Abstract
Background
There are limited data on femoropopliteal artery stent thrombosis (ST), which is a serious adverse outcome of peripheral artery interventions.
Methods and Results
Index procedures resulting in femoropopliteal ST were compared to stent procedures without subsequent ST in the Excellence in Peripheral Artery Disease (XLPAD) registry. The study data had a total of 724 cases of stent procedures and 604 unique patients. Femoropopliteal ST occurred in 26 of 604 patients (4.3%) over a median follow up of 6 months post-procedure. ST was more likely to occur in men (96.3% vs. 82.2%; p=0.026) and to have an initial intervention for chronic total occlusions (CTO) (88.5% vs. 64.0%; p=0.01). There was no significant difference in ST between drug-coated and bare-metal stents (BMS) (4.4% vs. 3.4%; p=0.55), but the rate of ST was significantly higher with self-expanding covered stent grafts compared to BMS (10.6% vs. 3.4%; p=0.02). ST was significantly associated with an increased risk of 12-month major adverse limb events (hazard ratio [HR]=4.99; 95% confidence interval [CI]: 2.31-10.77; p<0.001) compared to no-ST. On multivariate analysis treatment of CTO lesions (Odds Ratio [OR]=3.46; 95% CI: 0.98-12.20; p=0.05) and in-stent restenosis lesions (OR=5.30; 95% CI: 1.83-15.32; p=0.002) were independently associated with an increased risk of ST.
Conclusions
In a multicenter peripheral interventional registry, femoropopliteal ST occurred in 4.3% of patients who underwent stent procedures and was associated with treatment of CTO and in-stent restenosis lesions, and had higher 12-month major adverse limb events.
Keywords: stent thrombosis, peripheral artery disease, endovascular treatment
Endovascular interventions are often the first option for patients with symptomatic peripheral artery disease (PAD) who present with either claudication or critical limb ischemia (CLI) despite medical therapy.1 The majority of these procedures are performed in the infrainguinal arteries, primarily in the superficial femoral artery (SFA) and for the treatment of complex atherosclerotic disease.2 Over 70% of SFA PAD, especially in patients with diabetes mellitus, presents as complex lesions that include long (≥60 mm) diffusely diseased arterial segments or chronic total occlusions (CTO) and often require scaffolding with intravascular stent implants.3 Balloon angioplasty of such lesions has traditionally been associated with poor clinical outcomes.4 This has led to the widespread use of bare-mextal (nitinol) self-expanding stents (BMS) in the SFA.5-7 However, given the high rate of in-stent restenosis (ISR) with BMS, anti-proliferative drug (paclitaxel) coated metal stents (DCS) are being increasingly used in the SFA.8 The introduction of paclitaxel on DCS is directed towards suppression of smooth muscle cell proliferation, thereby lowering rates of BMS ISR.9 Given the dynamic nature of the SFA, which may make stents prone to fracture, and due to potential impairment of the antithrombotic functions of endothelial cells, both BMS and DCS implants could contribute to incomplete vascular healing and increased risk of stent thrombosis (ST).10,11 Self-expanding stent grafts (Viabahn™), on the other hand, may be resistant to ISR, but may also be prone to ST by virtue of collateral vessel occlusion and edge restenosis.12
Currently there are no published reports of systematic analysis of ST following stent implants in infrainguinal peripheral arteries. Herein, we present results from a contemporary multicenter registry on the occurrence and predictors of infrainguinal peripheral artery ST.
Methods
In this study, we report data from the Excellence in Peripheral Artery Disease (XLPAD) multi-center registry (NCT01904851) from 8 participating U.S. centers between May 2005 and January 2015. The registry received institutional review board (IRB) approval in 2013, and was built on an existing database at Dallas VA Medical Center. Each participating site has the provision to enter data prospectively (5.0%) or retrospectively (95.0%) from the year 2005 with deferred informed consent after approval from respective site IRBs. Currently, 43% of cases are from the Dallas VA Medical Center. A distinguishing feature of the registry includes a high level of data audit and verification performed by the study staff. The study staff has conducted on-site data audit and entry of 91% of procedure records and 93% of angiograms that have been downloaded at sites and made available for core laboratory analysis. Patient risk factors, co-morbid disease conditions, laboratory data, medications and procedural information were entered into the REDCap (Research Electronic Data Capture) online data capture software, an electronic data capture tool hosted at the University of Texas Southwestern Medical Center.13 This study selected 724 cases of stented procedures from the available 1,790 total procedures entered in the XLPAD registry at the time of the study, which consists of 1,428 femoropopliteal arterial revascularization procedures, 190 below-the-knee (BTK) artery, and 172 multi-level disease (femoropopliteal and BTK arteries).
Procedural angiograms were de-identified, mailed, and analyzed at the Veterans Affairs North Texas Angiography and Ultrasound core laboratory.14 Occurrence of ST was determined by acute onset of lower extremity pain in the form of claudication or acute limb ischemia and angiographic evidence of thrombus. The XLPAD criterion for establishing the diagnosis of ST was formulated by the study executive committee, based on ‘event certainty’ and the ‘time frame’.15 Based on ‘event certainty’, ST was divided into definite, probable and possible. The diagnosis of definite ST was made after confirmation of a thrombotic occlusion in the lower extremity peripheral artery involving a stented segment (±5 mm proximal and distal to a stent edge) during peripheral angiography, surgical exploration or autopsy in patients presenting with an acute onset of lower limb pain (Table 1). A case of definite ST is depicted in Figure 1. Probable ST was considered when an acute symptomatic occlusion involving the stented arterial segment was diagnosed by duplex ultrasonography, computed tomographic angiography, magnetic resonance imaging or angiography, in the absence of angiographic, surgical or an autopsy confirmation. Diagnosis of thrombus of a lower extremity peripheral artery with prior stent(s) by any imaging modality, surgical exploration or autopsy, however not involving the stented segment was adjudicated as possible ST. Though these criteria are applicable to iliac, femoropopliteal and BTK peripheral arteries, for the purposes of this analysis, iliac, common femoral and BTK arterial ST were excluded. Timing of ST after an index procedure was divided into early (≤30 days), late (≥31 days to 1 year), or very late (>1 year). Early ST was further subdivided into acute (≤48 hours) or sub-acute (>48 hours, but ≤30 days).
Table 1.
Peripheral artery stent thrombosis: Defining criteria
| Category | Definition |
|---|---|
| Based on event certainty: | |
| Definite stent thrombosis | Confirmation of a thrombotic occlusion of the lower extremity peripheral artery involving the stented segment (±5 mm proximal and distal to a stent edge) during peripheral angiography, surgical exploration or autopsy in patients presenting with an acute onset of lower limb pain |
| Probable stent thrombosis | Acute symptomatic occlusion involving the stented arterial segment was diagnosed by duplex ultrasonography, computed tomographic angiography, magnetic resonance imaging or angiography, in the absence of angiographic, surgical or an autopsy confirmation |
| Possible stent thrombosis | Diagnosis of thrombus of a lower extremity peripheral artery with prior stent placement by any imaging modality, surgical exploration or autopsy, however not involving the stented segment |
| Based on event timing: | |
| Early stent thrombosis | ≤30 days |
| Acute stent thrombosis | ≤48 hours |
| Sub-acute stent thrombosis | >48 hours, but ≤30 days |
| Late stent thrombosis | ≥31 days to 1 year |
| Very late stent thrombosis | >1 year |
Figure 1.
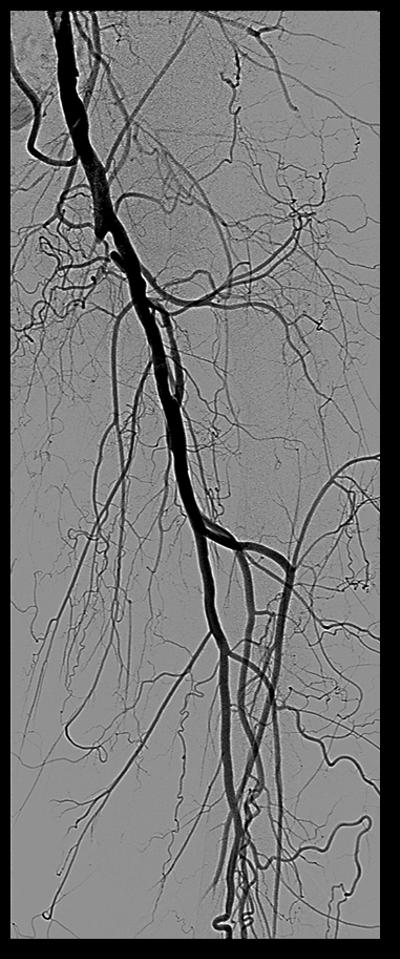
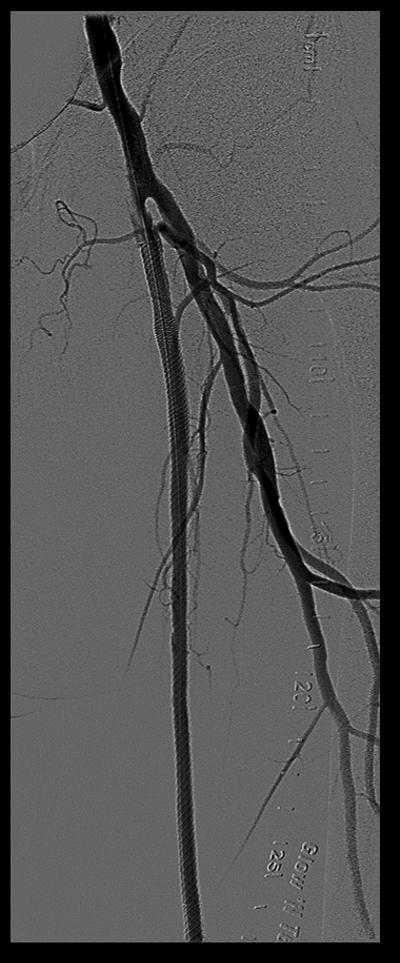
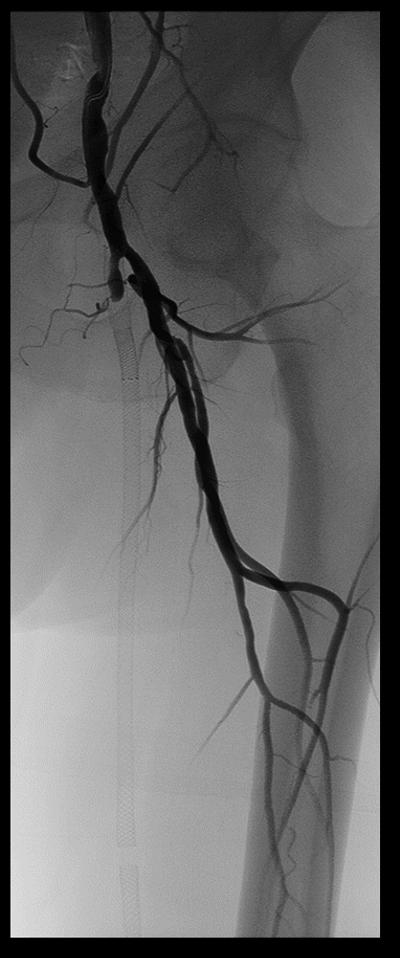
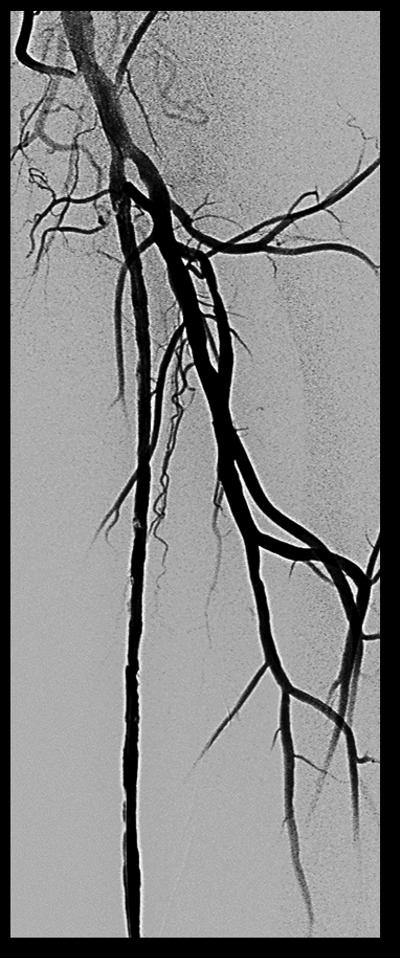
Case of definite stent thrombosis
Selection of lesion crossing strategy, type of guidewires, support catheters, stent type and dimensions, pre- and post-dilation strategies, need for atherectomy, anticoagulation regimen and anti-platelet therapy for the index and ST revascularization procedures were at the discretion of the operator. Patient outcome measures following ST included: procedural success, peri-procedural complications and major adverse events (MAE). Procedural success was defined as successful revascularization of the occluded lesion and restoration of ≥1 vessel flow to the foot with either planned percutaneous or surgical revascularization. Peri-procedural complications following endovascular revascularization for ST were defined as the occurrence of a flow-limiting dissection, arterial perforation, access site hematoma ≥5 cm in diameter, retroperitoneal hematoma, distal embolization, major bleed requiring blood transfusion, acute renal failure, need for emergency surgery, or death. Peri-procedural complications of surgical revascularization included: death, non-fatal myocardial infarction (MI), major bleed requiring blood transfusion, infection requiring intravenous antibiotics, amputation, or need for surgical re-exploration. Follow-up MAE were classified as major adverse limb (MALE) and major adverse cardiovascular or cerebrovascular (MACE) events. The endpoint of MALE was defined as a composite of repeat unplanned endovascular or surgical revascularization, definite ST, or major (above ankle) amputation of the target limb. The endpoint of MACE was a composite of all-cause mortality, MI, ischemic stroke or any coronary revascularization with percutaneous coronary intervention, or coronary artery bypass surgery. The follow-up of MALE and MACE were censored at 12 months post index procedure.
We identified all non-drug coated stents as BMS, except for the Supera™ mimetic stent (Abbott Vascular, Santa Clara, CA) and Viabahn™ stent graft (Gore Medical, Newark, DE), given their conceptually different design and deployment.16,17 Zilver PTX™ (Cook Medical, Bloomington, IN) is the only DCS used in the registry.18
To adequately compare procedures complicated by the occurrence of a first index limb ST event, comparisons were made to a cohort of cases with stented femoropopliteal lesions that did not subsequently develop ST. Therefore, procedures treating BTK lesions and those using non-stent based treatment strategies for femoropopliteal lesions were excluded from analysis. Twenty-eight percent of the cases included in the analysis included stent-based contralateral limb revascularizations. Baseline and index procedure characteristics were compared between ST and no-ST groups using two-tailed Wilcoxon rank sum test for continuous variables and the Cochran-Mentel-Haenszel (CMH) test for categorical variables. Means and standard deviations were reported as descriptive statistics for continuous variables and frequencies and percentages for categorical variables. Kaplan-Meier curves (SAS Proc Lifetest) with log-rank test and Maximum likelihood-based random effect Cox proportional hazard model (Frailty; SAS Proc Phreg) were used to conduct time-to-event analysis and to compare median survival time to MALE and MACE censored at 12 months between the ST and no-ST groups. A random coefficient was included in the models to capture within-subject correlations for subjects with more than one stent procedure. Hazard ratios (HR) with 95% confidence intervals (CI) and p-values were reported for time-to-event analysis.
To identify predictive factors for ST, we utilized a step-wise linear regression approach for a final model selection. First, multiple univariate generalized linear mixed models (GLMMs) (Proc Glimmix with logit link) were conducted to estimate ST odds ratios (OR) of each candidate factor, separately. A random coefficient in the GLMM addressed within subject variance in OR estimates of each variable. In GLMM, continuous variables were categorized into a binary value separated by the median thus; all variables of interest for GLMMs were dichotomized. We then used forward step-wise logistic regression and Akaike Information Criterion (smaller is a better fit) as model selection criteria for a multivariate GLMM. Step-wise logistic regressions used a significance level of 0.30 to allow a variable into the model, and 0.35 for a variable to stay in the model. The Hosmer and Lemeshow goodness-of-fit test for the final selected model was also presented. OR with 95% CI and p-values were reported. All statistical tests were 2-tailed and a p-value ≤0.05 was considered to be statistically significant. SAS 9.4 (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Figure 2 shows the distribution of cases with and without ST and timing and diagnosis of ST. There are a total of 604 patients with 724 cases in the XLPAD registry involving stent-based revascularization of a femoropopliteal artery. Overall, ST occurred in 26 out of 604 individual patients (4.3%). Definite ST occurred in 21 (3.5%), probable ST in 1 (0.2%), and possible ST in 4 (0.7%). The mean survival time to ST event from the index procedure in patients who had ST was 2.3±3.8 months (median=60 days, quartiles=30-150 days) and Kaplan-Meier curve for ST is shown in Figure 3. Very late ST occurred in 2 (0.3%), late ST in 12 (2.0%), and early ST in 13 (2.2%), with acute ST in 7 (1.2%) and sub-acute ST in 6 (1.0%). Description of ST event for each patient is provided in Table 2.
Figure 2.
Flowchart describing stent thrombosis
Figure 3.
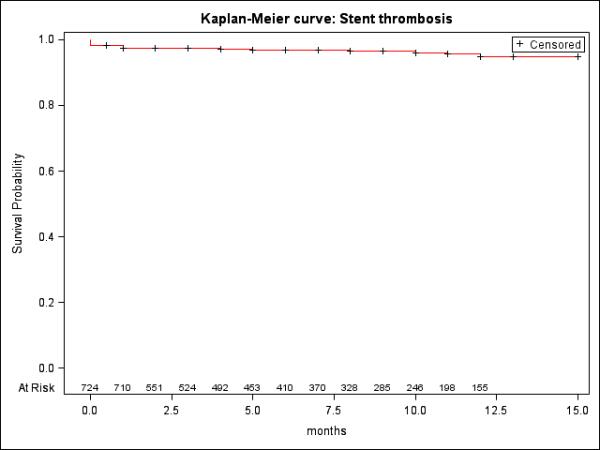
Kaplan-Meier curve: Stent Thrombosis.
• Censored at death and follow-up 15 months.
Table 2.
Description of stent thrombosis by patient
| Event ID | ST type | ST timing | Stent type | Index lesion characteristics |
*DAPT (m) | Stent fracture | Stent thrombosis therapy | |
|---|---|---|---|---|---|---|---|---|
| Index lesion type | BTK vessel runoff | |||||||
| 1 | Definite | Late 11m | BMS | Mid SFA CTO | 3 | 1 | Yes | Surgical intervention |
| 2 | Definite | Acute 2d | BMS | Popliteal CTO | 2 | 12 | No | Thrombectomy, balloon angioplasty |
| 3 | Definite | Late 5m | BMS | Distal SFA, diffuse | 3 | 1 | No | Balloon angioplasty, BMS |
| 4 | Definite | Late 6m | BMS | Mid SFA CTO | 2 | 12 | No | Thrombectomy, balloon angioplasty |
| 5 | Possible | Very Late 13m | BMS | Distal SFA CTO | 3 | 1 | No | Balloon angioplasty |
| 6 | Definite | Late 10m | BMS | Ostial SFA CTO | 1 | 1 | No | Thrombectomy, thrombolytics, BMS |
| 7 | Definite | Late 2m | BMS | Ostial SFA ISR, CTO | 3 | 12 | No | Thrombectomy, balloon angioplasty, laser |
| 8 | Definite | Sub-Acute 1m | Viabahn™ | Proximal SFA CTO | 3 | 12 | No | Surgical intervention |
| 9 | Definite | Late 5m | Viabahn™ | Mid SFA CTO | 1 | 1 | No | Thrombectomy, balloon angioplasty |
| 10 | Definite | Sub-Acute 3d | Supera™ | Mid SFA CTO | 1 | 0 | No | Surgical intervention |
| 11 | Definite | Late 3m | Viabahn™ | Ostial SFA CTO | 3 | 1 | No | Thrombectomy, thrombolytics, Viabahn™ |
| 12 | Possible | Acute 2d | BMS | Mid SFA ISR, CTO | 3 | 3 | No | Cutting balloon angioplasty |
| 13 | Definite | Acute 1d | BMS | Proximal SFA ISR, CTO | 3 | 0 | No | Surgical intervention |
| 14 | Definite | Sub-Acute 1m | BMS | Distal SFA | 2 | 3 | No | Balloon angioplasty, BMS |
| 15 | Definite | Acute 1d | BMS | Ostial SFA CTO | 2 | 12 | No | Thrombectomy, BMS |
| 16 | Probable | Sub-Acute 1m | DCS | Proximal CTO | 3 | 12 | No | Balloon angioplasty, DCS |
| 17 | Definite | Late 2m | Viabahn™ | Proximal SFA CTO | 3 | 1 | No | Thrombectomy, BMS |
| 18 | Definite | Late 2m | DCS | Proximal SFA CTO | 2 | 12 | No | Surgical intervention |
| 19 | Definite | Late 2m | Viabahn™ | Distal SFA CTO | 3 | 1 | No | Thrombectomy, thrombolytics, Viabahn™ |
| 20 | Definite | Sub-Acute 1wk | DCS | Proximal SFA CTO | 3 | 3 | No | Thrombectomy, balloon angioplasty |
| 21 | Definite | Acute 2d | Viabahn™ | Ostial SFA CTO | 2 | 1 | No | Thrombectomy, thrombolytics, Viabahn™ |
| 22 | Definite | Acute 2d | BMS | Ostial SFA CTO | 2 | 6 | No | Laser, balloon angioplasty |
| 23 | Definite | Sub-Acute 3wk | BMS | Proximal SFA Diffuse | 3 | 3 | No | Thrombectomy, balloon angioplasty, BMS |
| 24 | Possible | Acute 2d | BMS | Mid SFA Diffuse | 3 | 3 | No | Balloon angioplasty, BMS |
| 25 | Possible | Late 7m | Supera™ | Ostial SFA CTO | 2 | 3 | No | Laser, BMS, balloon angioplasty |
| 26 | Definite | Sub-Acute 1m | Supera™ | Popliteal CTO | 1 | 0 | No | Thrombectomy, thrombolytics |
BMS – bare metal stent, BTK – below the knee, CTO – chronic total occlusion, DAPT – dual antiplatelet therapy, DCS – drug coated stent, ISR – in-stent restenosis, SFA – superficial femoral artery, ST – stent thrombosis
Planned duration; m – months; d – days; wk - week
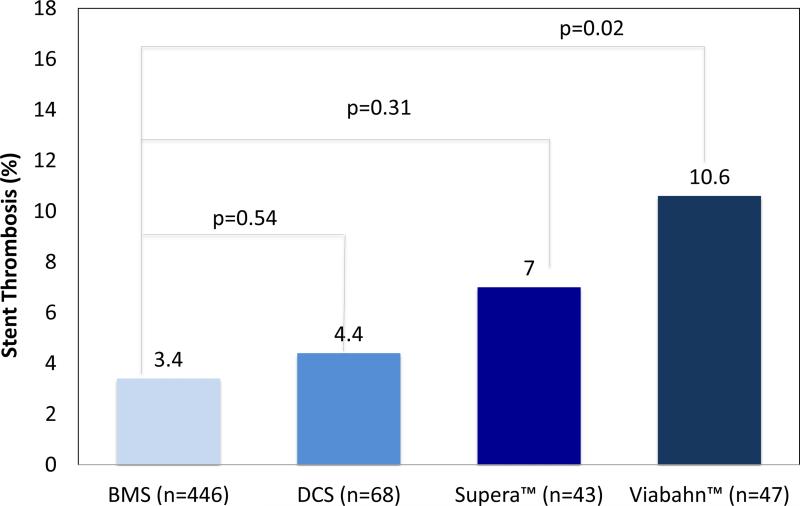
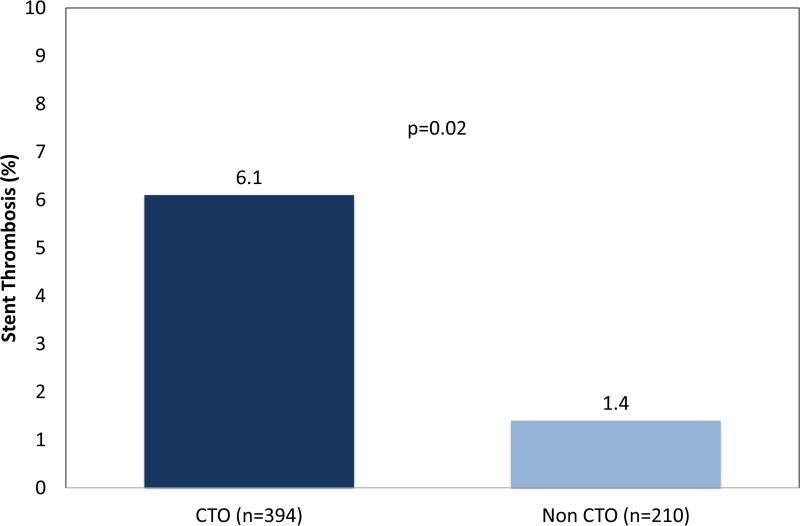
Comparisons of baseline characteristics, procedural characteristics, and outcomes between the ST and no-ST groups are shown in Table 3. Cardiovascular risk factors were not significantly different between ST and no-ST groups (Table 3). Compared to no-ST groups, ST groups were more likely to involve treatment of a CTO (88.5% vs. 64.0%; p=0.01), and longer stent lengths (446.2±124.8 mm vs. 194.7±124.7 mm; p=0.04) and lesion lengths (171.0±86.9 mm vs. 126.8±99.9 mm; p=0.01). Dual antiplatelet therapy (DAPT) with aspirin and a thienopyridine was prescribed at similar rates (81.5% vs. 90.6%; p=0.16) between ST and no-ST groups, respectively. ST occurred in 3 of 68 Zilver PTX™ drug coated stents cases (4.4%) compared to 15 of 446 BMS cases (3.4%; p=0.55), while Viabahn™ stent grafts experienced ST in 5 of 47 cases (10.8%), which was significantly higher compared to BMS (p=0.02). ST with Supera™ mimetic stents occurred in 3 of 43 cases (7.0%), and was similar to BMS (p=0.31; Figure 4a). Also, ST was more frequent in stented CTO lesions (Figure 4b). Twelve-month Kaplan-Meier curve did not indicate significant differences in median time to ST event among stent types and presence or absence of CTO lesion (supplementary Figures 1 and 2).
Table 3.
Comparison of stent thrombosis and non-stent thrombosis groups
| ST (n=26)** | No-ST (n=578) | p-value*** | |
|---|---|---|---|
| Baseline characteristics | |||
| Age (years) | 64.2±9.4 | 62.4±6.8 | 0.204 |
| Male | 25 (96.2%) | 474 (82.0%) | 0.063 |
| Caucasian | 19 (70.4%) | 376 (65.2%) | |
| Black | 7 (25.9%) | 180 (25.7%) | 0.924 |
| Hispanic | 1 (3.7%) | 56 (8.3%) | |
| Diabetes mellitus | 13 (50.0%) | 315 (54.5%) | 0.652 |
| Hyperlipidemia | 23 (88.5%) | 501 (86.7%) | 0.722 |
| Hypertension | 23 (88.5%) | 536 (92.7%) | 0.418 |
| Current smoker | 18 (69.2%) | 351 (60.7%) | 0.385 |
| Coronary artery disease | 20 (76.9%) | 382 (66.09%) | 0.253 |
| Prior myocardial infarction | 11 (42.3%) | 162 (28.0%) | 0.115 |
| Prior stroke | 1 (3.8%) | 55(9.5%) | 0.329 |
| Heart failure | 6 (23.1%) | 103 (17.8%) | 0.495 |
| Chronic kidney disease | 2 (7.4%) | 94 (16.3%) | 0.177 |
| Ankle brachial index | 0.56±0.27 | 0.53±0.41 | 0.553 |
| Rutherford category | |||
| Rutherford 2 | 1 (3.9%) | 35 (6.1%) | 0.6687 |
| Rutherford 3 | 18 (69.2%) | 382 (66.5%) | |
| Rutherford 4 | 7 (26.9%) | 62 (10.8%) | |
| Rutherford 5 | 0 (0%) | 69 (12.0%) | |
| Critical limb ischemia* | 7 (25.9%) | 131 (22.7%) | 0.701 |
| Dual antiplatelet therapy | 22 (81.5%) | 523 (90.6%) | 0.155 |
| Index procedure characteristics | |||
| Superficial femoral artery | 27 (92.6%) | 528 (91.7%) | 1.000 |
| Popliteal artery | 2 (7.7%) | 48 (8.3%) | 0.910 |
| Ostial SFA lesion | 6 (23.1%) | 81 (14.0%) | 0.199 |
| Distal SFA lesion | 3 (11.5%) | 140 (24.2%) | 0.133 |
| Lesion length (mm) | 171.0±86.9 | 126.8±99.9 | 0.011 |
| Chronic total occlusion | 23 (88.5%) | 370 (64.0%) | 0.010 |
| In stent restenosis | 5 (19.2%) | 35 (6.1%) | 0.008 |
| BTK vessel runoff | 2.50±0.71 | 2.26±0.81 | 0.714 |
| Bare metal stents | 15 (55.6%) | 431 (74.8%) | 0.041 |
| Supera™ mimetic stents | 3 (11.1%) | 40 (6.9%) | 0.441 |
| Viabahn™ stent grafts | 6 (22.2%) | 41 (7.1%) | 0.016 |
| Zilver PTX™ drug coated stents | 3 (11.1%) | 64 (11.1%) | 1.000 |
| Stent length (mm) | 246.2±124.8 | 194.7±124.7 | 0.035 |
| Atherectomy | 8 (30.8%) | 141 (24.4%) | 0.461 |
| Procedure success | 25 (96.2%) | 566 (98.4%) | 0.374 |
BTK – below the knee vessels, SFA – superficial femoral artery, ST – stent thrombosis
Includes Rutherford category 4 and 5
If patients had multiple stent procedures and experienced at least one ST following any of stent procedures, the individual patient was counted as having had ST.
Wilcoxon rank sum test for continuous variables; Cochran-Mentel-Haenszel test for categorical variables
Figure 4a.
Rate of stent thrombosis (ST) by stent type (overall ST rate = 4.3%)
Note: P-values are Tukey-Kramer adjusted p-values.
Figure 4b.
Rate of stent thrombosis (ST) by chronic total occlusion
Among the 26 patients with ST, 21 patients underwent endovascular revascularization and 5 underwent surgical intervention. Twelve patients (44.4%) presented with rest pain and 1 (3.7%) with progressive tissue loss. Angiography confirmed stent fracture in one patient (4.8%). The ostial SFA stent was the most common site for ST, with 6 (23.1%) occurrences. During endovascular intervention, aspiration and thrombectomy was performed in 16 (76.2%) cases of ST, and thrombolytic therapy was utilized in 6 (23.1%). Additional stenting was performed in 12 (57.1%) procedures, laser atherectomy in 2 (9.5%), and embolic protection was used in 10 (47.6%). Of surgical interventions, three were femoral-popliteal bypass with polytetrafluoroethylene graft, one femoral-popliteal bypass with greater saphenous vein graft, and two common femoral endarterectomies with patch angioplasty. Table 4 displays outcomes for patients treated for ST. Complications associated with endovascular treatment of ST were: 1 (4.8%) each of flow limiting dissection, distal embolization, need for emergency surgery, and acute renal failure. One peri-procedural MI occurred followed surgical revascularization; there were no major bleeds requiring transfusion, surgical re-interventions, amputations, infections requiring antibiotics, or deaths. After endovascular treatment for ST, 3 (11.1%) MALE occurred within 30 days, including 1 (3.7%) repeat stent thrombosis. A total of 10 (38.5%) MALE occurred within 1 year, with 2 (7.4%) repeat stent thrombosis events. No MACE occurred within 30 days, but 2 (7.7%) occurred within 1 year following endovascular or surgical revascularization for ST. Figures 5 a and 5b present Kaplan-Meier curves for 12-month MALE and MACE endpoints following stent implant, respectively, for both ST and no-ST groups. The 12-month time censored event rate for MALE since the index procedure was higher for patients with ST compared to those without (42.3% vs. 19.03%; p=0.004). The frailty model, after adjusting for age, gender, race, baseline ABI, diabetes mellitus, coronary artery disease, CLI, and smoking, showed that ST groups had higher MALE rate compared to no-ST patients (HR: 4.99; 95% CI: 2.31-10.77; p<0.001). The 12-month time censored event rate for MACE was not statistically different between ST and no ST groups.
Table 4.
Outcomes following treatment for stent thrombosis
| Peri-procedural outcomes | |
|---|---|
| Endovascular (n=21) | |
| Procedural complications | 4 (19.0%) |
| Flow-limiting dissection | 1 (4.8%) |
| Arterial perforation | 0 (0%) |
| Access site hematoma ≥5 cm in diameter | 0 (0%) |
| Retroperitoneal hematoma | 0 (0%) |
| Distal embolization | 1 (4.8%) |
| Major bleed requiring blood transfusion | 0 (0%) |
| Acute renal failure | 1 (4.8%) |
| Emergency surgery | 1 (4.8%) |
| Death | 0 (0%) |
| Surgical (n=5) | |
| Procedural complications | 1 (16.7%) |
| Death | 0 (0%) |
| Major bleed requiring blood transfusion | 0 (0%) |
| Infection requiring IV antibiotics | 0 (0%) |
| Amputation | 0 (0%) |
| Surgical re-exploration | 0 (0%) |
| Post-operative myocardial infarction | 1 (16.7%) |
| 30-day outcomes | |
| Death | 0 (0%) |
| Myocardial infarction | 0 (0%) |
| Stroke | 0 (0%) |
| Overall MACE | 0 (0%) |
| Repeat endovascular revascularization | 2 (7.4%) |
| Repeat stent thrombosis | 1 (3.7%) |
| Surgical revascularization | 1 (3.7%) |
| Amputation in target limb | 0 (0%) |
| Overall MALE | 3 (11.1%) |
| 12-month outcomes (aggregate) | |
| Death | 0 (0%) |
| Myocardial infarction | 2 (7.7%) |
| Stroke | 0 (0%) |
| Overall MACE | 2 (7.7%) |
| Repeat endovascular revascularization | 6 (23.1%) |
| Repeat stent thrombosis | 2 (7.7%) |
| Surgical revascularization | 3 (11.5%) |
| Amputation in target limb | 0 (0%) |
| Overall MALE | 10 (38.5%) |
MACE – major adverse cardiovascular events, MALE – major adverse limb events
Figure 5a.

Kaplan-Meier curves of MALE between stent thrombosis (ST) and no ST groups
Note: Censored at death and follow up months < 12 months; HR=4.99 95% CI = 2.31-10.77; p < 0.001
Figure 5b.
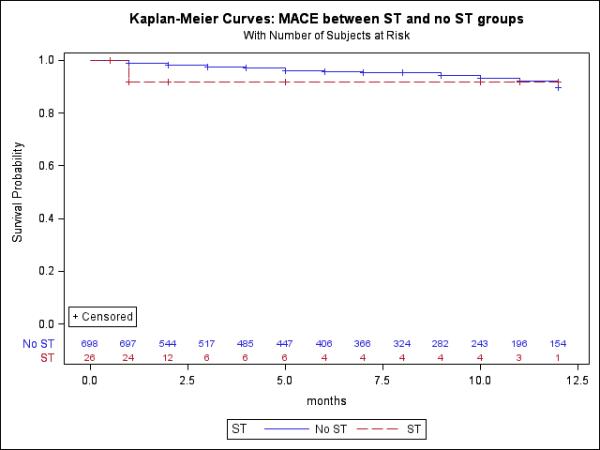
Kaplan-Meier curves of MACE between stent thrombosis (ST) and no ST groups
Note: Censored at death and follow up months < 12 months; HR= 2.92 95% CI = 0.69-12.33; p=0.1444
Table 5 presents GLMM results testing all clinical, angiographic, and procedural variables of interest as predictive factors for ST event, separately, and as a final multivariate GLMM which includes all variables that were retained after step-wise logistic regression. These variables are prior MI, CTO, ISR, stent types, and length of lesion greater than 130 mm. Multivariate GLMM results showed that CTO (OR=3.46; 95% CI: 0.98-12.20; p=0.05) and ISR lesions (OR=5.30; 95% CI: 1.83-15.32; p=0.002) are predictors of ST (Table 5), however not statistically significant for Viabahn™ stent grafts compared to BMS (p=0.105).
Table 5.
Predictors of stent thrombosis: Generalized Linear mixed Model (GLMM) estimates
| Variable | OR [95% CI] | P-value |
|---|---|---|
| Univariate | ||
| Age > 63 years | 1.02 [0.46-2.24] | 0.965 |
| Ankle brachial index > 0.66 | 1.13 [0.50-2.54] | 0.772 |
| Diabetes mellitus | 1.22 [0.55-2.70] | 0.615 |
| Hypertension | 1.85 [0.53-6.47] | 0.333 |
| Hyperlipidemia | 1.12 [0.33-3.86] | 0.856 |
| Smoking | 1.37 [0.58-3.22] | 0.469 |
| Critical limb ischemia | 1.32 [0.54-3.24] | 0.536 |
| Coronary artery disease | 1.55[0.61-3.96 | 0.352 |
| Prior myocardial infarction | 1.70 [0.76-3.80] | 0.191 |
| Congestive heart failure | 1.27 [0.50-3.27] | 0.611 |
| Chronic kidney disease | 0.43 [0.10-1.88] | 0.260 |
| Ostial SFA | 2.33 [0.95-5.76] | 0.065 |
| Distal SFA | 0.44 [0.13-1.50] | 0.186 |
| Popliteal | 1.06 [0.24-4.66] | 0.942 |
| Lesion length >133 mm | 2.22 [0.99-5.01] | 0.054 |
| Chronic total occlusion | 4.33 [1.27-14.75] | 0.019 |
| In stent restenosis | 5.07 [1.91-13.48] | 0.001 |
| Stent type | ||
| Bare metal stents | 1.00 | --- |
| Zilver PTX™ drug-coated stents | 1.48 [0.41-5.30] | 0.545 |
| Viabahn™ stent graft | 3.72 [1.28-10.81] | 0.006 |
| Supera™ mimetic stent | 1.94 [0.54-7.02] | 0.307 |
| Stent length >180 mm | 2.01 [0.91-4.41] | 0.082 |
| Multivariate | ||
| Prior myocardial infarction | 1.85 [0.80-4.27] | 0.150 |
| Chronic total occlusion | 3.46 [0.98-12.20] | 0.054 |
| In stent restenosis | 5.30 [1.83-15.32] | 0.002 |
| Stent type | ||
| Bare metal stents | 1.00 | --- |
| Zilver PTX™ drug-coated stents | 1.15 [0.31-4.23] | 0.710 |
| Viabahn™ stent graft | 2.57 [0.82-5.05] | 0.105 |
| Supera™ mimetic stent | 2.25 [0.60-8.37] | 0.224 |
| Lesion length >130 mm | 1.86 [0.80-4.31] | 0.149 |
CI – confidence interval, OR – odds ratios, SFA – superficial femoral artery, OR – Odds Ratios, CI- Confidence Intervals
*For Dual antiplatelet therapy (DAPT) variable, while convergence criterion is met but a solution is questionable because a covariance (G) matrix is not positive.
Stepwise logistic regression used to allow a variable into the model with a significance level of 0.30 and 0.35 for a variable to stay in the model to select GLMM to predict ST. The final selected predictors were prior myocardial infarction, chronic total occlusion, in stent restenosis, stent types, and lesion length >130 mm, simultaneously. The Hosmer and Lemeshow goodness-of-fit test for the final selected model was χ2=8.638 (degree of freedom=7) p=0.2796. The model's fit statistics AIC=216.36.
Discussion
The results of the present study are the first systematically reported experience of lower extremity peripheral artery ST to date, and provide an independent clinical and angiographic core laboratory assessment of infrainguinal artery ST events, early and late clinical outcomes, and factors associated with heightened risk of ST. The most important observations of this study include: (1) femoropopliteal peripheral artery ST is observed in 4.3% of patients; (2) treatment of CTO and in-stent restenosis lesions are independently associated with an increased risk of future ST, and (3) femoropopliteal peripheral artery ST is associated with an increased risk for future adverse limb, but not cardiovascular events.
In this study, ST events were most frequent in the first month following the index procedure, and most frequently involved stenting of CTO target lesions. The majority of stents implanted were BMS (73.8%), while Zilver PTX™, Viabahn™, and Supera™ use in the study were 11.1%, 7.8% and 7.1%, respectively. The relatively low use of non-BMS can be attributed to their more recent clinical introduction, limited availability at participating sites, or to higher cost. The registry, being observational in nature, is limited in its ability to account for all the variables leading to the selection of a particular stent type, and therefore subject to selection bias. The mechanism of ST could vary based on stent type, as all forms of non-BMS exhibited numerically higher ST rates compared to BMS, with Viabahn™ achieving statistical significance. Loss of collaterals and edge restenosis are known factors for Viabahn™ stent graft thrombosis.12 ST with DCS may be secondary to delayed endothelialization and endothelial cell dysfunction leading to upregulation of pro-thrombotic genes with antiproliferative drug (primarily paclitaxel) exposure.19,20 In support of this possibility, first generation paclitaxel drug-eluting stents were found to be associated with higher rates of ST than BMS in the randomized percutaneous coronary intervention trials.21 Due to a relatively small number of ST events in the study, the 12-month Kaplan Meier time to event analyses did not find significant differences for ST amongst stent types and presence or absence of CTO lesion. It is important to emphasize that the ST rates reported for Viabahn™ and Zilver PTX™ in this study are higher than those reported in more recent studies.12,22-24 The increased rate of ST in this registry could be due to the more complex lesions treated as well as heterogeneity in DAPT duration. Stent fractures may also contribute to both BMS and non-BMS thrombosis. Only 1 (4.8%) stent fracture was detected among patients referred for angiography and endovascular treatment of ST, higher than that reported in contemporary studies.25-27 However, given the observational nature of the registry and lack of mandatory screening for stent fracture, its actual incidence cannot be estimated. These factors, together with a significantly higher prevalence of CTO and a trend for longer stent lengths in the ST cohort may account for the higher risk associated with ISR lesions after multivariate adjustment.28 Although ST occurred significantly more often in males, this could result from the large sample of veteran patients comprising the registry. It is important to indicate that non-stented femoropopliteal arterial vessel thrombosis rates of 1.4% and 3.7% have recently been reported in the INPACT SFA study at 12 months with drug-coated balloon and conventional balloon angioplasty, respectively (p=0.096).29
Ex vivo imaging and histopathological studies of femoropopliteal CTO have indicated to extensive collagen deposition, especially at the proximal and distal caps of the CTO lesions.30 This observation, along with presence of heavy intramural calcification may limit optimal stent expansion and/or distort the architecture of deployed stents.31 Moreover, the large lipid and thrombotic material in the central core of CTO and ISR lesions may also contribute to distal embolization and poor run-off.32 These histopathological features, together with technical factors associated with lesion crossing, treatment and residual or unrecognized dissections (Figure 1) may contribute to the higher hazard of ST associated with stenting of femoropopliteal CTO and ISR lesions.
Short (<1 month) DAPT could play a significant role in ST of any stent type.33,34 Stents can be a nidus for thrombus formation, and the use of stents are an important predictor of DAPT use in the XLPAD registry overall.35 The benefit of DAPT in preventing clinical events has been clearly demonstrated following coronary artery stenting.36 However, the efficacy of DAPT use and duration is still unclear in the realm of PAD interventions, and as a result guidelines only recommend antiplatelet monotherapy following peripheral artery interventions.37 Only the MIRROR (Follow-up management of peripheral artery intervention with clopidogrel) randomized controlled trial (n=40 patients) has shown superior benefit of DAPT over antiplatelet monotherapy in reducing repeat endovascular intervention rates following a peripheral intervention.38 The results of two currently ongoing randomized controlled trials will add to the evidence base in support or against antiplatelet use following peripheral interventions (A Study Comparing Cardiovascular Effects of Ticagrelor and Clopidogrel in Patients With Peripheral Artery Disease (EUCLID) available at http://clinicaltrials.gov/show/NCT0173282; Antiplatelet Strategy for Peripheral Arterial Interventions for Revascularization of Lower Extremities (ASPIRE) available at http://clinicaltrials.gov/ct2/show/NCT02217501).
A significantly higher hazard ratio for ST was observed in univariate analysis with ostial SFA stents. Although its statistical significance diminished in the multivariate model, there are features of ostial femoropopliteal lesions that are worth highlighting. Generally these lesions have contiguous atherosclerotic disease involving the inflow vessels, the common femoral artery, or the profunda femoral artery (PFA). Precise placement of self-expanding stents at this location can be challenging and may lead to unintended coverage of the PFA origin, which may be diseased. These factors may lead to sluggish inflow, inadequate stent expansion and/or impediment of collateral supply via the PFA, and may therefore contribute to ST. Importantly, distal SFA stent location accounted for 14.8% of ST occurrences. This could be due to contiguous popliteal and infrapopliteal disease and the fact that distal SFA locations of stents are generally considered to be at a greater risk of fracture. Larger studies will be needed to explore fully the relationship between risk of ST and stent location along with quality of distal run-off vessels.
Aspiration thrombectomy, thrombolytic therapy, adjunctive debulking, and stenting were employed in treating patients with ST, and referral for surgical revascularization was infrequent. Importantly, occurrence of femoropopliteal ST was associated with worse long-term MALE, although we did not observe that peripheral artery ST portends an excess risk for MI. These observations highlight the need for careful consideration of the initial revascularization strategy for femoropopliteal lesions. This includes use of non-stent strategies such as atherectomy and/or balloon angioplasty, selection of stent type, efforts to optimize stent results, as well as DAPT use and its duration. The introduction of drug-coated balloons into the U.S. marketplace has provided yet another option to endovascular specialists when choosing a non-stent strategy.8 The impact of either one or more of the above treatments will need to be carefully assessed by future studies.
Important limitations of the present study include its observational nature, possible selection bias, high proportion of cases contributed by a single center, limited duration of follow-up, lack of a consistent DAPT regimen, inability to account for DAPT adherence, and lack of radiographic follow-up of all patients for evaluating stent fracture. However, many of these limitations reflect the challenges in conducting a systematic post-peripheral artery intervention ST study and may need to be considered as future studies are planned for this area.39
Conclusion
These findings from the XLPAD registry are the first to report that femoropopliteal peripheral artery ST occurred in 4.3% of patients and was associated with stenting of CTO lesions, and treatment of in-stent restenosis lesions. Finally, femoropopliteal ST is associated with not only high periprocedural complications, but also 12-month MALE.
Supplementary Material
What is known
Minimally invasive endovascular treatment of patients with symptomatic peripheral artery disease is highly prevalent.
Although information regarding restenosis and durability of peripheral interventions is widely available, there is little or no recognition of peripheral artery stent or vessel thrombosis, a rare, but serious complication of endovascular intervention.
What the study adds
This study is first to propose standard definition for peripheral artery stent and vessel thrombosis and present data on its incidence and outcomes.
The incidence of peripheral artery stent thrombosis is reported as 4.3%.
This information may guide providers caring for patients with peripheral artery disease in diagnosing and treating this potentially serious complication of endovascular intervention.
Acknowledgements
The authors of this manuscript would like to acknowledge the contributions of Tayo Addo, MD, Michael Luna, MD, and M. Ishti Ali, MD for their contributions to the XLPAD registry. We also acknowledge the support of the University of Texas Southwestern Medical Center for their support in establishing and managing the RedCap database software utilized in the XLPAD registry (Academic Information Systems NIH grant UL1-RR024982).
Sources of Funding
The study was funded by NIH grant UL1-RR024982 awarded to the University of Texas Southwestern Medical Center Academic Information Systems in establishing and managing the XLPAD registry using the RedCap database software.
Footnotes
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01904851.
Disclosures
Subhash Banerjee, MD: Research grants: Boston Scientific, Medicines Company; consultant/speaker honoraria: Gilead, St Jude, Cordis, Boehinger Ingerheim, Sanofi, Medtronic; ownership: Mdcare Global (spouse); intellectual property: HygeiaTel; Karan Sarode, MA: none; Atif Mohammad, MD: none; Osvaldo S. Gigliotti, MD: speaker income from Janssen; research contracts with Abbott Vascular, Medtronic, and AstraZeneca; Mirza S. Baig, MD: none; Shirling Tsai, MD: none; Nicolas W. Shammas, MD: Research grants: Boston Scientific, Possis, Edwards, the Medicines Co, ev3, Schering-Plough, Fox-Hollow, Spectranetics, Atrium, Gilead, Medtronic, Genesis Foundation, CSI, Bayer; Educational grants: Abbott Vascular, AGA Medical, Astellas Pharmaceuticals, AstraZeneca, Boehringer-Ingelheim, Boston Scientific, Cordis Vascular, Daiichi Sankyo, ev3, Gilead Sciences, IDEV Technologies, Lilly USA, Pfizer, BMS, The Medicines Company, Medtronic Cardiovascular, Spectranetics, St. Jude Medical, Takeda Pharmaceuticals, Terumo Medical, and Zoll Lifevest; Consultant: CSI, Medicines Company, Covidien/ev3, NAMSA; Promotional programs: Boehringer Ingelheim, Forest Pharmaceuticals, Lilly/Daichii, Astra Zeneca, Pfizer/BMS, Gilead; Anand Prasad, MD: Speaker honoraria: AstraZeneca, Gore, Abbott Vascular; Consultant: St Jude Medical; Mazen Abu-Fadel, MD: speaker income from Abbott Vascular; Andrew Klein, MD: none; Ehrin J. Armstrong, MD: consultant for Abbott Vascular, Medtronic, Merck, Pfizer and Spectranetics; Emmanouil S. Brilakis, MD, PhD: Research grants: consulting honoraria/speaker fees from Sanofi, Janssen, St Jude Medical, Terumo, Asahi, Abbott Vascular, and Boston Scientific; research grant from Guerbet; spouse is an employee of Medtronic. Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Get With The Guidelines Steering Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor); Research Funding: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Site Co-Investigator: Biotronik, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda.
References
- 1.Hong MS, Beck AW, Nelson PR. Emerging national trends in the management and outcomes of lower extremity peripheral arterial disease. Annals of vascular surgery. 2011;25:44–54. doi: 10.1016/j.avsg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, Golzarian J, Gornik HL, Halperin JL, Jaff MR, Moneta GL, Olin JW, Stanley JC, White CJ, White JV, Zierler RE. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2011;58:2020–2045. doi: 10.1016/j.jacc.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee S, Das TS, Abu-Fadel MS, Dippel EJ, Shammas NW, Tran DL, Zankar A, Varghese C, Kelly KC, Weideman RA, Little BB, Reilly RF, Addo T, Brilakis ES. Pilot trial of cryoplasty or conventional balloon post- dilation of nitinol stents for revascularization of peripheral arterial segments: the COBRA trial. Journal of the American College of Cardiology. 2012;60:1352–1359. doi: 10.1016/j.jacc.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 4.Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, Schlager O, Cejna M, Lammer J, Minar E. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. The New England journal of medicine. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 5.Vardi M, Novack V, Pencina MJ, Doros G, Burke DA, Elmariah S, Cutlip DE, Mauri L, Yeh RW. Safety and efficacy metrics for primary nitinol stenting in femoropopliteal occlusive disease: a meta-analysis and critical examination of current methodologies. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2014;83:975–983. doi: 10.1002/ccd.25179. [DOI] [PubMed] [Google Scholar]
- 6.Kasapis C, Henke PK, Chetcuti SJ, Koenig GC, Rectenwald JE, Krishnamurthy VN, Grossman PM, Gurm HS. Routine stent implantation vs. percutaneous transluminal angioplasty in femoropopliteal artery disease: a meta-analysis of randomized controlled trials. European heart journal. 2009;30:44–55. doi: 10.1093/eurheartj/ehn514. [DOI] [PubMed] [Google Scholar]
- 7.Cho L, Roffi M, Mukherjee D, Bhatt DL, Bajzer C, Yadav JS. Superficial femoral artery occlusion: nitinol stents achieve better flow and reduce the need for medications than balloon angioplasty alone. The Journal of invasive cardiology. 2003;15:198–200. [PubMed] [Google Scholar]
- 8.Sarode K, Spelber DA, Bhatt DL, Mohammad A, Prasad A, Brilakis ES, Banerjee S. Drug delivering technology for endovascular management of infrainguinal peripheral artery disease. JACC. Cardiovascular interventions. 2014;7:827–839. doi: 10.1016/j.jcin.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M, Jr., Nater C, Hruban RH, Rezai B, Abella BS, Bunge KE, Kinsella JL, Sollott SJ, Lakatta EG, Brinker JA, Hunter WL, Froehlich JP. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001;103:2289–2295. doi: 10.1161/01.cir.103.18.2289. [DOI] [PubMed] [Google Scholar]
- 10.Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation. 2003;107:2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- 11.Wood NB, Zhao SZ, Zambanini A, Jackson M, Gedroyc W, Thom SA, Hughes AD, Xu XY. Curvature and tortuosity of the superficial femoral artery: a possible risk factor for peripheral arterial disease. Journal of applied physiology (Bethesda, Md. : 1985) 2006;101:1412–1418. doi: 10.1152/japplphysiol.00051.2006. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. Journal of vascular surgery. 2013;58:386–395. e384. doi: 10.1016/j.jvs.2013.01.050. [DOI] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee S, Hadidi O, Mohammad A, Alsamarah A, Thomas R, Sarode K, Garg P, Baig MS, Brilakis ES. Blunt microdissection for endovascular treatment of infrainguinal chronic total occlusions. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2014;21:71–78. doi: 10.1583/12-4009MR.1. [DOI] [PubMed] [Google Scholar]
- 15.Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 16. [December 30, 2015];Gore Viabahn Instructions for Use. http://www.goremedical.com/resources/dam/assets/AP3360ML2.V3LD18.US_IFU.pdf.
- 17. [December 30, 2015];Abbott Vascular Supera Instructions for Use. http://www.abbottvascular.com/docs/ifu/peripheral_intervention/eIFU_Supera.pdf.
- 18. [December 30, 2015];Zilver PTX Drug Eluting Stent: IFU0063-0. 2011 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM273810.pdf.
- 19.Axel DI, Kunert W, Goggelmann C, Oberhoff M, Herdeg C, Kuttner A, Wild DH, Brehm BR, Riessen R, Koveker G, Karsch KR. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636–645. doi: 10.1161/01.cir.96.2.636. [DOI] [PubMed] [Google Scholar]
- 20.Creel CJ, Lovich MA, Edelman ER. Arterial paclitaxel distribution and deposition. Circulation research. 2000;86:879–884. doi: 10.1161/01.res.86.8.879. [DOI] [PubMed] [Google Scholar]
- 21.Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. The American journal of medicine. 2006;119:1056–1061. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Lammer J, Dake MD, Bleyn J, Katzen BT, Cejna M, Piquet P, Becker GJ, Settlage RA. Peripheral arterial obstruction: prospective study of treatment with a transluminally placed self-expanding stent-graft. International Trial Study Group. Radiology. 2000;217:95–104. doi: 10.1148/radiology.217.1.r00se0595. [DOI] [PubMed] [Google Scholar]
- 23.Lammer J, Zeller T, Hausegger KA, Schaefer PJ, Gschwendtner M, Mueller-Huelsbeck S, Rand T, Funovics M, Wolf F, Rastan A, Gschwandtner M, Puchner S, Ristl R, Schoder M. Heparin-bonded covered stents versus bare-metal stents for complex femoropopliteal artery lesions: the randomized VIASTAR trial (Viabahn endoprosthesis with PROPATEN bioactive surface [VIA] versus bare nitinol stent in the treatment of long lesions in superficial femoral artery occlusive disease). Journal of the American College of Cardiology. 2013;62:1320–1327. doi: 10.1016/j.jacc.2013.05.079. [DOI] [PubMed] [Google Scholar]
- 24.Dake M. Leipzig Interventional Course. Leipzig, Germany: 2015. Zilver PTX® Paclitaxel-Eluting Stents for Femoropopliteal Disease: 24-Month Results of the Randomized Study. [Google Scholar]
- 25.Dake MD, Ansel GM, Jaff MR, Ohki T, Saxon RR, Smouse HB, Zeller T, Roubin GS, Burket MW, Khatib Y, Snyder SA, Ragheb AO, White JK, Machan LS. Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results. Circulation. Cardiovascular interventions. 2011;4:495–504. doi: 10.1161/CIRCINTERVENTIONS.111.962324. [DOI] [PubMed] [Google Scholar]
- 26.Duda SH, Bosiers M, Lammer J, Scheinert D, Zeller T, Oliva V, Tielbeek A, Anderson J, Wiesinger B, Tepe G, Lansky A, Jaff MR, Mudde C, Tielemans H, Beregi JP. Drug-eluting and bare nitinol stents for the treatment of atherosclerotic lesions in the superficial femoral artery: long-term results from the SIROCCO trial. Journal of endovascular therapy : an official journal of the International Society of Endovascular Specialists. 2006;13:701–710. doi: 10.1583/05-1704.1. [DOI] [PubMed] [Google Scholar]
- 27.Laird JR, Katzen BT, Scheinert D, Lammer J, Carpenter J, Buchbinder M, Dave R, Ansel G, Lansky A, Cristea E, Collins TJ, Goldstein J, Jaff MR. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circulation. Cardiovascular interventions. 2010;3:267–276. doi: 10.1161/CIRCINTERVENTIONS.109.903468. [DOI] [PubMed] [Google Scholar]
- 28.Scheinert D, Scheinert S, Sax J, Piorkowski C, Braunlich S, Ulrich M, Biamino G, Schmidt A. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. Journal of the American College of Cardiology. 2005;45:312–315. doi: 10.1016/j.jacc.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Tepe G, Laird J, Schneider P, Brodmann M, Krishnan P, Micari A, Metzger C, Scheinert D, Zeller T, Cohen DJ, Snead DB, Alexander B, Landini M, Jaff MR. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131:495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munce NR, Yang VX, Standish BA, Qiang B, Butany J, Courtney BK, Graham JJ, Dick AJ, Strauss BH, Wright GA, Vitkin IA. Ex vivo imaging of chronic total occlusions using forward-looking optical coherence tomography. Lasers in surgery and medicine. 2007;39:28–35. doi: 10.1002/lsm.20449. [DOI] [PubMed] [Google Scholar]
- 31.Miki K, Fujii K, Fukunaga M, Kawasaki D, Shibuya M, Imanaka T, Tamaru H, Masutani M, Ohyanagi M, Masuyama T. Impact of post-procedural intravascular ultrasound findings on long-term results following self- expanding nitinol stenting in superficial femoral artery lesions. Circulation journal : official journal of the Japanese Circulation Society. 2013;77:1543–1550. doi: 10.1253/circj.cj-12-1182. [DOI] [PubMed] [Google Scholar]
- 32.Zankar A, Brilakis E, Banerjee S. Use of embolic capture angioplasty for the treatment of occluded superficial femoral artery segments. The Journal of invasive cardiology. 2011;23:480–484. [PubMed] [Google Scholar]
- 33.Linnemann B, Thalhammer A, Wolf Z, Tirneci V, Vogl TJ, Edelgard Lindhoff-Last A. Late peripheral stent thrombosis due to stent fracture, vigorous exercise and hyporesponsiveness to clopidogrel. VASA. Zeitschrift fur Gefasskrankheiten. 2012;41:136–144. doi: 10.1024/0301-1526/a000177. [DOI] [PubMed] [Google Scholar]
- 34.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Sarode K, Mohammad A, Spelber DA, Pershwitz G, R. T, Hadidi OF, Mody P, Luna M, Addo T, Brilakis ES, Banerjee S. Society for Coronary Angiography and Interventions. Las Vegas, NV.: May 28-31, 2014. Optimal Dual Antiplatelet Therapy after Lower Extremity Revascularization. [Google Scholar]
- 36.Hanna EB. Dual antiplatelet therapy in peripheral arterial disease and after peripheral percutaneous revascularization. The Journal of invasive cardiology. 2012;24:679–684. [PubMed] [Google Scholar]
- 37.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of vascular surgery. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 38.Tepe G, Bantleon R, Brechtel K, Schmehl J, Zeller T, Claussen CD, Strobl FF. Management of peripheral arterial interventions with mono or dual antiplatelet therapy--the MIRROR study: a randomised and double-blinded clinical trial. European radiology. 2012;22:1998–2006. doi: 10.1007/s00330-012-2441-2. [DOI] [PubMed] [Google Scholar]
- 39.Subherwal S, Patel MR, Chiswell K, Tidemann-Miller BA, Jones WS, Conte MS, White CJ, Bhatt DL, Laird JR, Hiatt WR, Tasneem A, Califf RM. Clinical trials in peripheral vascular disease: pipeline and trial designs: an evaluation of the ClinicalTrials.gov database. Circulation. 2014;130:1812–1819. doi: 10.1161/CIRCULATIONAHA.114.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.