Abstract
Infertility is a prevalent condition that has insidious impacts on the infertile individuals, their families and society that extend far beyond the inability to have a biological child. Lifestyle changes, fertility treatments and assisted reproductive technologies are available to help many infertile couples achieve their reproductive goals. All of these technologies require that the infertile individual is able to produce at least a small number of functional gametes (eggs or sperm). It is not possible for a person who does not produce gametes to have a biological child. This review focuses on the infertile man and describes several stem cell-based methods and gene therapy approaches that are in the research pipeline and may lead to new fertility treatment options for azoospermic men.
Keywords: fertility, infertility, male infertility, azoospermia, stem cells, spermatogonial stem cells, transplantation, grafting, xenografting, culture, organ culture, de novo testicular morphogenesis, induced pluripotent stem cells, germ cells, gene therapy
Introduction
In vitro fertilization (IVF) was pioneered in the United Kingdom by Drs. Patrick Steptoe (physician) and Robert Edwards (researcher)1 and led to the birth of Louise Brown (born July 25, 1978), the world's first baby conceived in a petri dish. This technology has now led to the birth of nearly five million babies worldwide and the 2010 Nobel Prize in Medicine for Dr. Edwards. Despite this progress treating infertile couples, many still remain beyond the reach of current assisted reproductive technologies because they are not able to produce mature sperm or eggs. For those couples, there are several methods in the research pipeline that may expand fertility options and lead to the next renaissance in the field of assisted reproduction. This review will focus on experimental options to preserve male fertility and/or treat male infertility.
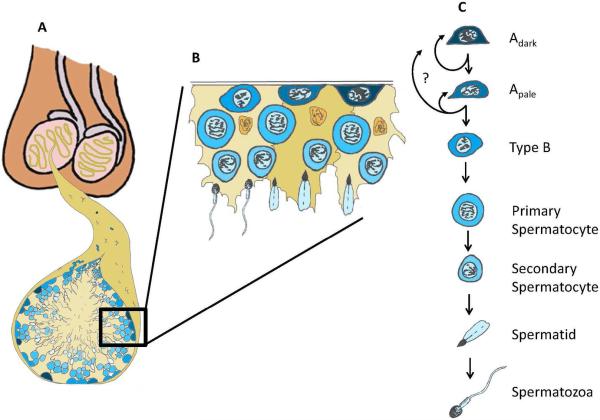
Spermatogenesis is an extraordinarily productive process that produces millions of sperm each day throughout the postpubertal life of men2. Spermatogenesis arises from a relatively small pool of Spermatogonial stem cells (SSCs) that are located in the seminiferous tubules of the testis3–5. These adult tissue stem cells (designated Adark and Apale spermatogonia in humans) balance self-renewing divisions that maintain the stem cell pool with differentiating divisions that insure continuous sperm production (Fig. 1)6–8. Therefore, SSCs are essential for spermatogenesis and male fertility. Conditions that compromise the stem cell pool, the differentiation process or the testicular environment (e.g., genetic, environmental, medical, age, injury or other) can lead to sub-fertility or infertility. Refinements in assisted reproductive technologies, including testicular sperm extraction (TESE) and intracytoplasmic sperm injection (ICSI), now allow many men with azoospermia (no sperm in their ejaculate) to father biological children from rare sperm that are biopsied directly from the testes (Fig. 2A)9–11. There are currently no options for men with azoospermia and failed TESE to have biological children.
Figure 1. Human spermatogonial stem cells and spermatogenesis.
(A) Spermatogenesis occurs inside the seminiferous tubules of the testis. (B) Cut-out of the basement membrane of the seminiferous Tubule. (B and C) The basement membrane of the seminiferous epithelium contains undifferentiated (Adark and Apale) spermatogonia and differentiating Type B spermatogonia. Type B spermatogonia give rise to primary spermatocytes that enter meiosis and migrate off the basement membrane. Subsequent meiotic divisions and spermiogenesis give rise to secondary spermatocytes, spermatids and the terminally differentiated spermatozoa that are released into the lumen of the seminiferous tubules. Figure reprinted from Valli et al., 201414 with permission from Elsevier. Artwork is by Dr. Bart Phillips, National Institute of Environmental Health Sciences.
Figure 2. Standard and experimental options to treat male infertility.
(A) Sperm obtained from ejaculated semen or by testicular sperm extraction (TESE) of infertile men, can be used to achieve pregnancy by intrauterine insemination (IUI), in vitro fertilitzation (IVF) or IVF with intracytoplasmic sperm injection (ICSI). (B) When it is not possible to obtain sperm, testicular tissue containing spermatogonial stem cells (SSCs) can be obtained by biopsy. Testicular tissue can be digested with enzymes to produce a cell suspension from which SSCs can be expanded in culture and/or transplanted into the testes of the patient. This method has the potential to regenerate spermatogenesis and possibly natural fertility. Heterogeneous testicular cell suspensions also have the potential undergo de novo testicular morphogenesis with seminiferous tubules and a polarized epithelium surrounded by a basement membrane with germ cells inside and interstitial cells outside the tubules. Sperm generated in the “rebuilt” testes can be used to fertilize eggs by ICSI. Intact testicular tissues from prepubertal animals can be grafted or xenografted under the skin or in the scrotum and produce mature sperm that can be used to fertilize eggs by ICSI. Sperm can also be generated when immature testicular tissues are maintained in organ culture and used to fertilize eggs by ICSI. (C) Patient-specific induced pluripotent stem (iPS) cells can be derived from patient somatic tissues (e.g., skin or blood) and differentiated into germline stem cells (GSCs) to be transplanted into patient testes. This method may have the potential to regenerate spermatogenesis and natural fertility. It may also be possible to differentiate iPS cells into sperm that can be used to fertilize eggs by ICSI.
Several technologies have emerged during the past two decades that may substantially increase the number of reproductive options available to men who do not produce any sperm and desire to have biological children: SSC transplantation; SSC culture; testicular tissue grafting; testicular tissue organ culture, induced pluripotent stem cells (iPSCs); the $1,000 genome and gene therapy (Fig. 2). This review will utilize two patient scenarios to illustrate how these technologies could be used to preserve fertility and generate or regenerate spermatogenesis in men with azoospermia. The first scenario is the prepubertal cancer patient who cannot preserve a semen sample prior to initiation of treatment and who grows up to become an infertile adult survivor of childhood cancer. The second scenario is a man with idiopathic non-obstructive azoospermia and no previous co-mobidities.
Patient Scenario 1: Medically induced (iatrogenic) azoospermia
Chemotherapy and radiation treatments for cancer and other conditions can cause permanent infertility. Adult men have the option to cryopreserve a semen sample prior to the initiation of treatment and use this sample in the future to achieve a pregnancy with their partner using assisted reproductive technologies (Fig. 2A)1,12,13. This option is not available to prepubertal boys who are not making sperm or to adult survivors who did not preserve sperm prior to treatment. This is a significant human health problem because we estimate that each year in the United States, there are over 4,000 patients who will receive treatments that put them at risk of permanent azoospermia and did not or could not save a semen sample (reviewed in Valli et al., 2015)14. TESE may be an option for azoospermic adult survivors of childhood cancers who did not save semen or testicular tissue. This is possible because a few SSCs may survive the gonadotoxic therapy and produce focal areas of spermatogenesis in the seminiferous tubules that can be retrieved by biopsy. Picton and colleagues surveyed results from a total of five centers and reported an overall sperm recovery rate of 44% in azoospermic patients undergoing TESE after chemotherapy15–20. Prepubertal boys cannot save a semen sample prior to therapy, but they do have Adark and Apale spermatogonial stem cells in their testes (Fig. 1)21 that are poised to initiate sperm production during puberty. Several centers in the United States and around the world are collecting (via biopsy) and cryopreserving testicular tissue or cells with anticipation that experimental SSC-based therapies will be available in the future (experimental options are reviewed below and in Fig. 2)22–26,20,27.
Patient Scenario 2: Idiopathic Non-Obstructive Azoospermia
About 1% of men in the general population and 10–15% of infertile men are azoospermic (no sperm in the ejaculate)28–31. Azoospermia can be classified as obstructive (OA, 15–20% of cases) or nonobstructive (NOA, 80–85% of cases), which indicates a problem with spermatogenesis30. NOA is characterized by spermatogenic failure and can be sub-classified as Sertoli cell only, early or late maturation arrest (MA), mixed atrophy or complete hyalinization of the seminiferous tubules. The chances of sperm recovery by TESE from the testes of men with “true” Sertoli cell only or complete hyalinization phenotypes are very low. Sperm recovery rates from men with uniform early maturation arrest (spermatogonia or spermatocytes) are considerably lower (23–41%) than patients with late maturation arrest and/or mixed atrophy (54–90%)32,29,33. Review of our own records and four retrospective studies revealed that about 15% of NOA patients and 2% of all infertile men have a uniform maturation arrest phenotype32,34,29,33. A diagnosis of non-obstructive azoospermia with maturation arrest suggests that germ cells are present, but fail to progress through meiosis and/or spermiogenesis. Maturation arrest could be due to intrinsic germ cell defects or extrinsic somatic/endocrine environment defects or both. If an underlying genetic cause of a somatic defect can be identified, NOA-MA male infertility might be amenable to gene therapy, using methods that have already been described in mice (see below). Improved knowledge about the genetic basis of infertility will aid in the counseling of infertile men; justify the development of diagnostic screens; and may lead to the development of patient-specific treatment options.
Stem Cell Therapies for Male Infertility
Spermatogonial Stem Cell Transplantation
Spermatogonial stem cell transplantation was described in 1994 by Ralph Brinster and Colleagues. In the initial reports, testicular tissue was obtained from the testes of transgenic mice with ubiquitous expression of the lacZ transgene. The tissue was digested with enzymes and the resulting cell suspension was transplanted into the seminiferous tubules of infertile recipient mice. A few months after transplantation, donor-derived spermatogenesis could be recognized in the testes of recipient animals by X-gal staining, which generated a blue color due to the activity of the lacZ encoded β-galactosidase activity35,36. One of those classic studies reported that fertility was restored and the lacZ transgene was transmitted to progeny, providing unequivocal evidence that SSCs could engraft the basement membrane of recipient seminiferous tubules and regenerate spermatogenesis with functional sperm35. This approach for homologous species SSC transplantation has now been recapitulated in rats, pigs, goats, bulls, sheep, dogs and monkeys with the production of donor-derived embryos or offspring in mice, rats, goats, sheep and monkeys37–48. Functional SSCs can be obtained from the testes of all ages from newborn to adult39,49,48 and SSCs retain their biological function after freezing and thawing50–52,48,53,54. Wu and colleagues demonstrated that mouse SSCs were competent to regenerate spermatogenesis and produce offspring after 14 years of cryostorage55. Based on these observations, it should be possible to cryopreserve testicular tissue or cells for prepubertal boys before they initiate cancer treatment and thaw those cells years later for transplantation and regeneration of spermatogenesis (Fig. 2B).
Translating Spermatogonial Stem Cell Transplantation to the Clinic
It is not widely known that Radford and colleagues already biopsied and cryopreserved testicular cell suspensions for 12 adult non-Hodgkin's lymphoma patients in 199956. This was before the method of SSC transplantation had been translated to any large animal species. Testicular cells were later re-introduced into the testes of seven of those patients57,58 after completion of their cancer treatments, but their fertility status has not been reported. Nonetheless, those reports demonstrate that patients are willing to enroll in experimental stem cell protocols to preserve and potentially restore their fertility. As indicated above, homologous species stem cell transplants have now been performed in several large animal models42–47, including our report that nonhuman primate SSCs could be frozen, thawed and transplanted to regenerate spermatogenesis with functional sperm48. Furthermore, we estimate from published reports and personal communications that testicular tissues or cells have been cryopreserved for several hundred patients worldwide14,20,59. Therefore, translation of the SSC transplantation technique to the clinic appears imminent.
While some centers are freezing testicular cell suspensions57,60–63, most are freezing intact pieces of testicular tissue for patients because this preserves the option for both tissue-based and cell-based therapies in the future (see Fig. 2)22,23,25,26,20,59. Biopsied testicular tissues are typically cut into small pieces (1–9 mm3); suspended in a DMSO based freezing medium and frozen at a controlled slow rate using a programmable freezing machine64,22,61,23,25,20,59,27. Some centers have reported using ethylene glycol based freezing medium instead of DMSO57,60,65 and some centers have reported that viability of vitrified testicular tissue is similar to tissue frozen at a controlled slow rate66–69.
SSC Culture
The size of testicular biopsy that can be obtained from the testes of prepubertal boys is relatively small and may contain a small number of SSCs. The number of SSCs that will be required to regenerate spermatogenesis and fertility in humans is not known, but it is reasonable to assume that SSC numbers will need to be expanded in culture before transplantation to ensure robust engraftment and spermatogenesis. Methods for maintaining mouse SSCs in culture were established in 2003/2004 and these methods were translated to rats in 2005. Cultured rodent SSCs can be maintained in long-term culture with exponential expansion in numbers and retain their biological potential to produce spermatogenesis and restore fertility when transplanted into the testes of infertile recipient mice70–73. Langenstroth and colleagues have reported maintaining nonhuman primate SSCs in short-term culture74 and several groups have reported culturing human SSCs for short- or long-term75–81,24,82,83. Each group reporting human SSC culture used different methods to isolate cells for culture, different feeder or matrix substrates, different growth factor cocktails and different methods to assess progress. To date, no human SSC culture method has be independently replicated by another group and this needs to happen to move the field forward (reviewed in Valli et al., 2015)59. Furthermore, while transplantation to regenerate spermatogenesis with functional sperm and offspring is the gold standard assay for rodent SSCs, there is not equivalent assay of human SSCs. Molecular markers and human to mouse xenotransplantation may be reasonable surrogate assays, but there is no gold standard that is universally agreed and deployed for human SSC experimentation. Perhaps de novo testicular morphogenesis and/or decellularized testes can be developed into tools to assay complete human spermatogenesis (see below)
De Novo Testicular Morphogenesis
Heterogeneous testicular cell suspensions (including germ cells, Sertoli cells, peritubular myoid cells, Leydig and other interstitial cells) have the remarkable ability to reorganize into normal looking seminiferous tubules when grafted under the skin of immune-deficient recipient mice84–88. Dobrinski's laboratory reported complete spermatogenesis when neonatal pig and sheep testis cells were pelleted and grafted under the skin of SCID mice86,88. Kita and colleagues obtained similar results with fetal or neonatal mouse, rat and pig testis cells87. Furthermore, this group mixed cultured green fluorescent protein (GFP)+ mouse SSCs with GFP- neonatal mouse or rat testis cells. Complete spermatogenesis was observed in reorganized seminiferous tubules 7–10 weeks after grafting, including GFP+ round spermatids that were recovered and used to fertilize mouse eggs by round spermatid injection. The resulting embryos were transferred to pseudopregnant females and gave rise to live offspring87. In principle a similar experimental design could be used to assay cultured human SSCs by mixing them with human testis cells (e.g., obtained from organ donors) and grafting under the skin of immune deficient mice. The method could also be used to generate sperm for clinical application. However, de novo testicular morphogenesis with human testis cells has not been reported to our knowledge. Furthermore, de novo testicular morphogenesis has not been achieved using adult cells from any species and access to human fetal or neonatal cells is likely to be very limited. Baert and colleagues recently reported decellularizing human testes and observed that human testis cells could infiltrate the three dimensional scaffold89. Perhaps in future studies, human testis cells can be infused into decellularized human testis scaffold and grafted under the skin of mice to facilitate de novo testicular morphogenesis.
Testicular Tissue Grafting and Xenografting
Honaramooz and colleagues reported that testicular tissue from newborn mice, rats, pigs and goats, in which spermatogenesis was not yet established, could mature and produce complete spermatogenesis when grafted under the skin of immune into nude mice90. The same group later reported the production of live offspring from sperm obtained from mouse testicular tissue grafts91. Fertilization competent sperm was also produced from xenografts of prepubertal nonhuman primate testicular tissue transplanted into mice92. These results suggest that it may be possible to obtain fertilization competent sperm by xenografting small pieces of testicular tissue from a prepubertal cancer patient under the skin of mice or other animal recipients such as pigs that are already an established source for human food consumption, replacement heart valves93,94 and potentially other organs95. Xenografting would also circumvent the issue of malignant contamination in cases such as leukemia where it would be unsafe to return testicular tissue or cells back into the body of a cancer survivor. However, the xenografting approach raises concerns about transmission of viruses from mice, pigs and other species to human cells96,97. Also, there is no evidence to date that xenografted human testicular tissue can produce spermatogenesis or sperm in mice98–103.
If malignant contamination of the testicular tissue is not a concern, autologous testicular tissue grafting can be considered. Luetjens and colleagues demonstrated that fresh autologous testicular tissue grafts from prepubertal marmosets could produce complete spermatogenesis when transplanted into the scrotum, but not under the skin104. Frozen and thawed grafts did not produce complete spermatogenesis in that study, but those grafts were only transplanted under the skin. Therefore, additional experimentation is merited. Testicular tissue grafting or xenografting will not restore natural fertility, but could generate haploid sperm, that can be used to fertilize oocytes by ICSI.
Testicular Tissue Organ Culture
There is some limited evidence that haploid germ cells can be produced in culture without the supporting structure of the seminiferous tubules105–107. However, fertilization has never been reported with those putative haploid cells. In contrast, Sato and colleagues reported that intact testicular tissue pieces from newborn mice could be maintained in organ culture and matured to produce complete spermatogenesis with fertilization competent haploid germ cells108,109. Testicular tissues were minced into pieces (1–3 mm3) and placed in culture at the gas/liquid interface on a slab of agarose that was soaked in medium. Haploid round spermatids and sperm were recovered from the tissue after 3–6 weeks in culture and used to fertilize mouse eggs by ICSI. The resulting embryos were transferred to pseudopregnant females and gave rise to healthy offspring that matured to adulthood and were fertile. If testicular tissue organ culture can be translated to humans, it will provide an alternative to autologous SSC transplantation or autologous grafting in cases where there is concern about malignant contamination of the testicular tissue. The same authors were also successful in producing haploid germ cells in organ culture of frozen and thawed testicular tissues, which is particularly relevant to the cancer survivor paradigm. However, the fertilization potential of those sperm were not tested108. To our knowledge, human testicular tissue organ culture with production of haploid gametes has not been reported. As with de novo testicular morphogenesis, one of the challenges to developing human testicular tissue organ culture is the limited access to fetal, newborn and/or prepubertal tissues.
Pluripotent stem cell technology
Induced pluripotent stem (iPS) cells can be derived from any tissue of the body using a cocktail of reprogramming factors110,111. Several groups have now reported that rodent, monkey and human pluripotent embryonic stem (ES) cells or iPS cells can be differentiated into germ cells112,113,105,114–117,107,118–123. Hayashi and coworkers reported that it is possible to differentiate ESCs or iPSCs into epiblast like cells (EpiLCs) that then give rise to primordial germ cell-like cells (PGCLCs) when cultured in the presence of BMP4116. The resulting germ cells were transplanted into the seminiferous tubules of infertile recipient mice where they regenerated spermatogenesis and haploid gametes that were used to fertilize mouse oocytes by ICSI. The embryos were transferred to recipient females and gave rise to live offspring. However, some of the offspring developed tumors in the neck area and died prematurely, suggesting that further optimization of the culture and differentiation protocols will be required116. The same groups also reported generation of EpiLCs and PGCLCs from female iPSCs. The resulting PGCLCs were transplanted into recipients and gave rise to functional eggs and live offspring124. Two groups recently reported the differentiation of human pluripotent stem cells into putative hPGCLCs exhibiting gene expression patterns similar to bona fide human PGCs121,123. Unfortunately, the human studies cannot be validated by transplantation or the production of offspring. As mentioned above, a surrogate assay of human spermatogenic potential is needed.
Currently the recommended and best approach to preserve fertility is to obtain and freeze gametes or tissue prior to the initiation of therapy that can damage or eliminate germ cells125,126. However, if the iPS cell to germ cell differentiation technology is responsibly developed and translated to the human clinic, this fertility preservation paradigm could change. An adult survivor of a childhood cancer who desires to start his family and discovers that he is infertile could theoretically produce sperm and biological offspring from his own skin, blood or other somatic cell type. This scenario applies not only to childhood cancer survivors, but all survivors or other infertile patients who cannot preserve or produce functional gametes.
Gene Therapies for Male Infertility
The Thousand Dollar Genome and Gene Therapy
It took eleven years, more than 200 scientists and 3 billion dollars to sequence and publish the first draft of the human genome in 2001127,128. This was a monumental achievement, but the exorbitant cost precluded sequencing of thousands or millions of genomes that would be necessary to uncover the genetic basis of many human diseases. In 2004, the National Human Genome Research Institute (NHGRI) initiated the $1,000 genome project to stimulate unprecedented academic/industrial collaboration to improve speed and reduce cost of human genome sequencing128. In 2015, the program has nearly achieved its goal with the cost of sequencing the whole human genome of about $5,000 and whole exome sequencing considerably less than that. This progress has accelerated the discovery of genes associated with human male infertility, which may in turn lead to the development of diagnostic screens and personalized treatment plans, perhaps including gene therapy.
Germline Gene Therapy
Methods for genetic modification of germline stem cells and Sertoli cells of the testis are well-established and used routinely in the basic research laboratory38,129–138. Germ cell gene therapy is technically feasible, but mired in ethical concerns that the genetic modification would be passed to progeny, thereby treating not only the infertile patient, but all subsequent generations. This subject is actively debated139–144 and was a key topic for discussion at an International Summit on Human Gene Therapy that was jointly sponsored by the National Academies of Science, the National Academy of Medicine, the Chinese National Academy and the Royal Society in December 2015 (http://www.nationalacademies.org/gene-editing/index.htm).
Sertoli Cell Gene Therapy
In 2002, three groups independently demonstrated that in vivo Sertoli cell gene therapy could reverse the infertile phenotype in “Steel” mice that lack the Kit Ligand in Sertoli cells129,130,132. Steel mice are infertile with small testes that are completely devoid of spermatogenesis. The testes of Steel mice do contain rare undifferentiated spermatogonia that fail to differentiate in the absence of Kit ligand, similar to the human phenotype of azoospermia with early maturation arrest. Reciprocal transplantation experiments revealed that the residual spermatogonia in Steel mouse testes are competent to produce complete spermatogenesis when transplanted into a permissive environment37. Adenovirus130, lentivirus129 and electroporation132 were used respectively, to introduce a functional Kit ligand gene into the Sertoli cells of Steel mice. In all cases, spermatogenesis was partially restored and in two cases (adenovirus and lentivirus) sperm were recovered and used to produce offspring by ICSI and embryo transfer129,130. Importantly, the corrective transgene was not transmitted to offspring in either of those studies, suggesting that it may be possible to implement Sertoli cell gene therapy without risk of germline modification. However, a combined total of only 33 pups were evaluated in those two studies, so more rigorous assessments of germline transmission risk are needed.
In humans, mutations in the Kit signaling pathway lead to the Piebald condition145, which is characterized by patches of pale hair or skin, but is not associated with infertility. However, with the increasing accessibility of whole genome and whole exome sequencing technology genes associated with human male infertility are being revealed at an increasingly rapid pace. Some examples include the germ cell genes, SOHLH1146,147 and TEX11148,149 and somatic cell genes, androgen receptor (AR)150–153 and NR5A1154,155. The successes in the Steel mouse model may suggest that in vivo Sertoli cell gene therapies could be developed to treat infertility of men with somatic defects, including AR and NR5A1. These examples are complicated because they have multiple endocrine phenotypes. However, Bashamboo and colleagues154 identified Nr5a1 mutations in men with spermatogenic failure, but who were otherwise healthy and commented that this broadens the range Nr5a1 phenotypes, which had previously been reported in more severe forms of gonadal dysgenesis. Similarly, AR mutations generate a range of phenotypes from complete sex reversal to mild phenotypes characterized primarily by male infertility150–153. Compelling progress in basic research investigations may justify comprehensive genetic screening of infertility patients to identify causative genes to facilitate counseling and possibly develop individualized treatments.
Safety and feasibility studies will be needed in rodents, nonhuman primates and human cells to confirm that Sertoli cell gene therapy can be achieved without risk of germline transmission and to carefully map genomic integrations of the therapeutic transgene. The risks of insertional mutagenesis156,157 may be reduced by using non-integrating adenoviral vectors158 or integration-deficient lentiviral vectors159. Safety and feasibility studies will be particularly important for the human gene therapy field that has already suffered serious setbacks due to unexpected adverse outcomes in previous trials156,160,161.
Conclusions
Approximately 10–15% of couples and 12% of men in the United States are subfertile or infertile162,163. Infertility is an insidious condition that impacts not only the ability to have biological children, but has broader implications for psychological well-being, relationships, finances (assisted reproduction and adoption can be expensive), general health and life expectancy164–166,163,167. Assisted reproductive technologies are available for men that produce even a small number of sperm in their ejaculates or testes. It is not possible for men that do not have cryopreserved or endogenous sperm to have biological children. This review describes several technologies that are currently in the research pipeline and may expand fertility options for men in the future. Each technology described in this review has produced functional sperm and progeny in at least one animal model, but none except SSC transplantation has been deployed in the human fertility clinic yet. In all cases, fundamental translational and preclinical studies of safety and feasibility are still needed. Nonhuman primates (NHPs) are expensive, but are amenable to transplantation/grafting studies that produce spermatogenesis and assisted reproductive technologies to produce progeny. Furthermore, stem cell dynamics, spermatogenic lineage development and testis anatomy in nonhuman primates are similar to human168,8. Human tissue/cell studies are equally important, but challenged by limited availability of tissues and biological assays of spermatogenic potential and sperm function. With responsible basic, translational and preclinical development, we believe it is reasonable to expect that one or more of the experimental reproductive technologies described in this review will impact the male fertility clinic in the next decade.
Acknowledgements
The authors would like to thank the Scaife Foundation, the Richard King Mellon Foundation, the Magee-Womens Research Institute and Foundation, the Children's Hospital of Pittsburgh Foundation and the University of Pittsburgh Departments of Obstetrics, Gynecology & Reproductive Sciences and Urology, which have generously provided funds to support the Fertility Preservation Program in Pittsburgh (http://www.mwrif.org/220). It is in this context that we have had the opportunity to meet the infertile patients that fuel our passion for fertility research. The Orwig lab is supported by the Magee-Womens Research Institute and Foundation, the Eunice Kennedy Shriver National Institute of Child Health and Human Development grants HD075795 and HD076412, the US-Israel Binational Science Foundation and gift funds from Montana State University, Sylvia Bernassoli and Julie and Michael McMullen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 2.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. Raven Press, Ltd.; New York: 1994. pp. 1363–1434. [Google Scholar]
- 3.Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the c3h/101 f1 hybrid mouse. Mutation research. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- 4.de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Current opinion in cell biology. 1998;10:694–701. doi: 10.1016/s0955-0674(98)80109-9. [DOI] [PubMed] [Google Scholar]
- 5.Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1663–1678. doi: 10.1098/rstb.2010.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clermont Y. Renewal of spermatogonia in man. The American journal of anatomy. 1966;118:509–524. doi: 10.1002/aja.1001180211. [DOI] [PubMed] [Google Scholar]
- 7.Clermont Y. Kinetics of spermatogenesis in mammals: Seminiferous epithelium cycle and spermatogonial renewal. Physiol Rev. 1972;52:198–236. doi: 10.1152/physrev.1972.52.1.198. [DOI] [PubMed] [Google Scholar]
- 8.Valli H, Phillips BT, Gassei K, Nagano MC, Orwig KE. Spermatogonial stem cells and spermatogenesis. In: Plant TM, Zeleznik AJ, editors. Knobil and neill's physiology of reproduction. Fourth ed Elsevier; San Diego: 2015. pp. 595–635. [Google Scholar]
- 9.Kim ED, Gilbaugh JH, Iii, Patel VR, Turek PJ, Lipshultz LI. Testis biopsies frequently demonstrate sperm in men with azoospermia and significantly elevated follicle-stimulating hormone levels. The Journal of Urology. 1997;157:144–146. [PubMed] [Google Scholar]
- 10.Silber SJ, Nagy Z, Devroey P, Tournaye H, van Steirteghem AC. Distribution of spermatogenesis in the testicles of azoospermic men: The presence or absence of spermatids in the testes of men with germinal failure. Human Reproduction. 1997;12:2422–2428. doi: 10.1093/humrep/12.11.2422. [DOI] [PubMed] [Google Scholar]
- 11.Chan PTK, Schlegel PN. Nonobstructive azoospermia. Current Opinion in Urology. 2000;10:617–624. doi: 10.1097/00042307-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Allamaneni SR. Artificial insemination. In: Falcone T, Hurd W, editors. Clinical reproductive medicine and surgery. Elsevier; Philadelphia: 2007. pp. 539–548. [Google Scholar]
- 14.Valli H, Phillips BT, Shetty G, Byrne JA, Clark AT, Meistrich ML, Orwig KE. Germline stem cells: Toward the regeneration of spermatogenesis. Fertility and Sterility. 2014;101:3–13. doi: 10.1016/j.fertnstert.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damani MN, Master V, Meng MV, Burgess C, Turek P, Oates RD. Postchemotherapy ejaculatory azoospermia: Fatherhood with sperm from testis tissue with intracytoplasmic sperm injection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:930–936. doi: 10.1200/JCO.2002.20.4.930. [DOI] [PubMed] [Google Scholar]
- 16.Meseguer M, Garrido N, Remohi J, Pellicer A, Simon C, Martinez-Jabaloyas JM, Gil-Salom M. Testicular sperm extraction (tese) and icsi in patients with permanent azoospermia after chemotherapy. Hum Reprod. 2003;18:1281–1285. doi: 10.1093/humrep/deg260. [DOI] [PubMed] [Google Scholar]
- 17.Zorn B, Virant-Klun I, Stanovnik M, Drobnic S, Meden-Vrtovec H. Intracytoplasmic sperm injection by testicular sperm in patients with aspermia or azoospermia after cancer treatment. Int J Androl. 2006;29:521–527. doi: 10.1111/j.1365-2605.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 18.Hibi H, Ohori T, Yamada Y, Honda N, Hashiba Y, Asada Y. Testicular sperm extraction and icsi in patients with post-chemotherapy non-obstructive azoospermia. Arch Androl. 2007;53:63–65. doi: 10.1080/01485010600915152. [DOI] [PubMed] [Google Scholar]
- 19.Hsiao W, Stahl PJ, Osterberg EC, Nejat E, Palermo GD, Rosenwaks Z, Schlegel PN. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: The weill cornell experience. Journal of Clinical Oncology. 2011;29:1607–1611. doi: 10.1200/JCO.2010.33.7808. [DOI] [PubMed] [Google Scholar]
- 20.Picton HM, Wyns C, Anderson RA, Goossens E, Jahnukainen K, Kliesch S, Mitchell RT, Pennings G, Rives N, Tournaye H, van Pelt AM, Eichenlaub-Ritter U, Schlatt S. A european perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boysdagger. Hum Reprod. 2015;30:2463–2475. doi: 10.1093/humrep/dev190. [DOI] [PubMed] [Google Scholar]
- 21.Paniagua R, Nistal M. Morphological and histometric study of human spermatogonia from birth to the onset of puberty. Journal of anatomy. 1984;139(Pt 3):535–552. [PMC free article] [PubMed] [Google Scholar]
- 22.Keros V, Hultenby K, Borgstrom B, Fridstrom M, Jahnukainen K, Hovatta O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum Reprod. 2007;22:1384–1395. doi: 10.1093/humrep/del508. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg JP, Carlson CA, Lin K, Hobbie WL, Wigo E, Wu X, Brinster RL, Kolon TF. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: A report of acceptability and safety. Hum Reprod. 2010;25:37–41. doi: 10.1093/humrep/dep371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA : the journal of the American Medical Association. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- 25.Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, Donnez J. Management of fertility preservation in prepubertal patients: 5 years' experience at the catholic university of louvain. Hum Reprod. 2011;26:737–747. doi: 10.1093/humrep/deq387. [DOI] [PubMed] [Google Scholar]
- 26.Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Human Reproduction. 2013;28:897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 27.Fertility preservation program of magee-womens hospital in pittsburgh. http://www.mwrif.org/220. at http://www.mwrif.org/220.
- 28.Lee JY, Dada R, Sabanegh E, Carpi A, Agarwal A. Role of genetics in azoospermia. Urology. 2011;77:598–601. doi: 10.1016/j.urology.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Weedin JW, Bennett RC, Fenig DM, Lamb DJ, Lipshultz LI. Early versus late maturation arrest: Reproductive outcomes of testicular failure. The Journal of Urology. 2011;186:621–626. doi: 10.1016/j.juro.2011.03.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudeloglu A, Parekattil SJ. Update in the evaluation of the azoospermic male. Clinics. 2013;68:27–34. doi: 10.6061/clinics/2013(Sup01)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esteves SC. Clinical management of infertile men with nonobstructive azoospermia. Asian journal of andrology. 2015;17:459–470. doi: 10.4103/1008-682X.148719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hung AJ, King P, Schlegel PN. Uniform testicular maturation arrest: A unique subset of men with nonobstructive azoospermia. The Journal of Urology. 2007;178:608–612. doi: 10.1016/j.juro.2007.03.125. [DOI] [PubMed] [Google Scholar]
- 33.Tsai M-C, Cheng Y-S, Lin T-Y, Yang W-H, Lin Y-M. Clinical characteristics and reproductive outcomes in infertile men with testicular early and late maturation arrest. Urology. 2012;80:826–832. doi: 10.1016/j.urology.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan RI, Rajpert-De Meyts E, Hoei-Hansen CE, de Kretser DM, Skakkebaek NE. Histological evaluation of the human testis—approaches to optimizing the clinical value of the assessment: Mini review. Human Reproduction. 2007;22:2–16. doi: 10.1093/humrep/del279. [DOI] [PubMed] [Google Scholar]
- 35.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–11302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Transplantation of male germ line stem cells restores fertility in infertile mice. Nature medicine. 2000;6:29–34. doi: 10.1038/71496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagano M, Brinster CJ, Orwig KE, Ryu BY, Avarbock MR, Brinster RL. Transgenic mice produced by retroviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2001;98:13090–13095. doi: 10.1073/pnas.231473498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proceedings Of The National Academy Of Sciences Of The United States Of America. 2001;98:6186–6191. doi: 10.1073/pnas.111158198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamra FK, Gatlin J, Chapman KM, Grellhesl DM, Garcia JV, Hammer RE, Garbers DL. Production of transgenic rats by lentiviral transduction of male germ-line stem cells. Proc Natl Acad Sci U S A. 2002;99:14931–14936. doi: 10.1073/pnas.222561399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinster CJ, Ryu BY, Avarbock MR, Karagenc L, Brinster RL, Orwig KE. Restoration of fertility by germ cell transplantation requires effective recipient preparation. Biol Reprod. 2003;69:412–420. doi: 10.1095/biolreprod.103.016519. [DOI] [PubMed] [Google Scholar]
- 42.Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69:1260–1264. doi: 10.1095/biolreprod.103.018788. [DOI] [PubMed] [Google Scholar]
- 43.Izadyar F, Den Ouden K, Stout TA, Stout J, Coret J, Lankveld DP, Spoormakers TJ, Colenbrander B, Oldenbroek JK, Van der Ploeg KD, Woelders H, Kal HB, De Rooij DG. Autologous and homologous transplantation of bovine spermatogonial stem cells. Reproduction. 2003;126:765–774. [PubMed] [Google Scholar]
- 44.Mikkola M, Sironen A, Kopp C, Taponen J, Sukura A, Vilkki J, Katila T, Andersson M. Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reproduction in domestic animals = Zuchthygiene. 2006;41:124–128. doi: 10.1111/j.1439-0531.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim Y, Turner D, Nelson J, Dobrinski I, McEntee M, Travis AJ. Production of donor-derived sperm after spermatogonial stem cell transplantation in the dog. Reproduction. 2008;136:823–831. doi: 10.1530/REP-08-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81:898–905. doi: 10.1095/biolreprod.109.078279. [DOI] [PubMed] [Google Scholar]
- 47.Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. doi: 10.1093/humrep/der160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, Peterson K, Masterson K, Ramsey C, Ward T, Lienesch M, Volk A, Cooper DK, Thomson AW, Kiss JE, Penedo MC, Schatten GP, Mitalipov S, Orwig KE. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ryu BY, Orwig KE, Avarbock MR, Brinster RL. Stem cell and niche development in the postnatal rat testis. Dev Biol. 2003;263:253–263. doi: 10.1016/j.ydbio.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 50.Dobrinski I, Avarbock MR, Brinster RL. Transplantation of germ cells from rabbits and dogs into mouse testes. Biol Reprod. 1999;61:1331–1339. doi: 10.1095/biolreprod61.5.1331. [DOI] [PubMed] [Google Scholar]
- 51.Dobrinski I, Avarbock MR, Brinster RL. Germ cell transplantation from large domestic animals into mouse testes. Mol Reprod Dev. 2000;57:270–279. doi: 10.1002/1098-2795(200011)57:3<270::AID-MRD9>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 52.Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, Schatten GP, Orwig KE. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dovey SL, Valli H, Hermann BP, Sukhwani M, Donohue J, Castro CA, Chu T, Sanfilippo JS, Orwig KE. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. The Journal of clinical investigation. 2013;123:1833–1843. doi: 10.1172/JCI65822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102:566–580. doi: 10.1016/j.fertnstert.2014.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu X, Goodyear SM, Abramowitz LK, Bartolomei MS, Tobias JW, Avarbock MR, Brinster RL. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Human Reproduction. 2012;27:1249–1259. doi: 10.1093/humrep/des077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radford JA, Shalet SM, Lieberman BA. Fertility after treatment for cancer. BMJ. 1999;319:935–936. doi: 10.1136/bmj.319.7215.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril. 2001;75:269–274. doi: 10.1016/s0015-0282(00)01721-0. [DOI] [PubMed] [Google Scholar]
- 58.Radford J. Restoration of fertility after treatment for cancer. Hormone research. 2003;59(Suppl 1):21–23. doi: 10.1159/000067840. [DOI] [PubMed] [Google Scholar]
- 59.Valli H, Gassei K, Orwig KE. Stem cell therapies for male infertility: Where are we now and where are we going? In: Carrell DT, Schlegel PN, Racowsky C, Gianaroli L, editors. Biennial review of infertility. Springer International Publishing; Switzerland: 2015. pp. 17–39. [Google Scholar]
- 60.Kvist K, Thorup J, Byskov AG, Hoyer PE, Mollgard K, Yding Andersen C. Cryopreservation of intact testicular tissue from boys with cryptorchidism. Hum Reprod. 2006;21:484–491. doi: 10.1093/humrep/dei331. [DOI] [PubMed] [Google Scholar]
- 61.Wyns C, Curaba M, Martinez-Madrid B, Van Langendonckt A, Francois-Xavier W, Donnez J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Human Reproduction. 2007;22:1603–1611. doi: 10.1093/humrep/dem062. [DOI] [PubMed] [Google Scholar]
- 62.Yango P, Altman E, Smith JF, Klatsky PC, Tran ND. Optimizing cryopreservation of human spermatogonial stem cells: Comparing the effectiveness of testicular tissue and single cell suspension cryopreservation. Fertil Steril. 2014;102:1491–1498. e1491. doi: 10.1016/j.fertnstert.2014.07.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wyns C, Collienne C, Shenfield F, Robert A, Laurent P, Roegiers L, Brichard B. Fertility preservation in the male pediatric population: Factors influencing the decision of parents and children. Hum Reprod. 2015 doi: 10.1093/humrep/dev161. [DOI] [PubMed] [Google Scholar]
- 64.Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: Comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Human Reproduction. 2005;20:1676–1687. doi: 10.1093/humrep/deh797. [DOI] [PubMed] [Google Scholar]
- 65.Unni S, Kasiviswanathan S, D'Souza S, Khavale S, Mukherjee S, Patwardhan S, Bhartiya D. Efficient cryopreservation of testicular tissue: Effect of age, sample state, and concentration of cryoprotectant. Fertil Steril. 2012;97:200–208. e201. doi: 10.1016/j.fertnstert.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 66.Curaba M, Poels J, van Langendonckt A, Donnez J, Wyns C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertility and Sterility. 2011;95:2123.e2129–2123.e2112. doi: 10.1016/j.fertnstert.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Sa R, Cremades N, Malheiro I, Sousa M. Cryopreservation of human testicular diploid germ cell suspensions. Andrologia. 2012;44:366–372. doi: 10.1111/j.1439-0272.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 68.Baert Y, Van Saen D, Haentjens P, In't Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Human Reproduction. 2013 doi: 10.1093/humrep/det100. [DOI] [PubMed] [Google Scholar]
- 69.Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28:578–589. doi: 10.1093/humrep/des455. [DOI] [PubMed] [Google Scholar]
- 70.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- 71.Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. Proc Natl Acad Sci U S A. 2005;102:14302–14307. doi: 10.1073/pnas.0506970102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langenstroth D, Kossack N, Westernströer B, Wistuba J, Behr R, Gromoll J, Schlatt S. Separation of somatic and germ cells is required to establish primate spermatogonial cultures. Human Reproduction. 2014;29:2018–2031. doi: 10.1093/humrep/deu157. [DOI] [PubMed] [Google Scholar]
- 75.Chen B, Wang YB, Zhang ZL, Xia WL, Wang HX, Xiang ZQ, Hu K, Han YF, Wang YX, Huang YR, Wang Z. Xeno-free culture of human spermatogonial stem cells supported by human embryonic stem cell-derived fibroblast-like cells. Asian journal of andrology. 2009;11:557–565. doi: 10.1038/aja.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadri-Ardekani H, Mizrak SC, van Daalen SK, Korver CM, Roepers-Gajadien HL, Koruji M, Hovingh S, de Reijke TM, de la Rosette JJ, van der Veen F, de Rooij DG, Repping S, van Pelt AM. Propagation of human spermatogonial stem cells in vitro. JAMA : the journal of the American Medical Association. 2009;302:2127–2134. doi: 10.1001/jama.2009.1689. [DOI] [PubMed] [Google Scholar]
- 77.Wu X, Schmidt JA, Avarbock MR, Tobias JW, Carlson CA, Kolon TF, Ginsberg JP, Brinster RL. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc Natl Acad Sci U S A. 2009;106:21672–21677. doi: 10.1073/pnas.0912432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82:363–372. doi: 10.1095/biolreprod.109.078550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term culture of human ssea-4 positive spermatogonial stem cells (sscs) Journal of stem cell research and therapy. 2011;S2:003. doi: 10.4172/2157-7633.S2-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu S, Tang Z, Xiong T, Tang W. Isolation and characterization of human spermatogonial stem cells. Reproductive biology and endocrinology : RB&E. 2011;9:141. doi: 10.1186/1477-7827-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nowroozi MR, Ahmadi H, Rafiian S, Mirzapour T, Movahedin M. In vitro colonization of human spermatogonia stem cells: Effect of patient's clinical characteristics and testicular histologic findings. Urology. 2011;78:1075–1081. doi: 10.1016/j.urology.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44:41–55. doi: 10.1111/j.1439-0272.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 83.Smith JF, Yango P, Altman E, Choudhry S, Poelzl A, Zamah AM, Rosen M, Klatsky PC, Tran ND. Testicular niche required for human spermatogonial stem cell expansion. Stem cells translational medicine. 2014;3:1043–1054. doi: 10.5966/sctm.2014-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dufour JM, Rajotte RV, Korbutt GS. Development of an in vivo model to study testicular morphogenesis. J Androl. 2002;23:635–644. [PubMed] [Google Scholar]
- 85.Gassei K, Schlatt S, Ehmcke J. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl. 2006;27:611–618. doi: 10.2164/jandrol.05207. [DOI] [PubMed] [Google Scholar]
- 86.Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: Formation of functional testis tissue after transplantation of isolated porcine (sus scrofa) testis cells. Biol Reprod. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- 87.Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, Nagashima Y, Aoki I, Taniguchi H, Noce T, Inoue K, Miki H, Ogonuki N, Tanaka H, Ogura A, Ogawa T. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biology of Reproduction. 2007;76:211–217. doi: 10.1095/biolreprod.106.056895. [DOI] [PubMed] [Google Scholar]
- 88.Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ER, Dobrinski I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008;136:85–93. doi: 10.1530/REP-07-0433. [DOI] [PubMed] [Google Scholar]
- 89.Baert Y, Stukenborg JB, Landreh M, De Kock J, Jornvall H, Soder O, Goossens E. Derivation and characterization of a cytocompatible scaffold from human testis. Hum Reprod. 2015;30:256–267. doi: 10.1093/humrep/deu330. [DOI] [PubMed] [Google Scholar]
- 90.Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- 91.Schlatt S, Honaramooz A, Boiani M, Scholer HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biology of Reproduction. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- 92.Honaramooz A, Li MW, Penedo MCT, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biology of Reproduction. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- 93.Jamieson SW, Madani MM. The choice of valve protheses*. Journal of the American College of Cardiology. 2004;44:389–390. doi: 10.1016/j.jacc.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 94.Andreas M, Wallner S, Ruetzler K, Wiedemann D, Ehrlich M, Heinze G, Binder T, Moritz A, Hiesmayr MJ, Kocher A, Laufer G. Comparable long-term results for porcine and pericardial prostheses after isolated aortic valve replacement. European Journal of Cardio-Thoracic Surgery. 2014 doi: 10.1093/ejcts/ezu466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cozzi E, White DJ. The generation of transgenic pigs as potential organ donors for humans. Nature medicine. 1995;1:964–966. doi: 10.1038/nm0995-964. [DOI] [PubMed] [Google Scholar]
- 96.Weiss RA. The discovery of endogenous retroviruses. Retrovirology. 2006;3:67. doi: 10.1186/1742-4690-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kimsa MC, Strzalka-Mrozik B, Kimsa MW, Gola J, Nicholson P, Lopata K, Mazurek U. Porcine endogenous retroviruses in xenotransplantation--molecular aspects. Viruses. 2014;6:2062–2083. doi: 10.3390/v6052062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Geens M, De Block G, Goossens E, Frederickx V, Van Steirteghem A, Tournaye H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Human Reproduction. 2006;21:390–396. doi: 10.1093/humrep/dei412. [DOI] [PubMed] [Google Scholar]
- 99.Schlatt S, Honaramooz A, Ehmcke J, Goebell PJ, Rubben H, Dhir R, Dobrinski I, Patrizio P. Limited survival of adult human testicular tissue as ectopic xenograft. Human Reproduction. 2006;21:384–389. doi: 10.1093/humrep/dei352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goossens E, Geens M, De Block G, Tournaye H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil Steril. 2008;90:2019–2022. doi: 10.1016/j.fertnstert.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 101.Wyns C, Van Langendonckt A, Wese FX, Donnez J, Curaba M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Human Reproduction. 2008;23:2402–2414. doi: 10.1093/humrep/den272. [DOI] [PubMed] [Google Scholar]
- 102.Sato Y, Nozawa S, Yoshiike M, Arai M, Sasaki C, Iwamoto T. Xenografting of testicular tissue from an infant human donor results in accelerated testicular maturation. Human Reproduction. 2010;25:1113–1122. doi: 10.1093/humrep/deq001. [DOI] [PubMed] [Google Scholar]
- 103.Van Saen D, Goossens E, Bourgain C, Ferster A, Tournaye H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Human Reproduction. 2011;26:282–293. doi: 10.1093/humrep/deq321. [DOI] [PubMed] [Google Scholar]
- 104.Luetjens CM, Stukenborg J-B, Nieschlag E, Simoni M, Wistuba J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology. 2008;149:1736–1747. doi: 10.1210/en.2007-1325. [DOI] [PubMed] [Google Scholar]
- 105.Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human dazl, daz and boule genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abu Elhija M, Lunenfeld E, Schlatt S, Huleihel M. Differentiation of murine male germ cells to spermatozoa in a soft agar culture system. Asian journal of andrology. 2011;14:285–293. doi: 10.1038/aja.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Easley CAt, Phillips BT, McGuire MM, Barringer JM, Valli H, Hermann BP, Simerly CR, Rajkovic A, Miki T, Orwig KE, Schatten GP. Direct differentiation of human pluripotent stem cells into haploid spermatogenic cells. Cell Rep. 2012;2:440–446. doi: 10.1016/j.celrep.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- 109.Sato T, Katagiri K, Kubota Y, Ogawa T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat Protocols. 2013;8:2098–2104. doi: 10.1038/nprot.2013.138. [DOI] [PubMed] [Google Scholar]
- 110.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 111.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 112.Kee K, Gonsalves JM, Clark AT, Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15:831–837. doi: 10.1089/scd.2006.15.831. [DOI] [PubMed] [Google Scholar]
- 113.Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, Matsumoto K, Saeki K, Iritani A, Sagawa N, Hosoi Y. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells. 2007;9:144–156. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- 114.Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE, Plath K, Clark AT. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yamauchi K, Hasegawa K, Chuma S, Nakatsuji N, Suemori H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS ONE. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146:519–532. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 117.Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, Reijo Pera RA. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dominguez AA, Chiang HR, Sukhwani M, Orwig KE, Reijo Pera RA. Human germ cell formation in xenotransplants of induced pluripotent stem cells carrying x chromosome aneuploidies. Scientific reports. 2014;4:6432. doi: 10.1038/srep06432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Durruthy Durruthy J, Ramathal C, Sukhwani M, Fang F, Cui J, Orwig KE, Reijo Pera RA. Fate of induced pluripotent stem cells following transplantation to murine seminiferous tubules. Hum Mol Genet. 2014;23:3071–3084. doi: 10.1093/hmg/ddu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramathal C, Durruthy-Durruthy J, Sukhwani M, Arakaki JE, Turek PJ, Orwig KE, Reijo Pera RA. Fate of ipscs derived from azoospermic and fertile men following xenotransplantation to murine seminiferous tubules. Cell Rep. 2014;7:1284–1297. doi: 10.1016/j.celrep.2014.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Irie N, Weinberger L, Tang WW, Kobayashi T, Viukov S, Manor YS, Dietmann S, Hanna JH, Surani MA. Sox17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–268. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ramathal C, Angulo B, Sukhwani M, Cui J, Durruthy-Durruthy J, Fang F, Schanes P, Turek PJ, Orwig KE, Reijo Pera R. Ddx3y gene rescue of a y chromosome azfa deletion restores germ cell formation and transcriptional programs. Scientific reports. 2015;5:15041. doi: 10.1038/srep15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sasaki K, Yokobayashi S, Nakamura T, Okamoto I, Yabuta Y, Kurimoto K, Ohta H, Moritoki Y, Iwatani C, Tsuchiya H, Nakamura S, Sekiguchi K, Sakuma T, Yamamoto T, Mori T, Woltjen K, Nakagawa M, Yamamoto T, Takahashi K, Yamanaka S, Saitou M. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17:178–194. doi: 10.1016/j.stem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 124.Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Science. 2012;338:971–975. doi: 10.1126/science.1226889. [DOI] [PubMed] [Google Scholar]
- 125.Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertility and Sterility. 2013;100:1214–1223. doi: 10.1016/j.fertnstert.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 126.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K. American Society of Clinical O. Fertility preservation for patients with cancer: American society of clinical oncology clinical practice guideline update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 128.Hayden EC. Technology: The $1,000 genome. Nature. 2014;507:294–295. doi: 10.1038/507294a. [DOI] [PubMed] [Google Scholar]
- 129.Ikawa M, Tergaonkar V, Ogura A, Ogonuki N, Inoue K, Verma IM. Restoration of spermatogenesis by lentiviral gene transfer: Offspring from infertile mice. Proc Natl Acad Sci U S A. 2002;99:7524–7529. doi: 10.1073/pnas.072207299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kanatsu-Shinohara M, Ogura A, Ikegawa M, Inoue K, Ogonuki N, Tashiro K, Toyokuni S, Honjo T, Shinohara T. Adenovirus-mediated gene delivery and in vitro microinsemination produce offspring from infertile male mice. Proc Natl Acad Sci U S A. 2002;99:1383–1388. doi: 10.1073/pnas.022646399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Orwig KE, Avarbock MR, Brinster RL. Retrovirus-mediated modification of male germline stem cells in rats. Biol Reprod. 2002;67:874–879. doi: 10.1095/biolreprod.102.005538. [DOI] [PubMed] [Google Scholar]
- 132.Yomogida K, Yagura Y, Nishimune Y. Electroporated transgene-rescued spermatogenesis in infertile mutant mice with a sertoli cell defect. Biol Reprod. 2002;67:712–717. doi: 10.1095/biolreprod.101.001743. [DOI] [PubMed] [Google Scholar]
- 133.Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. Journal of Andrology. 2007;28:353–360. doi: 10.2164/jandrol.106.001511. [DOI] [PubMed] [Google Scholar]
- 134.Izsvak Z, Frohlich J, Grabundzija I, Shirley JR, Powell HM, Chapman KM, Ivics Z, Hamra FK. Generating knockout rats by transposon mutagenesis in spermatogonial stem cells. Nat Meth. 2010;7:443–445. doi: 10.1038/nmeth.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sheng Y, Lin CC, Yue J, Sukhwani M, Shuttleworth J, Chu T, Orwig K. Generation and characterization of a tet-on (rtta-m2) transgenic rat. BMC Developmental Biology. 2010;10:17. doi: 10.1186/1471-213X-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ivics Z, Izsvák Z, Chapman KM, Hamra FK. Sleeping beauty transposon mutagenesis of the rat genome in spermatogonial stem cells. Methods. 2011;53:356–365. doi: 10.1016/j.ymeth.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chapman Karen M, Medrano Gerardo A, Jaichander P, Chaudhary J, Waits Alexandra E, Nobrega Marcelo A, Hotaling James M, Ober C, Hamra FK. Targeted germline modifications in rats using crispr/cas9 and spermatogonial stem cells. Cell Rep. 2015;10:1828–1835. doi: 10.1016/j.celrep.2015.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sato T, Sakuma T, Yokonishi T, Katagiri K, Kamimura S, Ogonuki N, Ogura A, Yamamoto T, Ogawa T. Genome editing in mouse spermatogonial stem cell lines using talen and double-nicking crispr/cas9. Stem Cell Reports. 2015;5:75–82. doi: 10.1016/j.stemcr.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, Corn JE, Daley GQ, Doudna JA, Fenner M, Greely HT, Jinek M, Martin GS, Penhoet E, Puck J, Sternberg SH, Weissman JS, Yamamoto KR. A prudent path forward for genomic engineering and germline gene modification. Science. 2015;348:36–38. doi: 10.1126/science.aab1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ishii T. Germline genome-editing research and its socioethical implications. Trends in molecular medicine. 2015;21:473–481. doi: 10.1016/j.molmed.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 141.Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J. Don't edit the human germ line. Nature. 2015;519:410–411. doi: 10.1038/519410a. [DOI] [PubMed] [Google Scholar]
- 142.Miller HI. Germline gene therapy: We're ready. Science. 2015;348:1325. doi: 10.1126/science.348.6241.1325-a. [DOI] [PubMed] [Google Scholar]
- 143.Pollack R. Eugenics lurk in the shadow of crispr. Science. 2015;348:871. doi: 10.1126/science.348.6237.871-a. [DOI] [PubMed] [Google Scholar]
- 144.Porteus MH, Dann CT. Genome editing of the germline: Broadening the discussion. Mol Ther. 2015;23:980–982. doi: 10.1038/mt.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spritz RA. Molecular basis of human piebaldism. The Journal of investigative dermatology. 1994;103:137S–140S. doi: 10.1111/1523-1747.ep12399455. [DOI] [PubMed] [Google Scholar]
- 146.Choi Y, Jeon S, Choi M, Lee M-h, Park M, Lee DR, Jun K-Y, Kwon Y, Lee O-H, Song S-H, Kim J-Y, Lee K-A, Yoon TK, Rajkovic A, Shim SH. Mutations in sohlh1 gene associate with nonobstructive azoospermia. Human Mutation. 2010;31:788–793. doi: 10.1002/humu.21264. [DOI] [PubMed] [Google Scholar]
- 147.Song B, Zhang Y, He X-j, Du W-d, Ruan J, Zhou F-s, Wu H, Zha X, Xie X-s, Ye L, Wei Z-l, Zhou P, Cao Y-x. Association of genetic variants in sohlh1 and sohlh2 with non-obstructive azoospermia risk in the chinese population. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2015;184:48–52. doi: 10.1016/j.ejogrb.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 148.Yang F, Silber S, Leu NA, Oates RD, Marszalek JD, Skaletsky H, Brown LG, Rozen S, Page DC, Wang PJ. Tex11 is mutated in infertile men with azoospermia and regulates genome-wide recombination rates in mouse. EMBO Molecular Medicine. 2015;7:1198–1210. doi: 10.15252/emmm.201404967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yatsenko AN, Georgiadis AP, Röpke A, Berman AJ, Jaffe T, Olszewska M, Westernströer B, Sanfilippo J, Kurpisz M, Rajkovic A, Yatsenko SA, Kliesch S, Schlatt S, Tüttelmann F. X-linked tex11 mutations, meiotic arrest, and azoospermia in infertile men. New Engl J Med. 2015;372:2097–2107. doi: 10.1056/NEJMoa1406192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Goglia U, Vinanzi C, Zuccarello D, Malpassi D, Ameri P, Casu M, Minuto F, Foresta C, Ferone D. Identification of a novel mutation in exon 1 of androgen receptor gene in an azoospermic patient with mild androgen insensitivity syndrome: Case report and literature review. Fertility and Sterility. 2011;96:1165–1169. doi: 10.1016/j.fertnstert.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 151.Mirfakhraie R, Kalantar S-M, Mirzajani F, Montazeri M, Salsabili N, Houshmand M, Hashemi-Gorji F, Pourmand G. A novel mutation in the transactivation-regulating domain of the androgen receptor in a patient with azoospermia. Journal of Andrology. 2011;32:367–370. doi: 10.2164/jandrol.110.010645. [DOI] [PubMed] [Google Scholar]
- 152.Massin N, Bry H, Vija L, Maione L, Constancis E, Haddad B, Morel Y, Claessens F, Young J. Healthy birth after testicular extraction of sperm and icsi from an azoospermic man with mild androgen insensitivity syndrome caused by an androgen receptor partial loss-of-function mutation. Clinical endocrinology. 2012;77:593–598. doi: 10.1111/j.1365-2265.2012.04402.x. [DOI] [PubMed] [Google Scholar]
- 153.Chen Y-H, Xu H-Y, Wang Z-Y, Zhu Z-H, Li C-D, Wu Z-G, Chen B-C. An insertion mutation in the androgen receptor gene in a patient with azoospermia. Asian journal of andrology. 2015;17:857–858. doi: 10.4103/1008-682X.148724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bashamboo A, Ferraz-de-Souza B, Lourenço D, Lin L, Sebire NJ, Montjean D, Bignon-Topalovic J, Mandelbaum J, Siffroi J-P, Christin-Maitre S, Radhakrishna U, Rouba H, Ravel C, Seeler J. Achermann JC, McElreavey K. Human male infertility associated with mutations in nr5a1 encoding steroidogenic factor 1. The American Journal of Human Genetics. 2010;87:505–512. doi: 10.1016/j.ajhg.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ropke A, Tewes A-C, Gromoll J, Kliesch S, Wieacker P, Tuttelmann F. Comprehensive sequence analysis of the nr5a1 gene encoding steroidogenic factor 1 in a large group of infertile males. Eur J Hum Genet. 2013;21:1012–1015. doi: 10.1038/ejhg.2012.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, Sorensen R, Forster A, Fraser P, Cohen JI, de Saint Basile G, Alexander I, Wintergerst U, Frebourg T, Aurias A, Stoppa-Lyonnet D, Romana S, Radford-Weiss I, Gross F, Valensi F, Delabesse E, Macintyre E, Sigaux F, Soulier J, Leiva LE, Wissler M, Prinz C, Rabbitts TH, Le Deist F, Fischer A, Cavazzana-Calvo M. Lmo2-associated clonal t cell proliferation in two patients after gene therapy for scid x1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 157.Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of hsc gene therapy. The Journal of clinical investigation. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Stephen SL, Montini E, Sivanandam VG, Al-Dhalimy M, Kestler HA, Finegold M, Grompe M, Kochanek S. Chromosomal integration of adenoviral vector DNA in vivo. J Virol. 2010;84:9987–9994. doi: 10.1128/JVI.00751-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu P, Li Y, Sands MS, McCown T, Kafri T. Generation of a stable packaging cell line producing high-titer ppt-deleted integration-deficient lentiviral vectors. Molecular therapy Methods & clinical development. 2015;2:15025. doi: 10.1038/mtm.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wilson JM. A history lesson for stem cells. Science. 2009;324:727–728. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]
- 161.Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Molecular Genetics and Metabolism. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 162.Chandra A, Copen CE, Stephen EH. National health statistics reports. 2013. Infertility and impaired fecundity in the united states, 1982–2010: Data from the national survey of family growth; pp. 1–18. [PubMed] [Google Scholar]
- 163.Louis JF, Thoma ME, Sorensen DN, McLain AC, King RB, Sundaram R, Keiding N, Buck Louis GM. The prevalence of couple infertility in the united states from a male perspective: Evidence from a nationally representative sample. Andrology. 2013;1:741–748. doi: 10.1111/j.2047-2927.2013.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Slade P, O'Neill C, Simpson AJ, Lashen H. The relationship between perceived stigma, disclosure patterns, support and distress in new attendees at an infertility clinic. Hum Reprod. 2007;22:2309–2317. doi: 10.1093/humrep/dem115. [DOI] [PubMed] [Google Scholar]
- 165.Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: A cohort study of 43,277 men. Am J Epidemiol. 2009;170:559–565. doi: 10.1093/aje/kwp168. [DOI] [PubMed] [Google Scholar]
- 166.Bak CW, Seok HH, Song SH, Kim ES, Her YS, Yoon TK. Hormonal imbalances and psychological scars left behind in infertile men. J Androl. 2012;33:181–189. doi: 10.2164/jandrol.110.012351. [DOI] [PubMed] [Google Scholar]
- 167.Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: The hidden hardship of building a family. Fertil Steril. 2013;99:2025–2030. doi: 10.1016/j.fertnstert.2013.01.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hermann BP, Sukhwani M, Hansel MC, Orwig KE. Spermatogonial stem cells in higher primates: Are there differences from those in rodents? Reproduction. 2010;139:479–493. doi: 10.1530/REP-09-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]