Abstract
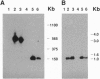
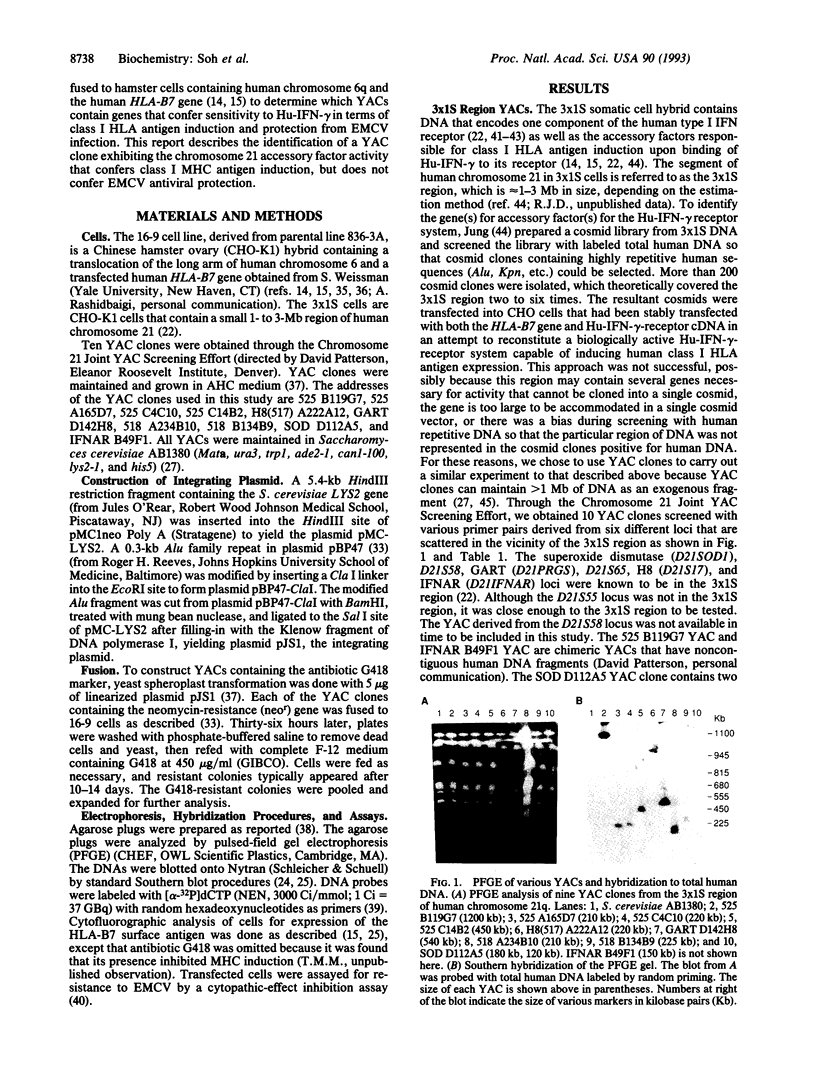
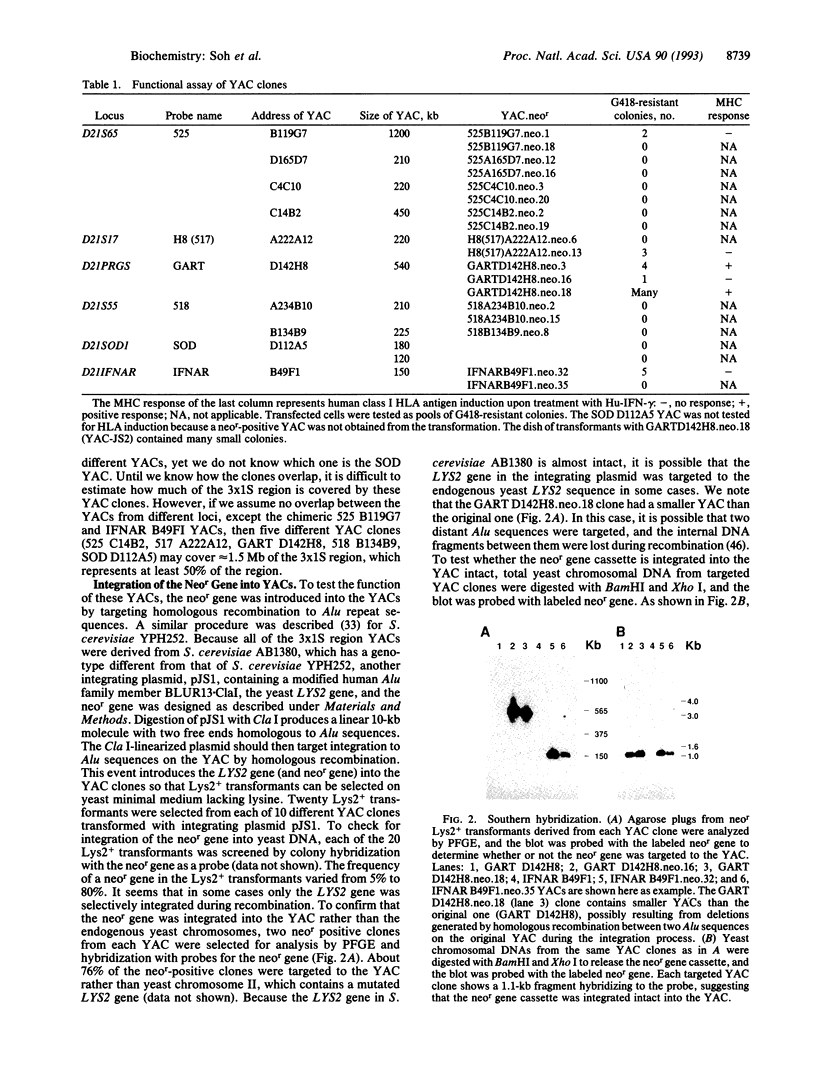
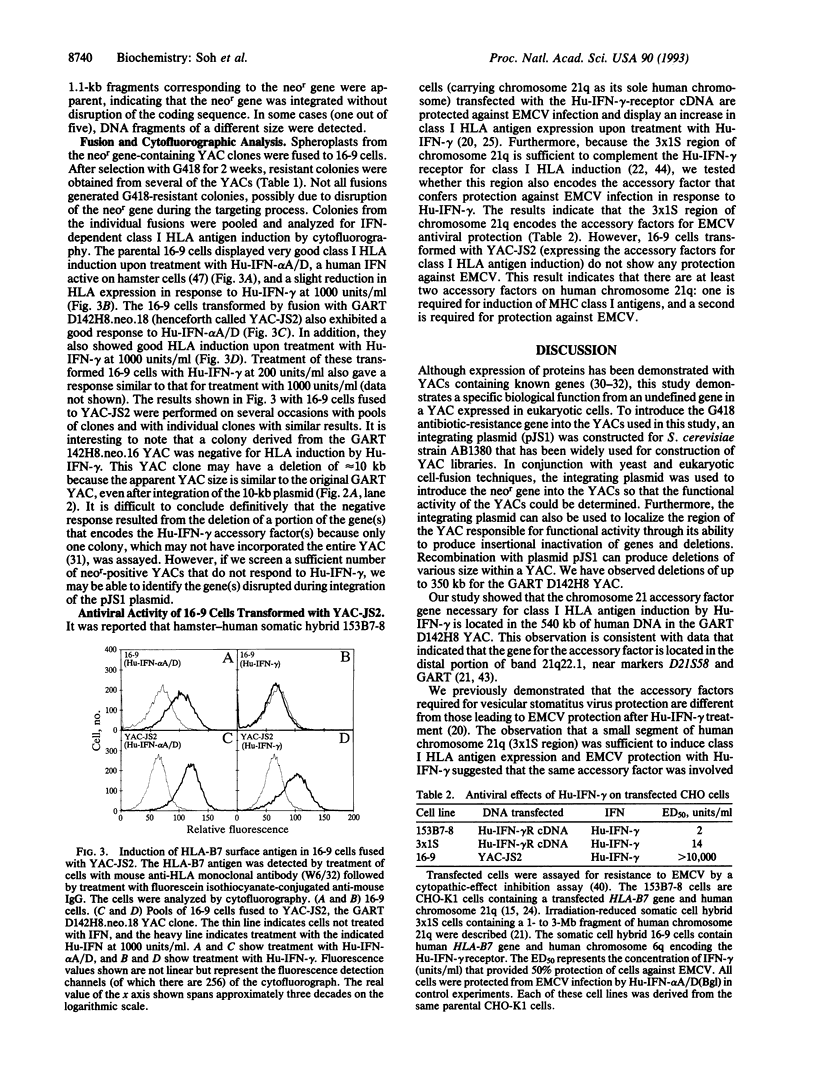
Human chromosomes 6 and 21 are both necessary to confer sensitivity to human interferon gamma (Hu-IFN-gamma), as measured by the induction of human HLA class I antigen. Human chromosome 6 encodes the receptor for Hu-IFN-gamma, and human chromosome 21 encodes accessory factors for generating biological activity through the Hu-IFN-gamma receptor. A small region of human chromosome 21 that is responsible for encoding such factors was localized with hamster-human somatic cell hybrids carrying an irradiation-reduced fragment of human chromosome 21. The cell line with the minimum chromosome 21-specific DNA is Chinese hamster ovary 3x1S. To localize the genes further, 10 different yeast artificial chromosome clones from six different loci in the vicinity of the 3x1S region were fused to a human-hamster hybrid cell line (designated 16-9) that contains human chromosome 6q (supplying the Hu-IFN-gamma receptor) and the human HLA-B7 gene. These transformed 16-9 cells were assayed for induction of class I HLA antigens upon treatment with Hu-IFN-gamma. Here we report that a 540-kb yeast artificial chromosome encodes the necessary species-specific factor(s) and can substitute for human chromosome 21 to reconstitute the Hu-IFN-gamma-receptor-mediated induction of class I HLA antigens. However, the factor encoded on the yeast artificial chromosome does not confer antiviral protection against encephalomyocarditis virus, demonstrating that an additional factor encoded on human chromosome 21 is required for the antiviral activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Georgiades J. A., Langford M. P., Johnson H. M. Purified human immune interferon has more potent anticellular activity than fibroblast or leukocyte interferon. Cell Immunol. 1980 Feb;49(2):390–394. doi: 10.1016/0008-8749(80)90041-6. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Chumakov I., Rigault P., Guillou S., Ougen P., Billaut A., Guasconi G., Gervy P., LeGall I., Soularue P., Grinas L. Continuum of overlapping clones spanning the entire human chromosome 21q. Nature. 1992 Oct 1;359(6394):380–387. doi: 10.1038/359380a0. [DOI] [PubMed] [Google Scholar]
- Cook J. R., Jung V., Schwartz B., Wang P., Pestka S. Structural analysis of the human interferon gamma receptor: a small segment of the intracellular domain is specifically required for class I major histocompatibility complex antigen induction and antiviral activity. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11317–11321. doi: 10.1073/pnas.89.23.11317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Urso M., Zucchi I., Ciccodicola A., Palmieri G., Abidi F. E., Schlessinger D. Human glucose-6-phosphate dehydrogenase gene carried on a yeast artificial chromosome encodes active enzyme in monkey cells. Genomics. 1990 Aug;7(4):531–534. doi: 10.1016/0888-7543(90)90196-2. [DOI] [PubMed] [Google Scholar]
- Duyk G. M., Kim S. W., Myers R. M., Cox D. R. Exon trapping: a genetic screen to identify candidate transcribed sequences in cloned mammalian genomic DNA. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8995–8999. doi: 10.1073/pnas.87.22.8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin P., Slynn G., Black D., Graham A., Butler R., Riley J., Anand R., Markham A. F. Isolation of cDNA clones using yeast artificial chromosome probes. Nucleic Acids Res. 1990 Jul 11;18(13):3913–3917. doi: 10.1093/nar/18.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familletti P. C., Rubinstein S., Pestka S. A convenient and rapid cytopathic effect inhibition assay for interferon. Methods Enzymol. 1981;78(Pt A):387–394. doi: 10.1016/0076-6879(81)78146-1. [DOI] [PubMed] [Google Scholar]
- Farrar M. A., Campbell J. D., Schreiber R. D. Identification of a functionally important sequence in the C terminus of the interferon-gamma receptor. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11706–11710. doi: 10.1073/pnas.89.24.11706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Katona I. M., Mosmann T. R., Coffman R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988 Feb 15;140(4):1022–1027. [PubMed] [Google Scholar]
- Gao J., Erickson P., Gardiner K., Le Beau M. M., Diaz M. O., Patterson D., Rowley J. D., Drabkin H. A. Isolation of a yeast artificial chromosome spanning the 8;21 translocation breakpoint t(8;21)(q22;q22.3) in acute myelogenous leukemia. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4882–4886. doi: 10.1073/pnas.88.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs V. C., Williams S. R., Gray P. W., Schreiber R. D., Pennica D., Rice G., Goeddel D. V. The extracellular domain of the human interferon gamma receptor interacts with a species-specific signal transducer. Mol Cell Biol. 1991 Dec;11(12):5860–5866. doi: 10.1128/mcb.11.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnirke A., Barnes T. S., Patterson D., Schild D., Featherstone T., Olson M. V. Cloning and in vivo expression of the human GART gene using yeast artificial chromosomes. EMBO J. 1991 Jul;10(7):1629–1634. doi: 10.1002/j.1460-2075.1991.tb07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi S., Merlin G., Aguet M. Functional characterization of a hybrid human-mouse interferon gamma receptor: evidence for species-specific interaction of the extracellular receptor domain with a putative signal transducer. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2737–2741. doi: 10.1073/pnas.89.7.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino Y., Kumar C. S., Mariano T. M., Lai D. H., Pestka S. Chimeric interferon-gamma receptors demonstrate that an accessory factor required for activity interacts with the extracellular domain. J Biol Chem. 1992 Feb 25;267(6):3741–3749. [PubMed] [Google Scholar]
- Hibino Y., Mariano T. M., Kumar C. S., Kozak C. A., Pestka S. Expression and reconstitution of a biologically active mouse interferon gamma receptor in hamster cells. Chromosomal location of an accessory factor. J Biol Chem. 1991 Apr 15;266(11):6948–6951. [PubMed] [Google Scholar]
- Huxley C., Hagino Y., Schlessinger D., Olson M. V. The human HPRT gene on a yeast artificial chromosome is functional when transferred to mouse cells by cell fusion. Genomics. 1991 Apr;9(4):742–750. doi: 10.1016/0888-7543(91)90369-p. [DOI] [PubMed] [Google Scholar]
- Jung V., Jones C., Kumar C. S., Stefanos S., O'Connell S., Pestka S. Expression and reconstitution of a biologically active human interferon-gamma receptor in hamster cells. J Biol Chem. 1990 Feb 5;265(4):1827–1830. [PubMed] [Google Scholar]
- Jung V., Jones C., Rashidbaigi A., Geyer D. D., Morse H. G., Wright R. B., Pestka S. Chromosome mapping of biological pathways by fluorescence-activated cell sorting and cell fusion: human interferon gamma receptor as a model system. Somat Cell Mol Genet. 1988 Nov;14(6):583–592. doi: 10.1007/BF01535312. [DOI] [PubMed] [Google Scholar]
- Jung V., Rashidbaigi A., Jones C., Tischfield J. A., Shows T. B., Pestka S. Human chromosomes 6 and 21 are required for sensitivity to human interferon gamma. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4151–4155. doi: 10.1073/pnas.84.12.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina U., Ozmen L., Di Padova K., Gentz R., Garotta G. The human gamma interferon receptor accessory factor encoded by chromosome 21 transduces the signal for the induction of 2',5'-oligoadenylate-synthetase, resistance to virus cytopathic effect, and major histocompatibility complex class I antigens. J Virol. 1993 Mar;67(3):1702–1706. doi: 10.1128/jvi.67.3.1702-1706.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C. S., Muthukumaran G., Frost L. J., Noe M., Ahn Y. H., Mariano T. M., Pestka S. Molecular characterization of the murine interferon gamma receptor cDNA. J Biol Chem. 1989 Oct 25;264(30):17939–17946. [PubMed] [Google Scholar]
- Langer J. A., Pestka S. Interferon receptors. Immunol Today. 1988 Dec;9(12):393–400. doi: 10.1016/0167-5699(88)91241-8. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Rashidbaigi A., Lai L. W., Patterson D., Jones C. Sublocalization on chromosome 21 of human interferon-alpha receptor gene and the gene for an interferon-gamma response protein. Somat Cell Mol Genet. 1990 May;16(3):231–240. doi: 10.1007/BF01233359. [DOI] [PubMed] [Google Scholar]
- Levine F., Erlich H., Mach B., Leach R., White R., Pious D. Deletion mapping of HLA and chromosome 6p genes. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3741–3745. doi: 10.1073/pnas.82.11.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett M., Kere J., Hinton L. M. Direct selection: a method for the isolation of cDNAs encoded by large genomic regions. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9628–9632. doi: 10.1073/pnas.88.21.9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfalla G., Gardiner K., Proudhon D., Vielh E., Uzé G. The structure of the human interferon alpha/beta receptor gene. J Biol Chem. 1992 Feb 5;267(4):2802–2809. [PubMed] [Google Scholar]
- Lutfalla G., Roeckel N., Mogensen K. E., Mattei M. G., Uzé G. Assignment of the human interferon-alpha receptor gene to chromosome 21q22.1 by in situ hybridization. J Interferon Res. 1990 Oct;10(5):515–517. doi: 10.1089/jir.1990.10.515. [DOI] [PubMed] [Google Scholar]
- Mariano T. M., Kozak C. A., Langer J. A., Pestka S. The mouse immune interferon receptor gene is located on chromosome 10. J Biol Chem. 1987 Apr 25;262(12):5812–5814. [PubMed] [Google Scholar]
- Pachnis V., Pevny L., Rothstein R., Costantini F. Transfer of a yeast artificial chromosome carrying human DNA from Saccharomyces cerevisiae into mammalian cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5109–5113. doi: 10.1073/pnas.87.13.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Generation of deletion derivatives by targeted transformation of human-derived yeast artificial chromosomes. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1300–1304. doi: 10.1073/pnas.87.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavan W. J., Hieter P., Reeves R. H. Modification and transfer into an embryonal carcinoma cell line of a 360-kilobase human-derived yeast artificial chromosome. Mol Cell Biol. 1990 Aug;10(8):4163–4169. doi: 10.1128/mcb.10.8.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Langer J. A., Zoon K. C., Samuel C. E. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Rashidbaigi A., Langer J. A., Jung V., Jones C., Morse H. G., Tischfield J. A., Trill J. J., Kung H. F., Pestka S. The gene for the human immune interferon receptor is located on chromosome 6. Proc Natl Acad Sci U S A. 1986 Jan;83(2):384–388. doi: 10.1073/pnas.83.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehberg E., Kelder B., Hoal E. G., Pestka S. Specific molecular activities of recombinant and hybrid leukocyte interferons. J Biol Chem. 1982 Oct 10;257(19):11497–11502. [PubMed] [Google Scholar]
- Rothermel C. D., Rubin B. Y., Murray H. W. Gamma-interferon is the factor in lymphokine that activates human macrophages to inhibit intracellular Chlamydia psittaci replication. J Immunol. 1983 Nov;131(5):2542–2544. [PubMed] [Google Scholar]
- Smith C. L., Lawrance S. K., Gillespie G. A., Cantor C. R., Weissman S. M., Collins F. S. Strategies for mapping and cloning macroregions of mammalian genomes. Methods Enzymol. 1987;151:461–489. doi: 10.1016/s0076-6879(87)51038-2. [DOI] [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S., Klimpel G. R., Fleischmann W. R., Jr, Baron S. Direct cytolysis by partially-purified preparations of immune interferon. Int J Cancer. 1982 Jul 15;30(1):59–64. doi: 10.1002/ijc.2910300111. [DOI] [PubMed] [Google Scholar]
- Wheelock E. F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science. 1965 Jul 16;149(3681):310–311. [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Lengyel P., Reddy E. S., Morgan W. R., Weissman S. M. Transcripts of human HLA gene fragments lacking the 5'-terminal region in transfected mouse cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):649–653. doi: 10.1073/pnas.81.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O., Schmidt H., Reddy E. S., Weissman S., Lengyel P. Mouse interferons enhance the accumulation of a human HLA RNA and protein in transfected mouse and hamster cells. J Biol Chem. 1982 Nov 25;257(22):13169–13172. [PubMed] [Google Scholar]