Abstract
Prediabetes is associated with low-grade chronic inflammation that increases the risk for developing type 2 diabetes (T2D) and cardiovascular disease (CVD). An elevated lipopolysaccharide concentration, associated with dysbiosis of the intestinal microbiota, has been implicated in the development of both T2D and CVD. Selective modulation of the intestinal microbiota with prebiotics reduces intestinal permeability and endotoxin concentrations, inflammation, and metabolic dysfunction in rodents. The effect of prebiotic supplementation on cardio-metabolic function in those at risk for T2D is not known. The primary aim of this trial is to determine the influence of prebiotic supplementation with inulin on insulin sensitivity and skeletal muscle metabolic flexibility in adults at risk for T2D. We hypothesize that prebiotic supplementation with inulin will improve insulin sensitivity and skeletal muscle metabolic flexibility. We will randomize 48 adults (40–75 yrs) with prediabetes or a score ≥5 on the American Diabetes Association (ADA) risk screener to 6 weeks of prebiotic supplementation with inulin (10 g/day) or placebo. Subjects will be provided with all food for the duration of the study, to avoid potential confounding through differences in dietary intake between individuals. Intestinal permeability, serum endotoxin concentrations, insulin sensitivity, skeletal muscle metabolic flexibility, endothelial function, arterial stiffness, and fecal bacterial composition will be measured at baseline and following treatment. The identification of prebiotic supplementation with inulin as an efficacious strategy for reducing cardio-metabolic risk in individuals at risk of T2M could impact clinical practice by informing dietary recommendations and increasing acceptance of prebiotics by the scientific and medical community.
Keywords: prebiotics, diabetes, cardiovascular disease, diet, microbiome
1. Introduction
In 2012, approximately 86 million U.S. adults aged 20 years and older had prediabetes.1 Low-grade chronic inflammation plays an integral role in the pathogenesis of atherosclerosis,2–4 and may increase risk for development of type 2 diabetes (T2D)5–8 and cardiovascular disease (CVD)-related events.4,5 Numerous studies in animal models 9–13 and humans 14–18 have implicated the intestinal microbiota (i.e., bacteria residing in the gastrointestinal tract) in the pathophysiology of obesity and T2D.
Studies in both human and rodent models suggest that the consumption of a high fat/high sugar, westernized diet may lead to changes in the composition/activity of the gut microbiota (e.g., depletion of Bifidobacteria), increase intestinal permeability to luminal antigens, and cause metabolic endotoxemia, i.e., elevated endotoxin concentrations.9,11,19,20 In turn, metabolic endotoxemia is associated with the development of a low-grade chronic inflammatory state, obesity and insulin resistance in rodents.9,11 In humans, endotoxin concentrations are higher in prediabetes and T2D compared with normoglycemic individuals. 21,22 In addition, elevated endotoxin concentrations are associated with an increased risk of incident T2D.23 Importantly, Frisard et al. 24 demonstrated that skeletal muscle, the primary site of insulin stimulated glucose disposal, is a target for circulating endotoxin. Furthermore, low dose endotoxin concentrations, consistent with metabolic endotoxemia, activate skeletal muscle TLR4 resulting in state of metabolic inflexibility consistent with that observed in insulin resistance and T2DM.25,26
Several lines of evidence implicate the gut microbiota in mediating CVD risk.27 First, serum endotoxin increases progressively with the number of metabolic syndrome components 23 and with the 10-yr CVD risk score.28 Second, endothelial dysfunction, an early step in atherogenesis, occurs following endotoxin exposure in humans.29–31 In addition, elevated serum endotoxin is independently associated with arterial stiffness.32 Finally, chronic elevations in endotoxin have been associated with carotid intima media thickening and incident CVD.33,34 These findings suggest that gut dysbiosis and the subsequent increases in circulating endotoxins may lead to adverse changes in vascular function and structure, and elevated CVD risk.
Consumption of non-digestible polysaccharides (i.e. prebiotics) is an effective way to improve overall gut health.35,36 Prebiotics are “selectively fermented ingredients that result in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring health benefit(s) upon host health.”37 Commonly used prebiotics include inulin-type fructans, fructo-oligosaccharides, xylo-oligosaccharides and galacto-oligosaccharides.38 In rodents, the proliferation of a targeted bacterial species (i.e. Bifiodobacterium spp. and Lactobacillus spp.) contributes to host cardio-metabolic health by reducing intestinal permeability, endotoxin concentration, and pro-inflammatory cytokines.38–41 In humans, the prebiotic inulin appears to be particularly efficacious in increasing the abundance of Gram-positive Bifidobacteria, while decreasing the proportions of gram negative bacteria (see reviews 42,43). However, the potential benefits of prebiotic supplementation on cardio-metabolic dysfunction in humans is unclear.
The identification of inulin supplementation as a simple and efficacious strategy for reducing cardio-metabolic risk in individuals at risk for T2D could impact clinical practice by informing dietary recommendations and increasing acceptance of prebiotics by the scientific and medical community. In turn, our findings could lead to enhanced adoption and maintenance of inulin supplementation as a cardio-metabolic risk-reducing behavior in individuals at risk for T2D.
2. Aims and Hypotheses
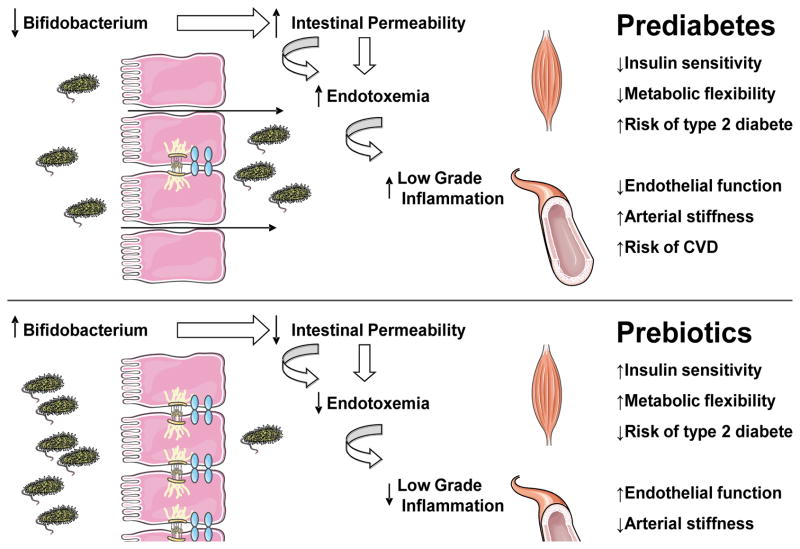
The general aim of this clinical efficacy trial is to determine the effect of prebiotic supplementation with inulin on cardio-metabolic function in those at risk for T2D. For reference, a conceptual framework figure has been provided (Figure 1) along with the specific aims and hypotheses:
Figure 1.
Conceptual Framework for Hypothesis Testing
Aim 1: To determine whether prebiotic supplementation with inulin improves insulin sensitivity and skeletal muscle metabolic flexibility in individuals at elevated risk of developing T2D. We hypothesize that prebiotic supplementation with inulin will improve insulin sensitivity and skeletal muscle metabolic flexibility in these individuals.
Aim 2: To determine whether prebiotic supplementation with inulin will reduce arterial stiffness and improve endothelial function in individuals at elevated risk of developing T2D. We hypothesize that prebiotic supplementation with inulin will reduce arterial stiffness and improve endothelial function in these individuals.
Aim 3: To determine the relationship between changes in the gut microbiota (i.e., the abundance of important groups of bacteria such as Bifidobacteria), intestinal permeability, and endotoxin concentration with prebiotic supplementation. We hypothesize that the magnitude of change in Gram-positive gut microbiota, intestinal permeability, and endotoxin concentrations with prebiotic supplementation will be correlated with the magnitude of change observed in the aforementioned metabolic and cardiovascular variables. The primary outcome is change in insulin sensitivity following treatment. Secondary outcomes include skeletal muscle metabolic flexibility, arterial stiffness, and endothelial function.
3. Study Design
3.1 Overview
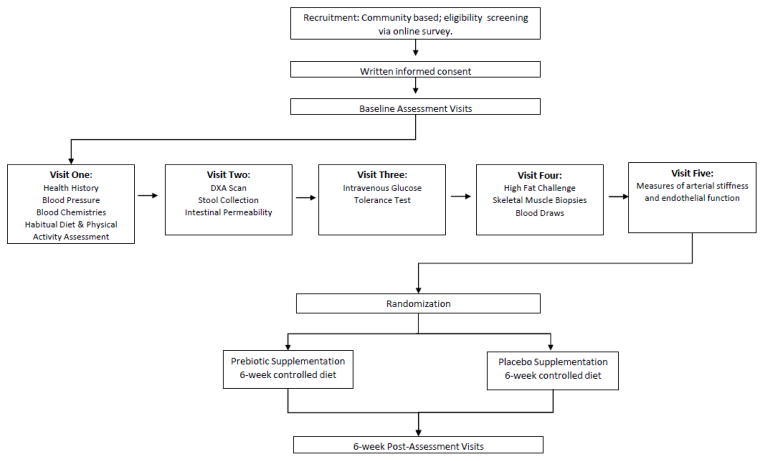
The Virginia Tech Institutional Review Board has approved the study protocol. The nature, purpose, risks and benefits of the study will be explained to each potential participant before obtaining written and verbal informed consent. We will include 48 adults with prediabetes or at increased risk for T2D, as determined by the American Diabetes Association (ADA) Diabetes Risk Screener.44 Eligibility and exclusion criteria are presented in Table 1. Participants will be randomized to 6 weeks of prebiotic supplementation (inulin) or a placebo (maltodextrin), and will be provided with all of their food and beverages for the 6-week intervention period to avoid the potential confounding effects of differences in dietary intake on the gut microbiome. Measurements of outcome variables will be performed at baseline and following 6 weeks of treatment (Figure 2).
Table 1.
Prebiotic Supplementation and Cardiometabolic Health: Participant Eligibility Criteria
Inclusion Criteria:
Exclusion Criteria:
|
Note: BMI=body mass index; ADA=American Diabetes Association; FBG=fasting blood glucose; OGT=oral glucose tolerance; CHD=coronary heart disease; NSAID= non-steroidal anti-inflammatory drugs
Figure 2.
Study Design Schematic
A double-blind, placebo-controlled, parallel group design will be utilized for the present study. All participants who successfully complete the screening process will be enrolled in the study. Following baseline measurements, individuals will be randomized to one of two groups: supplementation with 10g/d of inulin, or with 10g/d of maltodextrin (placebo) for 6 weeks. Product details are provided in the following section. The supplements will be mixed into water and provided with the supervised breakfast meal consumed in the laboratory dining facility. An inulin does of 10g/d was selected as the prebiotic type and dose because it is well-tolerated 45 and increases fecal Bifidobacteria within as few as 2 weeks.42,43 Importantly, Bifidobacteria are the most common prebiotic target 42,43 and have been shown to reduce endotoxin concentrations and improve gut barrier function.46–49 The 6-week feeding period duration was selected to allow adequate time for changes in the gut microbiota to exert its hypothesized effects and to improve the feasibility of diet adherence while considering the participant burden associated with a controlled feeding study. Randomization will be stratified by gender under the supervision of an individual not involved in the collection or analysis of the study data. Separate randomization schemes will be developed for males and females to ensure equal numbers within each gender strata. Individuals performing outcome measurements will be appropriately blinded.
3.2 Product Standardization and Palatability Testing
Participants will be supplemented with 10g/d of either inulin (Frutafit® IQ, Sensus American, Inc, Lawrenceville, NJ; 2 kcal/g) or placebo (maltodextrin; 4 kcal/g) for 6 weeks. All participants will begin taking inulin or the placebo at a 5g loading dose the first 7 days prior to taking to full 10 g amount. Frutafit® IQ is comprised of 100% inulin from chicory root. The nutrient composition of the supplements will be accounted for in the standardized diets (section 3.3) by using an average of 30 kcal and 10g carbohydrate per day. By comparison, usual intake of inulin in the US population is 2.6g/d, primarily from wheat products and onions.50 Fruitafit IQ was obtained in a single lot for the entire study along with a certificate of analysis documenting the composition and purity of the product.
Prior to the onset of the trial, the palatability and solubility of both supplements (10g dose) in water and in orange juice (one cup) were assessed by the research staff to determine the optimal supplement delivery mode. When mixed into either water or orange juice, both supplements were tasteless and acceptable, and both dissolved well in either beverage. Due to the calorie-controlled nature of the study diets, water was selected as the beverage for supplement delivery.
3.3 Diet Design and Standardization
All participants will be fed a standardized diet (55% carbohydrate, 30% fat [8% saturated fat], 15% protein) isocaloric to their individual energy requirements for 6 weeks to avoid the potential confounding effect of individual differences in dietary intake on the gut microbiota. The standardized diets will be prepared in the Metabolic Kitchen and Dining Laboratory at Virginia Tech, by ServSafe®-certified research assistants, and consist of a 7-day cycle of menus with 3 meals and a snack. Menus will be developed for each day for each of the following 5 calorie levels: 1500 kcal, 2000 kcal, 2500 kcal, 3000 kcal, 3500 kcal. An example of a one-day menu for the 2000 kcal level with nutrient information is provided in Table 2. Probiotic foods (e.g., yogurt) will be excluded from the diet. Daily soluble fiber intake will be maintained at ≤2g/1000 kcal and total dietary fiber will be maintained at or below the US daily average intake of 8g/1000 kcal.51–54 Sodium intake will be maintained at less than 3000mg/d, except for the 3500 kcal level, which will be under 3500mg/d.55,56
Table 2.
Sample 2000 kcal Menu
| Food Item (Approximate Volumetric Quantity) | Gravimetric Quantity (g) | Energy (kcal) | Protein (g) | Carbohydrate (g) | Fat (g) | Saturated Fat (g) | Fiber (g) | Sodium (mg) |
|---|---|---|---|---|---|---|---|---|
| BREAKFAST [Bagel with Cream Cheese, and Apple Juice] | ||||||||
| Plain, White Bagel (1 bagel) | 81 | 207.7 | 8.1 | 40.9 | 1.3 | 0.3 | 1.8 | 418.8 |
| Strawberry Cream Cheese (1.8 Tbs) | 28 | 78.8 | 0.9 | 4.4 | 6.4 | 4.0 | 0.04 | 97.4 |
| 100% Apple Juice, from Concentrate (1 cup) | 240 | 137.1 | 0.2 | 33.4 | 0.3 | 0.1 | 0.2 | 10 |
| LUNCH [Sandwich with Cookies, and Lemonade] | ||||||||
| White Bread (2 slices) | 86 | 231.4 | 6.6 | 43.6 | 3.4 | 0 | 2 | 586 |
| Low Sodium Roast Beef, deli slices (4 slices) | 100 | 189.4 | 36.1 | 0 | 5 | 1.7 | 0 | 44.6 |
| Low Sodium Swiss Cheese, deli slices (1 slice) | 28 | 106.2 | 7.5 | 1.5 | 7.8 | 5.0 | 0 | 4 |
| Mayonnaise (2 Tbs) | 25 | 182.2 | 0.2 | 0.8 | 19.8 | 3.0 | 0 | 142.3 |
| Vanilla Wafer Cookies, Mini (19 cookies) | 33 | 149 | 1.2 | 20.3 | 7.0 | 2.0 | 0.6 | 82 |
| Lemonade (2 cups) | 480 | 213 | 0.4 | 52.4 | 0.2 | 0 | 0 | 20 |
| DINNER [Pasta with Alfredo Sauce] | ||||||||
| Penne Pasta, cooked in unsalted water (1 cup) | 139 | 215.7 | 8.1 | 42.9 | 1.3 | 0.2 | 2.5 | 1 |
| Alfredo Sauce (0.75 cup) | 61 | 94.5 | 3.9 | 5.1 | 6.5 | 4.0 | 0.8 | 282 |
| SNACK | ||||||||
| Graham Crackers, Honey-Flavored (2 crackers) | 30 | 129 | 2.3 | 23.2 | 3.0 | 0.5 | 1.2 | 183.8 |
| SUPPLEMENT | ||||||||
| Inulin/Placebo | 10 | 30 | 0 | 10 | 0 | 0 | 0 | 0 |
| TOTAL | 1341 | 1944 | 75.5 | 278.5 | 62.0 | 20.8 | 9.1 | 1872 |
| CONTROLLED DIET TARGETS (% total energy) | - | 2000 | 75 (15%) | 275 (55%) | 67 (30%) | 18 (8%) | 16 (<8g/ 1000 kcal) | <3,000 |
The process used for menu development was as follows. First, a list of readily available food items (i.e., from local grocery stores, commercial food suppliers) was selected for inclusion in the controlled diets by a research dietitian. The label information for each food item selected was then matched to a comparable item in the nutritional analysis software’s database (Nutrition Data Systems for Research; NDS-R, Nutrition Coordinating Center, University of Minnesota), that provided detailed nutrient composition information on each food item used in the controlled diet. The research dietitian then developed 7 days of menus to meet the daily calorie and nutrient targets for each of the 5 kcal levels. Individual macronutrients were considered acceptable if they were ±5 g of the daily targeted amount, with the exception of soluble fiber, that was ±1 g of the daily targeted amount. The menus were then reviewed by a second research dietitian to verify that the daily energy and nutrient target levels were achieved, and that the proposed food portions were reasonable. After verification that daily energy and nutrient target levels were achieved, daily food preparation forms were developed for each day of the controlled diet that provided gram amounts for each food item and preparation instructions for the metabolic kitchen research assistants.
Energy requirements for each participant will be determined using estimated resting energy expenditure based upon age, weight, height, and sex multiplied by an activity factor based on self-reported physical activity levels.57 Participants will consume breakfast in our dining facility and be given their daily dose of inulin or placebo (mixed into water) at this supervised meal for each day of the controlled diet. The remainder of their meals for the day will be taken with them in a large portable cooler bag. The menu and instructions (i.e., for food preparation; to consume all foods provided, etc.) for the day will be included. Any uneaten items will be returned to the metabolic kitchen the following day, weighed by the research assistants, and recorded on the food preparation sheet for that participant. Participants will be blinded from their weight and weighed at each visit during the controlled feeding period, and any trend of >1.0 kg weight loss or gain over a 3-day period will be countered by the addition or subtraction of 250 kcal food modules (e.g., 45g low-sodium saltines, 20g Swiss cheese) with the same macronutrient composition as the overall diet. Participants will be permitted to consume no more than three 6 fl oz. caffeinated beverages daily.58,59 Caffeinated beverages allowed during the 6-week controlled feeding period will consist of unsweetened tea and coffee, which will be brewed in the metabolic kitchen. At each visit, participants will be asked to report if any non-study food or beverage were consumed since the preceding laboratory visit. Participants were instructed not to consume food and beverages (excluding water) outside the study diet. Participants who repeatedly (>3d/week) fail to consume 100% of the prescribed diet will be excluded.
3.4 Participant recruitment and screening
Recruitment will take place over a 2-year period. Direct mailers, advertisements in local newspapers, campus email list servs, and posted flyers will be utilized as recruitment methods. Individuals who contact the research coordinator will be emailed a link to an online screening survey to determine if they meet basic eligibility criteria (age, body mass index and medical/supplement use). Those who meet these basic eligibility criteria will be sent the informed consent form that provides details about the study requirements and an initial in-person visit will be scheduled. During this visit verbal and written consent and, subsequently, a detailed health history will be obtained from each eligible volunteer. If a participant meets all eligibility criteria (Table 1), subsequent baseline testing visits will be scheduled (Figure 2).
3.5 Procedures
Participants will be instructed to arrive for laboratory testing between 7:00 am and 10:00 am after a 12 hour overnight fast (including abstinence from caffeine containing foods/ beverages) and having performed no vigorous physical activity for the previous 48 hours. In addition, participants will report being free of acute illness/infection for the prior 2 weeks and a supplementary infection and inflammation questionnaire will be answered. Participants will undergo 5 laboratory visits at baseline and again following the 6-week intervention (Figure 2).
3.5.1 Body Mass and Composition
Participants will be weighed on a digital scale accurate to ±0.1 kg, and height will be determined using a scale-mounted stadiometer (SCALE-TRONIX Inc.; White Planes, New York). Body composition will be assessed using dual energy x-ray absorptiometry (Prodigy Advance, GE Healthcare) by a limited licensed radiologic technician as required by the Virginia Department of Health.
3.5.2 Plasma Lipids and Lipoproteins Concentrations
Plasma lipid and lipoprotein concentrations (i.e., total cholesterol, high- and low-density lipoprotein cholesterol, and triglycerides) will be measured in a Clinical Laboratory Improvement Amendments-certified laboratory (Solstas Lab Partners) (Table 1).
3.5.3 Resting Blood Pressure
Mercury sphygmomanometry will be used to measure blood pressure (BP) according to American Heart Association guidelines.60 Participants will be instructed to remain seated and resting for 10 minutes prior to the first BP measurement with a minimum of 1 minute between each BP measurement. Participants will be instructed to keep both feet on the floor without crossing legs, and the right arm will be supported at heart level. Blood pressure will be measured twice on participants who display a normal or pre-hypertensive value on the first measurement. The average of two additional measurements will be used for participants with an initial blood pressure in the hypertensive range.
3.5.4 Habitual Physical Activity and Dietary Intake
Physical activity levels will be assessed during screening via the Godin Leisure Time Questionnaire to insure participants are sedentary to minimally active.61 Upon study enrollment, habitual physical activity level will be assessed at baseline and follow-up using the ActiGraph GT3x accelerometer (ActiGraph LLC, Pensacola, Florida, USA). The ActiGraph GT3x is a triaxial accelerometer designed to measure physical activity for extended periods of time and will be utilized in this study to monitor activity over four consecutive days (3 weekdays and 1 weekend day) to capture 60% of the workweek and 50% of the weekend. The ActiGraph will be initialized to record continuously at 15-second epochs (i.e. time intervals) and will be analyzed using the Freedson cut-point equations.62 In addition to wearing the accelerometer, participants will be sent home with a form and instructions to note the wear time and non-wear time and any pertinent notes regarding why the accelerometer was removed (i.e. sleeping, showering, etc.). All non-wear times will be excluded from the analyses conducted in the accompanying manufacturer software. Participants will wear the accelerometer for 4 consecutive days on the right hip at each assessment point to ensure compliance with our instructions to maintain baseline levels of habitual physical activity.63 Assessment will occur concurrently with habitual dietary intake assessment.
Baseline dietary intake, including total energy and macronutrient intake, will be assessed using detailed 4-day food intake records. Participants will be instructed on procedures for measuring and recording food intake for 4 consecutive days. Participants will be provided with detailed recording forms, and a booklet of 2-dimensional food models to assist with accurate portion size determination. Returned records will be reviewed with the participant by research staff to ensure clarity and completeness of the food intake record. Records will be analyzed by trained research assistants using the NDS-R software (v. 2014) to determine participant’s habitual dietary energy and macronutrient intake. Participants with who are taking prebiotic supplementation will be asked to discontinue for a minimum of 3-months prior to participating in the study.
3.5.5 Intestinal Permeability
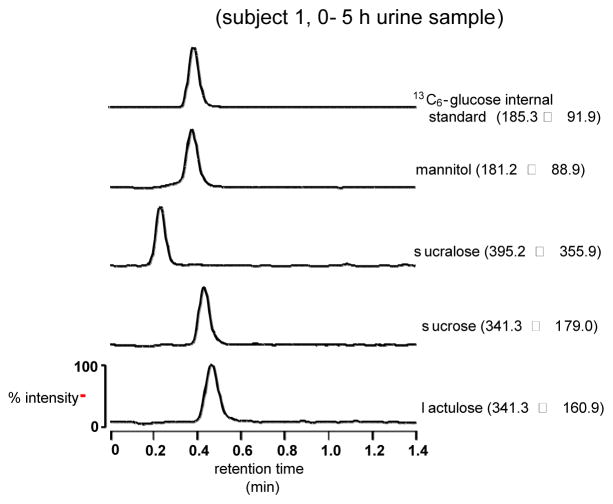
Intestinal permeability will be assessed using the four-sugar probe procedure.64–66 Following an overnight fast, participants will ingest 40 g sucrose (Spectrum Chemical MFG. Corp., Gardena CA and New Brunswick, NJ), 5 g lactulose (Qualitest Pharmaceuticals, Huntsville, AL), 1 g mannitol (Spectrum), and 1g sucralose (Spectrum) in 500 mL water.64–66 Participants will be instructed to consume an additional 500 ml of water within a 2-hour timeframe following consumption of the sugar probe. In addition, participants will be provided a standardized sucrose-free meal to consume within the first 5 hours of the urine collection period. All of the food consumed that day will be free of artificial sweeteners. Urine will be collected from 0–5 and 6–24 hours in containers with 5 ml 10% thymol (VWR, Radnor, PA) to inhibit bacterial growth. Total urine volume will be recorded, and aliquots frozen at −80°C for later analysis. Urinary sugars will be measured on a Waters Acquity UPLC-TQD.67 Permeability will be defined as % urinary excretion and excretion ratios for sugars from hours 0–5 (upper GI) and hours 6–24 (lower GI).64–67 Figure 3 shows LC-MS chromatogram of an internal standard and each of the 4 sugars recovered from urine of 1 of the Co-I’s (AN) 5 hours after ingestion.
Figure 3. Gut Permeability UPLC-MS Assay.
Liquid Chromotography-Mass Spectrometry Chromatogram of Internal Standard and 4 Sugars Recovered From Urine of Co-I (AN) 5 Hours After Ingestion
3.5.6 Plasma Endotoxin and Inflammatory Cytokine Concentrations
Plasma endotoxin concentration will be determined using the PyroGeneTM Recombinant Factor C Endotoxin Detection Assay (Lonza International). Pro-inflammatory cytokines (TNFα, IL-6, and MCP-1) in plasma will be measured by ELISA (American Diagnostica Inc, New York, NY).
3.5.7 Glucose tolerance and insulin sensitivity
Oral glucose tolerance tests (OGTT) will be used to determine eligibility during baseline screening. Following baseline blood sampling, participants will consume a single 10 fluid ounce beverage containing 75g glucose (Sun-Dex, Fisherbrand, Fisher Scientific, Hanover Park, IL). A second blood sample will be obtained 120 min following consumption of the beverage. Thresholds for normal, prediabetic and diabetic at the 2 hour time point are: <140 mg/dl, 140–200 mg/dl, and >200 mg/dl, respectively.68
Whole body insulin sensitivity will be estimated using Bergman’s minimal model (MINMOD Millenium) during a modified frequently-sampled Intravenous Glucose Tolerance Test (IVGTT).69 Intravenous catheters will be inserted in an antecubital vein of each arm for blood collection or glucose and insulin injection. Two baseline plasma blood samples ((t) = −10 and −1 min) will be drawn. Glucose (0.3 g/kg; 50% solution) will be injected at time 0 and insulin (0.025 U/kg) will be injected at (t) = 20 min. Additional blood samples (3 mL) will collected at (t) = 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 14, 16, 18, 22, 23, 24, 25, 27, 30, 40, 50, 60, 70, 80, 90, 100, 120, 150, and 180 min during the 3-hr protocol. Plasma glucose concentration (mg/dl) will be analyzed immediately using a YSI Glucose Analyzer (Yellow Springs, OH). Insulin (μU/mL) will be determined later via an ELISA (ALPCO Diagnostics, Salem, NH). Samples will analyzed in duplicate.
3.5.8 Skeletal Muscle Biopsies, Substrate Metabolism, and Metabolic Flexibility
Participants will arrive for testing after a 12-hour overnight fast. Muscle biopsies in the vastus lateralis will be obtained using a modified Bergstrom needle technique. Following local anesthesia (2% lidocaine), a 1.0 cm incision will be made through the skin and fascia. Biopsy tissue obtained will be obtained using a multi-pass approach. The incision will be closed with a steri-strip.
A catheter will be inserted in an antecubital vein for a baseline blood sample following the first biopsy. Participants will then be provided with a high-fat test meal (Breakfast-style sausage biscuits; 850 kcal; 63% of energy from fat; 21% saturated fat), which will be consumed within a 10-minute period. The test meal will be followed by hourly blood sampling for 4 hours, in addition to the initial baseline blood sample, and a second muscle biopsy will occur at the 4-hour mark. After the second biopsy, participants will be given a snack and standardized discharge instructions.
Fatty acid, glucose, and pyruvate oxidation will be assessed in skeletal muscle homogenates, prepared from ~100 mg of biopsied tissue, using [1-14C]-palmitic acid, [U-14C]-glucose, and [1-14C]-pyruvate, respectively, as previously described. 24,70,71 Briefly, complete and incomplete fatty acid oxidation will be assessed by measuring 14C-CO2 and acid soluble metabolites, respectively. Glucose and pyruvate oxidation will be assessed by measuring 14C-CO2 production. Metabolic flexibility will be assessed by measuring 14C-CO2 production from the oxidation of [1-14C]-pyruvate with/without the presence of non-labeled palmitic acid. Reductions in pyruvate oxidation in the presence of palmitic acid will be examined to assess metabolic flexibility.
3.5.9 Cardiovascular Outcomes
Flow mediated dilation (FMD) of the brachial artery will be assessed using duplex ultrasonography (HP Sonos 7500) with a high resolution linear array transducer according to published guidelines 72 and recent recommendations 73. Reactive hyperemia will be produced by inflation of a pediatric BP cuff around the forearm for 5 minutes. Off line analysis of baseline and post-reactive hyperemic diameters and velocities will be performed using edge detection software (Vascular Analysis Tools, Medical Imaging Applications, Inc). Endothelium independent vasodilation (EID) will be assessed by measuring brachial arterial dilation for 10 min following administration of 0.4 mg of sublingual nitroglycerine. Both FMD and EID will be expressed as mm and % change from baseline diameter.
Carotid-femoral (C-F) pulse wave velocity (PWV), our primary measure of arterial (aortic) stiffness, will be obtained by serially measuring carotid and femoral artery waveforms using a high fidelity, non-invasive applanation tonometer (NIHem, Cardiovascular Engineering, Inc.) as previously described.74,75 Briefly, subjects will be studied in the supine position after ~10 minutes of rest. A semi-automated computed controlled device will be used to auscultate brachial arterial pressure between 3–5 times at 2-minute intervals, in order to obtain BP stability (± 5 mm Hg difference for both systolic and diastolic blood pressure). Next, a high fidelity finger probe tonometer will be used to obtain carotid, brachial, radial, and, femoral artery waveforms over 10–20 cardiac cycles. Arterial waveforms will then be saved to a computer device for later analysis. Body surface measurements will be made from the suprasternal notch (SSN) to the carotid, brachial, and radial recording sited using a Gulik tape measure and to the femoral recording site using a large caliper.
Tonometry waveforms will be signal-averaged and the electrocardiogram R wave will serve as a fiducial point.75 BP values will be read by an experienced reviewer and the average of the systolic and diastolic BP will be used to calibrate the peak and trough of the signal averaged brachial waveform. Brachial diastolic and mean arterial pressures will then be used to calibrate the other arterial waveforms. C-F PWV will be calculated by dividing the travel distance (SSN to carotid recording site – SSN to the femoral recording site) by the travel time obtained from the foot to foot of the signal-averaged carotid and femoral pulse waves.
β-Stiffness index, a relatively BP independent index of carotid artery stiffness, will be measured using an ultrasound unit (Sonos 7500, Phillips Medical Systems) equipped with a high-resolution linear array transducer (3–11 MHz) and applanation tonometry (NIHem, Cardiovascular Engineering, Inc.) as previously described.76 Briefly, after resting in the supine position for ~20 minutes, longitudinal B mode images of the cephalic portion of left common carotid artery, 1–2 cm proximal to the carotid bulb, will be obtained over 15 cardiac cycles by placing the transducer at a 90° angle over the artery. When clear visibility of the near and fall walls are obtained, the images will be stored on an optical disk for offline quantification. The maximal and minimal carotid artery diameters of 3 consecutive cardiac cycles will be acquired with commercially available software (Vascular Research Tools 5, Medical Imaging Applications, LLC). Carotid artery BP will be acquired from applanation tonometry of the carotid artery and brachial artery auscultation as described above. β-Stiffness index will be calculated as: , where P1 represents carotid artery systolic pressure, P0 represents carotid artery diastolic pressure, D1 represents the maximal diameter recorded during systole, and D0 represents the minimal diameter recorded during diastole.
3.5.10 Assessment of Gut Microbiota
Stool samples will be collected daily for 3 days at baseline and the final 3 days of supplementation during the intervention. Participants will be provided with sterile plastic containers intended for stool sampling (Omnigene gut for microbiome, Owatonna, ON, Canada). Samples will be kept in a portable refrigerated cooler, and delivered to the Human Integrative Physiology Laboratory within 24 hours of collection. The sample will be immediately frozen at −80°C until final processing and analysis. Total bacterial DNA will be extracted from fecal samples using QIAamp DNA Stool Mini kit (QIAGEN California). Total fecal bacterial copies will be assessed using real-time quantitative polymerase chain reaction (qPCR) of the housekeeping gene rpoD and aliquots from the 3 composite days mixed equally. Abundance of select bacteria (Bifidobacterium, Lactobacillus), previously shown to be modulated by inulin will also be quantified by qPCR with previously published primers. Fecal bacterial community composition will be assessed using Tag-Encoded Pyrosequencing. The V4 region of the bacterial 16S rRNA gene will be amplified from fecal microbial DNA using barcoded PCR primers.77 Additionally a specific barcoded primer for Bifidobacterium will be used to amplify this group of bacteria, which has been shown to be under-represented by standard primer sets.78 The amplicons from each reaction will be mixed in equal amounts based on concentration and will be subjected to sequencing using the Illumina MiSeq platform. Baseline fecal bacterial compositions will be compared to fecal samples obtained after 6 weeks supplementation with inulin and a detailed characterization of the gut microbiota performed via bioinformatics pipelines including MG-RAST.79
3.5.11 Side effect monitoring
Participants will be asked to report any side effects they experience during the intervention period. Gastrointestinal side effects related to the diet and/or treatment will be recorded using a standardized questionnaire, and addressed by study personnel. The side effect questionnaire will rate gas/bloating, nausea, flatulence, cramping, diarrhea, constipation, and GI rumbling from none (rating of 0) to severe (rating of 3).80,81
4. Data Analysis
4.1 Power Analysis
Sample size/power calculations were based on the number of participants needed to detect statistically (P<0.05) and physiologically/clinically significant differences in the magnitude of change in insulin sensitivity and aortic PWV with prebiotic supplementation compared with placebo. With 2 groups, 2 repeated measures, and α=0.05 we will have greater than 80% power to detect significant group by time interactions for an effect size as small as 0.50 with minimum sample of n=20 participants per group. Our conservative estimate is for 20% attrition and hence we plan for n=24 per group or a total n=48 participants. Using our prior published and unpublished data, we estimated an effect size (prebiotic-placebo/S.D.) of 0.61 for insulin sensitivity (+20±32%) and 0.54 for aortic PWV (−20±37% difference in the reduction). As such, we will have greater than 90% power to detect significant group by time interactions. If dropouts exceed the 20% level, we will recruit additional participants to achieve our desired sample size.
4.2 Statistical Analysis
We will conduct descriptive univariate analyses on all study variables. Data will be examined for the presence of outliers, violations of normality and missing data. Major violations of normality will be corrected with an appropriate transformation procedure. In case of an outlier, rather than transform the data, the outlier will be “Winsorized,” that is, replaced by the most extreme value in the tail of the distribution.
To test our hypothesis that prebiotic supplementation will improve insulin sensitivity (primary outcome) and skeletal muscle metabolic flexibility in prediabetic individuals, we will use a multiple-sample repeated measure analysis of variance with between-subject factors approach. This is a common design in randomized controlled trials, where subjects are randomized to different treatment and control groups and followed across time. Because the data are repeated, we will treat the multiple observations as nested within individuals. This will allow us to make a direct comparison between the time points while accounting for the correlation in the data to make the correct inference regarding group differences. For our analysis, the group by time interaction will be of primary interest. A compound symmetry error structure will be chosen for this model. We will use an identical approach for testing our hypothesis associated with specific aim 2. Our study is not designed nor is it powered to detect significant differences in intervention efficacy across the stratification variables (i.e., age, gender, etc.). However, these subsamples will be compared on the main dependent variables at baseline. If the groups are found to be different from each other then they will be entered in the model as a covariate.
Aim 3 is exploratory in nature so we will begin with correlational analyses. We will use analysis of covariance (ANCOVA) with insulin sensitivity (or arterial stiffness) as the dependent variable, the treatment as the independent variable, and endotoxin concentration, serving as the covariate. If changes in endotoxin concentration with inulin supplementation are: 1) directly correlated with changes in insulin sensitivity among the individual subjects, and 2) the treatment is no longer significant in the ANCOVA, this will be interpreted as support for the concept that changes in insulin sensitivity (or arterial stiffness) with inulin supplementation are mediated, at least in part, by reductions in endotoxin concentration. We will use the same approach using percent abundance of bacterial taxonomic groups and intestinal permeability as covariates. Similarly, we will explore whether total fecal bacteria or changes in abundance of other bacterial members are significant covariates. We will also explore this using other approaches, such as mediation analysis or structural equation modeling.
4.3 Missing Data
We will use an intention-to-treat analysis as our primary analytic approach. We will examine the missing data patterns and utilize maximum likelihood algorithms with the mixed linear model ANOVAs to longitudinally compare our outcomes across the three groups. Maximum likelihood algorithm estimations use all available data to construct weighted averages across the different patterns of missing data to provide valid point estimates and confidence intervals for population parameters.82 As a secondary analysis, we will conduct a completers-only analysis and restrict the analysis to only those individuals who complete the interventions. In our experience, the two approaches yield similar results. However, if the results differ we will interpret the findings based on the intention-to-treat analysis, but report both so that the readers can reach their own conclusion.
4.4 Data Management and Quality Control
The Principal Investigator (PI) will ultimately be responsible for the quality of the data. The project coordinator will be responsible for handling all data, entering data on the study computer, performing data editing, and maintaining a secure filing system for the study data forms that will serve as an ultimate backup (and the source for data random re-entry). Before a form is entered, the data entry staff and PI will inspect the form for completeness and legibility. Each form will be logged into a microcomputer-based system for tracking, validation of assignment, and checked against duplication of visits or forms. This will identify problems in subject records and enable clarification. The data will then be entered into a data entry system that will be constructed to check (1) the validity of the subject identification, (2) each field entered for allowable response (range checks), and (3) validity of examination dates. Data will be duplicate keyed, verified for accuracy, and accumulated and managed using MS Access. Once all data are received, entered, and completeness verified, analysis will proceed with a listing of all data for each subject, summary statistics for all variables at each measurement period, a listing of subjects who are in noncompliance with the study protocol, and statistical analysis.
The PI will ultimately be responsible for quality control of study procedures and measurements. He will supervise performance of all of the various study protocols, questionnaires, forms, and measurements. An operations manual will be developed and the procedures strictly followed. Training sessions will cover all study procedures, including recruitment, informed consent, measurements, and specimen handling. Adherence to the procedures in the operations manual will be assured by periodic assessment and retraining.
5. Discussion
There is currently little information regarding if or how prebiotics improve cardio-metabolic function in humans, particularly in prediabetic individuals who are at high risk for developing T2D and experiencing cardiovascular events. Although the concept that dysbiosis of the gut microbiota leads to metabolic endotoxemia and increased risk of cardio-metabolic disease is not novel, very little information is available in humans. The significance of this trial includes providing proof of concept efficacy of prebiotic supplementation with inulin on cardio-metabolic dysfunction and assessing its relation with changes in gut bacterial communities, intestinal permeability and metabolic endotoxemia in individuals at increased risk for T2D. Our findings could lead to identification of inulin supplementation as a simple and efficacious adjunctive therapy for reducing cardio-metabolic risk in prediabetes, which could change clinical practice by informing dietary recommendations and increasing acceptance of prebiotics by the scientific and medical community.
There are several innovative aspects of this clinical trial. First, we are testing hypotheses involving a novel concept for which there are little data in humans. Second, we have linked our ideas to the important physiological problem of metabolic endotoxemia that has been implicated in T2D and CVD. Third, we are focusing on individuals at high risk for T2D, including those with prediabetes, who are a growing segment of the population at high risk for adverse CVD-related events that may precede T2D onset. Fourth, hundreds of prebiotic products are available, yet little is known about their cardio-metabolic health benefits. Finally, we may identify a simple and efficacious adjunctive lifestyle approach to reduce T2D and CVD risk. Importantly, if our hypotheses are supported, our findings could have a significant impact on clinical practice and public health.
Potential Challenges and Limitations
A primary challenge of our study will be participant recruitment, enrollment, and retention. Recruitment will be ongoing and challenges will be addressed and managed via weekly research team meetings. Participant enrollment and retention will be managed by study personnel and the project coordinator. All participants will visit with study personnel a minimum of 3 times/week and any concerns or issues will be immediately addressed by the project coordinator.
Strict adherence to the controlled diet may also pose a challenge to enrollment and retention. To overcome barriers related to the controlled diet, daily menus are designed to reflect the “average U.S. diet.” Therefore, the foods consumed during this study will be similar in composition and volume to the participant’s habitual intake. All participants will be given menus to review prior to initiating the controlled diet, in order to familiarize them with what they will be expected to consume. Any questions or concerns related to the diet will be addressed at that time by the project coordinator. All participants will be informed of the expectations of the controlled diet component prior during the consenting process. For example, all food must be consumed each day and difficulty consuming any of the food must be reported to study personnel. Strategies such as re-portioning the pre-designated amounts may be utilized. Participants who cannot or will not comply with the controlled diet will be excluded from participating.
Blinding subjects to inulin may be difficult if there are gastrointestinal side effects. However, the nature of the outcomes (biochemical and physiological) and the utilization of a controlled feeding paradigm will minimize any potential for this as a limitation. It is possible that any observed improvement in certain outcomes (e.g., blood lipids) could be attributed to the low saturated fat content of the diet provided. However, we are employing a randomized controlled trial design, and all of the participants will be receiving the same diet. Thus, the impact of the treatment on our outcomes should be preserved.
This investigation should provide vital preliminary data for a larger, more comprehensive trial that would serve to establish the efficacy on the effects of inulin supplementation on cardio-metabolic function in prediabetic individuals. One of the hallmarks of science is replication of study findings. As such, it will be important to replicate the findings of the proposed small clinical trial that may have limited generalizability to a broader context.
6. Conclusions
To date, the potential benefits of prebiotic supplementation on cardio-metabolic dysfunction in humans has received little attention. This trial will address this important research gap, by exploring the role of the prebiotic inulin in modifying cardio-metabolic risk among adults at increased risk for T2D. The results of this trial could impact clinical treatment approaches, and contribute to the evidence base for developing dietary guidelines that address the amount and types of dietary fiber to consume to maximize health benefits.
Acknowledgments
Funding
This work is supported by the National Institutes of Health R21 HL118668 and R21 HL118668S
ABBREVIATIONS
- T2D
Type 2 Diabetes
- CVD
Cardiovascular Disease
- ADA
American Diabetes Association
- BP
Blood Pressure
- OGTT
Oral Glucose Tolerance Test
- IVGTT
Intravenous Glucose Tolerance Test
- FMD
Flow Mediated Dilation
- EID
Endothelial Independent Vasodilation
- C-F
Carotid-Femoral
- PWV
Pulse Wave Velocity
- SSN
Suprasternal Notch
- ANCOVA
Analysis of Covariance
- CHD
Coronary Heart Disease
- FBG
Fasting blood glucose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Diabetes A. Statistics About Diabetes. National Diabetes Statistics Report, 2014. 2014;2014 http://www.diabetes.org/diabetes-basics/statistics/ [Google Scholar]
- 2.Moslehi J, Libby P. You can’t run from inflammation: lower extremity ischemia, hypoxia signaling, and macrophage subtypes. Circulation research. 2012 Apr 13;110(8):1045–1046. doi: 10.1161/RES.0b013e318255409e. [DOI] [PubMed] [Google Scholar]
- 3.Tuttolomondo A, Di Raimondo D, Pecoraro R, Arnao V, Pinto A, Licata G. Atherosclerosis as an inflammatory disease. Current pharmaceutical design. 2012;18(28):4266–4288. doi: 10.2174/138161212802481237. [DOI] [PubMed] [Google Scholar]
- 4.Moore KJ, Fisher EA. Macrophages, atherosclerosis and the potential of netrin-1 as a novel target for future therapeutic intervention. Future cardiology. 2012 May;8(3):349–352. doi: 10.2217/fca.12.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol. 2011 Aug 2;108(3 Suppl):3B–24B. doi: 10.1016/j.amjcard.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004 Mar;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 7.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998 Oct;41(10):1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 8.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006 Jul;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007 Jul;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 10.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. American journal of physiology. Gastrointestinal and liver physiology. 2010 Aug;299(2):G440–448. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD, Bibiloni R, Knauf C, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008 Jun;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006 Dec 21;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 13.Parnell JA, Reimer RA. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br J Nutr. 2012 Feb;107(4):601–613. doi: 10.1017/S0007114511003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006 Dec 21;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 15.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009 Jan 22;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS one. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Ma C, Han L, et al. Molecular characterisation of the faecal microbiota in patients with type II diabetes. Current microbiology. 2010 Jul;61(1):69–78. doi: 10.1007/s00284-010-9582-9. [DOI] [PubMed] [Google Scholar]
- 18.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009 Jun;89(6):1751–1759. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erridge C, Attina T, Spickett CM, Webb DJ. A high-fat meal induces low-grade endotoxemia: evidence of a novel mechanism of postprandial inflammation. Am J Clin Nutr. 2007 Nov;86(5):1286–1292. doi: 10.1093/ajcn/86.5.1286. [DOI] [PubMed] [Google Scholar]
- 20.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012 May;142(5):1100–1101. e1102. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Creely SJ, McTernan PG, Kusminski CM, et al. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab. 2007 Mar;292(3):E740–747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 22.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010 Feb;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011 Feb;34(2):392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisard MI, McMillan RP, Marchand J, et al. Toll-like receptor 4 modulates skeletal muscle substrate metabolism. Am J Physiol Endocrinol Metab. 2010 May;298(5):E988–998. doi: 10.1152/ajpendo.00307.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000 May;49(5):677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 26.Galgani JE, Moro C, Ravussin E. Metabolic flexibility and insulin resistance. American journal of physiology. Endocrinology and metabolism. 2008 Nov;295(5):E1009–1017. doi: 10.1152/ajpendo.90558.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010 Dec;31(6):817–844. doi: 10.1210/er.2009-0030. [DOI] [PubMed] [Google Scholar]
- 28.Miller MA, McTernan PG, Harte AL, et al. Ethnic and sex differences in circulating endotoxin levels: A novel marker of atherosclerotic and cardiovascular risk in a British multi-ethnic population. Atherosclerosis. 2009 Apr;203(2):494–502. doi: 10.1016/j.atherosclerosis.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Engelberger RP, Pittet YK, Henry H, et al. Acute endotoxemia inhibits microvascular nitric oxide-dependent vasodilation in humans. Shock. 2011 Jan;35(1):28–34. doi: 10.1097/SHK.0b013e3181ec71ab. [DOI] [PubMed] [Google Scholar]
- 30.Hingorani AD, Cross J, Kharbanda RK, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000 Aug 29;102(9):994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 31.Draisma A, Bemelmans R, van der Hoeven JG, Spronk P, Pickkers P. Microcirculation and vascular reactivity during endotoxemia and endotoxin tolerance in humans. Shock. 2009 Jun;31(6):581–585. doi: 10.1097/SHK.0b013e318193e187. [DOI] [PubMed] [Google Scholar]
- 32.Amar J, Ruidavets JB, Bal Dit Sollier C, et al. Soluble CD14 and aortic stiffness in a population-based study. J Hypertens. 2003 Oct;21(10):1869–1877. doi: 10.1097/00004872-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Current pharmaceutical design. 2006;12(32):4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 34.Wiedermann CJ, Kiechl S, Dunzendorfer S, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck Study. J Am Coll Cardiol. 1999 Dec;34(7):1975–1981. doi: 10.1016/s0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 35.Gibson GR. Prebiotics as gut microflora management tools. Journal of clinical gastroenterology. 2008 Jul;42(Suppl 2):S75–79. doi: 10.1097/MCG.0b013e31815ed097. [DOI] [PubMed] [Google Scholar]
- 36.Rastall RA, Gibson GR, Gill HS, et al. Modulation of the microbial ecology of the human colon by probiotics, prebiotics and synbiotics to enhance human health: an overview of enabling science and potential applications. FEMS microbiology ecology. 2005 Apr 1;52(2):145–152. doi: 10.1016/j.femsec.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995 Jun;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 38.Delzenne NM, Neyrinck AM, Cani PD. Modulation of the gut microbiota by nutrients with prebiotic properties: consequences for host health in the context of obesity and metabolic syndrome. Microb Cell Fact. 2011 Aug;10(Suppl 1):S10. doi: 10.1186/1475-2859-10-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brownawell AM, Caers W, Gibson GR, et al. Prebiotics and the health benefits of fiber: current regulatory status, future research, and goals. J Nutr. 2012 May;142(5):962–974. doi: 10.3945/jn.112.158147. [DOI] [PubMed] [Google Scholar]
- 40.Manning TS, Gibson GR. Microbial-gut interactions in health and disease. Prebiotics. Best practice & research. Clinical gastroenterology. 2004 Apr;18(2):287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2012 Aug;104(Suppl 2):S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 42.Kolida S, Tuohy K, Gibson GR. Prebiotic effects of inulin and oligofructose. Br J Nutr. 2002 May;87(Suppl 2):S193–197. doi: 10.1079/BJNBJN/2002537. [DOI] [PubMed] [Google Scholar]
- 43.Meyer D, Stasse-Wolthuis M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur J Clin Nutr. 2009 Nov;63(11):1277–1289. doi: 10.1038/ejcn.2009.64. [DOI] [PubMed] [Google Scholar]
- 44.Bang H, Edwards AM, Bomback AS, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009 Dec 1;151(11):775–783. doi: 10.1059/0003-4819-151-11-200912010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnema AL, Kolberg LW, Thomas W, Slavin JL. Gastrointestinal tolerance of chicory inulin products. J Am Diet Assoc. 2010 Jun;110(6):865–868. doi: 10.1016/j.jada.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 46.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007 Nov;50(11):2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths EA, Duffy LC, Schanbacher FL, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Digestive diseases and sciences. 2004 Apr;49(4):579–589. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]
- 48.Guarner F. Studies with inulin-type fructans on intestinal infections, permeability, and inflammation. J Nutr. 2007 Nov;137(11 Suppl):2568S–2571S. doi: 10.1093/jn/137.11.2568S. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. The Journal of trauma. 2006 Sep;61(3):650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- 50.Moshfegh AJ, Friday JE, Goldman JP, Ahuja JK. Presence of inulin and oligofructose in the diets of Americans. J Nutr. 1999 Jul;129(7 Suppl):1407S–1411S. doi: 10.1093/jn/129.7.1407S. [DOI] [PubMed] [Google Scholar]
- 51.Suitor CW, Meyers Linda D Dietary Reference Intakes Research Synthesis Workshop (2006 Institute of Medicine (US)), Institute of Medicine (US) Food and Nutrition Board. In: Dietary Reference Intakes Research Synthesis Workshop summary. Suitor Carol West, Meyers Linda D., editors. Food and Nutrition Board, Institute of Medicine of the National Academies; United States: Washington, DC: National Academies Press; 2007. p. c2007. [Google Scholar]
- 52.Bazzano LA, He J, Ogden LG, et al. Dietary fiber intake and reduced risk of coronary heart disease in US men and women: the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Archives of internal medicine. 2003 Sep 8;163(16):1897–1904. doi: 10.1001/archinte.163.16.1897. [DOI] [PubMed] [Google Scholar]
- 53.Ma Y, Hebert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition. 2008 Oct;24(10):941–949. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King DE, Mainous AG, 3rd, Lambourne CA. Trends in dietary fiber intake in the United States, 1999–2008. Journal of the Academy of Nutrition and Dietetics. 2012 May;112(5):642–648. doi: 10.1016/j.jand.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 55.Cogswell ME, Zhang Z, Carriquiry AL, et al. Sodium and potassium intakes among US adults: NHANES 2003–2008. Am J Clin Nutr. 2012 Sep;96(3):647–657. doi: 10.3945/ajcn.112.034413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fulgoni VL, 3rd, Agarwal S, Spence L, Samuel P. Sodium intake in US ethnic subgroups and potential impact of a new sodium reduction technology: NHANES Dietary Modeling. Nutrition journal. 2014;13(1):120. doi: 10.1186/1475-2891-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990 Feb;51(2):241–247. doi: 10.1093/ajcn/51.2.241. [DOI] [PubMed] [Google Scholar]
- 58.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997 Apr 17;336(16):1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 59.Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005 Nov 16;294(19):2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 60.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005 Feb;7(2):102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godin G, Shephard RJ. Godin leasiure-time exercise questionnaire. Medicine and science in sports and exercise. 1997;29(6s):S36. [Google Scholar]
- 62.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Medicine and science in sports and exercise. 1998 May;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? The international journal of behavioral nutrition and physical activity. 2011;8:62. doi: 10.1186/1479-5868-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dastych M, Dastych M, Jr, Novotna H, Cihalova J. Lactulose/mannitol test and specificity, sensitivity, and area under curve of intestinal permeability parameters in patients with liver cirrhosis and Crohn’s disease. Dig Dis Sci. 2008 Oct;53(10):2789–2792. doi: 10.1007/s10620-007-0184-8. [DOI] [PubMed] [Google Scholar]
- 65.Hilsden RJ, Meddings JB, Sutherland LR. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn’s disease. Gastroenterology. 1996 May;110(5):1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- 66.Farhadi A, Gundlapalli S, Shaikh M, et al. Susceptibility to gut leakiness: a possible mechanism for endotoxaemia in non-alcoholic steatohepatitis. Liver international : official journal of the International Association for the Study of the Liver. 2008 Aug;28(7):1026–1033. doi: 10.1111/j.1478-3231.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. Jan;22(1):e15–26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.American Diabetes A. Standards of medical care in diabetes--2013. Diabetes care. 2013 Jan;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes technology & therapeutics. 2003;5(6):1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 70.Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003 Apr;284(4):E741–747. doi: 10.1152/ajpendo.00514.2002. [DOI] [PubMed] [Google Scholar]
- 71.Hulver MW, Berggren JR, Carper MJ, et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell metabolism. 2005 Oct;2(4):251–261. doi: 10.1016/j.cmet.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corretti M, Plotnick G, Vogel R. Technical aspects of evaluating brachial artery vasodilation using high-frequency ultrasound. The American journal of physiology. 1995;37:H1397–H1404. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- 73.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology. Heart and circulatory physiology. 2011 Jan;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanaka H, Safar ME. Influence of lifestyle modification on arterial stiffness and wave reflections. Am J Hypertens. 2005 Jan;18(1):137–144. doi: 10.1016/j.amjhyper.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 75.Segers P, Rietzschel ER, De Buyzere ML, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007 Jun;49(6):1248–1255. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 76.Vyas M, Izzo JL, Jr, Lacourciere Y, et al. Augmentation index and central aortic stiffness in middle-aged to elderly individuals. Am J Hypertens. 2007 Jun;20(6):642–647. doi: 10.1016/j.amjhyper.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proceedings of the National Academy of Sciences of the United States of America. 2011 Mar 15;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sim K, Cox MJ, Wopereis H, et al. Improved detection of bifidobacteria with optimised 16S rRNA-gene based pyrosequencing. PLoS One. 7(3):e32543. doi: 10.1371/journal.pone.0032543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Meyer F, Paarmann D, D’Souza M, et al. The metagenomics RAST server - a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC bioinformatics. 2008;9:386. doi: 10.1186/1471-2105-9-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bonnema AL, Kolberg LW, Thomas W, Slavin JL. Gastrointestinal tolerance of chicory inulin products. J Am Diet Assoc. 2010 Jun;110(6):865–868. doi: 10.1016/j.jada.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 81.Grabitske HA, Slavin JL. Gastrointestinal effects of low-digestible carbohydrates. Critical reviews in food science and nutrition. 2009 Apr;49(4):327–360. doi: 10.1080/10408390802067126. [DOI] [PubMed] [Google Scholar]
- 82.Enders CK. A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling. 2001;8:128–141. [Google Scholar]