Abstract
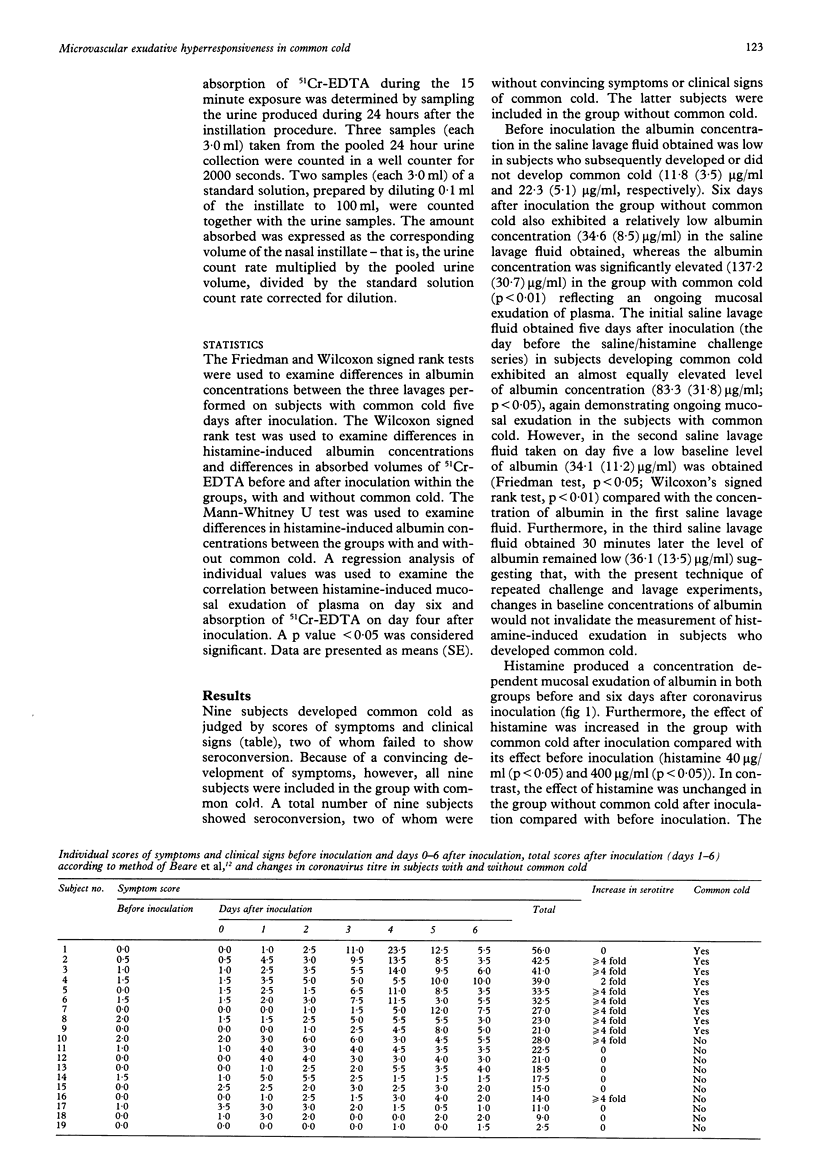
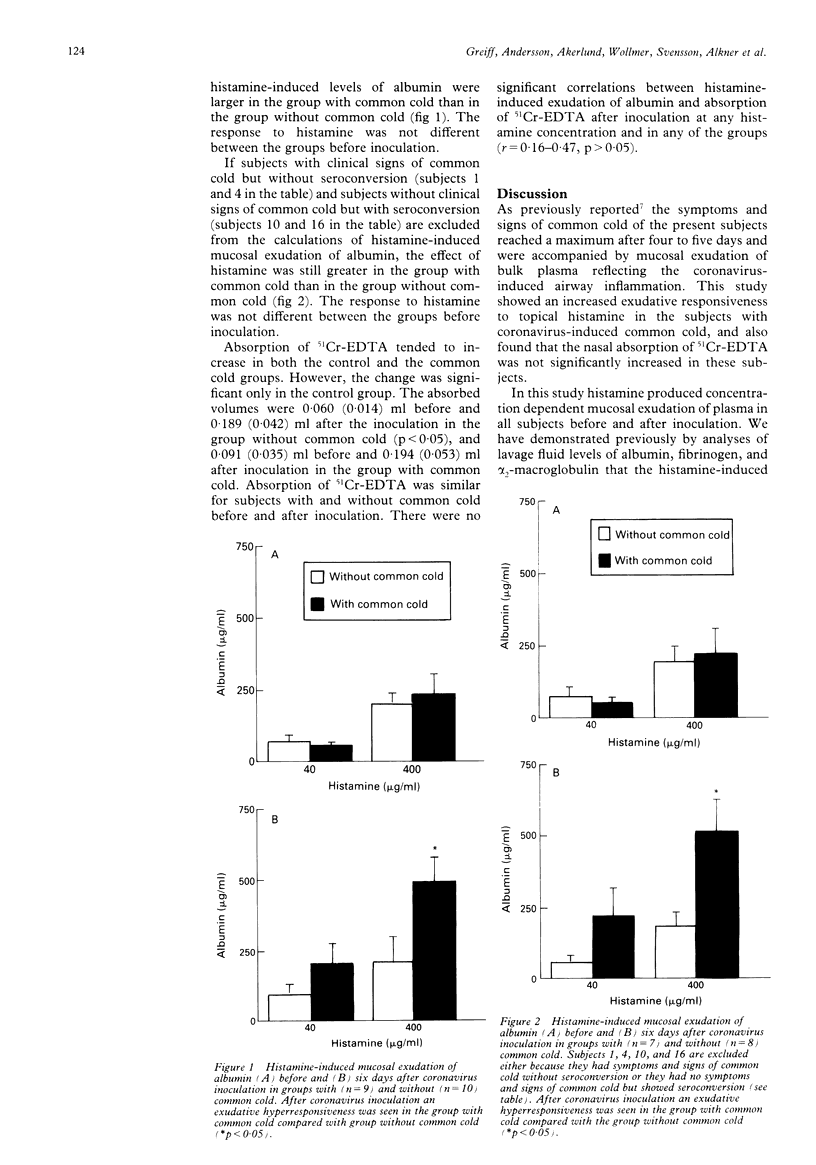
BACKGROUND--The inflammatory response of the airway microcirculation in rhinitis and asthma may be recorded as luminal entry of plasma macromolecules (mucosal exudation). This study examines the exudative responsiveness of the subepithelial microvessels in subjects with and without common cold after inoculation with coronavirus. METHODS--The airway mucosa was exposed to exudative concentrations of histamine (40 and 400 micrograms/ml) before and six days after inoculation. To assess whether mucosal penetration of a topically applied agent was altered, nasal absorption of chromium-51 labelled ethylene diamine tetraacetic acid (51Cr-EDTA, MW 372) was also examined. A nasal pool technique kept the challenge and tracer solutes in contact with the same ipsilateral mucosal surface. Concentrations of albumin in lavage fluids were measured as an index of mucosal exudation of plasma. Nasal absorption of 51Cr-EDTA was determined by the cumulated 24 hour urinary excretion of radioactivity. RESULTS--Nine subjects developed common cold after coronavirus inoculation and 10 remained healthy. Histamine produced concentration dependent mucosal exudation of plasma in all subjects before and after coronavirus inoculation. In subjects with common cold, however, the histamine-induced mucosal exudation was significantly augmented compared with the group without common cold. This exudative hyperresponsiveness is not explained by an increased baseline exudation because the lavage regimen used produced comparably low baseline exudation in both groups of subjects, nor is it explained by an increased penetration of topical histamine because the ability of the nasal mucosa to absorb 51Cr-EDTA was not significantly increased in the subjects with common cold. CONCLUSIONS--An increased proclivity of the airway subepithelial microcirculation to respond with plasma exudation develops during coronavirus-induced common cold. This specific exudative hyperresponsiveness may be a feature of inflammatory airway diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund A., Greiff L., Andersson M., Bende M., Alkner U., Persson C. G. Mucosal exudation of fibrinogen in coronavirus-induced common colds. Acta Otolaryngol. 1993 Sep;113(5):642–648. doi: 10.3109/00016489309135878. [DOI] [PubMed] [Google Scholar]
- Bardin P. G., Johnston S. L., Pattemore P. K. Viruses as precipitants of asthma symptoms. II. Physiology and mechanisms. Clin Exp Allergy. 1992 Sep;22(9):809–822. doi: 10.1111/j.1365-2222.1992.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckle F. G., Cohen A. B. Nasal mucosal hyperpermeability to macromolecules in atopic rhinitis and extrinsic asthma. J Allergy Clin Immunol. 1975 Apr;55(4):213–221. doi: 10.1016/0091-6749(75)90139-6. [DOI] [PubMed] [Google Scholar]
- Elwood R. K., Kennedy S., Belzberg A., Hogg J. C., Paré P. D. Respiratory mucosal permeability in asthma. Am Rev Respir Dis. 1983 Sep;128(3):523–527. doi: 10.1164/arrd.1983.128.3.523. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Luts A., Persson C. G. Appearance of airway absorption and exudation tracers in guinea pig tracheobronchial lymph nodes. J Appl Physiol (1985) 1993 Feb;74(2):817–824. doi: 10.1152/jappl.1993.74.2.817. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Allergen, bradykinin, and capsaicin increase outward but not inward macromolecular permeability of guinea-pig tracheobronchial mucosa. Clin Exp Allergy. 1991 Mar;21(2):217–224. doi: 10.1111/j.1365-2222.1991.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Erjefält I., Persson C. G. Inflammatory passage of plasma macromolecules into airway wall and lumen. Pulm Pharmacol. 1989;2(2):93–102. doi: 10.1016/0952-0600(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Greiff L., Erjefält I., Wollmer P., Pipkorn U., Persson C. G. Effects of histamine, ethanol, and a detergent on exudation and absorption across guinea pig airway mucosa in vivo. Thorax. 1991 Oct;46(10):700–705. doi: 10.1136/thx.46.10.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L., Pipkorn U., Alkner U., Persson C. G. The 'nasal pool' device applies controlled concentrations of solutes on human nasal airway mucosa and samples its surface exudations/secretions. Clin Exp Allergy. 1990 May;20(3):253–259. doi: 10.1111/j.1365-2222.1990.tb02680.x. [DOI] [PubMed] [Google Scholar]
- Greiff L., Wollmer P., Erjefält I., Pipkorn U., Persson C. G. Clearance of 99mTc DTPA from guinea pig nasal, tracheobronchial, and bronchoalveolar airways. Thorax. 1990 Nov;45(11):841–845. doi: 10.1136/thx.45.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L., Wollmer P., Pipkorn U., Persson C. G. Absorption of 51Cr EDTA across the human nasal airway barriers in the presence of topical histamine. Thorax. 1991 Sep;46(9):630–632. doi: 10.1136/thx.46.9.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiff L., Wollmer P., Svensson C., Andersson M., Persson C. G. Effect of seasonal allergic rhinitis on airway mucosal absorption of chromium-51 labelled EDTA. Thorax. 1993 Jun;48(6):648–650. doi: 10.1136/thx.48.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg J. C., Eggleston P. A. Is asthma an epithelial disease? Am Rev Respir Dis. 1984 Feb;129(2):207–208. [PubMed] [Google Scholar]
- Laitinen L. A., Heino M., Laitinen A., Kava T., Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985 Apr;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich M., Dirks R., Roberts R. S., Newhouse M. T. Lung epithelial permeability: relation to nonspecific airway responsiveness. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jul;57(1):77–84. doi: 10.1152/jappl.1984.57.1.77. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Alkner U., Baumgarten C., Greiff L., Gustafsson B., Luts A., Pipkorn U., Sundler F., Svensson C. Plasma exudation as a first line respiratory mucosal defence. Clin Exp Allergy. 1991 Jan;21(1):17–24. doi: 10.1111/j.1365-2222.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Gustafsson B., Luts A. Subepithelial hydrostatic pressure may regulate plasma exudation across the mucosa. Int Arch Allergy Appl Immunol. 1990;92(2):148–153. doi: 10.1159/000235206. [DOI] [PubMed] [Google Scholar]
- Persson C. G. Role of plasma exudation in asthmatic airways. Lancet. 1986 Nov 15;2(8516):1126–1129. doi: 10.1016/s0140-6736(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Svensson C., Greiff L., Anderson M., Wollmer P., Alkner U., Erjefält I. The use of the nose to study the inflammatory response of the respiratory tract. Thorax. 1992 Dec;47(12):993–1000. doi: 10.1136/thx.47.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomonsson P., Grönneberg R., Gilljam H., Andersson O., Billing B., Enander I., Alkner U., Persson C. G. Bronchial exudation of bulk plasma at allergen challenge in allergic asthma. Am Rev Respir Dis. 1992 Dec;146(6):1535–1542. doi: 10.1164/ajrccm/146.6.1535. [DOI] [PubMed] [Google Scholar]
- Svensson C., Baumgarten C. R., Pipkorn U., Alkner U., Persson C. G. Reversibility and reproducibility of histamine induced plasma leakage in nasal airways. Thorax. 1989 Jan;44(1):13–18. doi: 10.1136/thx.44.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]


