Abstract
Objective
To describe the burden of pediatric tuberculosis (TB) in a human immunodeficiency virus (HIV)-infected population and explore the demographic and clinical factors associated with the occurrence of pediatric TB.
Design
Longitudinal analysis of a cohort of HIV-infected children.
Methods
The endpoint of the study was clinically diagnosed TB. Cox proportional hazard regression was used to explore the predictors of incident TB among HIV-infected children under age 15 years after enrollment into the HIV program.
Results
The cohort comprised of 5040 children [median age: 5 years, interquartile range (IQR): 1–9 years]. During a median follow-up of 0.8 (IQR: 0.1–2.5) years, 376 out of 5040 children met the case definition for TB. The overall incidence of TB was 5.2/100 person-years. In multivariate analyses, older age at enrollment [relative risk (RR): 1.7, 95%, confidence interval (CI): 1.5–1.8], severe wasting (RR: 1.8, 95% CI: 1.3 –2.5), severe immune-suppression (RR: 2.6, 95% CI: 1.8–3.8), anemia (RR: 1.4, 95% CI: 1.0–1.9) and World Health Organization (WHO) stage IV (RR: 4.5, 95% CI: 2.4–8.5) were all independently associated with a higher risk of TB. In addition, the use of antiretroviral drugs for more than 180 days reduced the risk of TB by 70% (RR: 0.3, 95% CI: 0.2–0.4).
Conclusions
ART use is strongly associated with a reduced risk of tuberculosis among HIV-infected children, and should therefore be included in HIV care and treatment programs. Trials of interventions designed to improve the nutritional and hematologic status of these children should also be performed.
Keywords: Africa, childhood tuberculosis, antiretroviral therapy, nutrition, HIV/AIDS
Introduction
Extensive geographic, biologic, and epidemiologic overlap exists between infections with human immunodeficiency virus (HIV) and tuberculosis (TB) in sub-Saharan Africa. The World Health Organization (WHO) estimates that one-third of the world’s population is infected with Mycobacterium tuberculosis, [1] a condition referred to as latent tuberculosis infection (LTBI). Although the bacterium often remains latent, people with intact immune systems have a lifetime risk for development of active TB of about 5–10%, [1] while those infected with HIV have a lifetime risk of approximately 30–40%. [2, 3] TB manifestations are more severe and progression to death is more rapid in HIV positive patients. [4] In addition, TB can accelerate the course of HIV disease, increasing morbidity, mortality, and the frequency of opportunistic infections. [5, 6]
Childhood TB contributes at least 10%–15% to the global burden of this disease. [7] In areas where TB is under good control, the incidence of TB is low among HIV-infected children, [8] while the incidence is high where TB control is poor. [9, 10] This may be explained in part by the shifting of the age distribution of TB in HIV endemic areas. In HIV-affected communities, the highest TB incidence rate occurs among men and women in their reproductive years, as opposed to the historical scenario of a higher incidence in elderly men. [11] The higher TB prevalence among parents and other caregivers, and the subsequent higher risk for children to be exposed to M. tuberculosis, help contribute to a higher TB burden for children in HIV-endemic areas.
The diagnosis of childhood TB presents a major challenge. In children, use of the bacteriologic culture, the accepted “gold standard”, [12] is limited due to the paucibacillary nature of the illness in children, [13] the difficulty in specimen collection among young patients, [14] as well as limited laboratory capacity in resource-constrained environment. [15] Diagnostic problems are even more pronounced in HIV-infected children because the symptoms, disease progression and interpretation of chest radiographs may all be confounded by HIV-related comobidities. [16]
In spite of these obstacles, there is an urgent need for data on the burden of TB and risk factors that could help identify effective interventions. We preformed an observational, prospective cohort study of children participating in an HIV treatment program in Tanzania. The objectives of this study were (1) to describe the burden of pediatric TB in an HIV-infected population; and (2) to describe the demographic and clinical factors associated with the occurrence of pediatric TB.
Methods
Study population
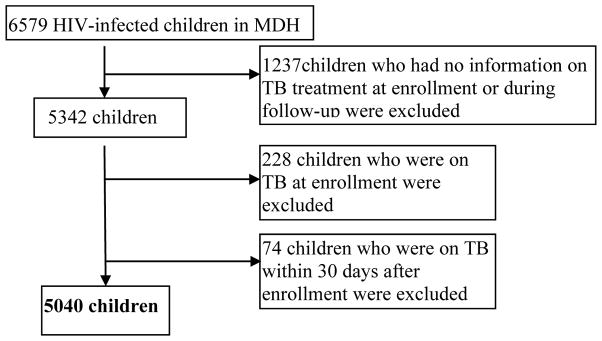
Based in Dar es Salaam, Tanzania, the Management and Development for Health (MDH) program was initiated in 2004 as a joint partnership between Muhimbili University of Health and Allied Sciences, Dar es Salaam City Council and Harvard University. With financial support from the President’s Emergency Plan for AIDS Relief (PEPFAR), MDH provides support to HIV care and treatment, laboratory, integrated prevention of mother-to-child transmission and TB services in Dar es Salaam. Between November 2004 to September 2011, 6579 HIV-infected patients were enrolled in MDH pediatric (<15 years) program from 28 clinics in urban Dar es Salaam. Doctors and nurses provided care and treatment to HIV-infected patients in these clinics. Figure 1 presents the exclusion criteria for the study and final sample size. The institutional review boards for human research at the Harvard School of Public Health and the ethics review committees at Muhimbili University of Health and Allied Sciences in Dar es Salaam approved the study.
Figure 1.
Study flow of patients
Standard of care
Following enrollment, clinic visits were scheduled monthly for children receiving antiretroviral therapy (ART). For children not receiving ART, visits were scheduled monthly for those aged 5 years of age and under, and at 3-monthly intervals for children above age 5 years. In general, children were screened for tuberculosis based on clinical symptoms, sputum smear (for children 5 years of age or older) or chest x-ray (in younger children). Sputum culture was not available. For HIV-infected children in the program, ART initiation criteria were for children between 12–18 months, CD4+% <25; for children between 19–59 months, CD4+% <20; for children 5 years old and above, CD4+% <15 (or CD4+ count <200 cells/mm3). For children under 1 year old, the criteria were changed in the year of 2007. Before 2007, for those who were at WHO stage III/IV, ART were initiated regardless of CD4+%; for those who were at WHO stage I/II, ART were initiated if CD4+% <25%. After 2007, all children under age 1 year old were initiated on ART regardless of CD4+% or WHO stage.
Data collection
At each patient visit, physicians and nurses completed standardized forms capturing demographic and clinical information, as well as medications prescribed. Anthropometric measures, including height, weight, and mid upper arm circumference (MUAC), were obtained by nurses using standardized techniques. [17] Blood samples were collected and immunologic, biochemical, and hematologic tests were performed. The data assurance process included several stages of review and editing for checking completeness and consistency, double data entry, supervisory checks of the inconsistencies discovered at second data entry, and finally “retirement” and archiving of the form.
Study variables
Incident TB cases were defined as children who were prescribed anti-TB medications during follow-up and who were not diagnosed with TB at enrollment or within 30 days after enrollment. Demographic, clinical and nutritional characteristics were considered as independent variables. Z scores were calculated using growth references published by the WHO in 2007. [18] Wasting was defined as weight for length Z-score (≤ 2 years old) or body mass index (BMI) Z-score (> 2 years old) < −2. Stunting was defined as height for age Z-score < −2. Moderate and severe wasting/stunting was defined as −3 ≤ Z-score <−2 and Z-score < −3, respectively. [19] Low MUAC was defined as <11.5 cm for children under age 5 years, <12.9 cm for children between age 5 – 9 years, and <16.0 cm for children age 10 years and above. [20] Elevated alanine aminotransferase (ALT) was defined as >40 (U/L) for children aged 5 years or under, [21] >30 (U/L) for children above 5 years old. [22] For children age 5 years or under, immune suppression was defined none (CD4+ % ≥ 25), advanced (15 ≤ CD4+ % <25) or severe (CD4+ %<15); for children above 5 years, immune suppression was defined none (CD4+ count ≥ 500 cells/mm3), advanced (200 cells/mm3 ≤ CD4+ count <500 cells/mm3) or severe (CD4+ count <200 cells/mm3). [23] Anemia was measured by age-specific hemoglobin level: <2 years, hemoglobin <9.5 g/dL; 2 to <7 years, hemoglobin <11.0 g/dL; 7 to < 11 years, hemoglobin <11.5 g/dL; ≥ 11 years, hemoglobin <12.0 g/dL. [24]
Statistical methods
The Kaplan-Meier method was used to estimate the incidence of TB after enrollment. [25] The incidence was calculated from the date of enrollment in the program. Results are presented per 100 child-years of follow up and 95% confidence intervals (CI) were calculated using exact Poisson probabilities. The primary outcome was time to first TB diagnosis after enrollment. The Andersen-Gill formulation of the Cox proportional hazards model [26] was applied to evaluate the association between each characteristic measured at enrollment and selected time-varying parameters with the time to first TB diagnosis after enrollment. Children who did not experience incident TB were censored at death or loss to follow-up. A missing indicator was created when there were missing values. [27] Multivariate models were fit including all variables that were associated with the primary outcome with p <0.20 in the univariate analysis. The possibility of non-linear relations between continuous covariates and the risk of incident TB was examined non-parametrically with restricted cubic splines. [28] All statistical tests were two-sided, with p <0.05 considered significant. All analyses were carried out using the statistical software package SAS, Release 9.2 (Cary, NC).
Results
There were 5,040 HIV-infected children under age 15 years eligible for the analysis (Figure 1). At enrollment, median age was 5 years and approximately one half were male. The median length/height for age Z-score was −2.0 [interquartile range (IQR): −3.1 to −0.9]. The median MUAC was 15 (IQR: 13–17) cm; 17% of children had low MUAC. 28% of patients were wasted at enrollment. For children ≤ 2 years old, the median weight for length Z-score was −1.1 (IQR: −2.4 to −0.2), while for children older than 2 years, the median BMI Z-score was −1.0 (IQR: −2.1 to −0.1). The median hemoglobin level was 9.9 (8.7–11.0) g/dL, and nearly three quarters of children were anemic. Other characteristics at enrollment are presented in Table 1.
Table 1.
Baseline characteristics of children enrolled in a human immunodeficiency virus (HIV) care and treatment program in Dar es Salaam, Tanzania.
| Characteristics | N (%) or Median (IQR) |
|---|---|
| Age – Median [interquartile range (IQR)] (n=5016) | 5 (1–9) |
| Sex (n=5016) | |
| Female | 2556 (51) |
| Male | 2460 (49) |
| District (n=4984) | |
| Ilala | 2225 (45) |
| Kinondoni | 1428 (29) |
| Temeke | 1331 (27) |
| Year of enrollment (n=5016) | |
| 2004–2006 | 762 (15) |
| 2007–2008 | 1943 (39) |
| 2009–2011 | 2311 (46) |
| Season (5016) | |
| Apr. – May. (Rainy) | 871 (17) |
| Oct. – Nov | 756 (15) |
| Dec.–Mar. | 1580 (32) |
| Jun. – Sep. (Dry) | 1809 (36) |
| Wasting 1 (n=3800) | |
| No | 2727 (72) |
| Moderate | 533 (14) |
| Severe | 540 (14) |
| Stunting 2 (n=4083) | |
| No | 2054 (50) |
| Moderate | 968 (24) |
| Severe | 1061 (26) |
| Low mid upper arm circumference (MUAC)3 (n=4705) | |
| No | 3899 (83) |
| Yes | 806 (17) |
| HIV-associated immunodeficiency 4 (n=3613) | |
| Not significant/mild | 982 (27) |
| Advanced | 1292 (36) |
| Severe | 1339 (37) |
| Anemia 5 (n=3582) | |
| No | 935 (26) |
| Yes | 2647 (74) |
| World Health Organization (WHO) stage (n=4674) | |
| I | 858 (18) |
| II | 1487 (32) |
| III | 1995 (43) |
| IV | 334 (7) |
| Elevated alanine aminotransferase (ALT) 6 (n=3248) | |
| No | 2532 (78) |
| Yes | 716 (22) |
For children under or equal age 2, wasting was measured by weight for length Z-score; for children above age 2, wasting was defined by body mass index (BMI) Z-score. Z-score ≥ −2 was defined as non-wasting; −3 ≤ Z-score ≤ −2 was defined as moderate wasting; Z-score ≤ −3 was defined as severe wasting.
Stunting was measured by height/length for age Z-score. Z-score ≥ −2 was defined as non- stunting; −3 ≤ Z-score ≤ −2 was defined as moderate stunting; Z-score ≤ −3 was defined as severe stunting.
Low MUAC was defined as mid-upper arm circumference<115 mm for children under age 5 years, <129 mm for children between age 5–9 years, <160 mm for children above 10 years.
For children under or equal age 5, immune suppression was measured by CD4%: no significant/mild (CD4% ≥ 25%), advanced (15% ≤ CD4% <25%) severe (CD4%<15); For children above age 5, immune suppression was defined by CD4 count: no significant/mild (CD4 count ≥500 cells/mm3), advanced (200 cells/mm3 ≤ CD4 count <500 cells/mm3) severe (CD4 count <200 cells/mm3);
Anemia was measured by age-specific hemoglobin level: <2 years, hemoglobin<9.5 g/dL; 2–7 years, hemoglobin<11.0 g/dL; 7–11 years, hemoglobin<11.5 g/dL; 11–15 years, hemoglobin<12.0 g/dL
For children under or equal age 5, elevated ALT was defined as ALT ≥ 40 (u/l); for children above age 5, elevated ALT was defined as ALT ≥ 30 (u/l).
During a median follow-up of 0.8 (IQR: 0.1–2.5) years, 376 out of 5040 children met the case definition for tuberculosis. There were 7220.6 person-years of follow-up, with a corresponding incidence rate of 5.2 (95% CI: 4.7–5.8) /100 person-years. The incidence rate was 15.0 (95% CI: 12.7–17.7) /100 person-years in the first 3 months after enrollment, 7.0 (95% CI: 5.8–8.5)/100 person-years during 3 to 6 months, and 2.3 (95% CI: 1.9–2.7) /100 person-years after 6 months of follow-up. The median time to incident TB case from enrollment was 121 days (IQR: 67–282 days)
Table 2 presents univariate and multivariate analyses identifying risk factors associated with incident TB. Multivariate analysis indicated that older children had a higher risk of developing TB after enrollment of the program. Children enrolled in the Kinondoni district had a 100% increased in the risk of developing TB than children enrolled in the Ilala district, and a 90% increased in the risk than children enrolled in the Temeke district [relative risk (RR) =1.9, 95% CI: 1.4, 2.5]. The risk of TB decreased significantly over the years of enrollment. Compared with the years 2004 to 2006, children who were enrolled in 2009–2011 had a 60% reduced risk of TB. There was no significant association between family size and having other children in the household with the risk of TB.
Table 2.
Risk factors associated with incident tuberculosis among 5040 HIV-infected children in Dar es Salaam, Tanzania.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| Relative risk (95% confidence interval) | p-value | Relative risk (95% confidence interval) | p-value | |
| Age (years) | 2.1 (1.9, 2.3) | <0.001 | 1.7 (1.5, 1.8) | <0.001 |
| Sex | 0.86 | |||
| Female | Ref.† | |||
| Male | 1.0 (0.8, 1.3) | |||
| District | <0.001 | <0.001 | ||
| Ilala | Ref. | Ref. | ||
| Kinondoni | 2.1 (1.7, 2.6) | 2.0 (1.6, 2.6) | ||
| Temeke | 1.1 (0.8, 1.4) | 1.1 (0.8, 1.4) | ||
| Other children in the household | 0.64 | |||
| No | Ref. | |||
| Yes | 1.1 (0.8, 1.4) | |||
| Family size | 0.63 | |||
| ≤3 family members | Ref. | |||
| > 3 family members | 0.9 (0.5, 1.4) | |||
| Year of enrollment | 0.001 | <0.001 | ||
| 2004–2006 | Ref. | Ref. | ||
| 2007–2008 | 0.8 (0.6, 1.0) | 0.9 (0.6, 1.2) | ||
| 2009–2011 | 0.6 (0.4, 0.8) | 0.4 (0.3. 0.5) | ||
| Season (time–varying) | 0.66 | |||
| Apr. – May. (Rainy) | Ref. | |||
| Oct. – Nov | 0.7 (0.5, 1.0) | |||
| Dec.–Mar. | 1.0 (0.8, 1.3) | |||
| Jun. – Sep. (Dry) | 0.8 (0.6, 1.1) | |||
| Wasting at enrollment1* | <0.001 | 0.003 | ||
| No | Ref. | Ref. | ||
| Moderate | 1.6 (1.1, 2.2) | 1.3 (0.9, 1.8) | ||
| Severe | 2.3 (1.7, 3.2) | 1.8 (1.3, 2.5) | ||
| Stunting at enrollment2* | <0.001 | 0.02 | ||
| No | Ref. | Ref. | ||
| Moderate | 1.4 (1.0, 1.9) | 1.2 (0.9, 1.6) | ||
| Severe | 2.0 (1.5, 2.7) | 1.5 (1.1, 2.0) | ||
| Low MUAC3* | <0.001 | <0.001 | ||
| No | Ref. | Ref. | ||
| Yes | 2.3 (1.8, 3.0) | 1.9 (1.4, 2.5) | ||
| WHO stage at enrollment | <0.001 | <0.001 | ||
| I | Ref. | Ref. | ||
| II | 1.0 (0.7, 1.4) | 2.2 (1.3, 3.6) | ||
| III | 1.9 (1.5, 2.6) | 4.2 (2.5, 6.8) | ||
| IV | 2.2 (1.4, 3.5) | 4.5 (2.4, 8.5) | ||
| Immune suppression4 (time-varying) | <0.001 | <0.001 | ||
| Not significant | Ref. | Ref. | ||
| Advanced | 2.4 (1.7, 3.4) | 1.6 (1.2, 2.4) | ||
| Severe | 5.9 (4.2, 8.4) | 2.6 (1.8, 3.8) | ||
| Anemia (time-varying) 5 | <0.001 | 0.05 | ||
| No | Ref. | Ref. | ||
| Yes | 2.0 (1.5, 2.8) | 1.4 (1.0, 1.9) | ||
| On Cotrimoxazole (time-varying) | 0.29 | |||
| No | Ref. | |||
| Yes | 1.2 (0.9, 1.6) | |||
| Elevated ALT6 (time-varying) | 0.78 | |||
| No | Ref. | |||
| Yes | 1.1 (0.6, 2.1) | |||
| Duration on antiretroviral therapy (ART) (days) (time-varying) | <0.001 | <0.001 | ||
| ≤30 | Ref. | Ref. | ||
| 30 – 180 | 1.3 (1.0, 1.7) | 0.8 (0.6, 1.1) | ||
| >180 | 0.2 (0.2, 0.3) | 0.3 (0.2, 0.4) | ||
For children under or equal age 2, wasting was measured by weight for length Z-score; for children above age 2, wasting was defined by BMI Z-score. Z-score ≥ −2 was defined as non-wasting; −3 ≤ Z-score ≤ −2 was defined as moderate wasting; Z-score ≤ −3 was defined as severe wasting.
Stunting was measured by height/length for age Z-score. Z-score ≥ −2 was defined as non- stunting; −3 ≤ Z-score ≤ −2 was defined as moderate stunting; Z-score ≤ −3 was defined as severe stunting.
Low MUAC was defined as mid-upper arm circumference<115 mm for children under age 5 years, <129 mm for children between age 5–9 years, <160 mm for children above 10 years.
For children under or equal age 5, immune suppression was measured by CD4%: no significant/mild (CD4% ≥ 25%), advanced (15% ≤ CD4% <25%) severe (CD4%<15); For children above age 5, immune suppression was defined by CD4 count: no significant/mild (CD4 count ≥ 500 cells/mm3), advanced (200 cells/mm3 ≤ CD4 count <500 cells/mm3) severe (CD4 count <200 cells/mm3);
Anemia was measured by age-specific hemoglobin level: <2 years, hemoglobin<9.5 g/dL; 2–7 years, hemoglobin<11.0 g/dL; 7–11 years, hemoglobin<11.5 g/dL; 11–15 years, hemoglobin<12.0 g/dL
For children under or equal age 5, elevated ALT was defined as ALT ≥ 40 (u/l); for children above age 5, elevated ALT was defined as ALT ≥ 30 (u/l).
Three nutrition indicators – wasting, stunting, low MUAC, were explored separately in multivariate models with the same covariates. Estimates for other covariates presented in Table 2 are from multivariate model for wasting. The estimates of the covariates from other two models are similar as this one.
Reference level.
The associations between children’s nutritional status at enrollment and risk of TB were explored. Three anthropometric indicators, weight-for-length/BMI Z-score, height-for-age Z-score, and MUAC, were separately examined in multivariate regression models. A similar pattern was noted for the three indicators: severe wasting was associated with a 1.8 times higher risk of TB than non-wasted children; severe stunting was associated with a 1.5 times higher risk, and low MUAC was associated with a 1.9 times higher risk (Table 2).
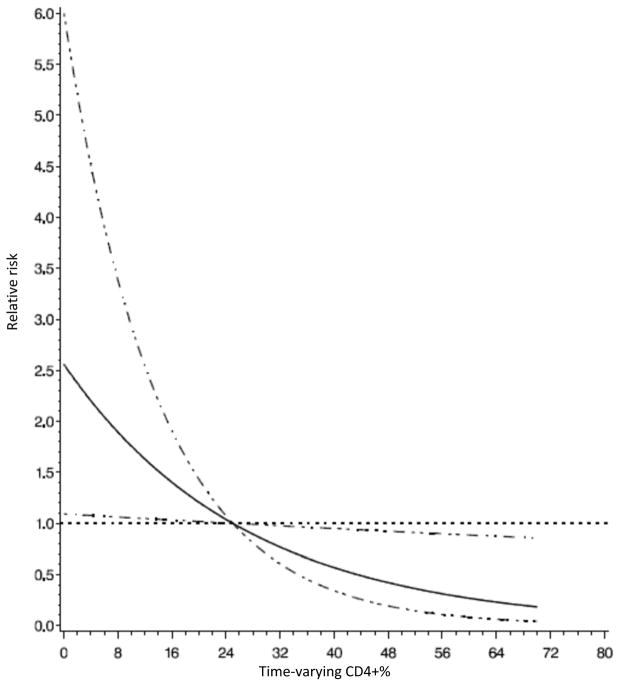
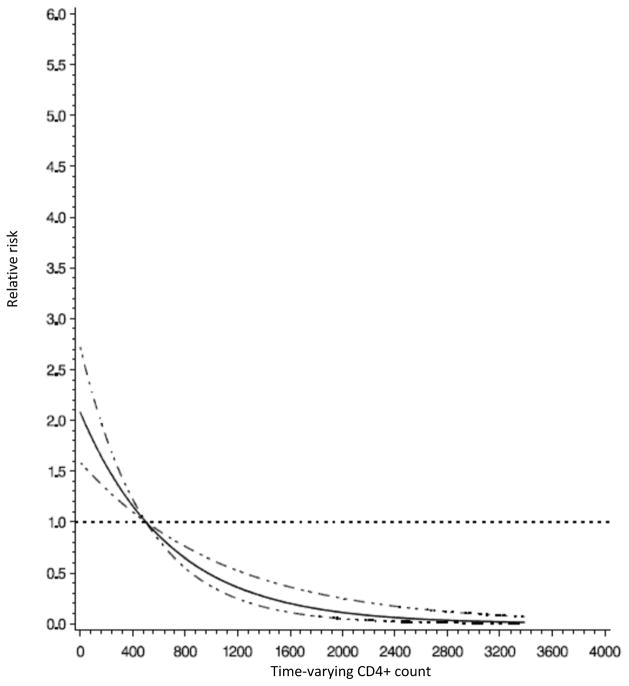
WHO HIV stage at enrollment, time-varying anemia and immune suppression were also significantly associated with the risk of TB (Table 2). Compared with children who were stage I at enrollment, children who were stage II, III and IV had 2.2, 4.2 and 4.5 times greater risk of developing TB, respectively (p-value for trend<0.001). Anemic children were 40% more likely to develop TB after controlling for other covariates. Figure 2 shows that the relative risk of TB decreased with an increase in CD4+ % among children aged 5 years or under (p-value for linear relationship <0.001). A similar pattern can be seen in Figure 3, where higher CD4+ count was associated with lower risk of TB among children above age 5 years (p-value for linear relationship = 0.03). Children with severe immune suppression were at approximately 3-fold higher risk of TB than children without significant immune suppression (Table 2).
Figure 2.
Relative risk of incident tuberculosis by CD4+ % among HIV-infected children under age 5 years in Dar es Salaam, Tanzania.
Note: The horizontal dot line shows where the relative risk equal to 1; the solid line shows relative risks; the dot-dash lines show the 95% confidence intervals for relative risks.
Figure 3.
Relative risk of incident tuberculosis by CD4+ count among HIV-infected children above age 5 years in Dar es Salaam, Tanzania.
Note: The horizontal dot line shows where the relative risk equal to 1; the solid line shows relative risks; the dot-dash lines show the 95% confidence intervals for relative risks.
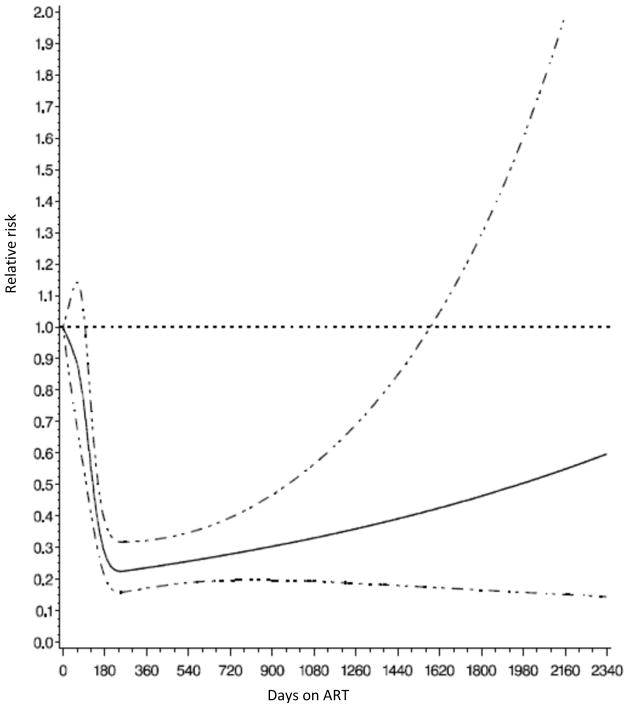
During follow-up, 2624 (51%) children were prescribed ART, and the median time between enrollment and ART initiation was 26 (IQR: 16–56) days. Frequently prescribed initial ART regimens were nevirapine (NVP), lamivudine (3TC) and zidovudine (AZT) in 57% of children, NVP, 3TC and stavudine (D4T) in 29% and efavirenz (EFV), 3TC and AZT in 7%. After controlling for other covariates, children who were on ART for 30 to 180 days had a 20% decreased risk of developing TB than children who were not on ART or on ART for fewer than 30 days. For children who were treated with ART for more than 180 days, the risk of TB decreased 70% (RR: 0.3, 95% CI: 0.2–0.4). Figure 4 shows an inverse linear relationship between the relative risk of TB and duration on ART (p-value for linear relationship <0.001). The relative risk decreased consistently to 0.3 at 180 days and then increased slightly. A separate model was fit to examine the effect of the duration between enrollment and ART initiation on the risk of TB. After controlling for the same covariates presented in Table 2 except for duration on ART, compared with those who initiated ART within 30 days, children who initiated between 30 to 90 days had a 2.2 times higher risk of developing TB (RR: 2.2, 95% CI: 1.7, 2.9), and children who initiated after 90 days had 1.9 times higher risk (RR: 1.9, 95% CI: 1.4, 2.6). Among children who were receiving ART, nucleoside reverse transcriptase inhibitors (NRTI) regimen (D4T vs. AZT) was not significantly associated with the risk of TB after controlling for other covariates. For non-nucleoside reverse transcriptase inhibitors (NNRTI), children who were initiated on EFV had an almost 4-time higher risk of developing TB then those initiated on NVP (RR=3.8, 95% CI: 2.5, 5.9).
Figure 4.
Relative risk of incident tuberculosis by duration on ART among HIV-infected children in Dar es Salaam, Tanzania.
Note: The horizontal dot line shows where the relative risk equal to 1; the solid line shows relative risks; the dot-dash lines show the 95% confidence intervals for relative risks.
Discussion
Our study of HIV-infected children in Tanzania strongly supports the role of ART use in reducing the burden of tuberculosis, and also confirms the critical importance of nutritional status as predictors of TB risk, even after controlling for this ART effect and multiple other risk factors. Whether expressed as wasting, stunting or lower mid-upper arm circumference, nutritional deficits were associated with 1.5 – 1.9 times the risk of incident TB in our cohort.
Consistent with the previous literatures, [6, 15, 29] our study observed a protective relationship between ART use and the risk of childhood TB. We also demonstrated a further reduction in TB burden with longer duration of ART use. Studies among HIV-infected adults suggested that starting ART at higher CD4 counts (<350/μl vs. <250/ μl) is an important public health strategy to reduce the risk of TB in resource-limited settings. [30] Further study is needed to evaluate the same strategy among children. In our study, children who were initiated on EFV appeared to be at higher risk of TB than those who were initiated on NVP. EFV is recommended over NVP for older children, who may have been immune-compromised for a while and have stronger immune reconstitution after ART initiation. Host’s enhanced immune reaction may itself cause more overt manifestations of some symptoms such as prolonged fever, respiratory symptoms and lymphadenopathy, [31] which makes these children more likely to be diagnosed with TB.
Our study also demonstrated that nutritional status is a strong predictor for the risk of TB among HIV-infected children. The relationship between undernutrition and TB could be bidirectional: having TB leads to undernutrition, and being undernourished is a risk factor for developing TB. In our analysis, we examined nutritional status at enrollment. Since children who had TB at enrollment and within 30 days after enrollment were excluded from the analysis, a temporal relationship between nutritional status and incident TB could be better established. We examined three indicators: stunting, wasting, and low MUAC separately and all were significantly associated with a higher risk of TB after controlling for the use of ART and several other risk factors. This suggests that both long term and short term malnutrition increases the risk of TB among HIV-infected children. However, this finding should be interpreted cautiously because of the possibility of TB immune reconstitution inflammatory syndrome (IRIS) secondarily leading to weight loss and malnutrition. In the MDH program, children with severe malnutrition are provided with ready to use therapeutic food. Nutritional rehabilitation could conceivably result in TB IRIS. Among adults, the triple burden of tuberculosis, HIV infection and malnutrition has been well documented. [32] More study is needed to assess the synergy of these diseases among children, as well as whether strategies to improve nutritional status reduce the risk of TB.
The risk of childhood TB decreased over the years of enrollment in this study, after controlling for other covariates. The reduction in childhood TB burden could be a reflection of Tanzanian success in TB control. Tanzania is one of the 22 high TB burden countries, which account for about 80% of all new cases each year and have been prioritized by WHO at global level since 2002 for TB control. [33] Today, the country has reached the target of halving the 1990 TB mortality rate by 2010, and TB incidence has decreased from 359/100,000 person-year in 2002[33] to 177/100,000 person-year in 2011 nationwide. [34] ART initiation criteria for children under age 1 year old changed in 2007. Early ART initiation among this age group may also contribute to the decrease in the childhood TB incidence over years. Children enrolled in the Kinondoni district had a higher risk of developing TB than children enrolled in other two districts. Compared with district Ilala, people in Kinondoni have lower social-economic status (SES)[35] which could lead to higher risk of TB. While based on Tanzania 2002 census, [36] the population density was much lower in Temeke (977 people/km2) than in Kinondoni (2041 people/ km2) and over-crowding may promote TB transmission. [15]
Our study identified several other risk factors for TB development among HIV-infected children. School attendance has been identified as a risk factor for childhood TB[15] which may contribute to the positive association between age and TB risk suggested by our study. Consistent with other published data, [15, 37] advanced immune suppression play a significant role as a risk factor for childhood TB. This suggests that establishing and maintaining virological suppression to maximize CD4 cell recovery is critical to reduce the risk of childhood TB. We found a strong association between anemia and the risk of childhood TB. One study conducted in Tanzania found that anemia with iron deficiency was associated with more than a 6-fold increase in the risk of TB recurrence among HIV-infected adults. [38] One possible explanation for this finding is the iron redistribution known to occur in anemia of inflammation in HIV-infected patients. [39] Anemic children often receive iron supplements which may also contribute to developing of TB. The sequestration and loading of iron could facilitate replication of Mycobacterium tuberculosis and inhibition of cellular defense systems. [38]
Our study documented a TB incidence rate of 5.2 (95% CI: 4.7–5.8) /100 person-years after enrolling in an HIV care and treatment program. Previous studies in Africa reported an incidence rate among HIV-infected children of 0.8 to 21.1/100 person-years. [15, 37, 40, 41] This wide variation could reflect a true difference in childhood disease burden in different countries, or alternatively the variation could be due to the difficulty in making the diagnosis in children, use of different TB case definitions, as well as variable access to ART.
There are a number of limitations to this observational study. Information on viral load and detailed socioeconomic data were not routinely collected in this study. In addition, there was a moderate amount of missing data. To minimize the unintended consequences of missing data, we applied the missing indicator method in our analyses. Perhaps most importantly, we relied on the TB treatment recorded by the physician as our case definition of TB, as opposed to more standard microbiologic confirmation of infection. Despite these limitations, however, the strength of our study lies in its large sample size and relatively long follow-up period. The comprehensive clinical information collected from this cohort enabled us to identify important novel risk factors.
Our findings demonstrate that ART use and poor underlying nutritional status are important predictors for incident TB in HIV-infected children. TB screening should be focused on children with evidence of immunosuppression, malnutrition or anemia. Further studies are needed to elucidate the optimal timing of ART initiation and/or nutritional rehabilitation for these children to optimize health outcomes. Although major diagnostic and therapeutic challenges remain, given sufficient political commitment and well designed interventions, the management of HIV-infected children at risk of TB can be substantially improved.
Acknowledgments
Financial support: This study is supported by U.S. President’s Emergency Plan for AIDS Relief (PEPFAR). Dr Duggan was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD K24HD058795).
The authors thank Management and Development for Health (MDH), Dar es Salaam City Council, Muhimbili University of Health and Allied Sciences (MUHAS), Harvard School of Public Health (HSPH), and the Ministry of Health and Social Welfare for guidance and collaboration in implementing this national HIV care and treatment program in Dar es Salaam, Tanzania. We thank all the patients and staff of the MDH-supported care and treatment sites who contributed to these findings. We thank Ellen Hertzmark for her guidance in biostatistics, and the efforts of Ester Mungure and Aveika Akum in data management.
Footnotes
Role of authors
Nan LI: data analysis, writing, literature search.
Karim P. MANJI: literature search, editing.
Donna SPIEGELMAN: data analysis, editing.
Aisa MUYA: literature search, editing
Ramdhani S MWIRU: editing.
Enju LIU: data analysis, editing.
Guerino CHALAMILLA: data collection, editing.
Wafaie W FAWZI: data analysis, editing.
Christopher DUGGAN: data analysis, literature search, editing
Contributor Information
Nan LI, Department of Global Health and Population, Harvard School of Public Health.
Karim P. MANJI, Department of Pediatrics, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
Donna SPIEGELMAN, Departments of Epidemiology and Biostatistics, Harvard School of Public Health.
Aisa MUYA, Management and Development for Health, Dar es Salaam, Tanzania.
Ramadhani S. MWIRU, Department of Nutrition, Harvard School of Public Health
Enju LIU, Department of Global Health and Population, Harvard School of Public Health.
Guerino CHALAMILLA, Management and Development for Health, Dar es Salaam, Tanzania.
Wafaie W. FAWZI, Departments of Global Health and Population, Epidemiology and Nutrition, Harvard School of Public Health
Christopher DUGGAN, Department of Nutrition, Harvard School of Public Health, Center for Nutrition, Division of GI/Nutrition, Boston Children’s Hospital.
References
- 1.Towards universal access : scaling up priority HIV/AIDS interventions in the health sector : progress report 2008. Geneva, Swizerland: World Health Organization, UNAIDS, UNICEF; 2008. [Google Scholar]
- 2.Corbett El WCJWN, et al. The growing burden of tuberculosis: Global trends and interactions with the hiv epidemic. Archives of Internal Medicine. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Djoba Siawaya JF, Ruhwald M, Eugen-Olsen J, Walzl G. Correlates for disease progression and prognosis during concurrent HIV/TB infection. Int J Infect Dis. 2007;11:289–299. doi: 10.1016/j.ijid.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Churchyard G. Epidemiology of HIV-associated tuberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesseling AC, Westra AE, Werschkull H, Donald PR, Beyers N, Hussey GD, et al. Outcome of HIV infected children with culture confirmed tuberculosis. Arch Dis Child. 2005;90:1171–1174. doi: 10.1136/adc.2004.070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar A, Upadhyay S, Kumari G. Clinical Presentation, treatment outcome and survival among the HIV infected children with culture confirmed tuberculosis. Curr HIV Res. 2007;5:499–504. doi: 10.2174/157016207781662434. [DOI] [PubMed] [Google Scholar]
- 7.Global tuberculosis control. World Health Organization; 2010. [Google Scholar]
- 8.Perez Mato S, Van Dyke RB. Pulmonary infections in children with HIV infection. Semin Respir Infect. 2002;17:33–46. doi: 10.1053/srin.2002.31685. [DOI] [PubMed] [Google Scholar]
- 9.Harries AD, Hargreaves NJ, Graham SM, Mwansambo C, Kazembe P, Broadhead RL, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6:424–431. [PubMed] [Google Scholar]
- 10.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 12.Eamranond P, Jaramillo E. Tuberculosis in children: reassessing the need for improved diagnosis in global control strategies. Int J Tuberc Lung Dis. 2001;5:594–603. [PubMed] [Google Scholar]
- 13.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis. 2006;42:e69–71. doi: 10.1086/502652. [DOI] [PubMed] [Google Scholar]
- 14.Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med. 2006;173:1078–1090. doi: 10.1164/rccm.200511-1809SO. [DOI] [PubMed] [Google Scholar]
- 15.Braitstein P, Nyandiko W, Vreeman R, Wools-Kaloustian K, Sang E, Musick B, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J. 2009;28:626–632. doi: 10.1097/INF.0b013e31819665c5. [DOI] [PubMed] [Google Scholar]
- 16.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis. 2007;196(Suppl 1):S76–85. doi: 10.1086/518659. [DOI] [PubMed] [Google Scholar]
- 17.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Ill: Human Kinetics Books; 1991. Abridged ed. [Google Scholar]
- 18.Nakayiwa S, Abang B, Packel L, Lifshay J, Purcell DW, King R, et al. Desire for children and pregnancy risk behavior among HIV-infected men and women in Uganda. AIDS Behav. 2006;10:S95–104. doi: 10.1007/s10461-006-9126-2. [DOI] [PubMed] [Google Scholar]
- 19.Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 20.Guidelines for an Integrated Approach to the Nutritional care of HIV-infected children. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 21.Behrman RE, Kliegman R, Jenson HB. Nelson textbook of pediatrics. 17. Philadelphia, PA: Saunders; 2004. [Google Scholar]
- 22.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and and children. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 24.Butensky E, Harmatz P, Lubin B. Nutrition in pediatrics : basic science, clinical application. 4. Hamilton: BC Decker; 2008. [Google Scholar]
- 25.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 26.Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Annals of Statistics. 1982;10:1100–1120. [Google Scholar]
- 27.Miettinen OS. Theoretical epidemiology : principles of occurrence research in medicine. New York: Wiley; 1985. [Google Scholar]
- 28.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 29.Kampmann B, Tena-Coki GN, Nicol MP, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS. 2006;20:1011–1018. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 30.Walensky RP, Wolf LL, Wood R, Fofana MO, Freedberg KA, Martinson NA, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–166. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fishman JE, Saraf-Lavi E, Narita M, Hollender ES, Ramsinghani R, Ashkin D. Pulmonary tuberculosis in AIDS patients: transient chest radiographic worsening after initiation of antiretroviral therapy. AJR Am J Roentgenol. 2000;174:43–49. doi: 10.2214/ajr.174.1.1740043. [DOI] [PubMed] [Google Scholar]
- 32.van Lettow M, Fawzi WW, Semba RD. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev. 2003;61:81–90. doi: 10.1301/nr.2003.marr.81-90. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization., Global Tuberculosis Programme. Global tuberculosis control : WHO report. Geneva: Global Tuberculosis Programme; 2002. [Google Scholar]
- 34.World Health Organization., Global Tuberculosis Programme. Global tuberculosis control : WHO report. Geneva: Global Tuberculosis Programme; 2011. [Google Scholar]
- 35.Kahabuka FK, Plasschaert A, van’t Hof M. Prevalence of teeth with untreated dental trauma among nursery and primary school pupils in Dar es Salaam, Tanzania. Dent Traumatol. 2001;17:109–113. doi: 10.1034/j.1600-9657.2001.017003109.x. [DOI] [PubMed] [Google Scholar]
- 36.2002 Population and Housing Census: General Report. Dar es Salaam: Central Census Office, National Bureau of Statistics, United Republic of Tanzania; 2003. [Google Scholar]
- 37.Martinson NA, Moultrie H, van Niekerk R, Barry G, Coovadia A, Cotton M, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis. 2009;13:862–867. [PMC free article] [PubMed] [Google Scholar]
- 38.Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142:350–357. doi: 10.3945/jn.111.144287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wisaksana R, Sumantri R, Indrati AR, Zwitser A, Jusuf H, de Mast Q, et al. Anemia and iron homeostasis in a cohort of HIV-infected patients in Indonesia. BMC Infect Dis. 2011;11:213. doi: 10.1186/1471-2334-11-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edmonds A, Lusiama J, Napravnik S, Kitetele F, Van Rie A, Behets F. Anti-retroviral therapy reduces incident tuberculosis in HIV-infected children. Int J Epidemiol. 2009;38:1612–1621. doi: 10.1093/ije/dyp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouakoussui A, Fassinou P, Anaky MF, Elenga N, Laguide R, Wemin ML, et al. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev. 2004;5:311–315. doi: 10.1016/j.prrv.2004.07.008. [DOI] [PubMed] [Google Scholar]