Abstract
Background:
Ovarian cancer is the third leading site of cancer among women, trailing behind cervix and breast cancer.
Aim:
This study was undertaken to analyze the immunohistochemical (IHC) profile of estrogen receptors (ER), progesterone receptors (PR), Ki-67, and p53 in various ovarian epithelial tumors and attempt correlation with clinical and histopathological findings.
Materials and Methods:
The present study was conducted over a period of 4 years. A technique of manual tissue array was employed for cases subjected for IHC. The primary antibodies used were ER, PR, p53, and Ki-67. A correlation was attempted between histopathological and IHC findings. Results were subjected to statistical analysis. Software program “the primer of biostatistics 5.0” was used for calculation of interrelationships between the analyzed ER, PR, p53, and Ki-67 expression and histological factors by Pearson's Chi-square test. The results were considered to be significant when the P < 0.05.
Results:
There were 110 cases of surface epithelial ovarian tumors (SEOT) encountered over the period of 4 years. The expression of ER was more in malignant tumors (13/16, 81.25%) than borderline (9/12, 75%) and benign (20/82, 24.39%). As compared to ER, the expression of PR was more in benign (51/82, 62.19%) than borderline (8/12, 66.67%) and malignant tumors (9/16, 56.25%). The expression of PR was more in benign tumors than borderline and malignant tumors. However, this was not statistically significant (Chi-square = 0.335 with 2 degrees of freedom; P = 0.846). The expression of p53 was less in benign (5/82, 6.1%) than borderline (9/12, 75%) and malignant tumors (13/16, 81.25%). The expression of Ki-67 was more in malignant (4/82, 4.88%) than borderline (10/12, 83.33%) and benign tumors (15/16, 93.75%). In all the above cases, the difference was statistically significant (P < 0.05). There was statistically significant difference in the expression of ER, PR, p53, and Ki-67 in the patients with age <40 years and above 40 years (P = 0.912). A positive correlation was observed in p53 expression and tumor grade. Similar correlation was seen in Ki-67 and tumor grade. It was also noted that mean Ki-67 labeling index (Li) had also increased with tumor grade. In the case of serous tumors, ER was expressed in all high- and low-grade tumors. The expression of PR was more in low-grade tumors than high-grade ones. P53 expression was seen in all high-grade tumors and 33.34% of low-grade tumor. The Ki-67 Li was more in high-grade tumors than low-grade tumors. Expression of ER, p53, and Ki-67 was higher in tumor showing metastasis. The mean Ki-67 Li was also higher in metastasizing tumors. However, PR expression was less in metastasizing tumors than nonmetastasizing tumors.
Conclusion:
IHC marker report of ER, PR status, and Ki-67 if included in each pathology report will pave the way for better understanding of biological behavior and modify treatment strategies.
Key Words: Estrogen receptors, immunohistochemical, Ki-67, p53, progesterone receptors, surface epithelial ovarian tumors
INTRODUCTION
Ovarian cancer is the sixth most common cancer and the seventh leading cause of cancer deaths among women worldwide. In most of the population-based cancer registries in India, ovarian cancer is the third leading site of cancer among women, trailing behind cervix and breast cancer. The age-adjusted incidence rates of ovarian cancer vary between 5.4 and 8.0/100,000 population in different parts of the country.[1]
World Health Organization (WHO) classifies ovarian tumors according to their most probable cell of origin and histomorphological features.[2] More than 90% of ovarian tumors are “epithelial” in origin. Some evidence suggest that the fallopian tube epithelial lining is the precursor lesion of some ovarian tumors.[3] However, the etiopathogenetic mechanisms are still under research.[2]
There have been persistent efforts in the investigation of molecular markers in epithelial ovarian tumors by immunohistochemical (IHC) studies.[2] Steroid hormones such as estrogen and progesterone are thought to play an important role in the process of carcinogenesis of ovarian tumors. Ovarian neoplasms are characterized by changes in their receptor status and consequently, tumor can be either primary receptor negative or as a result of their progression, they may lose the receptors.[4] On account of this, few workers have studied estrogen receptors (ER) and progesterone receptors (PR) status in ovarian neoplasms and correlated with various variables. Ki-67 is a proliferation marker helpful in predicting disease outcome in many types of malignancies including ovarian neoplasms.[5] Previous studies have shown that the p53 gene is mutated in 30-80% of ovarian carcinomas.[6] The role of immunostaining is now employed not only for diagnosis but also for other parameters including prognosis, microscopic tumor staging, prediction of response to therapy, and for the selection of therapeutic agents.
This study was undertaken to analyze the IHC profile of ER, PR, Ki-67, and p53 in various ovarian epithelial tumors and attempt correlation with clinicopathological and histopathological findings.
MATERIALS AND METHODS
This study included 110 cases of ovarian SEOT diagnosed histopathologically over a period of 4 years in a tertiary care hospital. The cases proved to be of primary epithelial ovarian origin as per radiological and pathological findings were included in the study. Available clinical data were analyzed. The gross examination details were obtained from files of surgical pathology section [Figure 1]. The criteria of “WHO histological classification of ovary” were followed for subtyping the lesions [Figure 2]. The representative sections were submitted for IHC staining. The following prediluted primary antibodies were used - ER (clone 6F11; Novacastra)[Figure 3b], PR (clone PGR312; Novacastra) [Figure 3c], p53 (clone DO-7; Dako [Figure 3a], and Ki-67 (clone MM-1; Novacastra) [Figure 3d]. Immunoreactivity for ER and PR was assessed in each case by estimating the percentage of cells showing nuclear staining. When at least 5% of the cells showed nuclear positivity, the case was considered positive. The reaction for p53 was recorded as either positive or negative. The nuclear staining for Ki-67 was graded by counting Ki-67 labeling index (Li) as a percent of positively stained tumor nuclei in 1000 tumor cells in the hot spot area of the tumor. The mean Ki-67 Li is recorded in every case. Software program “The Primer of Biostatistics 5.0” (manufactured by McGraw-Hill) was used for calculation of interrelationships among the analyzed ER, PR, Ki-67, and p53 expression and histological or clinical factors by Pearson's Chi-square test and Fisher exact test. The results were considered to be significant when the P < 0.05.
Figure 1.
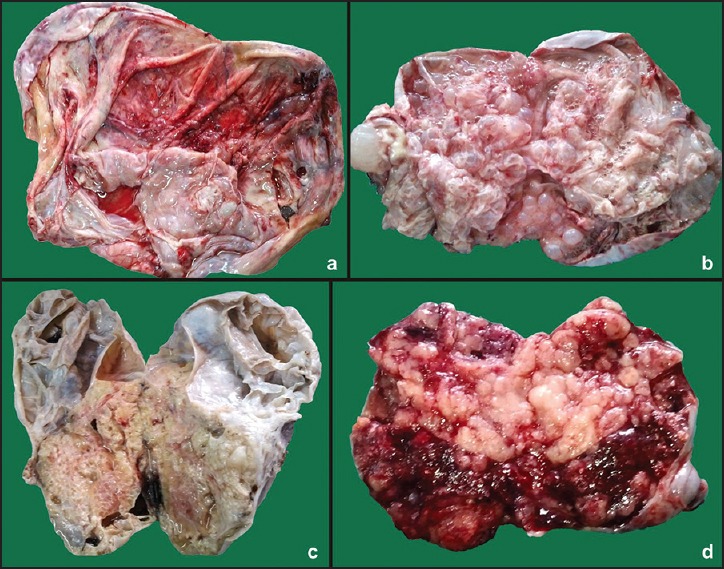
Gross photograph showing (a) Smooth glistening cut surface of serous cystadenoma, (b) Multilocular cut surface of malignant mucinous tumor with predominant solid and necrotic areas, (c) Multilocular cut surface of borderline mucinous tumor, and (d) Cut surface of endometrioid carcinoma having solid, hemorrhagic, and necrotic areas
Figure 2.
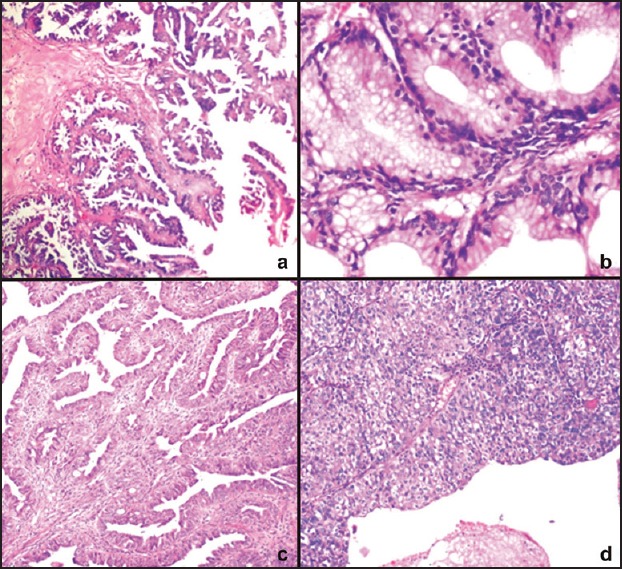
Photomicrograph of (a) Borderline serous tumor showing multiple papillary areas (H and E, ×100), (b) Borderline mucinous cystadenoma showing glandular budding (H and E, ×400), (c) Endometrioid carcinoma showing glandular and papillary areas (H and E, ×100), and (d) Transitional cell carcinoma showing papillary structures lined by urothelial-like lining (H and E, ×100)
Figure 3.
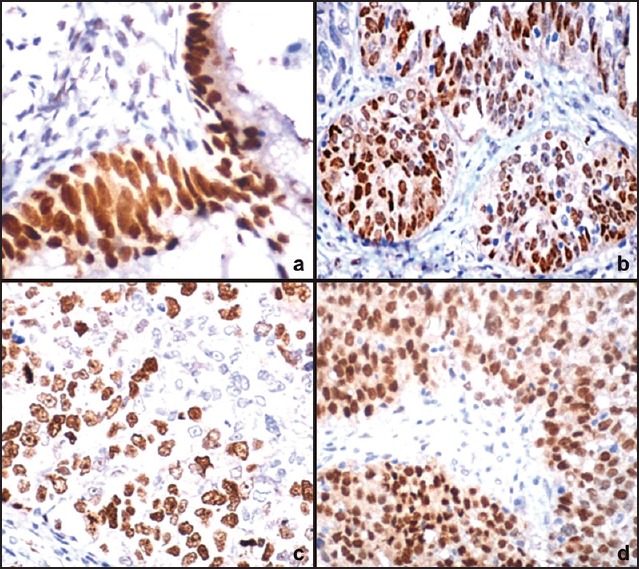
Photomicrograph showing (a) Strong p53 expression in borderline mucinous tumor (IHC, ×400), (b) Strong estrogen receptors immunoreactivity in endometrioid carcinoma (IHC, ×400), (c) Strong progesterone receptors immunoreactivity in endometrioid carcinoma (IHC, ×400), and (d) Strong nuclear positivity of Ki-67 in transitional cell carcinoma (IHC, ×400)
RESULTS
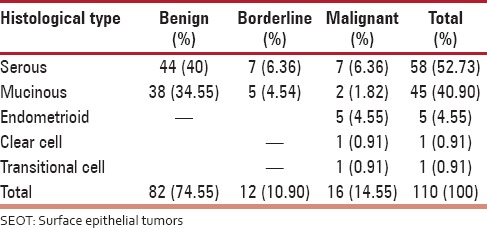
There were 110 cases of SEOT encountered over the period of 4 years [Table 1]. The peak incidence for benign tumors was in the third decade of life while for malignant tumors it was the fifth and sixth decades. In the present series, there were four high-grade serous malignant tumors and three were low grade. Out of total 9 malignant surface epithelial tumors (SET) (other than malignant serous tumors), 2 were Grade 1 tumors, 3 were Grade 2, and 4 were Grade 3 tumors. There were 11 tumors which belonged to FIGO stage I. The category of FIGO III and IV had two and three tumors, respectively. There were five tumors which showed metastasis accounting for 31.25% of all malignant tumors. Ascites was present in all the cases showing metastasis. Fluid cytological examination correlated well with four of them showing tumor cells in the fluid.
Table 1.
Distribution of SEOT according to histopathological subtypes (n = 110)
The expression of ER was more in malignant tumors (13/16, 81.25%) than borderline (9/12, 75%) and benign (20/82, 24.39%), and this was statistically significant (Chi-square = 26.073 with 2 degrees of freedom; P < 0.05) [Tables 2 and 3]. As compared to ER, the expression of PR was more in benign (51/82, 62.19%) than borderline (8/12, 66.67%) and malignant tumors (9/16, 56.25%). This correlation was statistically significant (Chi-square = 43.016 with 6 degrees of freedom; P < 0.05). The expression of PR was more in benign than borderline and malignant tumors. However, this was statistically not significant (Chi-square = 0.335 with 2 degrees of freedom; P = 0.846). The expression of p53 was less in benign (5/82, 6.1%) than borderline (9/12, 75%) and malignant tumors (13/16, 81.25%). This difference was statistically significant (Chi-square = 59.340 with 2 degrees of freedom; P < 0.05). The expression of Ki-67 was more in malignant (4/82, 4.88%) than borderline (10/12, 83.33%) and benign tumors (15/16, 93.75%). This difference was statistically significant (Chi-square = 76.986 with 2 degrees of freedom; P < 0.05).
Table 2.
Expression of immunohistochemical markers in benign, borderline, and malignant SEOT
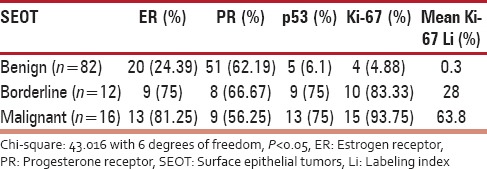
Table 3.
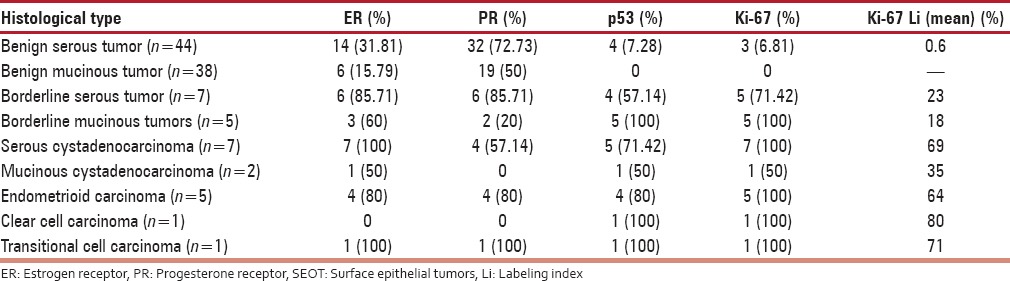
Expression of various immunohistochemical markers in histological subtypes of SEOT
There is statistically significant difference in the expression of ER, PR, p53, and Ki-67 in the patients with age <40 years and above 40 years (Chi-square = 1.571 with 3 degrees of freedom; P = 0.912) [Table 4]. There was no correlation found between the grade of tumor and expression of ER and PR status. However, a positive correlation was observed in expression of p53 and tumor grade. Similar correlation was seen in Ki-67 and tumor grade. It was also noted that mean Ki-67 Li had also increased with grade of tumor [Table 5].
Table 4.
Comparison of expression of immunohistochemical markers and age of patient*

Table 5.
Expression of immunohistochemical markers in Grade 1, Grade 2, and Grade 3 malignant SEOT

In the case of serous tumors, ER was expressed in all high- and low-grade tumors. The expression of PR was more in low-grade tumors than high-grade ones. p53 expression was seen in all high-grade tumors and 33.34% of low-grade tumor. The Ki-67 Li was more in high-grade tumors than low-grade tumors.
Expression of IHC markers ER, p53, and Ki-67 was higher in tumor showing metastasis. The mean Ki-67 Li was also higher in metastasizing tumors. However, expression of PR was less in metastasizing tumors than nonmetastasizing tumors [Table 6].
Table 6.
Expression of immunohistochemical markers and metastasis

DISCUSSION
In the present study, 110 cases of SET were distributed in age range of 14-75years similar to one of the Indian authors.[7] It can be observed that benign tumors occur in younger age group while malignant SET occur in the fifth and sixth decades. The age wise distribution of various tumor types is comparable with previous studies.[2,7,8] This could be explained by the suggestion that transformation from the benign to the malignant tumor type may occur over time occasionally in a few tumors by progressive increase in the degree of epithelial abnormality.
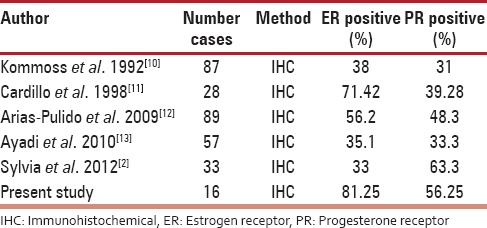
The expression of ER was more in malignant tumors (81.25%) than borderline (75%) and benign (24.39%). This is parallel to study done by Sylvia et al.[2] This may support the role of estrogen in oncogenesis. The expression of PR was more in benign than borderline and malignant tumors. This probably indicates the protective effect of progesterone in the development of ovarian carcinomas. Similar to other studies, the present series also showed higher expression of ER in malignant cases than PR. However, one of the studies showed more expression of PR than ER[2] [Table 7].
Table 7.
Studies done on expression of hormone receptors (estrogen receptor and progesterone receptor) in ovarian cancers
ER positivity was more frequently found in transitional cell carcinoma (100%), endometrioid carcinoma (80%), followed by serous tumors (46.55%) and mucinous tumors (26.66%). PR expression showed similar distribution. Expression of PR in transitional cell carcinoma, endometrioid carcinoma, serous tumors, and mucinous tumors was 100%, 80%, 72.41%, and 51.11%, respectively. From above findings, it can be interpreted that serous was the most common subtype among the epithelial ovarian tumors. Furhtermore, it showed more consistent expression of IHC markers. This may be the reason many studies have included only serous tumors in their studies.[4] It must be noted that, like other studies,[2,6,9] endometrioid tumor expressed positive ER and PR status in majority (80%) cases and the clear cell carcinoma had negative ER PR status.
In the present series, the expression of p53 was more in malignant [Figure 3a] than borderline and benign tumors. These results were in agreement with many other studies.[2] However, many authors suggest that the expression of p53 in benign tumors is virtually nonexistent, but in the present series, p53 immunoreactivity was seen in 6.1% cases.
Ki-67, a proliferative marker, has been studied by many workers in malignant ovarian tumors. The labeling index calculated by counting number of cells showing nuclear positivity in 1000 cells at the hot spot was used to compare the intensity of immunoreactivity of this marker. Majority of the studies in literature showed that the Ki-67 Li is the highest in malignant tumors which is followed by borderline tumors and the lowest in benign tumors.[2,6]
Gursan et al.[6] demonstrated that the mean Ki-67 Li in benign tumors was 14.9% (0.7-32.5%); in borderline tumors, it was 22.8% (0.2-61.2%); in malignant tumors, it was 42.8% (0.4-84.2%). When compared with the benign tumors, Ki-67 Li was found to be significantly increased in the malignant tumors. In the present study, similar results were obtained viz., the mean Ki-67 Li for benign, borderline, and malignant was 0.3%, 28%, and 63.8%, respectively.
There was no statistically significant correlation between age of patient and ER PR status; however, one of the study found such correlation. This may be because of distribution of cases as; the study had more number of malignant cases in the evaluation.[2] However, the present study had more number of benign cases.
In the present series, there was no correlation between expression of ER, PR, and grade of malignant tumor. Similar results were obtained by few workers.[2,6] In tumors showing metastasis, i.e., expression of ER was more as compared to tumors not showing metastasis and the expression of PR was less in metastasizing tumors than nonmetastasizing. Many studies in literature revealed a positive correlation of p53 expression with grading system of malignant ovarian tumors. Similar results were observed in this series. This may suggest that p53 can be used as a prognostic marker. The Ki-67 positivity correlates well with nuclear grade and aggressive behavior of the tumor. This may be helpful in predicting treatment outcomes in ovarian cancer.
It is felt that this important marker should be studied routinely in all cases of ovarian cancer. IHC marker report of ER, PR status and Ki-67 if included in each pathology report will greatly help treatment strategies. The findings in this study compared well in features and proportions to results of workers. If used in right perspective, IHC studies will impact the patient management. This interesting field needs to be shifted from research laboratories to clinical histopathology laboratories for better patient care.
SUMMARY AND CONCLUSION
It was also noted that the peak incidence for benign tumors was in the third decade of life while for malignant tumors it was the fifth and sixth decades. Expression of ER was more in malignant tumors than borderline and benign tumors. As compared to ER, the expression of PR was more in benign than borderline and malignant tumors. p53 was expressed more often in malignant tumors followed by borderline and benign tumors. The mean Ki-67 labeling index was the highest in malignant followed by borderline and benign tumors; it correlated well with aggressive cytomorphology. The findings of this study indicate that IHC marker report of ER, PR status and Ki-67 if included in each pathology report will pave the way for better understanding of biological behavior and modify treatment strategies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Basu P, De P, Mandal S, Ray K, Biswas J. Study of ‘patterns of care’ of ovarian cancer patients in a specialized cancer institute in Kolkata, Eastern India. Indian J Cancer. 2009;46:28–33. doi: 10.4103/0019-509x.48592. [DOI] [PubMed] [Google Scholar]
- 2.Sylvia MT, Kumar S, Dasari P. The expression of immunohistochemical markers estrogen receptor, progesterone receptor, Her-2-neu, p53 and Ki-67 in epithelial ovarian tumors and its correlation with clinicopathologic variables. Indian J Pathol Microbiol. 2012;55:33–7. doi: 10.4103/0377-4929.94852. [DOI] [PubMed] [Google Scholar]
- 3.Piek JM, van Diest PJ, Verheijen RH. Ovarian carcinogenesis: An alternative hypothesis. Adv Exp Med Biol. 2008;622:79–87. doi: 10.1007/978-0-387-68969-2_7. [DOI] [PubMed] [Google Scholar]
- 4.Buchynska LG, Iurchenko NP, Grinkevych VM, Nesina IP, Chekhun SV, Svintsitsky VS. Expression of the estrogen and progesterone receptors as prognostic factor in serous ovarian cancers. Exp Oncol. 2009;31:48–51. [PubMed] [Google Scholar]
- 5.Hall PA, Levison DA. Review: Assessment of cell proliferation in histological material. J Clin Pathol. 1990;43:184–92. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gursan N, Sipal S, Calik M, Gundogdu C. P53, bcl-2, ki-67 li (labeling index) status in benign, proliferative, and malignant ovarian surface epithelial neoplasms. Eurasian J Med. 2009;41:10–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Maheshwari V, Tyagi SP, Saxena K, Tyagi N, Sharma R, Aziz M, et al. Surface epithelial tumours of the ovary. Indian J Pathol Microbiol. 1994;37:75–85. [PubMed] [Google Scholar]
- 8.Merino MJ, Jaffe G. Age contrast in ovarian pathology. Cancer. 1993;71(2 Suppl):537–44. doi: 10.1002/cncr.2820710208. [DOI] [PubMed] [Google Scholar]
- 9.Soslow RA. Histologic subtypes of ovarian carcinoma: An overview. Int J Gynecol Pathol. 2008;27:161–74. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 10.Kommoss F, Pfisterer J, Thome M, Schäfer W, Sauerbrei W, Pfleiderer A. Steroid receptors in ovarian carcinoma: Immunohistochemical determination may lead to new aspects. Gynecol Oncol. 1992;47:317–22. doi: 10.1016/0090-8258(92)90133-4. [DOI] [PubMed] [Google Scholar]
- 11.Cardillo MR, Petrangeli E, Aliotta N, Salvatori L, Ravenna L, Chang C, et al. Androgen receptors in ovarian tumors: correlation with oestrogen and progesterone receptors in an immunohistochemical and semiquantitative image analysis study. J Exp Clin Cancer Res. 1998;17:231–7. [PubMed] [Google Scholar]
- 12.Arias-Pulido H, Smith HO, Joste NE, Bocklage T, Qualls CR, Chavez A, et al. Estrogen and progesterone receptor status and outcome in epithelial ovarian cancers and low malignant potential tumors. Gynecol Oncol. 2009;114:480–5. doi: 10.1016/j.ygyno.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayadi L, Chaabouni S, Khabir A, Amouri H, Makni S, Guermazi M, et al. Correlation between immunohistochemical biomarkers expression and prognosis of ovarian carcinomas in Tunasian patients. World J Oncol. 2010;1:118–28. doi: 10.4021/wjon2010.06.213w. [DOI] [PMC free article] [PubMed] [Google Scholar]


