Abstract
Background
Incretins are hormones produced by the intestine and can stimulate the secretion of insulin, helping to diminish the post-prandial glycemia. The administration of an emulsion of palm oil can help in the maintenance of the weight, and can increase circulating incretins levels. Glutamine increases the concentration of incretins in diabetic people. Both can help in metabolic syndrome.
Aim
To analyze the effects of ingestion of palm oil and glutamine in glycemia and in incretins in patients with diabetes submitted to surgical duodenojejunal exclusion with ileal interposition without gastrectomy.
Methods
Eleven diabetic type 2 patients were included and were operated. They were called to laboratory follow-up without eating anything between eight and 12 hours. They had there blood collected after the stimulus of the palm oil and glutamine taken in different days. For the hormonal doses were used ELISA kits.
Results
The glycemia showed a meaningful fall between the fast and two hours after the stimulus of the palm oil (p=0,018). With the glutamine the GLP-1 showed an increase between the fast and one hour (p=0,32), the PYY showed an important increase between the fast and one hour after the stimulus (p=0,06), the glycemia showed a meaningful fall after two hours of the administration of the stimulus (p=0,03).
Conclusion
Palm oil and glutamine can influence intestinal peptides and glucose
Keywords: Diabetes Mellitus, Palm oil, Glutamine
Abstract
Racional
A administração de óleo de palma auxilia na manutenção do peso e aumenta níveis de incretinas circulantes. A glutamina aumenta a concentração de incretinas em indivíduos diabéticos. Assim, eles podem influenciar no tratamento da síndrome metabólica.
Objetivo
Analisar os efeitos da ingestão de óleo de palma e glutamina na glicemia e incretinas em pacientes diabéticos que foram submetidos à operação de exclusão duodenojejunal com interposição ileal sem gastrectomia.
Métodos
Participaram 11 pacientes, portadores de diabete melito tipo 2, que foram operados com exclusão duodenojejunal com interposição ileal sem gastrectomia. Foram convocados para comparecer ao laboratório em jejum de oito a 12 horas e submetidos ao procedimento de coleta de sangue após os estímulos de óleo de palma e glutamina via oral em dias distintos. Para as dosagens hormonais foram utilizados kits de ELISA.
Resultados
A glicemia apresentou queda significativa entre o jejum e duas horas após o estímulo de óleo de palma (p=0,018). Com a glutamina, o GLP-1 apresentou aumento entre o jejum e uma hora (p=0,32); o PYY apresentou aumento entre o jejum e uma hora após o estímulo (p=0,06); a glicemia apresentou queda significativa após duas horas da administração do estímulo (p=0,03).
Conclusão
O óleo de palma e a glutamina podem influenciar os peptídeos intestinais e na glicemia.
INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a complex metabolic disorder whose clinical manifestations include hyperglycemia and its complications18. The disease is characterized by resistance to insulin and progressive failure of beta cells11 and hyperglycemia plays an essential role in the development of diabetic complications3. About 285 million adults worldwide are estimated to have diabetes, 85% to 95% of them with T2DM19. Conventional methods of bariatric surgery and new gastrointestinal surgical techniques can cause long-term remission of diabetes and improve other metabolic disorders such as hyperlipidemia and hypertension in non-obese patients22.
Metabolic surgery is currently defined as any modification of the gastrointestinal tract in which the passage and rerouting of food permits the improvement of T2DM, irrespective of mechanisms of weight loss. Although not the standard treatment for T2DM, this surgical procedure has become close to ideal. Studies have shown that metabolic surgery is a reasonable alternative for diabetic patients with a body mass index (BMI) <35 kg/m2 who do not respond to standard therapy25.
The exclusion of the duodenum and part of jejunum alters food transit, leading to early arrival of undigested or partially digested food in the ileum which, in turn, causes changes in the secretion of gastrointestinal hormones21.
Incretins are hormones produced by the intestine that stimulate the secretion of insulin and consequently reduce postprandial glycemia12. About two-thirds of the insulin response to oral glucose is the result of the potentiation of incretin action1. In normal subjects, insulin secretion is higher when glucose is ingested orally compared to the same load of intravenous glucose, an effect known as the incretin effect18. Incretins facilitate the uptake of glucose by the liver and simultaneously suppress the secretion of glucagon by pancreatic alpha-cells, thus reducing endogenous glucose production in the liver8. The main incretins are gastric inhibitory polypeptide (GIP), also known as glucose-dependent insulinotropic polypeptide, and glucagon-like peptide 1 (GLP-1). After the discovery of the actions of these hormones, advances occurred in the treatment of T2DM in terms of incretin-based glucose-lowering medications4.
Peptides produced by the intestine also regulate appetite and food intake and, through their effect on the hypothalamus, can induce a sensation of satiety20. GLP-1 is a prohormone consisting of 160 amino acids, which is produced by L-cells of the distal intestine, alpha-cells of the pancreatic islets, and the central nervous system26. This hormone is secreted in response to the ingestion of nutrients, with glucose and triacylglycerols being the main components stimulating this hormone. However, fructose and other proteins can also induce the secretion of GLP-112.
Peptide YY (PYY) consists of a chain of 36 amino acids and is produced by L-cells of the enteroendocrine epithelium of the intestine. Increased concentrations of this hormone are observed in the distal parts of the intestine such as colon and rectum. PYY acts on distant target tissues and its various functions include the delay of gastric emptying10.
Palm oil is derived from the mesocarp of fruits of the palm tree Elaeis guineensis24. The oil contains a fraction rich in vitamin E and tocotrienol. The latter is a potent antioxidant that contributes to the treatment of diabetes by reducing oxidative stress resulting from hyperglycemia3. Diepvens et al.7 observed that the administration of an emulsion of fractionated palm oil (40%) and fractional oat oil (2.5%) in water contributes to weight maintenance, in addition to increasing circulating GLP-1 levels.
Glutamine is an L-α-amino acid that can be synthesized by any tissue of the organism and is the most abundant free amino acid found in plasma, muscle and other body tissues. Glutamine is involved in cell proliferation and growth, particularly cells of the immune system. Other functions include regulation of the acid-base balance, transport of ammonia between tissues, and donation of carbon skeletons for glyconeogenesis6. Glutamine is a nutritional supplement that contributes to the maintenance of intestinal integrity. Oral administration of glutamine protects the intestine of patients undergoing chemoradiotherapy9. In addition, oral glutamine has been shown to increase the concentration of GLP-1 in lean, insulin-resistant, obese and diabetic patients23. Reimann15 showed that glutamine also stimulates the secretion of GIP in rat duodenal cell cultures
The objective of the present study was to evaluate the effects of ingestion of palm oil and glutamine on serum levels of GLP-1, PYY and glycemia in T2DM patients submitted to surgical duodenojejunal bypass with ileal interposition and without gastrectomy.
METHODS
The study was approved by the Ethics Committee of Universidade Federal do Triângulo Mineiro (Permit No. 1726). All patients signed a free informed consent form.
Eleven patients, five men (45.45%) and six women (54.54%), ranging in age from 21 to 60 years, participated in the study. All patients had T2DM, underwent surgery in 2010, and were followed up for 2 years. The values obtained for the patients served as their own control for descriptive analysis.
Patients fasted for a minimum period of 8 h and a maximum period of 12 h were invited for blood collection after oral ingestion of palm oil and glutamine on different days. On the first day, the patients ingested 9 g palm oil emulsion in encapsulated form to facilitate oral administration. On the second day, 30 g glutamine diluted in 200 ml water was administered orally. Two patients did not appear on the second day and blood was therefore collected from only nine patients.
Three blood samples were collected from one of the arms through a venous access device, one in the fasted state and the second and third 1 and 2 h after the ingestion of palm oil or glutamine, respectively. During each collection, blood was collected into two vacuum tubes with a yellow cap containing separation gel. In one tube, 10 µl dipeptidyl peptidase inhibitor 4 diluted in 1 ml blood was added to prevent the degradation of GLP-1 and PYY. The other tube was used for the measurement of glucose. The tubes were kept at low temperature and immediately centrifuged at 3500 rpm for 10 min at 4º C. The serum was stored frozen at -70ºC in sterile Eppendorf tubes until the time of analysis. Sandwich ELISA kits (Millipore) were used for hormone measurements.
The results were analyzed using the Statistica 10.0 and GraphPad Prism 6 programs. All variables were submitted to descriptive analysis consisting of the determination of the number of valid cases (n), mean, median, minimum and maximum values, variance, and standard deviation. Application of the Kolmogorov-Smirnov normality test revealed that none of the variables showed a normal distribution. Therefore, the variables were analyzed using the nonparametric Friedman test and Dunn's post-test for multiple comparisons. All non-normally distributed variables are reported as the median (minimum and maximum). A p value <0.05 was considered to be significant.
RESULTS
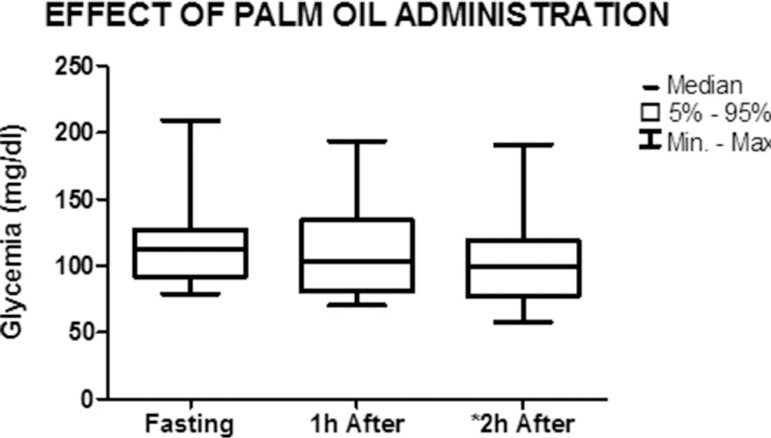
Figures 1, 2 and 3 show the effect of the administration of palm oil on the parameters studied. There was a significant decrease in blood glucose levels from 112.4 mg/dl (79.1-209) in the fasted state to 99.8 mg/dl (57.7-190.9) 2 h after the ingestion of palm oil (p=0.018). A decrease in blood glucose levels was also observed 1 h after ingestion of the stimulus, from 112.4 mg/dL (79.1-209) to 102.8 mg/dL (70.5-193.4), but the difference was not significant (p=0.09) (Figure 1).
Figure 1.
Graph showing the median and minimum and maximum values of blood glucose levels in the fasted state and 1 h and 2 h after the administration of palm oil (nonparametric Friedman test) *p=0.018
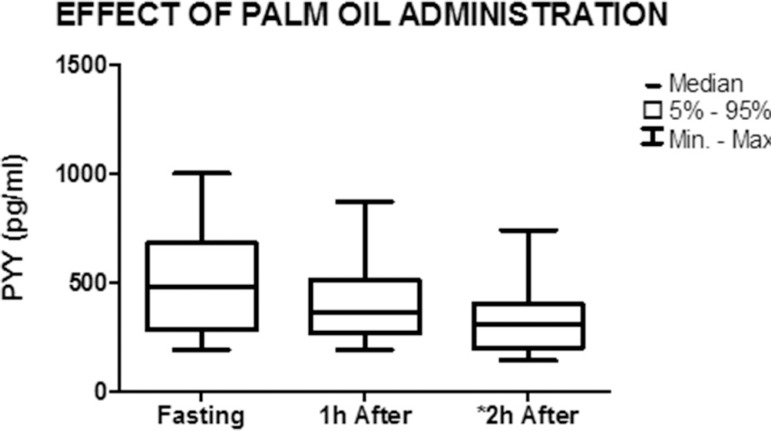
Figure 2.
Graph showing the median and minimum and maximum values of PYY concentration in the fasted state and 1 h and 2 h after the administration of palm oil (nonparametric Friedman test) *p=0.0002
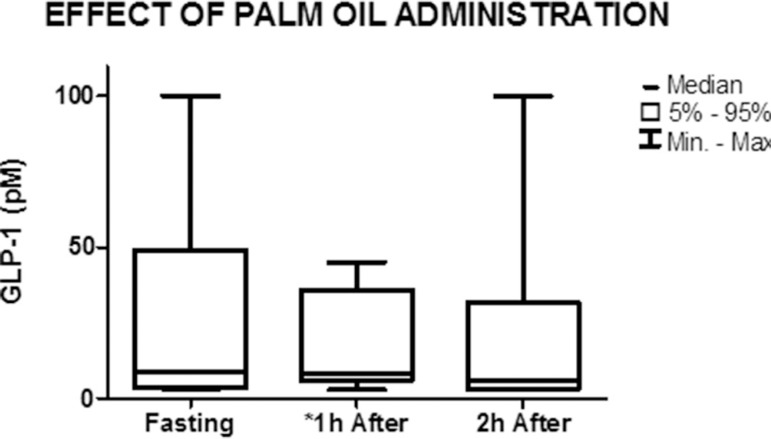
Figure 3.
Graph showing the median and minimum and maximum values of GLP-1 concentration in the fasted state and 1 h and 2 h after the administration of palm oil (nonparametric Friedman test) *p=0.04
A significant decrease in mean PYY levels was observed 2 h after the administration of palm oil, from 480 pg/mL (191-1000) to 310 pg/mL (140-740) (p=0.002). Comparison between the fasted state and 1 h after the administration of palm oil showed a decrease from 480 pg/mL (191-1000) to 360 pg/mL (191-870), but the difference was not significant (p=0.15) (Figure 2).
Figure 3 illustrates the effect of the administration of palm oil on GLP-1 levels. The levels of this hormone decreased significantly from 8.6 pM (3.1-100) in the fasted state to 8.4 pM (3-45) 1 h after the administration of palm oil (p=0.043). A slight decrease in GLP-1 levels was observed between 1 h and 2 h after administration of the stimulus, from 8.4 pM (3-45) to 6 pM (3-100), but the difference was not significant (p=0.72).
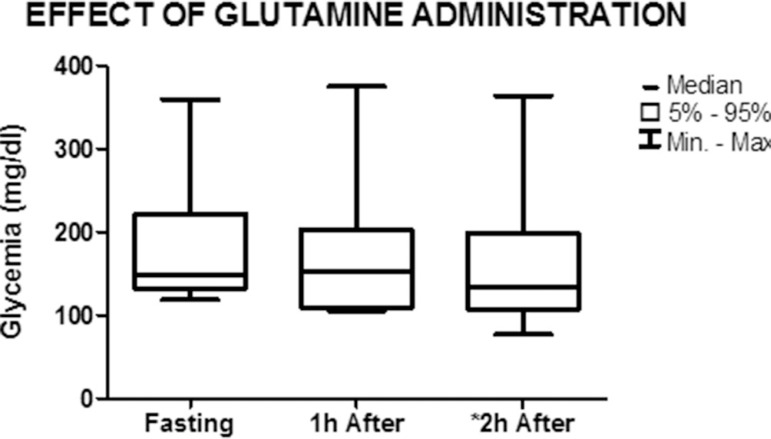
Figures 4, 5 and 6 show the effect of the administration of glutamine on the studied parameters. Blood glucose levels decreased from 148.6 mg/dL (118.7-359.4) in the fasted state to 133.5 mg/dL (76.7-364) 2 h after the ingestion of the stimulus (p=0.03). There was a nonsignificant decrease in blood glucose levels 1 h after the administration of glutamine (fasted state: 148.6 mg/dL (118.7-359.4), 1 h: 153.7 mg/dL (105.6-375.4); p = 0.25) (Figure 4).
Figure 4.
Graph showing the median and minimum and maximum values of blood glucose levels in the fasted state and 1 h and 2 h after the administration of glutamine (nonparametric Friedman test) *p=0.03
Figure 5.
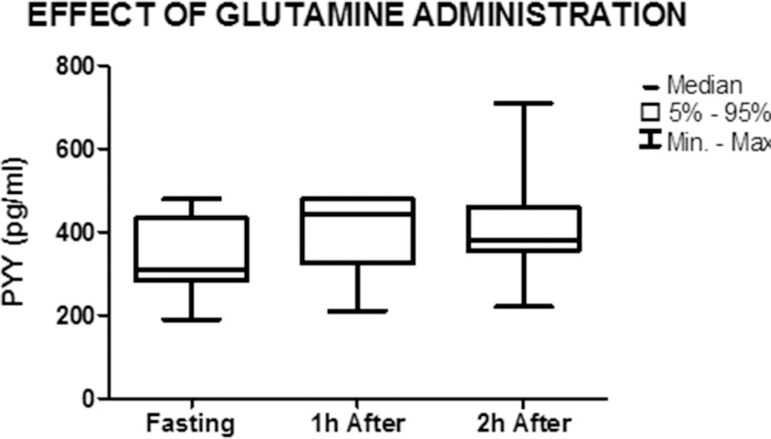
Graph showing the median and minimum and maximum values of PYY in the fasted state and 1 h and 2 h after the administration of glutamine (nonparametric Friedman test) p=0.06
Figure 6.
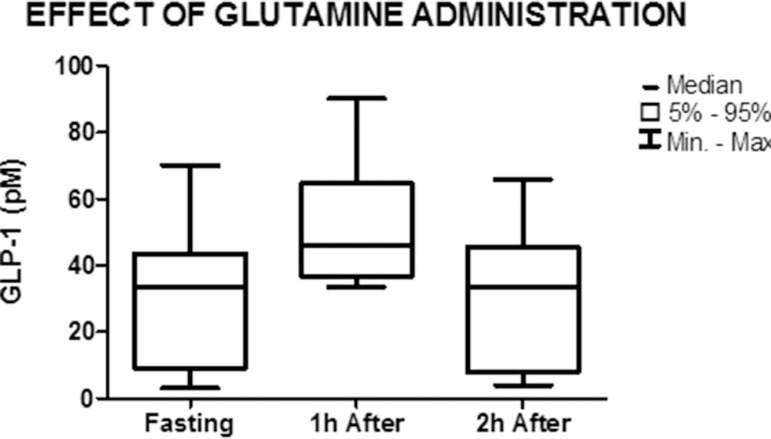
Graph showing the median and minimum and maximum values of GLP-1 in the fasted state and 1 h and 2 h after the administration of glutamine (nonparametric Friedman test) p=0.32
PYY concentration increased from 310 pg/mL (191-480) in the fasted state to 445 pg/mL (210-480) 1 h after the administration of glutamine, but the difference was not significant (p=0.06). The concentration of PYY (380 pg/mL (220-710)) remained elevated 2 h after the administration of glutamine when compared to the fasted state, but was lower when compared to 1 h after the stimulus. However, these differences were not significant (p=0.26) (Figure 5).
GLP-1 concentration increased from 33.5 pM (3-70) in the fasted state to 46 pM (33.5-90) 1 h after the administration of glutamine, but the difference was not significant (p=0.32). No significant difference in GLP-1 concentration was observed between the fasted state (33.5 pM (3-70)) and 2 h after the administration of glutamine (33.5 pM (3-65.9)) (p=0.87) (Figure 6).
DISCUSSION
The present study demonstrated a significant decline in blood glucose levels after ingestion of palm oil, suggesting that this compound can be used as an alternative for glycemic control in diabetic patients. Budin et al.3 treated streptozotocin-induced diabetic rats with the tocotrienol-rich fraction of palm oil and observed a significant reduction of serum glucose and glycated hemoglobin in treated rats14.
On the other hand, Sundram et al. 27 administered palm oil and other oleic compounds to 30 patients for four weeks and found no significant effect of palm oil on glucose concentration. The divergence between the results of the present study and those reported by Sundram et al. 27 might be explained by the fact that the patients studied here were submitted to duodenojejunal bypass with ileal interposition and without gastrectomy, a condition that permits food to reach L-cells of the ileum more rapidly, exerting a hypoglycemic effect, or by physiological differences between humans and rats3,14.
In the study of Robertson et al 17, healthy women consumed a fraction of palm oil containing other saturated fatty acids as part of their meal. Measurement of serum GLP-1 and PYY levels showed hormone peaks 30 min after ingestion of the meal. In the present study, a significant decrease in PYY was observed 2 h after the ingestion of palm oil. These results suggest that the peak in this peptide occurs immediately after administration of the oil17.
Wit et al.30 fed C57BL/6J mice (genetically modified to develop obesity) a high-fat and low-fat diet. The two diets had the same composition of proteins, carbohydrates and fats, but differed in the proportion of individual fatty acids. The low-fat diet contained 20 g palm oil and the high-fat diet contained 177.5 g palm oil, in addition to soybean oil. The animals were fed the diet for 2, 4 and 8 weeks. The authors suggested that the high-fat diet does not influence incretins such as GLP-1 to an extent that would cause alterations in the insulin response pattern. Despite the observation of similar GLP-1 levels, comparison with the present study is limited since the diet administered by Wit et al.30 contained different compounds that could influence the mechanism of incretin action. In the present study, palm oil was administered to patients previously submitted to duodenojejunal bypass with ileal interposition and without gastrectomy and these factors cited may behave differently depending on the organism30.
There are some factors that may explain the significant reduction in GLP-1 after the administration of palm oil, such as the route of administration. The palm oil emulsion was encapsulated to facilitate its ingestion and therefore may have not been intact when it reached the portion of the ileum located immediately after the stomach in operated patients. Another factor related to the reduction in GLP-1 is the higher concentration of the hormone seen in fasted patients, suggesting that the surgical procedure had increased the secretion of this incretin in the fasted state
The effect of glutamine on the three parameters evaluated agrees with the results of studies reported in the literature. A mild increase in GLP-1 was observed in the first hour after the administration of glutamine (p=0.32). Greenfield et al.9 and Tolhurst et al.29 showed that glutamine significantly increases the expression of GLP-1. The lack of a significant difference in the expression of GLP-1 observed in the present study might be due to the small number of patients. Another explanation are the high serum levels of GLP-1 seen in fasted patients, which were 33.5 pM (3-70), when compared to fasting plasma concentrations of this incretin of 2 to 15 pM reported in the literature13. These elevated GLP-1 levels may be a consequence of surgery since, according to Rubino et al.22, duodenal bypass can interfere with the anti-incretin effect22.
In the study of Breitman et al. 2, obese patients submitted to gastric bypass received 24 g of an oral supplement containing glutamine, leucine and arginine twice daily for eight weeks. The results showed that administration of the supplement caused a significant decrease in glucose over the period studied, even in non-diabetic patients. Insulin resistance improved despite the lack of a significant increase in GLP-1. The study of Breitman et al.2 shows some similarities with the procedures used in the present investigation. However, the surgical procedure adopted by these authors, gastric bypass, differs from that used here. In addition, the supplement administered by the authors was a combination of amino acids, whereas the present patients received only glutamine. It can be suggested that glutamine caused a decrease in glucose levels in the study of Breitman et al.2 or that other compounds have influenced the action of incretins.
The concentration of PYY increased in the first hour after the administration of glutamine, although the difference was not significant. This finding agrees with Reimann et al.16 who observed a significant increase in PYY after the administration of glutamine to rats submitted to resection of the small intestine. The lack of a significant increase in PYY might be due to the small number of patients studied.
In the present study, a significant decrease in blood glucose was observed 2 h after the ingestion of glutamine. Similar results have been reported by Samocha-Bonet et al.23 who found a significant reduction of glycemia in diabetic patients ingesting 30 g glutamine. Taken together, these results suggest that glutamine is a promising compound for short-term glycemic control.
GLP-1 was found to be increased in the fasted state. This finding suggests that surgery may have influenced the increase in this incretin. In this respect, Nauck et al.12 demonstrated an effect of deterioration of incretins in patients with T2DM. The authors reported that the plasma alterations seen after oral administration of glucose were the same as those observed when glucose was administered intravenously. These results indicate that this incretin does not act completely on glycemic control. Another fact that may suggest that surgery exerted an effect on GLP-1 concentration is the finding of Toft-Nielsen et al.28 who observed impaired secretion of GLP-1 in patients with T2DM characterized by a significant decrease in the response of this incretin. Similar results have been reported by Chacra4.
Cohen et al.5, studying 86 patients with a BMI of 22 to 34 kg/m2 submitted to duodenojejunal bypass, observed long-term remission of T2DM in 78% of the patients, but no significant increase in GLP-1. These results suggest that the surgical procedure performed in the present study promotes intestinal alterations that differ from those induced by duodenojejunal bypass, increasing fasting GLP-1 concentration and thus contributing to the improvement of T2DM. In contrast, no ileal interposition is performed in duodenojejunal bypass. As a consequence, stimuli do not pass through L-cells and no increase in GLP-1 occurs.
CONCLUSION
The present results suggest that palm oil and glutamine influence intestinal peptides and glycemia.
Footnotes
Conflicts of interest: none
Financial source: none
REFERENCES
- 1.Bandeira F, Graf H, Griz L, Faria M, Lazaretti-Castro M. Endocrinologia e Diabetes.2ed. Rio de Janeiro: MEDBOOK; 2009. [Google Scholar]
- 2.Breitman I, Saraf N, Kakade M, Yellumahanthi K, White M, Hackett JA, Clements RH. The Effects of an Amino Acid Supplement on Glucose Homeostasis, Inflammatory Markers, and Incretins after Laparoscopic Gastric Bypass. J Am Coll Surg. 2011;212(4):617–627. doi: 10.1016/j.jamcollsurg.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Budin SB, Othman F, Louis SR, Bakar Ma, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. CLINICS. 2009;64(3):235–244. doi: 10.1590/S1807-59322009000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chacra AR. Efeito Fisiológico Das Incretinas. Adv Stud Med. 2006;6(7B):S613–S617. [Google Scholar]
- 5.Cohen R, Torres M, Schiavon C. Cirurgia metabólica: mudanças na anatomia gastrointestinal e a remissão do diabetes mellitus tipo 2. ABCD Arq Bras Cir Dig. 2010;23(1):40–45. [Google Scholar]
- 6.Cruzat VF, Petry ÉR, Tirapegui JO. Glutamina: aspectos bioquímicos, metabólicos, moleculares e suplementação. Rev Bras Med Esporte. 2009;15:392–397. [Google Scholar]
- 7.Diepvens K, Soenen S, Steijns J, Arnold M, Westerterp-Plantenga M. Long-term effects on consumption of a novel fat emulsion in relation to body-weight management. Int J Obes (Lond) 2007;31:942–949. doi: 10.1038/sj.ijo.0803532. [DOI] [PubMed] [Google Scholar]
- 8.Drucker DJ. The role of gut hormones in glucose homeostasis. J. Clin Invest. 2007;117:24–32. doi: 10.1172/JCI30076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, Reimann F, Holst JJ, Gribble FM. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89:106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grenier E, Garofalo C, Delvin E, Levy E. Modulatory Role of PYY in Transport and Metabolism of Cholesterol in Intestinal Epithelial Cells. Plos One. 2012 Jul;7(7) doi: 10.1371/journal.pone.0040992. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Kashyap SR, Gatmaitan P, Brethauer S, Schauer P. Bariatric surgery for type 2 diabetes. Weighing the impact for obese patients. Cleve Clin J Med. 2010;77(7):468–476. doi: 10.3949/ccjm.77a.09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ. Secretion of glucagon-like pepetide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia. 2011;54(10):10–18. doi: 10.1007/s00125-010-1896-4. [DOI] [PubMed] [Google Scholar]
- 13.Peruzzo GM. Análise dos Níveis de GLP-1 nos Estados de Jejum e Pós prandial Durante a Gestação em Mulheres Não Diabéticas. Programa de Pós graduação em Saúde e Desenvolvimento na Região Centro-oeste, Universidade Federal de Mato Grosso do Sul, Campo Grande; http://www.cbc.ufms.br/tedesimplificado/tde_arquivos/14/TDE-2010-12-13T115508Z-636/Publico/Giselle.pdf [Google Scholar]
- 14.Pories WJ, Albrecht RJ. Etiology of type 2 diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–531. doi: 10.1007/s002680020348. [DOI] [PubMed] [Google Scholar]
- 15.Reimann F. Molecular mechanisms underlying nutrient detection by incretin-secreting cells. Int Dairy J. 2010;20:236–242. doi: 10.1016/j.idairyj.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimann F, Williams L, Xavier G da Silva, Rutter G. A, Gribble F.M. Diabetologia. Diabetologia. 2004;47:1592–1601. doi: 10.1007/s00125-004-1498-0. [DOI] [PubMed] [Google Scholar]
- 17.Robertson M, Jackson K, Fielding B, Morgan L, Williams C, Frayn K. Acute ingestion of a meal rich in n-3 polyunsaturated fatty acids results in rapid gastric emptying in humans. Am J Clin Nutr. 2002;76:232–238. doi: 10.1093/ajcn/76.1.232. [DOI] [PubMed] [Google Scholar]
- 18.Rocha H, Carvalho R. O papel das incretinas no tratamento da Diabetes Mellitus tipo 2. Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto; Porto: 2009. http://repositorio-aberto.up.pt/bitstream/10216/21023/2/tese%20final.pdf [Google Scholar]
- 19.Ross SA, Ekoé JM. Incretin agents in type 2 diabetes. Can Fam Physician. 2010;56:639–648. [PMC free article] [PubMed] [Google Scholar]
- 20.Roux CW, Aylwin SJB, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut Hormone Profiles Following Bariatric Surgery Favor an Anorectic State, Faciliate Weight Loss, and Improve Metabolic Parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubino F, Gagner M. Potencial of Surgery for Curing Type 2 Diabtes Mellitus. Ann Surg. 2002;236(5):554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino F, R'bibo SL, Genio F, Mazumdar M, Mcgraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6(2):102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, Holst JJ, Chisholm DJ, Greenfield JR. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr. 2011;141:1233–1238. doi: 10.3945/jn.111.139824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sen CK, Rink C, Khanna S. Palm Oil-Derived Natural Vitamin E a-Tocotrienol in Brain Health and Disease. J Am Coll Nutr. 2010;29(3) Suppl:314S–323S. doi: 10.1080/07315724.2010.10719846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimizu H, Tinratana P, Shauer PR, Rogula T. Review of Metabolic Surgery for Type 2 Diabetes in Patients with a BMI < 35 kg/m2. Journal of Obesity. 2012 2012 Mar; doi: 10.1155/2012/147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva AD, Bloom SR. Gut Hormones and Appetite Control: A Focus on PYY and GLP-1 as Therapeutic Targets in Obesity. Gut Liver. 2012;6:10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundram K, Karupaiah T, Hayes KC. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr Metab. 2007;4:3–3. doi: 10.1186/1743-7075-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 29.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–413. doi: 10.1210/en.2010-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wit NJ, Bosch-Vermeulen H, de Groot PJ, et al. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14–14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]