532 [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler JP (2014) Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiol 165: 37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounaris K, Barber J (1983) Monogalactosyldiacylglycerol: the most abundant polar lipid in nature. Trends Biochem Sci 8: 378–381 [Google Scholar]
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X (2012) The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geoscientific Model Development 5: 1471–1492 [Google Scholar]
- Härtel H, Lokstein H, Dörmann P, Trethewey RN, Benning C (1998) Photosynthetic light utilization and xanthophyll cycle activity in the galactolipid deficient dgd1 mutant of Arabidopsis thaliana. Plant Physiol Biochem 36: 407–417 [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Horton P, Wentworth M, Ruban A (2005) Control of the light harvesting function of chloroplast membranes: the LHCII-aggregation model for non-photochemical quenching. FEBS Lett 579: 4201–4206 [DOI] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Nakamoto H, Mishkind ML, Munnik T, Saidi Y, Goloubinoff P, Harwood JL, Vigh L (2012) Heat shock response in photosynthetic organisms: membrane and lipid connections. Prog Lipid Res 51: 208–220 [DOI] [PubMed] [Google Scholar]
- Ivanov AG, Hendrickson L, Krol M, Selstam E, Öquist G, Hurry V, Huner NPA (2006) Digalactosyl-diacylglycerol deficiency impairs the capacity for photosynthetic intersystem electron transport and state transitions in Arabidopsis thaliana due to photosystem I acceptor-side limitations. Plant Cell Physiol 47: 1146–1157 [DOI] [PubMed] [Google Scholar]
- Jacoby RP, Li L, Huang S, Pong Lee C, Millar AH, Taylor NL (2012) Mitochondrial composition, function and stress response in plants. J Integr Plant Biol 54: 887–906 [DOI] [PubMed] [Google Scholar]
- Jarvis P, Dörmann P, Peto CA, Lutes J, Benning C, Chory J (2000) Galactolipid deficiency and abnormal chloroplast development in the Arabidopsis MGD synthase 1 mutant. Proc Natl Acad Sci USA 97: 8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2010) Chloroplast proteomics highlights the subcellular compartmentation of lipid metabolism. Prog Lipid Res 49: 128–158 [DOI] [PubMed] [Google Scholar]
- Kaling M, Kanawati B, Ghirardo A, Albert A, Winkler JB, Heller W, Barta C, Loreto F, Schmitt-Kopplin P, Schnitzler JP (2015) UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant Cell Environ 38: 892–904 [DOI] [PubMed] [Google Scholar]
- Kanervo E, Aro EM, Murata N (1995) Low unsaturation level of thylakoid membrane lipids limits turnover of the D1 protein of photosystem II at high irradiance. FEBS Lett 364: 239–242 [DOI] [PubMed] [Google Scholar]
- Kirchhoff H, Haase W, Haferkamp S, Schott T, Borinski M, Kubitscheck U, Rögner M (2007) Structural and functional self-organization of photosystem II in grana thylakoids. Biochim Biophys Acta 1767: 1180–1188 [DOI] [PubMed] [Google Scholar]
- Kirchhoff H, Horstmann S, Weis E (2000) Control of the photosynthetic electron transport by PQ diffusion microdomains in thylakoids of higher plants. Biochim Biophys Acta 1459: 148–168 [DOI] [PubMed] [Google Scholar]
- Kirchhoff H, Mukherjee U, Galla HJ (2002) Molecular architecture of the thylakoid membrane: lipid diffusion space for plastoquinone. Biochemistry 41: 4872–4882 [DOI] [PubMed] [Google Scholar]
- Kiss AZ, Ruban AV, Horton P (2008) The PsbS protein controls the organization of the photosystem II antenna in higher plant thylakoid membranes. J Biol Chem 283: 3972–3978 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Narise T, Sonoike K, Hashimoto H, Sato N, Kondo M, Nishimura M, Sato M, Toyooka K, Sugimoto K, et al. (2013) Role of galactolipid biosynthesis in coordinated development of photosynthetic complexes and thylakoid membranes during chloroplast biogenesis in Arabidopsis. Plant J 73: 250–261 [DOI] [PubMed] [Google Scholar]
- Kouřil R, Dekker JP, Boekema EJ (2012) Supramolecular organization of photosystem II in green plants. Biochim Biophys Acta 1817: 2–12 [DOI] [PubMed] [Google Scholar]
- Kreuzwieser J, Scheerer U, Kruse J, Burzlaff T, Honsel A, Alfarraj S, Georgiev P, Schnitzler JP, Ghirardo A, Kreuzer I, et al. (2014) The Venus flytrap attracts insects by the release of volatile organic compounds. J Exp Bot 65: 755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumova SB, Laptenok SP, Kovács L, Tóth T, van Hoek A, Garab G, van Amerongen H (2010) Digalactosyl-diacylglycerol-deficiency lowers the thermal stability of thylakoid membranes. Photosynth Res 105: 229–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate MT, Ko K, Ko ZW, Pinto LS, Real MJ, Romano MR, Barja PR, Granell A, Friso G, van Wijk KJ, et al. (2004) Constitutive expression of pea Lhcb 1-2 in tobacco affects plant development, morphology and photosynthetic capacity. Plant Mol Biol 55: 701–714 [DOI] [PubMed] [Google Scholar]
- Lee AG. (2003) Lipid-protein interactions in biological membranes: a structural perspective. Biochim Biophys Acta 1612: 1–40 [DOI] [PubMed] [Google Scholar]
- Lee AG. (2004) How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta 1666: 62–87 [DOI] [PubMed] [Google Scholar]
- Leister D. (2012) Retrograde signaling in plants: from simple to complex scenarios. Front Plant Sci 3: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2007) Lipids in photosystem II: interactions with protein and cofactors. Biochim Biophys Acta 1767: 509–519 [DOI] [PubMed] [Google Scholar]
- Loreto F, Fineschi S (2014) Reconciling functions and evolution of isoprene emission in higher plants. New Phytol 206: 578–582 [DOI] [PubMed] [Google Scholar]
- Loreto F, Schnitzler JP (2010) Abiotic stresses and induced BVOCs. Trends Plant Sci 15: 154–166 [DOI] [PubMed] [Google Scholar]
- McLoughlin F, Testerink C (2013) Phosphatidic acid, a versatile water-stress signal in roots. Front Plant Sci 4: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mène-Saffrané L, Davoine C, Stolz S, Majcherczyk P, Farmer EE (2007) Genetic removal of tri-unsaturated fatty acids suppresses developmental and molecular phenotypes of an Arabidopsis tocopherol-deficient mutant: whole-body mapping of malondialdehyde pools in a complex eukaryote. J Biol Chem 282: 35749–35756 [DOI] [PubMed] [Google Scholar]
- Mène-Saffrané L, Dubugnon L, Chételat A, Stolz S, Gouhier-Darimont C, Farmer EE (2009) Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J Biol Chem 284: 1702–1708 [DOI] [PubMed] [Google Scholar]
- Minagawa J. (2011) State transitions: the molecular remodeling of photosynthetic supercomplexes that controls energy flow in the chloroplast. Biochim Biophys Acta 1807: 897–905 [DOI] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: a response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie EH, Niyogi KK (2011) Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol 155: 86–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustárdy L, Buttle K, Steinbach G, Garab G (2008) The three-dimensional network of the thylakoid membranes in plants: quasihelical model of the granum-stroma assembly. Plant Cell 20: 2552–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M, Kress E, Lambrev P, Miloslavina Y, Müller M, Holzwarth AR, Jahns P (2010) Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim Biophys Acta 1797: 466–475 [DOI] [PubMed] [Google Scholar]
- Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components calculation of qP and Fv’/Fm’ without measuring Fo’. Photosynth Res 54: 135–142 [Google Scholar]
- Pfannschmidt T. (2010) Plastidial retrograde signalling: a true “plastid factor” or just metabolite signatures? Trends Plant Sci 15: 427–435 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Pribil M, Labs M, Leister D (2014) Structure and dynamics of thylakoids in land plants. J Exp Bot 65: 1955–1972 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8: 546–553 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP (2012) The photoprotective molecular switch in the photosystem II antenna. Biochim Biophys Acta 1817: 167–181 [DOI] [PubMed] [Google Scholar]
- Sakurai I, Mizusawa N, Wada H, Sato N (2007) Digalactosyldiacylglycerol is required for stabilization of the oxygen-evolving complex in photosystem II. Plant Physiol 145: 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane PV. (2004) Thermoluminescence: a technique for probing photosystem II. In R Carpentier, ed, Photosynthesis Research Protocols. Humana Press, Totowa, NJ, pp 229–248 [DOI] [PubMed] [Google Scholar]
- Schmid-Siegert E, Loscos J, Farmer EE (2012) Inducible malondialdehyde pools in zones of cell proliferation and developing tissues in Arabidopsis. J Biol Chem 287: 8954–8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Chen X, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10: 368–375 [DOI] [PubMed] [Google Scholar]
- Testerink C, Munnik T (2011) Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot 62: 2349–2361 [DOI] [PubMed] [Google Scholar]
- Teuber M, Zimmer I, Kreuzwieser J, Ache P, Polle A, Rennenberg H, Schnitzler JP (2008) VOC emissions of Grey poplar leaves as affected by salt stress and different N sources. Plant Biol (Stuttg) 10: 86–96 [DOI] [PubMed] [Google Scholar]
- Tikkanen M, Nurmi M, Suorsa M, Danielsson R, Mamedov F, Styring S, Aro EM (2008) Phosphorylation-dependent regulation of excitation energy distribution between the two photosystems in higher plants. Biochim Biophys Acta 1777: 425–432 [DOI] [PubMed] [Google Scholar]
- Vanzo E, Ghirardo A, Merl-Pham J, Lindermayr C, Heller W, Hauck SM, Durner J, Schnitzler JP (2014) S-Nitroso-proteome in poplar leaves in response to acute ozone stress. PLoS ONE 9: e106886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Ghirardo A, Vanzo E, Merl J, Hauck SM, Schnitzler JP (2014) Genetic manipulation of isoprene emissions in poplar plants remodels the chloroplast proteome. J Proteome Res 13: 2005–2018 [DOI] [PubMed] [Google Scholar]
- Velikova V, Sharkey TD, Loreto F (2012) Stabilization of thylakoid membranes in isoprene-emitting plants reduces formation of reactive oxygen species. Plant Signal Behav 7: 139–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Várkonyi Z, Szabó M, Maslenkova L, Nogues I, Kovács L, Peeva V, Busheva M, Garab G, Sharkey TD, et al. (2011) Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol 157: 905–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5: 283–291 [DOI] [PubMed] [Google Scholar]
- Vidi PA, Kanwischer M, Baginsky S, Austin JR, Csucs G, Dörmann P, Kessler F, Bréhélin C (2006) Tocopherol cyclase (VTE1) localization and vitamin E accumulation in chloroplast plastoglobule lipoprotein particles. J Biol Chem 281: 11225–11234 [DOI] [PubMed] [Google Scholar]
- Way DA, Ghirardo A, Kanawati B, Esperschütz J, Monson RK, Jackson RB, Schmitt-Kopplin P, Schnitzler JP (2013) Increasing atmospheric CO2 reduces metabolic and physiological differences between isoprene- and non-isoprene-emitting poplars. New Phytol 200: 534–546 [DOI] [PubMed] [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37: 877–888 [DOI] [PubMed] [Google Scholar]
- Xu J, Chen D, Yan X, Chen J, Zhou C (2010) Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight mass spectrometry. Anal Chim Acta 663: 60–68 [DOI] [PubMed] [Google Scholar]
- Yamamoto HY, Higashi RM (1978) Violaxanthin de-epoxidase: lipid composition and substrate specificity. Arch Biochem Biophys 190: 514–522 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Furutera A, Seki K, Toyoda Y, Tanaka K, Sugimoto Y (2008) Malondialdehyde generated from peroxidized linolenic acid causes protein modification in heat-stressed plants. Plant Physiol Biochem 46: 786–793 [DOI] [PubMed] [Google Scholar]
- Zaks J, Amarnath K, Sylak-Glassman EJ, Fleming GR (2013) Models and measurements of energy-dependent quenching. Photosynth Res 116: 389–409 [DOI] [PMC free article] [PubMed] [Google Scholar]