Abstract
In this study, healthy volunteers were scanned using functional magnetic resonance imaging (fMRI) to investigate the neural systems involved in processing the threatening content conveyed via visually presented “threat words.” The neural responses elicited by these words were compared to those elicited by matched neutral control words. The results demonstrate that linguistic threat, when presented in written form, can selectively engage areas of lateral temporal and inferior frontal cortex, distinct from the core language areas implicated in aphasia. Additionally, linguistic threat modulates neural activity in visceral/emotional systems (amygdala, parahippocampal gyrus and periaqueductal gray), and at earlier stages of the visual-linguistic processing stream involved in visual word form representations (ventral occipitotemporal cortex). We propose a model whereby limbic activation modulates activity at multiple nodes along the visual-linguistic-semantic processing stream, including a perisylvian “semantic access network” involved in decoding word meaning, suggesting a dynamic interplay between feedforward and feedback processes.
Keywords: emotion, fMRI, threat, functional neuroimaging, neuroanatomy, reading
Graphical Abstract
1. Introduction
Humans are unique among animals for our ability to glean information about the world symbolically through the use of language. Much evidence suggests that the limbic system, and in particular, the amygdala, along with its afferent and efferent connections, plays an important role in the detection of threat and in the coordination of behavioral, neuroendocrine, and autonomic responses to threat (LeDoux, 2003; Nieuwenhuys, Voogd, & van Huijzen, 2008; Ohman, 2005). The limbic system is phylogenetically old relative to the more recently evolved neocortex (Mega, Cummings, Salloway, & Malloy, 1997), and yet many of the threats humans encounter are nuanced and indirect, and can be conveyed adequately only via language, an evolutionarily recent innovation believed to be neocortically-based. Nevertheless, we have previously demonstrated a role for the amygdala in the processing of linguistic threat utilizing positron-emission tomography (PET) imaging in an emotional stroop task (Isenberg et al., 1999). Over-activation of the amygdala and other mesial temporal lobe structures are also believed to be involved in the misperception of threat among patients with schizophrenia, for example (Holt et al., 2006). The limbic system must gain access to these threats by way of neocortical systems specialized for parsing language and accessing its symbolic meaning. Semantic representations must be activated so that threat can be identified, and the limbic system can be engaged. Pulvermüller (2002) has proposed a model of language representation in the brain in which words are represented as “functional webs”, which he defines as sets of strongly connected neurons that are distributed over specific sets of functional areas and work together as functional units. Articulatory and phonological components of word webs are likely distributed over the anterior and posterior portions of perisylvian language cortex, respectively. Links to semantic representations are likely distributed more broadly, incorporating brain areas functionally specialized for specific aspects of the word’s semantic content. Pulvermüller theorizes that when an emotional connotation for a word is learned, connections between the word web and limbic structures are strengthened, so that when the word web becomes activated in the future, a limbic extension, or “tail,” of the word web becomes activated, representing the word’s emotional association.
The present study builds on our prior PET study (Isenberg, et al., 1999) by utilizing a more naturalistic silent reading task with minimal cognitive load, and an imaging method with higher spatial resolution, functional magnetic resonance imaging (fMRI), to identify the neural structures involved in processing the threatening information of a paranoid nature conveyed via visually presented single words. Specifically, we sought to test the hypothesis that during silent reading of single words, even when no directed cognitive task is employed, words with connotations of paranoid threat can differentially engage limbic/paralimbic structures as well as visual and perisylvian cortices involved in reading. Dysfunction in the neural systems subserving detection and processing of threat are believed to be of particular importance in the paranoid delusions experienced by patients with schizophrenia and other psychotic disorders. Thus, a set of words was chosen with the assistance of psychiatrists experienced in evaluating and treating patients with schizophrenia that were consonant with paranoid ideology such that they might be particularly potent at probing this circuitry.
While in the scanner, 17 participants were asked to read single words and press a button to indicate that they completed reading the word. The neural responses elicited by reading threat words were compared with the responses to matched emotionally neutral control words. Threat-specific activations in response to single-word reading were seen in several portions of the limbic system, including the left amygdala, as well as in regions of visual association cortex associated with word-reading (C. J. Price, 2000) and regions of perisylvian language cortex involved with accessing word meanings. Thus, visually presented linguistic paranoid threat modulated limbic activity as well as the activity of several nodes along the processing stream involved in perception and comprehension of the written word.
2. Materials and Methods
2.1 Subjects
Participants were 17 healthy, right-handed volunteers (12 male). A written statement of informed consent was signed by each participant in accordance with the Weill Medical College Institutional Review Board and the Code of Ethics of the World Medical Association. All of the participants were free of major psychiatric, neurologic, and medical diagnoses. Mean age was 26.9 (range=18–39). Educational background varied among the participants with five possessing advanced degrees, four possessing bachelor’s degrees, and six reporting some college experience. Of the remaining two participants, one possessed a high school degree and the other was pursuing a GED. In order to participate in the study, subjects had to demonstrate English reading proficiency at or above an 8th grade level ascertained by a score of at least 42 on the Wide-Range Achievement Test (WRAT). Among the participants in this study, WRAT scores ranged from 48 to 57.
2.2 Stimuli
Stimuli consisted of 48 written words that participants viewed on an MRI compatible Sharp LCD screen inside the scanner. The stimuli were of two types: 24 words were selected for their threatening content and negative valence so as to be of particular relevance to patients with paranoid disorders, e.g., “blame,” “distrust,” and “sinister,” while the remaining 24 words were selected for their emotionally neutral content, e.g., ”navigate,” “inhabit,” and “folder.” The two categories of words were counterbalanced for the possible confounding variables of word length, frequency within the lexicon (Carroll, Davies, & Richman, 1971), and part of speech.
2.3 Paradigm
The stimuli were presented in a block design consisting of 4 blocks of 6 threat (T) words each and 4 blocks of 6 neutral (N) words each. The blocks were intermixed in a pseudorandom order (NTTNNTNT). Each word appeared for 2 seconds with an interstimulus interval jittered based on a uniform distribution on the range [1.8, 3.8] (i.e., around an average of 2.8 seconds), for a total of 28.8 seconds per block. Each block was followed by 24 seconds of rest, with the paradigm as a whole preceded and followed by two additional 12-second rest periods. Stimulus presentation and response collection were performed within the Integrated Functional Imaging System SA/E-Prime environment (MRI Devices Corporation, Waukesha, Wisc.; Psychology Software Tools, Inc., Pittsburgh, PA.). Subjects were instructed to read each word silently and then to immediately press a button under their right index finger. During rest periods, subjects were instructed to look at a dash at the center of the screen (fixation condition). There was no attempt to actually induce emotions in the subjects, but they were expected to read and comprehend the meaning of the words. To verify that this in fact happened, immediately after imaging was completed and subjects were removed from the scanner, their memory for the specific stimuli they saw in the scanner was tested with a list consisting of the 48 stimuli seen during scanning (targets) and 24 novel words (distractors) with which the targets were interspersed. Distractor words were divided equally into threat and neutral categories and balanced for the same qualities as the target words. The subjects were instructed to read each word and to indicate those words that they believed they had seen in the scanner. Following completion of this particular task, subjects were asked to rate the emotional valence of each word on a Likert-type scale of −3 to +3 to demonstrate that the threat words were in fact perceived as negatively valenced.
2.4 Image acquisition and processing
Image data were acquired on one of two research-dedicated GE-Signa 3 Tesla MRI scanners (scanner 1: eight participants; scanner 2: nine participants) (GE Healthcare, Waukesha, WI, USA) using blood-oxygen-level-dependent (BOLD) fMRI (max gradient strength 40mT/m; max slew rate 150T/m/s) with an MRI-compatible head holder. BOLD fMRI measures hemodynamic and oxygenation changes associated with localized neural activity in the brain (Logothetis, 2003). Because there were no detectable differences in imaging data acquired on the two scanners as confirmed via ANCOVA with the scanner ID as a covariate for intersubject variation, datasets were combined.
Three to five T1 weighted sagittal slices were collected to localize the anterior and posterior commissures, followed by a set of 17 coronal slices perpendicular to the AC-PC line to determine the location of the amygdala and hippocampus. A reference T1 weighted anatomical image with the same axial slice placement and thickness as the functional imaging was then acquired with two slices centered within the amygdala as determined by trained personnel based on visual identification of the amygdala from the localizer images (256x256 matrix size, 5mm in thickness, 1mm gap, TE/TR=14/500ms, FoV=240mm).
After shimming to maximize homogeneity, a series of functional scans was collected using a gradient echo EPI sequence (TR=1200ms, TE=30ms, flip angle=70, 21 slices with 5mm in thickness and 1mm gap, FoV=240mm, matrix=64x64, voxel size=3.75x3.75x6mm3), with a z-shimming algorithm (Gu et al., 2002) to reduce susceptibility artifact in ventral brain regions of interest.
A high-resolution T1 weighted anatomical image was acquired using a spoiled gradient (SPGR) recalled acquisition sequence (TR/TE=30/8msec, flip angle=45, field of view=220mm, 140 coronal slices with thickness=contiguous 1.5mm, number of averages=1, matrix=256x256, voxel size=0.8594x1.5x0.8594mm3).
Modified SPM software (Wellcome Department of Imaging Neuroscience) was used for processing the imaging data (Frackowiak et al., 2004), which consisted of the following steps carried out on UNIX workstations (Sun Microsystems, Mountainview, CA): reconstruction of echoplanar functional images using modified GE reconstruction software with off-resonance phase correction, slice-timing correction and Hanning-window apodization; extraction of physiological fluctuations such as cardiac and respiratory cycles from EPI image sequences (Frank, Buxton, & Wong, 2001); manual AC-PC re-orientation of all anatomical and EPI images; realignment to correct for slight head movement between scans and for differential spin excitation history based on intracranial voxels (data sets with movement of greater than 1/3 voxel over the study session were excluded); co-registration of functional EPI images to the corresponding high-resolution anatomical image based on the rigid body transformation parameters of the reference anatomical image to the latter for each individual subject; stereotactic normalization to a standardized coordinate space (Montreal MRI Atlas version of Talairach space with the Montreal Neurological Institute [MNI] average of 152 T1 brain scans as the template) based on the high-resolution anatomical image to normalize for individual differences in brain morphology; and spatial smoothing with an isotropic Gaussian kernel (full width at half maximum=7.5mm) to increase signal-to-noise ratio.
2.5 Functional Image analysis
Using customized fmristat software (Worsley et al., 2002), a two-level voxel-wise linear fixed-effects model was utilized to examine the effect sizes of the key condition contrasts in an ANCOVA setting. First, a voxel-wise multiple linear regression model was employed at the individual subject level. This was comprised of the block-wise regressors of interest, which consisted of the stimulus onset times convolved with a prototypical hemodynamic response function, and the covariates of no interest, which consisted of the temporal first-order derivative of the principal regressors (to compensate for slight latency differences in individual hemodynamic responses from the prototypical response function), global fluctuations, physiological fluctuations, realignment parameters, and scanning periods (McGonigle et al., 2000). Temporal filtering was performed to counter the effects of baseline shifts and higher frequency noise (than prototypical hemodynamic response), and an AR(1) model of the time course was used to accommodate temporal correlation in consecutive scans. Effects at every brain voxel were estimated using the EM (expectation maximization) algorithm, and regionally specific effects were then compared using linear contrasts. That is, for each subject, the effect image and its standard error image for each condition was calculated, and these are also combined in a series of linear contrasts to be entered into the second stage group-level analysis to assess within-group effect sizes of the key hypotheses. At the group level, a fixed-effects model was employed using multistat (Worsley, et al., 2002). The effects of the hypothesis-driven contrasts were estimated, with demographic variables (age and gender) incorporated as covariates of no interest. These group-level effect estimates generate statistical maps of the t statistic, and the statistical significance of the t-maps was then evaluated in the final step of inference. The statistical inference was based on random field theory as implemented in fmristat (Worsley, et al., 2002), where the p-values at peak voxels are corrected based on the family-wise error (FWE) rate over the entire brain or within a region of interest. The statistics of a particular contrast at a certain brain region were considered significant if they were part of a cluster with a spatial extent of no less than 250 mm3 with initial voxel-wise p-value less than 0.001, and the regional peak p-values corrected for multiple comparisons over the whole brain (pcorrwb) were less than 0.05. Voxels within a priori regions of interest (bilateral amygdalae, hippocampi/parahippocampal gyri, ventromedial prefrontal cortex (PFC), and left perisylvian cortex) were considered significant if the regional peak p-values corrected for multiple comparisons within the small volume masks of these regions (pcorrsv) was less than 0.05. A priori regions of interest were identified based on the expectation that brain regions most likely to be of importance in the processing of linguistic paranoid threat were limbic and paralimbic regions and language cortices. Anterior and posteiror language-related perisylvian cortical regions were considered separately. The anterior perisylvian mask comprised the AAL parcellation (Automated Anatomical Labeling) (Tzourio-Mazoyer et al., 2002) of the IFG pars opercularis, pars triangularis, and pars orbitalis with the orbitofrontal component removed. The posterior perisylvian parcellation comprised the left superior temporal gyrus (STG), middle temporal gyrus (MTG), and supramarginal gyrus (SMG). The ventromedial PFC region incorporated ventromedial, orbitofrontal, and anterior cingulate areas restricted to the posterior region of the ventromedial prefrontal cortex.
2.6 Functional Connectivity Analysis
As the amygdala was the focal point of the hypothesis regarding the detection and processing of linguistic threat, and the categorical analysis identified the left amygdala as a region that was significantly more active during the reading of threat words compared to neutral words, a seed analysis was performed to investigate which other brain regions function in concert with the amygdala during the processing of threat-related language. The aim was to explore the interactions between the amygdala and other limbic areas as well as visual and language areas during the processing of linguistic threat.
The seed voxel for this analysis was the local maximum within the left amygdala from the threat vs. neutral contrast (x=−18, y=0, z=−18; see results). (No threat-specific activity was seen in the right amygdala in that contrast.) The adjusted functional BOLD signal time course extracted from Left Amygdala was separated into block-wise regressors in a multiple linear regression model for each subject (Hampson, et al., 2002; Worsley, Chen, Lerch, & Evans, 2005), and the functional connectivity for Threat Words, Neutral Words, and Threat vs Neutral Words conditions was assessed via linear contrasts. Under the same framework of statistical modeling as in voxel-wise prototypical analysis, the effect image and its standard error image for each contrast (Threat Words, Neutral Words, and Threat vs Neutral Words conditions respectively) were calculated and entered into the second stage group-level analysis to assess within-group effect sizes of the key connectivity hypotheses. Again at the group level, a fixed-effects model was used to summarize the voxel-by-voxel correlation levels with the seed voxel. The resulting group-level t-statistic maps were subjected to the same family-wise error rate correction as well.
3. Results
3.1 Behavioral Results
Following the scanning session, subjects’ recognition was not significantly different for the threat words (mean d′ = 1.20, SE = 0.17) as compared to neutral words (mean d′ = 1.19, SE = 0.15) in a paired t-test t = 0.08(16), p = 0.94). Threat words were rated significantly more negative (mean rating = −1.65, SE = 0.16) than neutral words (mean rating = 0.40, SE = 0.10) on a scale of −3 to +3 (paired t-test t = −8.84(16), p < 0.001).
3.2 fMRI Results
The threat vs. neutral comparison identified a set of brain regions largely consisting of frontolimbic, visual, and higher order language processing areas (Table 1). Some of these findings are explored in more detail below.
Table 1.
Brain regions showing significant differential effects of linguistic paranoid threat on BOLD signal
| Region | MNI coordinates | z-score | pcorrwb | cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Threat > Neutral | ||||||
| L. Dorsomedial PFC (BA9) | −9 | 72 | 30 | 5.631 | <0.001 | 9315 |
| R. vOTC (BA19/37) | 42 | −78 | −21 | 5.819 | <0.001 | 2430 |
| R. MTG (BA21) | 63 | −3 | −27 | 4.95 | 0.001 | 1728 |
| L. PHG (BA28) | −21 | −24 | −15 | 4.577 | 0.007 | 918 |
| Periaqueductal region | −3 | −30 | −6 | 4.197 | 0.033 | 945 |
| L. PHG* | −12 | −6 | −27 | 3.838 | 0.004 | 729 |
| L. Amygdala* | −18 | 0 | −18 | 3.824 | 0.042 | ** |
| L. vOTC (BA19/37) | −48 | −69 | −18 | 4.38 | 0.017 | 1350 |
| L. MTG (BA21) | −69 | −27 | 0 | 4.775 | 0.003 | 1593 |
| L. IFG pars orbitalis (BA45/47)* | −54 | 33 | −9 | 3.891 | 0.04 | 378 |
| Neutral > Threat | ||||||
| R. Dorsolateral PFC (BA46/9) | 33 | 39 | 36 | 4.626 | 0.006 | 5373 |
| L. Calcarine (BA17) | −6 | −69 | 18 | 4.349 | 0.019 | 6453 |
| R. Parieto-occipital sulcus (BA18/19) | 15 | −69 | 30 | 4.177 | 0.036 | ** |
| R. Parieto-occipital sulcus (BA18/19) | 18 | −69 | 33 | 4.149 | 0.04 | ** |
| R. Insula, superior | 42 | 12 | 6 | 4.596 | 0.007 | 4752 |
| R. IFG pars opercularis (BA44) | 66 | 15 | 12 | 4.925 | 0.002 | 1215 |
| L. Cerebellum | −42 | −54 | −42 | 4.248 | 0.028 | 864 |
| L. MTG (BA21/37) | −36 | −54 | 12 | 4.128 | 0.043 | 405 |
| L. PrCG (BA4) | −66 | 6 | 15 | 4.415 | 0.014 | 432 |
Significance criteria: Initial voxel-wise threshold p<0.001, cluster spatial extent threshold > 250mm3, significance threshold = pcorrwb < 0.05, where pcorrwb = p-value at the peak coordinate FWE corrected for whole brain volume;
pcorrsv<0.05 via small volume correction in an a priori region of interest;
The peak voxel is a submaximum within the cluster above.
3.2.1 Limbic Regions
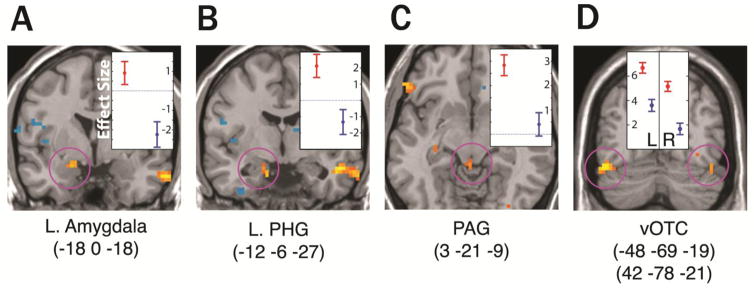
A significantly stronger signal was observed in the left amygdala (Fig. 1A) and in two areas of the left anterior parahippocampal gyrus (PHG) during the threat condition than during the neutral condition. One PHG cluster was strong enough to survive multiple comparison correction for the whole brain volume, while the other PHG cluster (Fig. 1B) and the amygdala effect survived a priori-identified small volume correction.
Figure 1.
Coronal (A,B,D) and axial (C) slices with group-level statistical results showing regions with significantly different BOLD response during the threat condition as compared to neutral (initial uncorrected voxel-wise threshold p<0.001 for the purpose of visualization). Highlighted findings survived correction for multiple comparisons at p<0.05 on peak voxels as described in the text. Plots show effect sizes with standard deviation error bars at cluster maxima (MNI coordinates below the image) in the threat vs. neutral contrast. Red = threat; Blue = neutral.
The area of greatest difference between the threat and neutral conditions within the brainstem was in the dorsal portion of the periaqueductal region (Fig. 1C). This region activated above baseline only during the threat condition, although a more ventral portion of the periaqueductal region was activated to a similar degree in both conditions.
3.2.2 Perisylvian Language Regions
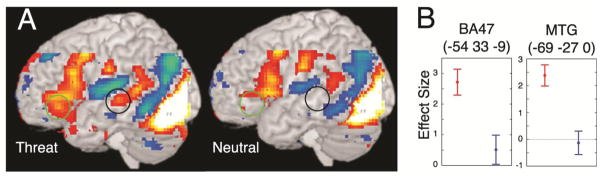
Within the left temporal lobe, the area of maximal activation was similar in both the threat and the neutral conditions, lying in the posterior portion of the STG/MTG (−60, −45, 9 and −57, −45,12 for threat and neutral respectively). The activations in these regions did not differ significantly between the two conditions. However, an additional region in the left MTG adjacent but anterior to this area activated in the threat condition alone (Fig. 2A, black circles; Fig. 2B). A region of the right MTG (63, −3, −27) was also significantly more active during the threat condition than during the neutral condition, but this region occupied a more anterior portion of the gyrus and the effect was driven by a signal decrease below the fixation level in the neutral condition rather than an increase in the threat condition as seen in the left MTG.
Figure 2.
(A) Significant signal increases (warm colors) and decreases (cold colors) in the threat and neutral conditions superimposed on a 3D rendering of the left cerebral hemisphere (initial uncorrected voxel-wise threshold p<0.001 for the purpose of visualization). Green and black circles indicate regions of the left IFG and left MTG respectively that were significantly more active during the threat condition than during neutral (p<0.05 on peak voxels when corrected for multiple comparisons). (B) Plots of effect sizes with standard deviations at the voxels of maximum significance within BA47 (indicated by the green circles in A) and the mid-MTG (indicated by the black circles in A). Red = threat; Blue = neutral.
Both the threat and the neutral conditions significantly activated the left IFG relative to fixation with the area of maximal activation in the posterior part of the gyrus (pars opercularis). While the neutral words activated the pars opercularis and pars triangularis but not the pars orbitalis, the threat condition activated the entire gyrus with a sub-maximum in the pars orbitalis (Fig. 2A, green circles; Fig. 2B). The difference in signal between the two conditions in the pars orbitalis did not survive whole brain correction, but was significant in the small volume correction for the IFG.
3.2.3 Visual Cortex
In both conditions, bilateral occipital cortex significantly activated relative to fixation. The activations incorporate the posterior portion of the medial occipital lobe as well as the lateral occipital cortex, and bilateral portions of ventral occipitotemporal cortex (vOTC) coursing anteriorly along the fusiform gyri. The vOTC activated significantly more during the threat condition than the neutral condition bilaterally (Fig. 1D).
3.2.4 Left Amygdala Functional Connectivity Analysis
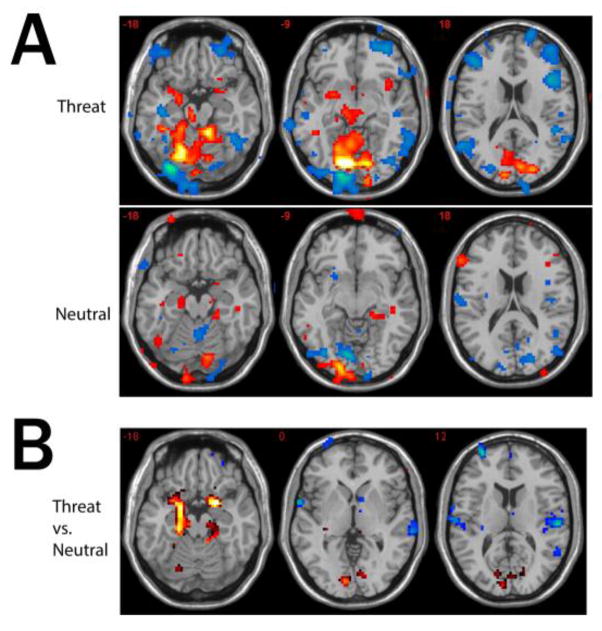
The voxel cluster that showed the most significant positive correlation with the left amygdala during the threat condition occupied a large region of ventromedial occipital cortex centered around bilateral lingual gyri and encompassing bilateral calcarine cortices, parieto-occipital sulci and cerebellum. The cluster extended along the ventral visual stream bilaterally, with sub-maxima in the left fusiform gyrus. A deep, largely sub-cortical cluster with local maxima in the left thalamus, left putamen, left amygdala, left midbrain in the regions incorporating ventral tegmentum, substantia nigra, superior colliculus and periaqueductal region was also positively correlated with the amygdala. Significant positive correlations were also seen in some cortical regions associated with language and some paralimbic areas (left PHG and right rostral agranular insula). The left amygdala correlated negatively with many other neocortical areas including some other regions associated with language processing (Fig. 3A, Table 2).
Figure 3.
Amygdala Connectivity Analysis
(A) Three axial slices showing voxels with significant (initial uncorrected voxel-wise threshold p<0.001 for the purpose of visualization) functional connectivity to the left amygdala during the threat and neutral conditions. Warm colors indicate positive correlations; cold colors indicate negative correlations. (B) Three axial slices showing significant differences in left amygdala functional connectivity between the threat and neutral conditions. Warm colors indicate positive differential connectivity, and cold colors indicate negative differential connectivity during the threat condition as compared to neutral.
Table 2.
Brain regions showing significant correlations with the left amygdala during the threat condition*
| Region | MNI coordinates | z-score | pcorrwb | cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlations | ||||||
| bilateral Inferior/medial occipital cortex and superior cerebellum | −12 | −78 | −12 | >8 | <0.001 | 89613 |
| L. Thalamus, putamen, amygdala, midbrain | −9 | −21 | 9 | 6.553 | <0.001 | 13662 |
| R. IFG pars opercularis | 63 | 21 | 27 | 5.465 | <0.001 | 1944 |
| L. Central sulcus, precentral gyrus | −48 | −3 | 39 | 4.992 | 0.001 | 1755 |
| L. Middle temporal gyrus | −48 | −42 | 9 | 6.601 | <0.001 | 837 |
| L. Supramarginal gyrus | −45 | −30 | 27 | 4.285 | 0.024 | 648 |
| R. Thalamus | 24 | −21 | 3 | 4.472 | 0.011 | 810 |
| L. Parahippocampal gyrus | −21 | −9 | −36 | 4.502 | 0.01 | 513 |
| L. Cerebellum/paramedian | −6 | −90 | −21 | 4.586 | 0.007 | 243 |
| b/l Cebellum, tonsil | 3 | −51 | −42 | 4.305 | 0.022 | 459 |
| R. Insula | 39 | 12 | −9 | 4.096 | 0.048 | 351 |
| Negative correlations | ||||||
| L. Cerebellum/inferior occipital cortex | −33 | −81 | −21 | >8 | <0.001 | 9018 |
| R. STS, posterolateral temporal cortex | 48 | −21 | −3 | 6.861 | <0.001 | 22086 |
| R. IFG/orbitofrontal cortex | 51 | 18 | 36 | 7.055 | <0.001 | 34047 |
| R. Inferior occipital cortex/superior cerebellum | 42 | −78 | −12 | >8 | <0.001 | 2916 |
| L. Posterior temporal/occipital cortex | −54 | −72 | 9 | 5.894 | <0.001 | 5913 |
| L. Mid occipital cortex | −30 | −78 | 27 | 5.75 | <0.001 | 2403 |
| L. STG/MTG | −63 | 3 | 0 | 6.042 | <0.001 | 5859 |
| R. Superior frontal gyrus | 27 | 69 | 0 | 6.101 | <0.001 | 6318 |
| L. VLPFC | −39 | 48 | −15 | 5.066 | 0.001 | 3240 |
| L. DLPFC | −42 | 36 | 27 | 6.617 | <0.001 | 6021 |
| R. Mid occipital cortex | 30 | −75 | 33 | 5.331 | <0.001 | 1431 |
| L. DLPFC | −30 | 63 | 12 | 6.411 | <0.001 | 3645 |
| L. Fusiform gyrus | −36 | −51 | −18 | 4.937 | 0.002 | 351 |
| L. Cerebellum | −33 | −90 | −36 | 4.302 | 0.023 | 2322 |
| L. Angular gyrus | −57 | −66 | 39 | 7.069 | <0.001 | 2727 |
| L. IFG | −60 | −3 | −33 | 5.294 | <0.001 | 1269 |
| L. ITG/fusiform gyrus | −42 | −18 | −18 | 5.583 | <0.001 | 972 |
| L. Cerebellum | −30 | −42 | −45 | 4.641 | 0.006 | 702 |
| L Mid cingulate gyrus | −3 | −12 | 27 | 5.033 | 0.001 | 729 |
| L DLPFC | −12 | 51 | 42 | 4.538 | 0.009 | 1107 |
| R DMPFC | 9 | 57 | 42 | 4.257 | 0.027 | 1161 |
| L precentral gyrus/DLPFC | −57 | 21 | 42 | 4.315 | 0.021 | 297 |
| R ITS | 66 | −6 | −30 | 4.473 | 0.011 | 378 |
IFG– inferior frontal gyrus; MTG – middle temporal gyrus; STS – superior temporal sulcus; STG- superior temporal gyrus; ITG – inferior temporal gyrus; ITS – inferior temporal sulcus; VLPFC – ventrolateral prefrontal cortex; DLPFC – dorsolateral prefrontal cortex;
Significance criteria are the same as in Table 1; submaxima are not shown.
The regions within left IFG and left MTG and right MTG that were shown in the categorical analysis to be significantly more active during the threat condition than the neutral condition did not correlate significantly with left amygdala activity during the threat condition. Both MTG regions did, however, show non-significant trends toward anti-correlation.
When left amygdala connectivity was contrasted between the threat and neutral conditions (Figure 3B, Table 3) a bilateral limbic network was highlighted: the right amygdala and left PHG were both significantly more connected with the left amygdala during the threat condition than during the neutral condition. In addition, bilateral calcarine cortex showed a positive correlation with the left amygdala during the threat condition and was anticorrelated during the neutral condition. On the other hand, the posterior planum temporale (primary auditory cortex) was anticorrelated with the left amydala to a greater degree during the threat condition than the neutral condition (significant on the right).
Table 3.
Brain regions showing significant differences in left amygdala connectivity between threat and neutral conditions
| Region | MNI coordinates | z-score | pcorrwb | cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Threat > Neutral | ||||||
| L. Amygdala | −21 | 0 | −15 | 4.87 | 0.002 | 5481 |
| L. Parahippocampal gyrus | −18 | −15 | −15 | 4.841 | 0.002 | ** |
| L. Parahippocampal gyrus | −21 | −27 | −18 | 4.828 | 0.003 | ** |
| L. Hippocampus/Amygdala junction | −18 | −3 | −18 | 4.791 | 0.003 | ** |
| L. Hippocampus | −21 | −21 | −15 | 4.79 | 0.003 | ** |
| L. Amygdala | −24 | 3 | −18 | 4.749 | 0.004 | ** |
| L. Hippocampus | −18 | −9 | −18 | 4.669 | 0.005 | ** |
| L. Olfactory cortex | −24 | 9 | −15 | 4.286 | 0.024 | ** |
| L. Temporal pole | −27 | 6 | −18 | 4.107 | 0.047 | ** |
| R. Amygdala | 18 | 3 | −15 | 5.593 | <0.001 | 3564 |
| R. Amygdala | 24 | 3 | −18 | 5.4 | <0.001 | ** |
| R. Olfactory cortex | 24 | 15 | −12 | 4.609 | 0.007 | ** |
| R. Claustrum/insula | 30 | 12 | −12 | 4.47 | 0.012 | ** |
| R. Claustrum/insula | 33 | 9 | −15 | 4.104 | 0.047 | ** |
| L. Calcarine | −9 | −90 | 0 | 4.169 | 0.037 | 3483 |
| R Calcarine | 12 | −81 | 3 | 4.289 | 0.024 | 1809 |
| L. Cuneus | −6 | −84 | 42 | 4.226 | 0.03 | 324 |
| L. Cuneus | −9 | −84 | 39 | 4.115 | 0.045 | ** |
| L. Inferior frontal gyrus/Inferior frontal sulcus | −60 | 33 | 18 | 4.791 | 0.003 | 324 |
| Neutral > Threat | ||||||
| R. Middle temporal gyrus | 63 | −3 | −30 | 4.938 | 0.002 | 2025 |
| R. Orbitofrotnal cortex | 30 | 54 | −9 | 4.495 | 0.01 | 2295 |
| R. Orbitofrotnal cortex | 30 | 51 | −12 | 4.392 | 0.016 | ** |
| R. Orbitofrotnal cortex | 21 | 60 | −12 | 4.219 | 0.031 | ** |
| R. Planum temporale | 57 | −21 | 12 | 4.654 | 0.005 | 2484 |
| L. Middle frontal gyrus (DLPFC) | −30 | 60 | 15 | 4.823 | 0.003 | 2052 |
| L. Middle frontal gyrus (DLPFC) | −30 | 63 | 12 | 4.702 | 0.004 | ** |
| R. Middle temporal gyrus | 69 | −30 | 0 | 4.143 | 0.041 | 1296 |
| R. Middle temporal gyrus | 60 | −51 | 18 | 4.209 | 0.032 | 1026 |
| L. Post central gyrus/parietal operculum | −60 | −18 | 15 | 4.841 | 0.002 | 1647 |
| L. Post central gyrus/parietal operculum | −63 | −15 | 15 | 4.693 | 0.005 | ** |
| L. Superior temporal gyrus | −63 | 3 | 0 | 4.421 | 0.014 | 675 |
Significance criteria are the same as in Table 1;
The peak voxel is a submaximum within the cluster above.
4. Discussion
These results suggest that connotations of threat contained in the written word can influence neural activity during the reading of such words at multiple stages of the visual-linguistic processing stream including visual word form representations (vOTC), semantic/conceptual representations (lateral temporal cortex and anterior inferior frontal cortex), and visceral/emotional systems involved in modulating behavioral and autonomic responses (amygdala, PHG and PAG). Portions of the left perisylvian cortex were activated by threat words and not neutral words, suggesting that there are regions within a network dedicated primarily to language processing that are engaged specifically by emotional aspects of language. In the following sections, the effects of linguistic threat on each of these neural systems are discussed individually in the context of the existing literature.
4.1 Limbic Structures
Several studies of visually presented emotional words have reported amygdala activations (Hamann & Mao, 2002; Herbert et al., 2009; Isenberg, et al., 1999; Kensinger & Corkin, 2004; Kensinger & Schacter, 2006; Landis, 2006; Naccache et al., 2005). Although most studies have focused on amygdala responses to negative stimuli, a variety of emotions have been shown to activate the amygdala, and this effect may depend more on how arousing or salient the stimuli are than on their valence (Costafreda, Brammer, David, & Fu, 2007; Kensinger & Schacter, 2006). While this effect can be seen with both linguistic and non-linguistic visual stimuli, linguistic stimuli have been associated with a left lateralization effect (Costafreda, et al., 2007; Kensinger & Schacter, 2006). The left amygdala finding in the present study is in line with these observations. The left anterior PHG, which showed a distinct peak in the threat vs. neutral contrast (Fig. 1B), is closely interconnected with the amygdala and hippocampus (Furtak, Wei, Agster, & Burwell, 2007) as well as most other paralimbic regions and appears to be involved in emotional processing as well (Gosselin et al., 2006; Meunier & Bachevalier, 2002).
The periaqueductal gray (PAG) is a gray matter structure surrounding the cerebral aqueduct that is continuous rostrally with the hypothalamus and has close connections with the amygdala and hypothalamus. Its functions include coordinating defensive behaviors and cardiovascular and respiratory responses to threat, as well as modulating pain perception. The PAG has not been previously implicated in processing of emotional language, but functional imaging studies have shown PAG activation in rats (Nephew, Caffrey, Felix-Ortiz, Ferris, & Febo, 2009) and in humans (Mobbs et al., 2009; Tuescher et al., 2011) when encountering situational threat. Stimulation of the PAG in humans produces a fear response, and aggressive or escape behavior can be elicited in non-human mammals with electrical stimulation in different parts of the PAG (Behbehani, 1995), while lesions to the PAG can abolish innate and learned defensive behaviors. Stimulation of the amygdala or the hypothalamus can also induce defensive reactions, and this effect is blocked by a lesion to the PAG, although the converse is not true, suggesting that the PAG is downstream from the amygdala and hypothalamus in the neural hierarchy governing defensive responses to threat (Vianna & Brandao, 2003).
4.2 Neocortical Structures
When language is read rather than heard, the language-specialized perisylvian cortical areas are engaged (C. J. Price, 2000), but linguistic information must access these multi-modal cortical areas via visual association cortex rather than auditory association cortex. A critical role has been argued for the left ventral occipitotemporal cortex in lexicosemantic transformations. This region of cortex lies in the so-called “ventral visual stream” believed to play an important role in representing higher order features of visual perception, such as object and face identification. Cohen and colleagues (2000) have identified a region of the left ventral occipitotemporal cortex that they have dubbed the “visual word form area” on the basis of imaging studies demonstrating activation peaks in this region in response to visual words as compared to visual fixation in individual subjects independent of the stimulus’ location on the retina (although this region’s specificity for the visual word form has been debated (C. J. Price & Devlin, 2003)). In the present study, when subjects read threat words, this area activated bilaterally to a significantly greater degree than when subjects read neutral words. This suggests that connotations of threat can modulate neural activity at the visual word form processing stage.
The vOTC presumably provides an access point by which visually presented words can activate the perisylvian language system. Language processing involves a large interconnected neural network encompassing much of the perisylvian cortex (Matsumoto et al., 2004; Pulvermüller, 2002). The anterior component in the left inferior frontal gyrus (Brodmann’s areas 44 and 45) and anterior insula is relatively specialized for representing the articulatory aspects of language due to its close connections with upper motor neurons controlling the muscles of the larynx, tongue and lips, and the posterior component in the left temporoparietal cortex is relatively specialized for representing the phonological aspects of language due to its close connections with auditory cortex, but both areas co-activate during many language tasks, due to their close connections with one another. Other areas of perisylvian cortex may participate as well, depending on the nature of the linguistic task (C. J. Price, 2000).
While the activation of the core anterior and posterior language areas classically known as Broca’s and Wernicke’s areas (posterior IFG/anterior insula and posterior STG/STS/angular gyrus respectively) was seen in response to silent reading of both neutral and threat words in the present study, threat words activated anterior extensions of both the anterior and posterior language areas that were not activated by neutral words: the left IFG pars orbitalis, which is roughly equivalent to Brodmann Area 47 (BA47) and the middle portion of the left MTG respectively. To our knowledge, this has not been previously shown. The concept that a neocortical region could play a specific role in processing emotional aspects of language is certainly not unprecedented. Right-hemisphere homologues of the core language areas have been widely believed to play an important role in paralinguistic components of affective speech (such as affective prosody) (Ross, 2000). These right-hemisphere regions were not engaged by the task employed in this study, which is not surprising, given that these paralinguistic elements of emotional expression (such as affective prosody) are not present in written language where emotional connotation can only be conveyed via semantic content. This study demonstrates that paranoid threat, when conveyed linguistically (in written form), can engage left-hemisphere structures in the perisylvian region that are not activated by neutral stimuli. Emotional word associations may be represented in association cortices anterior to the principle language areas in the perisylvian region. This raises the question is the function of these regions specific for processing linguistic emotion or are they involved in a more general process that is engaged by this emotional task? Various literature suggests that both BA47 and the middle portion of the MTG have a more general function related to accessing semantic content of words and are thus grouped together here under the term “semantic access network.”
Numerous functional imaging studies have suggested a role for BA47 in processing the semantic relationships between words or in retrieving semantic information to support both speech comprehension and production tasks (Bookheimer, 2002; Dapretto & Bookheimer, 1999; Fiez, 1997; C. J. Price, 2000; C.J. Price, 2010). For example, BA47 is selectively engaged when subjects generated a semantic association to a presented noun (Petersen, Fox, Posner, Mintun, & Raichle, 1988) and has been shown to be selectively active for words vs. pseudowords in a meta-analaysis of functional imaging studies examining neural responses to spoken words (Davis & Gaskell, 2009). The middle portion of the MTG is also highly associated with semantic representations of spoken (Davis & Gaskell, 2009) as well as written (Diaz & McCarthy, 2009) words. Patients with lesions in the MTG and the underlying white matter tend to show severe deficits in word comprehension and naming (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004). Thus both BA47 and the MTG are involved in modality-independent access to the semantic content of words. BA47 and the part of the MTG in question demonstrate significant resting functional connectivity to each other, and appear, based on diffusion tensor imaging, to be structurally linked via the inferior occipito-frontal fasciculus (Turken & Dronkers, 2011). The authors of that study proposed that the broad connectivity of the MTG indicates that it holds a key position within the language comprehension network, and that this may explain why injuries to this region are so particularly devastating. It appears to serve as a nexus for distributed semantic representations. BA47, as part of the PFC, may mediate executive aspects of semantic access (Bookheimer, 2002). Thus, the specific regions of perisylvian cortex modulated by paranoid threat in this study appear to be key nodes in a distributed network mediating access to semantic representation for language (labeled semantic access network in figure 4).
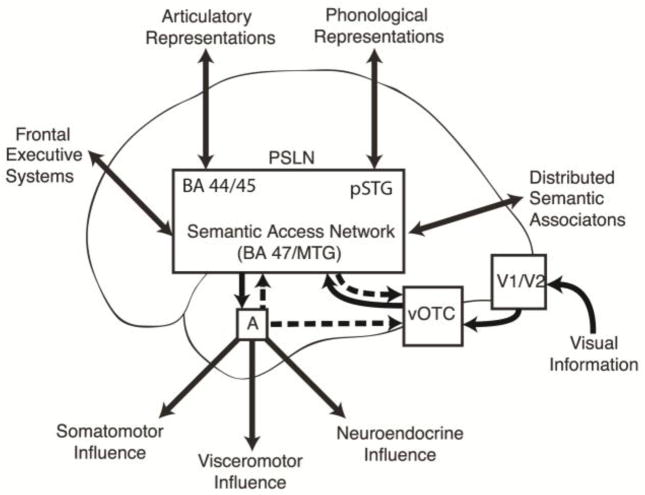
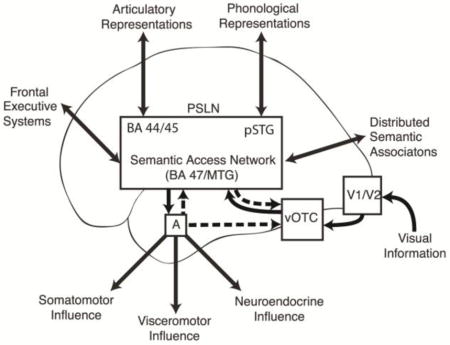
Figure 4.
A schematic diagram illustrating the proposed flow of information during visual processing of linguistic paranoid threat. Feedback projections illustrated with hashed arrows. Visual information enters the system via the ventral visual stream and accesses the PSLN via the vOTC. The MTG and BA 47 are considered here to be key nodes in a “semantic access network” subserving lexicosemantic transformations (and thus an exit point from the PSLN through which widely distributed conceptual representations can be activated). The amygdala is activated when the emotional content of a word is identified. Selective activation of the “semantic access network” during the threat condition could reflect an intrinsic sensitivity of these cortical regions to emotion, or it could be driven by feedback projections from the amygdala. Enhanced activity in the vOTC during the threat condition could result from feedback projections from the amygdala or from the perisylvian region. The increased activity in visual and semantic regions when subjects read threat words is presumed to enhance processing of these specific visuolexicosemantic associations.
Key: PSLN – perisylvian language network; BA 44/45 – Brodmann Areas 44 and 45; pSTG – posterior superior temporal gyrus; A – amygdala; vOTC – ventral occipitotemporal cortex; BA 47 – Brodmann Area 47; MTG – middle temporal gyrus
Previous functional imaging studies have demonstrated emotional modulation of anterior (Beauregard et al., 1997; Kuchinke et al., 2005; Maddock, Garrett, & Buonocore, 2003) and posterior (Beauregard, et al., 1997; Cato et al., 2004; Maddock, et al., 2003) perisylvian regions during language tasks, although the specific brain regions have varied, probably as a result of differing imaging methodologies, types of stimuli, and task designs. There is also considerable literature from single cell recordings in monkeys, and functional imaging and EEG studies in humans demonstrating that directed attention and emotion can modulate visual processing (Vuilleumier & Driver, 2007). When a task demands attention to specific attributes of a visual stimulus, neural responses are selectively enhanced in the parts of the visual processing stream specialized for that attribute (Corbetta, Miezin, Dobmeyer, Shulman, & Petersen, 1990). Since attention-related effects are driven by task demands or specific instructions to subjects, they are considered “top-down” effects and are likely mediated by higher order neural systems such as the fronto-parietal attention networks (Corbetta & Shulman, 2002), but emotion-related effects are typically driven by stimulus features and can be thought of as “bottom-up” effects in that sensory processing of a stimulus is necessary to characterize the stimulus, identify its emotional content and engage emotional circuitry before emotion-related modulation can be expected to occur. Such effects are likely mediated by interactions between amygdala and the visual cortices as evidenced, for example, by the fact that these effects can be abolished with amygdala, but not hippocampal damage (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). These emotion-related effects can be seen even in patients with right parietal lobe lesions when double-simultaneous stimulation interferes with awareness of the emotional stimuli (Vuilleumier et al., 2002), and are thus dissociable from consciously directed attention, arguing against the hypothesis that emotional modulation of visual processing is an indirect effect mediated by frontoparietal attention systems.
Langner (2011) has hypothesized that modulation of sensory cortex based on top-down voluntary direction of attention might allow for biasing of sensory processing to influence competition between concurrent sensory inputs in favor of the most relevant information. In that study, modality specific sensory cortex was modulated by modality expectancies in the absence of modality-specific stimuli allowing for an effect purely driven by the subjects’ expectations. In the present study, a combination of top-down and bottom-up effects may be at play, given the block design. Interpretation of sensory information must occur before the biasing signal can be sent, as it is only by reading and comprehending a word that one knows it is threatening. In this context, a “bottom-up” effect may be more plausible. It could be that this phenomenon represents a general property of neural dynamics in which activity is enhanced in neural systems actively processing information of particular relevance to the organism. For example, once identifying the threat content in a word, the brain may be primed to more readily detect the next threat word. This effect could be enhanced by the block design which can create some expectation (top-down) effects (as in Langner’s study) over the course of a block of similar stimuli, but a block design is apparently not necessary to observe this effect, as Herbert and colleagues (2009) found that silent reading of pleasant as compared to neutral words demonstrated increased activation of the same left vOTC region using an event-related paradigm in which stimuli of different emotional content were interspersed in a random order.
It may be the case that in the context of the present study, the greater engagement of a neural system (such as the visual word form area) serves not to enhance rapid detection of a stimulus but to activate word-associations to a greater degree. Presumably, word-associations are stored in components of the “word web” more loosely connected than those directly representing word meaning and thus requiring a stronger input to become engaged. The fact that not only sensory cortices but also brain regions involved in access to semantic content are selectively activated by threat lends support for this hypothesis.
In figure 4, a model is proposed for visual processing of linguistic threat incorporating both feed-forward and feed-back components. Visual information enters the system via the ventral visual stream and accesses the perisylvian language network via the vOTC. The MTG and BA47 are considered here to be key nodes in a “semantic access network” subserving lexicosemantic transformations (and thus an exit point from the perisylvian network through which widely distributed conceptual representations can be activated). The amygdala is reciprocally connected with much of the lateral and inferior temporal association cortex (Nieuwenhuys, et al., 2008), and when threat words are processed by these regions, the amygdala is activated, which can in turn feedback on the visual and semantic regions that activated it in order to enhance processing of visuolexicosemantic associations. The selective activation of the “semantic access network” during the threat condition could reflect an intrinsic property of these cortical regions to respond to emotional language, or it could be driven by the amygdala, as has been shown for emotional modulation of inferior temporal cortex during face processing (Vuilleumier, et al., 2004). Though the temporal resolution of fMRI is not sufficient to determine whether the limbic modulation of visual and language processing seen in this study is a monosynaptic or polysynaptic feedback process, a direct pathway is hypothesized on the basis of what is known about anatomical amygdala connectivity and the fact that EEG studies have shown that emotion-related enhancement of the P1 component of an evoked potential thought to be generated from extra-striate cortex occurs at approximately 120 ms and single cell recordings from face-selective neurons in monkeys can show modulation based on particular facial expressions within 50–100 ms of the initial response (Vuilleumier & Driver, 2007).
4.3 Left Amygdala Functional Connectivity Analysis
The correlational analysis revealed a network of largely left-sided limbic, paralimbic and subcortical regions whose activity correlated with left amygdala activity while the subjects viewed threat words and suggests a functional relationship during this task between the amygdala and the ventral visual processing stream and language cortices involved in processing the visually presented words and extracting their meanings. Many of the remaining neocortical areas were anticorrelated with the left amygdala. Thus, greater amygdala activation predicted a shift toward greater subcortical/limbic processing and less neocortical processing with the exception of those neocortical regions directly involved in the sensory/associative processing of the threat-related stimuli. This sort of reciprocal relationship between limbic/subcortical and neocortical systems has been observed previously in the context of emotion. For example, improvement in depressive symptoms correlate with increases in dorsal cortical regions and decreases in limbic, paralimbic and subcortical regions (Mayberg, 2003). The shift toward less neocortical and greater subcortical activity in the context of amygdala activation may be seen as a streamlining of neural resources under emotional conditions, which could be evolutionarily adaptive when a threat stimulus poses actual danger to the organism requiring a rapid and stereotypical defensive behavior rather than a complex cognitive response. Interestingly, the portion of the striatum associated with left amygdala activity in this analysis was the left putamen posterior to the anterior commisure, a region not typically associated with limbic function, but rather with automatic or overlearned behavior (Ashby, Turner, & Horvitz, 2010; Miyachi, Hikosaka, & Lu, 2002; Miyachi, Hikosaka, Miyashita, Karadi, & Rand, 1997).
5. Conclusions
In conclusion, this study has demonstrated that the threat content in visually presented words modulates the neural activity of a broadly distributed network of brain regions, some of which are traditionally associated with emotional processing, but many of which are generally associated with sensory and linguistic processing. Reading single words with connotations of threat can activate multiple limbic system structures, and linguistic threat can modulate activity at multiple nodes along the visuo-linguistic processing stream even when no explicit effort is made to induce emotional experience in subjects. While processing visually-presented threat words, amygdala activity is associated with a change in the pattern of distributed neural activity toward less neocortical and greater limbic/subcortical activity throughout the brain with the exception of visual cortical areas. The notion of a linear processing stream beginning with primary visual cortex and terminating in regions representing the semantic content of the written word becomes increasingly untenable. A certain degree of visual processing is obviously necessary before the emotional content of a written word can be recognized, but once it is, much of the processing stream is affected, likely because of a need to maintain efficiency while devoting increased resources to the most salient stimuli. It stands to reason that the limbic system would interact with regions of neocortex to represent emotional aspects of language, but it remains unclear whether the amygdala is necessary to drive the MTG and BA47 responses to threat. It is possible that sensitivity to emotional word content is an intrinsic property of these cortical regions. This question could be addressed by examining patients with amygdala lesions, as has been done to investigate the amygdala’s role in emotional modulation of visual processing (Vuilleumier, et al., 2004). In addition, electrophysiological studies using intracranial electrodes could help to identify the sequence in which the various cortical and subcortical structures are engaged in a linguistic threat task and may reveal whether the amygdala activates before or after these perisylvian cortical regions, thus helping to elucidate the causality within this circuit. Additional studies using different types of emotional words may also help to identify to what degree BA47 and the MTG are selectively responsive to threat as compared to other types of emotional stimuli.
Supplementary Material
Highlights.
fMRI was used to investigate the neural systems processing linguistic threat.
Linguistic threat selectively engages specific areas of perisylvian neocortex.
Threat modulates activity early in the visual-linguistic processing stream.
These findings suggest limbic feedback modulation of sensory-linguistic processing.
Acknowledgments
Funding:
This work was supported by a National Institutes of Mental Health grant for the study of psychotic symptoms in schizophrenia (R01 MH61825).
The authors would like to thank Eva Catenaccio for her assistance in preparing the figures for this manuscript.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14(5):208–215. doi: 10.1016/j.tics.2010.02.001. S1364-6613(10)00030-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Chertkow H, Bub D, Murtha S, Dixon R, Evans A. The Neural Substrate for Concrete, Abstract, And Emotional Word Lexica: A Positron Emission Tomography Study. J Cogn Neurosci. 1997;9(4):441–461. doi: 10.1162/jocn.1997.9.4.441. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46(6):575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. 112701.142946 [pii] [DOI] [PubMed] [Google Scholar]
- Carroll JB, Davies P, Richman B. The American Heritage word frequency book. Boston, MA: Houghton Mifflin Company; 1971. [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Briggs RW. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 2004;16(2):167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Attentional modulation of neural processing of shape, color, and velocity in humans. Science. 1990;248(4962):1556–1559. doi: 10.1126/science.2360050. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [pii] [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Brammer MJ, David AS, Fu CH. Predictors of amygdala activation during the processing of emotional stimuli: A meta-analysis of 385 PET and fMRI studies. Brain Res Rev. 2007;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Dapretto M, Bookheimer SY. Form and Content:: Dissociating Syntax and Semantics in Sentence Comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1536):3773. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MT, McCarthy G. A comparison of brain activity evoked by single content and function words: An fMRI investigation of implicit word processing. Brain research. 2009;1282:38–49. doi: 10.1016/j.brainres.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(1–2):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5(2):79–83. [PubMed] [Google Scholar]
- Frackowiak RSJ, Friston KJ, Frith CD, Dolan RJ, Price CJ, Zeki S. Human Brain Function. 2. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- Frank LR, Buxton RB, Wong EC. Estimation of respiration-induced noise fluctuations from undersampled multislice fMRI data. Magn Reson Med. 2001;45(4):635–644. doi: 10.1002/mrm.1086. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gosselin N, Samson S, Adolphs R, Noulhiane M, Roy M, Hasboun D, Peretz I. Emotional responses to unpleasant music correlates with damage to the parahippocampal cortex. Brain. 2006;129(Pt 10):2585–2592. doi: 10.1093/brain/awl240. [DOI] [PubMed] [Google Scholar]
- Gu H, Feng H, Zhan W, Xu S, Silbersweig DA, Stern E, Yang Y. Single-shot interleaved z-shim EPI with optimized compensation for signal losses due to susceptibility-induced field inhomogeneity at 3 T. Neuroimage. 2002;17(3):p1358–1364. doi: 10.1006/nimg.2002.1274. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15–19. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15(4):247–262. doi: 10.1002/hbm.10022. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert C, Ethofer T, Anders S, Junghofer M, Wildgruber D, Grodd W, Kissler J. Amygdala activation during reading of emotional adjectives--an advantage for pleasant content. Soc Cogn Affect Neurosci. 2009;4(1):35–49. doi: 10.1093/scan/nsn027. nsn027 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Kunkel L, Weiss AP, Goff DC, Wright CI, Shin LM, Heckers S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr Res. 2006;82(2–3):153–162. doi: 10.1016/j.schres.2005.09.021. S0920-9964(05)00465-2 [pii] [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattie B, Stern E. Linguistic threat activates the human amygdala. Proc Natl Acad Sci U S A. 1999;96(18):10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proc Natl Acad Sci U S A. 2004;101(9):3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Processing emotional pictures and words: effects of valence and arousal. Cogn Affect Behav Neurosci. 2006;6(2):110–126. doi: 10.3758/cabn.6.2.110. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage. 2005;28(4):1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Landis T. Emotional words: what’s so different from just words? Cortex. 2006;42(6):823–830. doi: 10.1016/s0010-9452(08)70424-6. [DOI] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Boers F, Sturm W, Willmes K, Eickhoff SB. Modality-Specific Perceptual Expectations Selectively Modulate Baseline Activity in Auditory, Somatosensory, and Visual Cortices. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr083. bhr083 [pii] [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23(4–5):727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23(10):3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, Luders HO. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127(Pt 10):2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65(1):193. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11(6 Pt 1):708–734. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Salloway S, Malloy P. The Limbic System: An Anatomic, Phylogenetic, and Clinical Perspective. In: Salloway S, Malloy P, Cummings JL, editors. The Neuropsychiatry of Limbic and Subcortical Disorders. Washington, DC: American Psychiatric Press; 1997. pp. 3–18. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J. Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion. 2002;2(2):147–161. doi: 10.1037/1528-3542.2.2.147. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Experimental brain research. 2002;146(1):122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Miyashita K, Karadi Z, Rand MK. Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res. 1997;115(1):1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Marchant JL, Hassabis D, Seymour B, Tan G, Gray M, Frith CD. From threat to fear: the neural organization of defensive fear systems in humans. J Neurosci. 2009;29(39):12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. 29/39/12236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache L, Gaillard R, Adam C, Hasboun D, Clemenceau S, Baulac M, Cohen L. A direct intracranial record of emotions evoked by subliminal words. Proc Natl Acad Sci U S A. 2005;102(21):7713–7717. doi: 10.1073/pnas.0500542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic ‘emotions’ circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30(5):934–945. doi: 10.1111/j.1460-9568.2009.06875.x. EJN6875 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, van Huijzen C. The Human Central Nervous System. 4. Springer; 2008. [Google Scholar]
- Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30(10):953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner M, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19(3):473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191(1):62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Pulvermüller Friedemann. The neuroscience of language: on brain circuits of words and serial order. Cambridge, U.K.; New York: Cambridge University Press; 2002. [Google Scholar]
- Ross ED. Affective Prosody and the Aprosodias. In: Mesulum MM, editor. Principles of Behavioral and Cognitive Neurology. 2. New York: Oxford University Press; 2000. [Google Scholar]
- Tuescher O, Protopopescu X, Pan H, Cloitre M, Butler T, Goldstein M, Stern E. Differential activity of subgenual cingulate and brainstem in panic disorder and PTSD. J Anxiety Disord. 2011;25(2):251–257. doi: 10.1016/j.janxdis.2010.09.010. S0887-6185(10)00192-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken U, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience. 2011:5. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. S1053811901909784 [pii] [DOI] [PubMed] [Google Scholar]
- Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Braz J Med Biol Res. 2003;36(5):557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Clarke K, Husain M, Driver J, Dolan RJ. Neural response to emotional faces with and without awareness: event-related fMRI in a parietal patient with visual extinction and spatial neglect. Neuropsychologia. 2002;40(12):2156–2166. doi: 10.1016/s0028-3932(02)00045-3. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):837–855. doi: 10.1098/rstb.2007.2092. 0M631H12296Q3431 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Chen JI, Lerch J, Evans AC. Comparing functional connectivity via thresholding correlations and singular value decomposition. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):913–920. doi: 10.1098/rstb.2005.1637. UJ978JFKLP5VP1JR [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.