Abstract
The term premature ovarian insufficiency (POI) describes a continuum of declining ovarian function in a young woman, resulting in an earlier than average menopause. It is a term that reflects the variable nature of the condition and is substantially less emotive than the formerly used “premature ovarian failure” which signaled a single event in time. Contrary to the decline in the age of menarche seen over the last 3-4 decades there has been no similar change in the age of menopause. In developed nations, the average age for cessation of menstrual cycles is 50-52 years. The age is younger among women from developing nations. Much has been written about POI despite a lack of good data on the incidence of this condition. It is believed that 1% of women under the age of 40 years and 0.1% under the age of 30 years will develop POI. Research is increasingly providing information about the pathogenesis and treatments are being developed to better preserve ovarian function during cancer treatment and to improve fertility options. This narrative review summarizes the current literature to provide an approach to best practice management of POI.
Key Words: Iatrogenic menopause, menopause hormone therapy, premature ovarian insufficiency
Dr. Anna Fenton, BHB, MBChB, CCD, PhD, FRACP

Anna a Gynaecological Endocrinologist working at Christchurch Women's Hospital and Southern Women's Health, Christchurch, New Zealand.
She has served on the board of Osteoporosis New Zealand and is currently the clinical leader for the Canterbury District Health Board Bone Density Service.
She has special interests in the management of premature ovarian insufficiency and the treatment of women after gynaecological cancer.
She is co-editor-in-chief of Climacteric, the journal of the International Menopause Society and Immediate Past President of the Australasian Menopause Society.
INTRODUCTION
The term premature ovarian insufficiency (POI) describes a continuum of declining ovarian function in a young woman, resulting in an earlier than average menopause. It is a term that reflects the variable nature of the condition and is substantially less emotive than the formerly used “premature ovarian failure” which signaled a single event in time.
Contrary to the decline in the age of menarche seen over the last 3-4 decades there has been no similar change in the age of menopause. In developed nations, the average age for cessation of menstrual cycles is 50-52 years. The age is younger among women from developing nations.
Much has been written about POI despite a lack of good data on the incidence of this condition. It is believed that 1% of women under the age of 40 years and 0.1% under the age of 30 years will develop POI. Research is increasingly providing information about the pathogenesis and treatments are being developed to better preserve ovarian function during cancer treatment and to improve fertility options.
METHODS
This is a narrative review of the evidence concerning the pathogenesis, long-term consequences, and management of POI. We did not attempt to summarize studies or data in a systematic way. An exhaustive literature search to identify all of the studies of interest was not conducted. Papers were selected based on their currency and relevance to the discussion. Likewise, we did not use statistical testing or concepts of statistical significance.
PATHOGENESIS
Genetic and chromosomal
The heritability of the age of menopause has been estimated at 30-85%. Fifteen to 30% of cases of POI are considered to be familial. A higher degree of heritability may be seen if a family history of early menopause between 40 and 45 years is included.[1,2] The normal ovarian function requires the presence of many intact genes functionally normally and in a coordinated fashion.
X chromosome
Two X chromosomes appear to be necessary for full and normal ovarian function. Both the short and long arms of the chromosome appear to play important roles. The proximal region of the short arm of the X chromosome is vital and terminal deletions originating in this region are usually associated with complete or premature ovarian failure. Almost all terminal deletions of the long arm of the X chromosome result in primary amenorrhea and lack of breast development.[3] Turner syndrome (45 X monosomy) affects 1 in 2500 live female births and is associated with a range of important health issues. Most women with Turner syndrome will present with primary amenorrhea, but 3-5% will menstruate and develop some secondary sexual characteristics. These young women typically demonstrate a Turner mosaic karyotype. Turner syndrome has a frequency of 4-5% in POI.[4,5]
Trisomy X affects 1 in 1000 women. Mosaic forms may present in 10% of cases. Microdeletions of the X chromosome have also been reported.
Fragile X syndrome is an X-linked dominant genetic condition associated with POI. Women exhibiting extended repeats of the CGG trinucleotide sequence may be classified as having the premutation (55-199 repeats) or the full condition (>200 repeats). Women carrying the premutation have a 23% rate of POI and typically experience menopause 5 years earlier than average. There is an association, although not linear, between the number of repeats and the severity of the condition. Frequencies of between 3% and 15% have been reported in POI.[6]
Bone morphogenetic protein 15 is a member of the transforming growth factor beta (TGF-β) superfamily and is located on the short arm of the X chromosome. It appears to have a vital role in fertility and oocyte quality and mutations in this gene may be linked to POI.[7]
Autosomal
Mutations affecting both estrogen receptors (ER-α and ER-β) and a variety of other targets have been described, including LHr, FSHr, INHA, forkhead box L2 FOXO3, steroidogenic factor 1, and CYP19A1.[3] Many of these mutations are rare. Some primarily affect gonadal function while others have more diverse effects on body systems.
Autoimmune
There is a strong association between POI and autoimmune dysfunction [Tables 1–3]. This may be linked to the role of the X chromosome in autoimmunity. Autoimmune POI is a member of the polyglandular deficiency syndrome family. It may be the first condition to be diagnosed, followed up to a decade later by potentially life-threatening conditions such as Addison's disease or type 1 diabetes. Two to 10% appear to later develop Addison's disease and 2.5% develop type 1 diabetes. Up to 25% of women with autoimmune POI will go on to develop hypothyroidism.[4]
Table 1.
Polyglandular autoimmune syndrome I
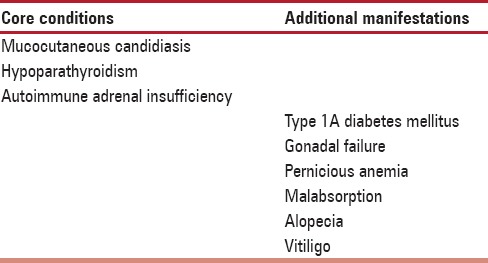
Table 3.
Polyglandular autoimmune syndrome III
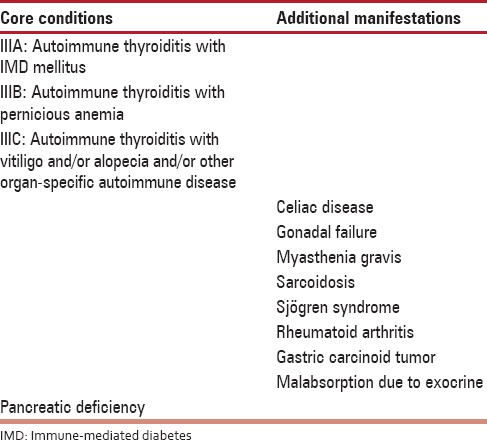
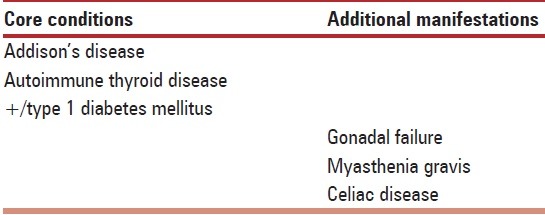
Table 2.
Polyglandular autoimmune syndrome II
Iatrogenic
Iatrogenic causes of POI are becoming more common as access increases to surgical management options as well as chemotherapy and radiotherapy. One in 49 women will be diagnosed with cancer before the age of 40 years. Eighty percent of those affected by childhood cancer will survive long-term. The risk of nonsurgical POI is 13 fold higher in cancer survivors compared with their noncancer affected siblings.[8]
Risk factors for chemotherapy-induce ovarian failure.
Patient age (>40 years).
Familial ovarian history.
Ovarian follicle reserve.
History of previous ovarian and pelvic surgery.
Previous chemotherapy.
Previous pelvic and abdominal radiotherapy.
Persistent high level of follicle stimulating hormone (FSH) [Table 4].
Class of the chemotherapeutic agent (alkylating vs. nonalkylating).
Dosing and length of chemotherapy.
Concomitant diseases.
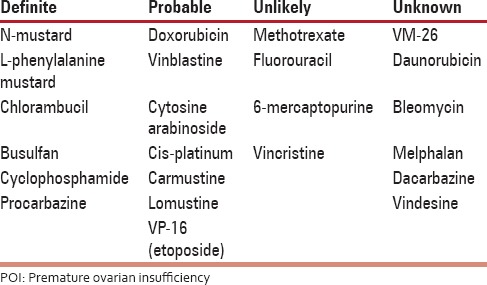
Table 4.
Risk of POI with chemotherapeutic medications
Chemotherapy induces apoptosis of mature ovarian follicles, and histological studies have shown fibrosis, vascular damage, and reduced follicle numbers. The use of alkylating agents will lead to POI in approximately 40% of women. A combination of chemotherapy and total body or abdominopelvic radiotherapy is particularly deleterious. Ovarian function may recover, but generally menopause will still occur early.
Women known to be carriers of the BRCA genes are presenting in increasing numbers for prophylactic surgery, and some forms of gynecological surgery considered, in the past, to be minor are now being reported to have greater consequences on ovarian function. A New Zealand prospective cohort of 257 women under the age of 46 years demonstrated that hysterectomy without oophorectomy brought forward the age of menopause by 3.7 years (confidence interval 1.5-6 years), and this was an effect independent of baseline FSH, body mass index (BMI), and smoking status.[9] A rapid decline in anti-Mullerian Hormone (AMH) is seen after hysterectomy.[10] Research has now also linked ovarian drilling for polycystic ovary syndrome and removal of endometriotic cysts to an earlier age at menopause.[11,12] The somewhat routine practice of removing healthy ovaries at the time of hysterectomy is, fortunately, diminishing with time.
Miscellaneous
On average smokers reach menopause 1.74 ± 0.46 years earlier than average. This effect is independent of weight. A residual effect can be seen for some time after smoking cessation. The age of starting to smoke is not linked to the risk after adjustment for a number of cigarettes currently smoked.[13]
The role of infections remains controversial with a potential link between mumps and a later increased risk of POI. Other potential causes of POI include tuberculosis, malaria, varicella, and shigella.[14] There are scanty data to make definitive statements about their role. Recently, there have been reports of an association between HIV infection or antiviral therapy and POI risk.[15]
Idiopathic
In many commentaries, unexplained POI is the most common diagnosis with reported incidence figures as high as 90%. This may reflect the nature of investigations performed or the referral source of the women.
We have maintained a POI database of women referred to the Gynecological Endocrinology clinics at Christchurch Women's Hospital and Southern Women's Health since 2003. Referrals come from the community and from within a large tertiary referral hospital. Of the 302 women with POI the average age of onset is 33 years of age. In just 25% of the women was the cause unidentified.
Causes of premature ovarian insufficiency in a Christchurch, New Zealand database
Idiopathic – 25%.
Iatrogenic – 37%.
Autoimmune – 19%.
Genetic – 19%.
Fifty percent of the women diagnosed with POI before the age of 20 years were shown to have a genetic cause. Twenty-five percent had an FMR premutation; 70% had other X chromosome abnormalities, and 5% had miscellaneous mutations.
Hormone results at the time of diagnosis: A New Zealand database
The mean FSH level at diagnosis was 64.8 u/L, range 14-122.8.
The AMH level was undetectable in all women at baseline but rose in 15 women after chemotherapy. This was associated with a return of regular menstrual cycles. The time to ovarian recovery was 6 months 5 years and was seen primarily among women with breast and hematological malignancies.
Consequences
There is accumulating evidence of a number of adverse health outcomes for women reaching menopause earlier than ideal.
Symptoms
In women experiencing menopause induced by surgery or cancer treatment, the symptoms of estrogen deficiency are often more severe and longer lasting than seen in women experiencing a natural menopause. This is likely to be related to the rapid decline in hormone levels.
Cardiovascular
The Framingham study was one of the first to show a higher incidence of cardiovascular disease among postmenopausal women compared to age-matched women who were premenopausal.[16] A number of studies have subsequently demonstrated higher rates of coronary artery disease, greater severity of symptoms, higher rates of heart failure, and higher rates of mortality in women reaching menopause before 40-45 years of age.[17] Some observational studies have indicated an increased risk of stroke in women with POI. However, this is not a universal finding in all studies.[18,19,20,21,22,23,24,25,26] Hormone therapy appears to reduce the risk of cardiovascular disease in young women with POI.[17]
Bone health
Greater rates of bone loss and higher markers of bone turnover have been documented in women with POI, particularly after a bilateral salpingo-oophorectomy (BSO). Although reported effects on fracture risk have been variable, there have been more consistent increases in fracture risk seen among young women with POI. The effect of early ovarian demise on fracture risk may diminish by age 70 years.[27,28,29,30,31,32,33,34]
Cognitive and neurological health
Surgical removal of the ovaries prior to the normal age of menopause has been shown to increase the risk of developing cognitive impairment or dementia nearly two-fold in the Mayo Clinic Cohort Study of Oophorectomy and Aging. A trend of increasing risk with earlier age at oophorectomy was noted. An increased risk of Parkinsonism has been linked to both unilateral and bilateral oophorectomy before the natural age of menopause. The risk of glaucoma is increased in women undergoing BSO prior to the age of 43 years, and the risk of macular degeneration is increased in women undergoing early menopause following removal of one or both ovaries before the age of 45 years. It has been suggested that early estrogen loss accelerates aging of the optic nerve and increases susceptibility to glaucomatous changes.[17]
MENTAL HEALTH
The diagnosis of POI is life-altering and may lead to significant changes in mood. Studies have demonstrated lower self-esteem and higher rates of depression and anxiety. The risk of changes in mental health may be greater among young women.[17] The response to estrogen therapy appears to be variable.
Metabolic and bone health in a New Zealand database of premature ovarian insufficiency
At the time of diagnosis, the women had few metabolic complications:
At baseline, 1 woman had triglyceride levels >1.7 mmol/L and 2 had high-density lipoprotein levels <1 mmol/L. Normal lipid profiles were seen in all other women.
Average blood pressure at baseline was 120/70. This did not alter over the period of follow-up.
Average weight at baseline was 74.2 kg (BMI 27).
However:
At baseline, 31% of women had bone mass outside the normal age-matched range.
Despite the use of hormone therapy, bone density decreased in 50% after 2 years of therapy.
MANAGEMENT
Diagnosis
The diagnosis of POI needs to be handled with care and empathy, as it can be devastating for a young woman.
There must be 4-6 months of amenorrhea in a woman under the age of 40 years with elevated gonadotrophins and a low estradiol. The hormonal changes need to be seen on at least 2, if not more, occasions separated by a month. Measurement of AMH can be helpful in confirming a diagnosis. Ultrasound scans may confirm small ovaries with few antral follicles. Excluding a pregnancy is a step often overlooked.
The differential diagnosis of amenorrhea includes:
Pregnancy.
Polycystic ovarian syndrome.
Hypothalamic amenorrhea.
Pituitary disease.
Hypothyroidism and hyperthyroidism.
Uterine abnormalities.
Chronic medical illness secondary to poorly controlled diabetes or celiac disease.
Lifestyle habits (excessive exercise and poor caloric intake).
Therefore appropriate tests may include:
Pregnancy test.
Thyroid hormones, prolactin, early morning cortisol, and androgen levels.
Karyotype.
FMR genotype, particularly if there is a family history of POI or the woman is <30 years.
Autoantibody screen, including tissue and thyroid antibodies.
Vitamin B 12 and folic acid.
Anti-adrenal antibodies.
Anti-ovarian antibodies are not specific, and controversy exists as to whether they are the cause or an effect of the POI.
AMH is a member of the TGFβ superfamily and is secreted by the granulosa cells of the preantral and small antral follicles within the ovaries. It is used as a marker of ovarian reserve and has been considered to be relatively unaffected by other hormonal or health influences.[35] However, AMH levels have recently been shown to be lower in some women with cancer and those who are systemically unwell. It is lowered by the use of Gonadotropin-releasing hormone (GnRH) agonists and the contraceptive pill. After cancer treatment, an unmeasurable AMH 2 years after cancer therapy is usually associated with a low chance of menstruation returning. Measurement of AMH may be useful in determining appropriate treatment after breast cancer and guidelines have recently been published using a combination of factors to determine if menopause has indeed occurred.[36]
In Turner syndrome, women who have reached menarche, many of whom had mosaic karyotypes, an AMH of <3 pmol/L (<−2 standard deviation) has both high specificity and sensitivity in predicting POI.[37]
HORMONE THERAPY
Unless, there are contraindications women with POI should receive hormone therapy until the normal age of menopause.[38] The results of the Women's Health Initiative studies do not apply to women who have experienced early menopause.[39] There is evidence from a number of long-term observational studies that hormone therapy improves the quality of life and reduces the serious health consequences of POI.
Because of the sudden loss of ovarian hormones in those women who have undergone a surgical or chemo/radiotherapy-induced menopause higher than average doses of estrogen may be required to restore the quality of life. However, there is no data investigating optimal doses of estrogen. There is a theoretical advantage in using transdermal estrogen to preserve sexual function by minimizing any impact on SHBG levels. Cyclical progestogen therapy will in most cases lead to a monthly withdrawal bleed. The absence of this bleed serves as a signal of potential pregnancy in the small group that manages to conceive naturally and, therefore, may have an advantage over continuous bleed-free progestogen regimes. However, every woman is unique in her requirements, so careful titration of doses and appropriate choice of mode of hormone delivery need to be undertaken for each woman.
The oral contraceptive pill is an option for those women with residual ovarian function and in whom pregnancy is not desired.
For some women, there may be extra challenges in restoring the quality of life and androgen supplements may be considered to maintain sexual function and energy. However, the use of androgen supplements is not routinely advocated. There is a dearth of research including long-term follow-up studies in this population of women, and there is no form of testosterone specifically designed for use in women.
Fertility
Because of the variable nature of POI, there is still a 5-10% chance of spontaneous conception. There are however no guidelines to inform us as to the preferred treatment or approach to fertility. Studies have examined the role of clomiphene, gonadotrophins, GnRH agonists and antagonists, glucocorticoids and menopause hormone therapy.[40] No clear advantage has been shown with GnRH agonists. The use of dehydroepiandrosterone (DHEA) supplements has been promoted to increase the number of oocytes retrieved in assisted conception cycles, improve pregnancy rates, and decrease miscarriage rates. Recent studies have shown improvements in follicle counts, and hormone levels in the DHEA-treated groups but larger, longer-term studies are required. The contraceptive pill has been used prior to ovarian stimulation to lower FSH levels, but data are mixed. Many of the studies examining other options are not adequately powered and often not randomized. Donor egg pregnancies remain the primary option for most women.
SUPPORT
The diagnosis of POI can be devastating for many women, and care and sensitivity are required. Referral to a support group can be very useful and provides a chance for the young woman to discuss her concerns with other women in a similar situation. Because of the return of ovarian function for some women it is important to talk through the possible outcomes. Early referral for fertility support can be important.
THE FUTURE
In addition to better understanding of the pathogenesis of POI, the future holds the possibility of restoring ovarian function with stem cell therapy. Endometrial mesenchymal stem cells have been shown to successfully restore fertility in a mouse model, and work is now underway to take this research into human trials.[41] Preservation of ovarian function before or during cancer treatment is also a focus of research. The role of in vitro oocyte maturation or oocyte/ovarian cryopreservation remains limited. The recent POEMS study examined the effect of goserelin injections prior to and during chemotherapy for ER-negative breast cancer.[42] Eight percent of those young women who received goserelin developed ovarian failure compared to 22% in the placebo group. The pregnancy rate in the treated group was twice that of the placebo group. It is plausible that gonadotropin releasing hormone agonists cause a reduction in ovarian blood flow and perfusion while activating GnRH receptors on oocytes or granulosa cells. In turn, these biochemical pathways may lead to an upregulation of anti-apoptotic pathways and prevention of accelerated follicular atresia through interruption of FSH secretion.[43]
SUMMARY
POI is a common condition that can have far-reaching consequences for a woman's physical and mental health. Early diagnosis and management are crucial. Increasingly, we are seeing improved access to new diagnostic tools and more effective options to preserve ovarian function in young women being treated for cancer. We need to be able to move on from the concerns about the safety of hormone therapy in older women and focus on effective management to improve health outcomes for these young women. Sharing of information by researchers and the new international database for women with POI will improve our understanding and management of this condition.[44]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman's reproductive life: A twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–80. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 2.Murabito JM, Yang Q, Fox C, Wilson PW, Cupples LA. Heritability of age at natural menopause in the Framingham heart study. J Clin Endocrinol Metab. 2005;90:3427–30. doi: 10.1210/jc.2005-0181. [DOI] [PubMed] [Google Scholar]
- 3.Simpson JL. Genetic and phenotypic heterogeneity in ovarian failure: Overview of selected candidate genes. Ann N Y Acad Sci. 2008;1135:146–54. doi: 10.1196/annals.1429.019. [DOI] [PubMed] [Google Scholar]
- 4.Luisi S, Orlandini C, Regini C, Pizzo A, Vellucci F, Petraglia F. Premature ovarian insufficiency: From pathogenesis to clinical management. J Endocrinol Invest. 2015;38:597–603. doi: 10.1007/s40618-014-0231-1. [DOI] [PubMed] [Google Scholar]
- 5.Cox L, Liu JH. Primary ovarian insufficiency: An update. Int J Womens Health. 2014;6:235–43. doi: 10.2147/IJWH.S37636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastore LM, Johnson J. The FMR1 gene, infertility, and reproductive decision-making: A review. Front Genet. 2014;5:195. doi: 10.3389/fgene.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otsuka F, McTavish KJ, Shimasaki S. Integral role of GDF-9 and BMP-15 in ovarian function. Mol Reprod Dev. 2011;78:9–21. doi: 10.1002/mrd.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hudson MM. Reproductive outcomes for survivors of childhood cancer. Obstet Gynecol. 2010;116:1171–83. doi: 10.1097/AOG.0b013e3181f87c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: A prospective cohort study. BJOG. 2005;112:956–62. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 10.Atabekoglu C, Taskin S, Kahraman K, Gemici A, Taskin EA, Ozmen B, et al. The effect of total abdominal hysterectomy on serum anti-Müllerian hormone levels: A pilot study. Climacteric. 2012;15:393–7. doi: 10.3109/13697137.2011.642426. [DOI] [PubMed] [Google Scholar]
- 11.Fenton A, Panay N. Does routine gynecological surgery contribute to an early menopause? Climacteric. 2012;15:1–2. doi: 10.3109/13697137.2012.647623. [DOI] [PubMed] [Google Scholar]
- 12.Api M. Is ovarian reserve diminished after laparoscopic ovarian drilling? Gynecol Endocrinol. 2009;25:159–65. doi: 10.1080/09513590802585605. [DOI] [PubMed] [Google Scholar]
- 13.McKinlay SM, Bifano NL, McKinlay JB. Smoking and age at menopause in women. Ann Intern Med. 1985;103:350–6. doi: 10.7326/0003-4819-103-3-350. [DOI] [PubMed] [Google Scholar]
- 14.Morrison JC, Givens JR, Wiser WL, Fish SA. Mumps oophoritis: A cause of premature menopause. Fertil Steril. 1975;26:655–9. [PubMed] [Google Scholar]
- 15.Ohl J, Partisani M, Demangeat C, Binder-Foucard F, Nisand I, Lang JM. Alterations of ovarian reserve tests in Human immunodeficiency virus (HIV)-infected women. Gynecol Obstet Fertil. 2010;38:313–7. doi: 10.1016/j.gyobfe.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: The Framingham study. Ann Intern Med. 1976;85:447–52. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 17.Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483–91. doi: 10.3109/13697137.2015.1020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, et al. Oophorectomy vs ovarian conservation with hysterectomy: Cardiovascular disease, hip fracture, and cancer in the women's health initiative observational study. Arch Intern Med. 2011;171:760–8. doi: 10.1001/archinternmed.2011.121. [DOI] [PubMed] [Google Scholar]
- 19.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A. On Behalf of the Collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive Disorders. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol. 2014:pii: 2047487314556004. doi: 10.1177/2047487314556004. [DOI] [PubMed] [Google Scholar]
- 20.Alonso de Leciñana M, Egido JA, Fernández C, Martínez-Vila E, Santos S, Morales A, et al. Risk of ischemic stroke and lifetime estrogen exposure. Neurology. 2007;68:33–8. doi: 10.1212/01.wnl.0000250238.69938.f5. [DOI] [PubMed] [Google Scholar]
- 21.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010;17:506–10. doi: 10.1097/gme.0b013e3181c7dd41. [DOI] [PubMed] [Google Scholar]
- 22.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: A population-based cohort study. Eur Heart J. 2011;32:745–50. doi: 10.1093/eurheartj/ehq477. [DOI] [PubMed] [Google Scholar]
- 23.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: The Framingham heart study. Stroke. 2009;40:1044–9. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113:1027–37. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD., Jr Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19:272–7. doi: 10.1097/gme.0b013e31822a9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D. Early menopause predicts future coronary heart disease and stroke: The multi-ethnic study of atherosclerosis. Menopause. 2012;19:1081–7. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida T, Takahashi K, Yamatani H, Takata K, Kurachi H. Impact of surgical menopause on lipid and bone metabolism. Climacteric. 2011;14:445–52. doi: 10.3109/13697137.2011.562994. [DOI] [PubMed] [Google Scholar]
- 28.Hadjidakis DJ, Kokkinakis EP, Sfakianakis ME, Raptis SA. Bone density patterns after normal and premature menopause. Maturitas. 2003;44:279–86. doi: 10.1016/s0378-5122(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 29.Francucci CM, Romagni P, Camilletti A, Fiscaletti P, Amoroso L, Cenci G, et al. Effect of natural early menopause on bone mineral density. Maturitas. 2008;59:323–8. doi: 10.1016/j.maturitas.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 30.van der Klift M, de Laet CE, McCloskey EV, Johnell O, Kanis JA, Hofman A, et al. Risk factors for incident vertebral fractures in men and women: The Rotterdam study. J Bone Miner Res. 2004;19:1172–80. doi: 10.1359/JBMR.040215. [DOI] [PubMed] [Google Scholar]
- 31.van Der Voort DJ, van Der Weijer PH, Barentsen R. Early menopause: Increased fracture risk at older age. Osteoporos Int. 2003;14:525–30. doi: 10.1007/s00198-003-1408-1. [DOI] [PubMed] [Google Scholar]
- 32.Gärdsell P, Johnell O, Nilsson BE. The predictive value of bone loss for fragility fractures in women: A longitudinal study over 15 years. Calcif Tissue Int. 1991;49:90–4. doi: 10.1007/BF02565127. [DOI] [PubMed] [Google Scholar]
- 33.Melton LJ, 3rd, Crowson CS, Malkasian GD, O'Fallon WM. Fracture risk following bilateral oophorectomy. J Clin Epidemiol. 1996;49:1111–5. doi: 10.1016/0895-4356(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 34.Vesco KK, Marshall LM, Nelson HD, Humphrey L, Rizzo J, Pedula KL, et al. Surgical menopause and nonvertebral fracture risk among older US women. Menopause. 2012;19:510–6. doi: 10.1097/gme.0b013e318239caeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenton A, Panay N. Anti-Müllerian hormone - Is it a clinically useful test? Climacteric. 2013;16:1–2. doi: 10.3109/13697137.2013.756623. [DOI] [PubMed] [Google Scholar]
- 36.De Vos FY, van Laarhoven HW, Laven JS, Themmen AP, Beex LV, Sweep CG, et al. Menopausal status and adjuvant hormonal therapy for breast cancer patients: A practical guideline. Crit Rev Oncol Hematol. 2012;84:252–60. doi: 10.1016/j.critrevonc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP, et al. AMH as predictor of premature ovarian insufficiency: A longitudinal study of 120 turner syndrome patients. J Clin Endocrinol Metab. 2015;100:E1030–8. doi: 10.1210/jc.2015-1621. [DOI] [PubMed] [Google Scholar]
- 38.de Villiers TJ, Gass ML, Haines CJ, Hall JE, Lobo RA, Pierroz DD, et al. Global consensus statement on menopausal hormone therapy. Climacteric. 2013;16:203–4. doi: 10.3109/13697137.2013.771520. [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women's health initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Nagi J, Panay N. Premature ovarian insufficiency: How to improve reproductive outcome? Climacteric. 2014;17:242–6. doi: 10.3109/13697137.2013.860115. [DOI] [PubMed] [Google Scholar]
- 41.Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Transl Med. 2015;13:155. doi: 10.1186/s12967-015-0516-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerber B, Ortmann O. Prevention of early menopause study (POEMS): Is it possible to preserve ovarian function by gonadotropin releasing hormone analogs (GnRHa)? Arch Gynecol Obstet. 2014;290:1051–3. doi: 10.1007/s00404-014-3493-0. [DOI] [PubMed] [Google Scholar]
- 43.Blumenfeld Z. How to preserve fertility in young women exposed to chemotherapy. The role of GnRH agonist cotreatment in addition to cryopreservation of embrya, oocytes, or ovaries? Oncologist. 2007;12:1044–54. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 44.Panay N, Fenton A. Premature ovarian insufficiency: Working towards an international database. Climacteric. 2012;15:295–6. doi: 10.3109/13697137.2012.700846. [DOI] [PubMed] [Google Scholar]


