Abstract
Background and Purpose: The appearance of the renal papillae in patients with nephrolithiasis can be quite variable and can range from entirely healthy to markedly diseased. The implications of such findings remain unknown. One potential reason is the lack of a standardized system to describe such features. We propose a novel grading scale to describe papillary appearance at the time of renal endoscopy.
Methods: Comprehensive endoscopic renal assessment and mapping were performed on more than 300 patients with nephrolithiasis. Recurring abnormal papillary characteristics were identified and quantified based on degree of severity.
Results: Four unique papillary features were chosen for inclusion in the PPLA scoring system— ductal Plugging, Pitting, Loss of contour, and Amount of Randall's plaque. Unique scores are calculated for individual papillae based on reference examples.
Conclusions: The description and study of renal papillary appearance in stone formers have considerable potential as both a clinical and research tool; however, a standardized grading system is necessary before using it for these purposes.
Introduction
Technologic advancements in the field of endourology have revolutionized the ability to treat nephrolithiasis. Whereas surgeons once had to remove stones from the ureter using a combination of blind basketing and fluoroscopy, it is now possible not only to visualize the entirety of the urinary tract but also to do so in high definition. Much of the clinical interest in modern scope technology has focused on enhanced optics for stone visualization and removal. The significance of improved visualization of the kidney and collecting system themselves, however, is often overlooked. Through correlation with stone analysis and papillary biopsy, our group has previously demonstrated that it is possible to distinguish specific stone forming phenotypes by endoscopic patterns of papillary appearance alone.1–4
Coincident with this finding is a separate important concept that certain types of stone forming pathologies appear to correspond to a greater degree of renal papillary injury. For example, patients with a history of brushite stones, renal tubular acidosis, and primary hyperparathyroidism all have diffuse evidence of abnormal papillary morphology that corresponds to tissue damage seen on biopsy specimens.1,5–7 Furthermore, this papillary injury is potentially measurable and distinct from one stone forming pathology to the next as demonstrated by the fact that patients with primarily calcium phosphate stones have greater degrees of visible papillary injury (flattening and erosion) as well as ductal plugs compared with idiopathic calcium stone formers.8
Despite these findings, there is a paucity of medical literature correlating the degree of visible renal injury to clinical outcomes. One possible reason that this concept has not matured is the inherent complexity in describing the multitude of possible variations in papillary appearance in a simplified yet meaningful fashion.
We believe that a standardized approach toward describing papillary appearance at the time of endourologic assessment potentially has profound implications in the management of stone disease. This is especially true considering that flexible ureteroscopy is one of the most commonly performed urologic surgical procedures and is growing at a rapid rate.9 As such, the potential diffusion of such a system is considerable and has tremendous potential for use as a clinical and research tool. We describe a novel grading system designed to standardize and simplify the description of renal papillary appearance in stone formers at the time of endoscopy.
Methods
Since 1999, 342 patients have been prospectively enrolled and given consent to be part of an NIH funded project (PO1 DK56788) studying the pathogenesis of stone formation at a single institution (Methodist Hospital, Indiana University Health). A standardized protocol for digital video mapping of the intrarenal collecting system has been described previously.10 All procedures, whether percutaneous or ureteroscopic, were initially performed using fiberoptic scopes. Over the past several years, there has been a shift to digital endoscopy because such equipment has become more readily available and offers superior optics.
We have used these observations to describe several mechanisms of human kidney stone formation11 and have characterized the phenotypes as they apply to stone formation in nine different disease states as well as those with no history of stones.12 The two predominant mechanisms of stone formation (growth on Randall's plaque and Bellini duct plugging) manifest themselves with uniquely different characteristics. Randall's plaque, evident as white suburothelial deposits, does not cause cellular injury.13 Bellini duct plugging on the other hand, evident as focal yellow mineral deposits and/or dilated ducts where the mineral deposits once existed, does cause progressive damage to the nephron.14 This progressive injury can eventually lead to other gross changes in the papilla including pitting (a crater-like erosion of the papillary tip) or loss of contour (a diffuse flattening of the papilla in its entirety).6
A grading system was subsequently devised based on the collective knowledge and experience of the primary research team to better characterize the presence and degree of abnormal papillary appearance in patients with nephrolithiasis (Table 1). It should be noted that persons with medullary sponge kidney are a unique population of stone formers and while they, too, are likely to demonstrate many of the abnormal features described by this scale, they represent a unique pathophysiology very unlike other common stone-forming conditions and should be considered separately.15
Table 1.
Scale for Abnormal Papillary Appearance
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Plugging | 0 yellow plaque deposits/dilated ducts | ≤5 yellow plaque deposits/dilated ducts | >5 yellow plaque deposits/dilated ducts |
| Pitting | None | ≤25% papillary surface involved | ≥25% papillary surface involved |
| Loss of contour | None | Depressed | Completely flattened |
| Grade | a | b | c |
| Amount of Randall's plaque | Mild | Moderate | Severe |
| Final PPLA score | Sum (letter) | ||
PPA = plugging, pitting, loss of contour, amount of Randall's plaque.
The grading system is applied uniquely to each papilla so that each one receives its own score. Scoring should not be performed until all stones overlying or attached to the papillary surface are removed. Each papilla is given a score in four separate domains: Plugging, pitting, loss of contour, and amount of Randall's plaque (PPLA). Plugging, pitting, and loss of contour are graded as 0, 1, or 2. Amount of Randall's plaque is graded as a, b, or c. A final score is calculated by adding the plugging, pitting, and loss of contour scores together and designating the grade of Randall's plaque separately.
For example, a perfectly healthy papilla would receive the lowest possible score of 0(a). The most diseased papilla would receive the highest possible score of 6(c). After identifying and assigning scores to all papillae within the renal unit, a mean PPLA score can be calculated by dividing the sum of the papillae scores by the number of papillae examined. In addition, the lowest and highest papillary scores are each identified to denote the range of pathology encountered.
The grading system is intended for use with a digital instrument because such scopes offer optimal visualization, although fiberoptic scopes do have the potential of capturing sufficient detail regarding the appearance of the papilla under ideal circumstances. Furthermore, it is intended for use only when the papilla can be fully visualized and should not be applied unless a comprehensive view of the papilla can be obtained. Finally, in instances of compound papillae, the entire papillary unit is considered a single papilla unless there is a discrete separation and minimal tissue bridging between the two structures.
Results
Randall's plaque
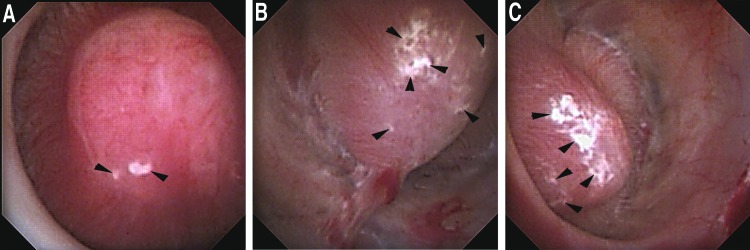
This plaque is characteristically white and can be visualized as irregular lesions most common near the tip of the papilla but with potential to appear anywhere on its surface. While frequently encountered in patients with nephrolithiasis, it has not been shown to cause injury at the level of the nephron13 and as such is designated with an alphabetic rather than numeric score. (Fig. 1 A–C)
FIG. 1.
(A) Healthy papilla with mild amount of Randall's plaque (black arrowheads) (Plugging = 0, Pitting = 0, Loss of Contour = 0, Randall's plaque = a [PPLA 0a]). (B) Healthy papilla with moderate amount of Randall's plaque (black arrowheads) (Plugging = 0, Pitting = 0, Loss of Contour = 0, Randall's plaque = b [PPLA 0b]). (C) Healthy papilla with extensive amount of Randall's plaque (black arrowheads) (Plugging = 0, Pitting = 0, Loss of Contour = 0, Randall's plaque = c [PPLA 0c]).
Plugging
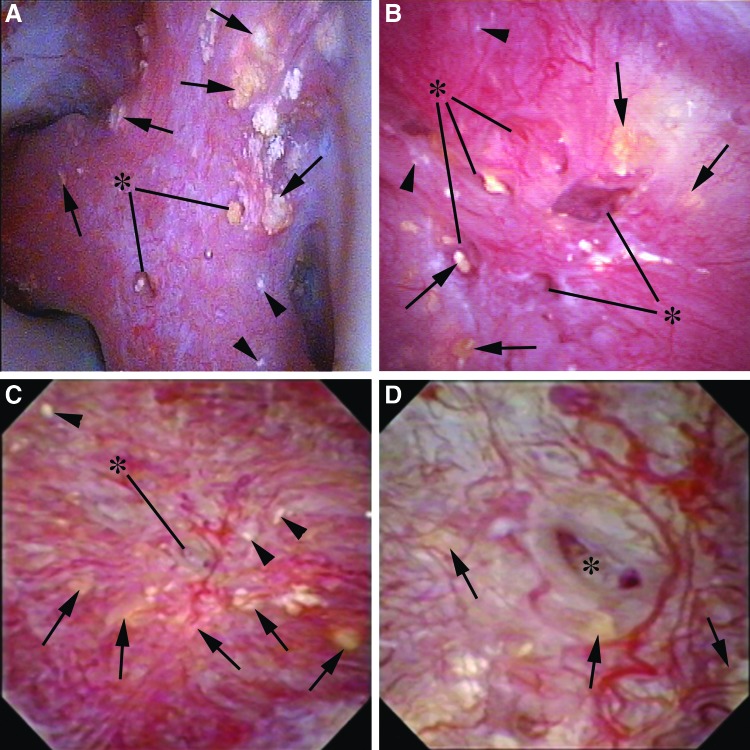
Bellini duct plugging can manifest in one of two ways. One is as yellow intraductal mineral deposits visualized just under the urothelial surface or protruding from the mouth of a dilated duct itself. The other manifestation is as an empty dilated duct where a plug once existed. A normal Bellini duct is only 300 to 600 micrometers on average, but a dilated, diseased duct can distend to many times that diameter (Fig. 2 A–D).
FIG. 2.
(A) This compound papilla demonstrates numerous deposits of yellow plaque, cumulatively more than 5 (black arrows). Dilated ducts with protruding yellow mineral deposits are designated with the asterisk. There is no evidence of pitting or loss of contour. A minute amount of Randall's plaque can be visualized by the arrowheads in the lower right corner (Plugging = 2, Pitting = 0, Loss of Contour = 0, Randall's plaque = a [PPLA 2a]). (B) This papilla also demonstrates plugging as evidenced by both yellow plaque (black arrows) and dilated ducts, some of which contain protruding yellow mineral deposits (asterisk). There is no evidence of pitting and an intermediate loss of contour. A minimal amount of Randall's plaque can be seen as designated by the black arrowheads (Plugging = 2, Pitting = 0, Loss of Contour = 1, Randall's plaque = a (PPLA 3a). (C) This papilla has many yellow plaque deposits (black arrows). One large dilated duct can be seen in the center of the papilla (asterisk) that is magnified in Figure 2D. There is no pitting, intermediate loss of contour, and minimal Randall's plaque (Plugging = 2, Pitting = 0, Loss of Contour = 1, Randall's plaque = a [PPLA 3a]). (D) This image is the magnification of the central region of Figure 2C. Yellow plaque deposits are again designated with black arrows. The prominent dilated duct of Bellini is designated by the asterisk within it.
Pitting
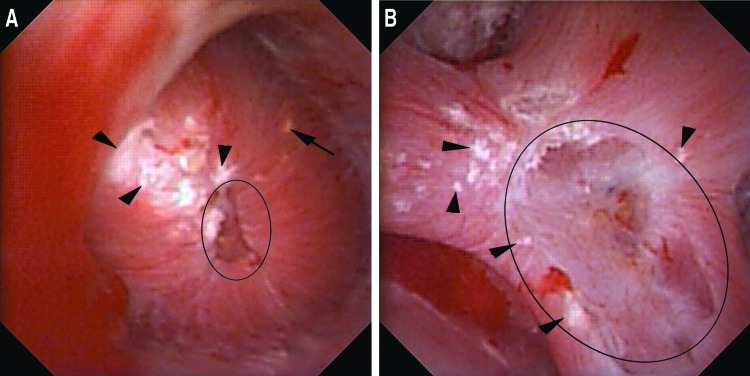
Pitting describes crater-like, focal erosion in the surface of the papilla. These changes are most frequently seen at the papillary tip and are likely a manifestation of progressive renal injury and damaged nephrons (Fig. 3 A,B). We suspect that pitting may arise either by one of two mechanisms. One is via progressive ductal plugging that leads to focal tubular atrophy and nephron loss.6,8 The other is by mechanical disruption and spontaneous passage of an attached stone whereby the stone pulls off part of the urothelium and underlying tubules. This has been demonstrated to occur naturally based on the finding of renal tubules adherent to spontaneously passed stones when imaged using scanning electron microscopy.16
FIG. 3.
(A) This papilla has pitting focused at the periphery of the papillary tip (black circle). Less than 25% of the papillary surface is affected. A single yellow plaque deposit is marked by a black arrow. The papilla has no loss of contour and a severe amount of Randall's plaque (black arrowheads) (Plugging = 1, Pitting = 1, Loss of Contour = 0, Randall's plaque = c [PPLA 2c]). (B) This compound papilla demonstrates pitting with more than 25% of the papillary surface affected (black circle). There is no evidence of plugging or loss of contour. Moderate Randall's plaque is seen (Plugging = 0, Pitting = 2, Loss of Contour = 0, Randall's plaque = b [PPLA 2b]).
Loss of contour
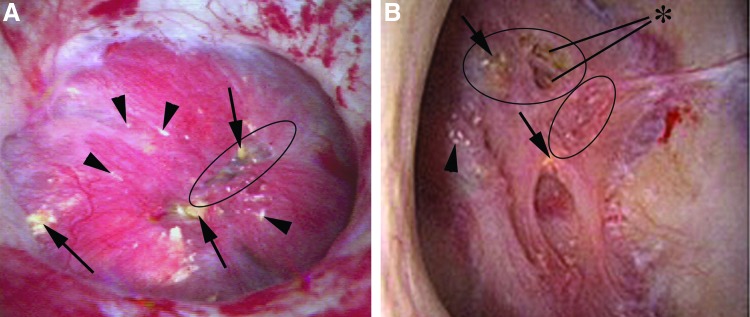
Loss of papillary contour is an advanced stage of papillary injury believed to be indicative of global loss of papillary volume (Fig. 4 A,B). A normal papilla should have the shape of a mountain peak or tall hill (grade 0) (Fig. 1). As loss of contour occurs, the peak becomes flattened into a more plateau like architecture (grade 1) (Fig. 4A) and ultimately demonstrates complete flattening relative to the surrounding tissue similar to a plain (grade 2) (Fig. 4B). Another potential etiology for loss of contour is obstruction, especially in instances of chronic hydronephrosis. As such, we do not recommend applying the scoring system to these patients at this time.
FIG. 4.
(A) This papilla has a number of abnormal findings including numerous deposits of yellow plaque (black arrows), <25% pitting (black circle), a moderate loss of contour, and several small foci of Randall's plaque (black arrowheads) (Plugging = 2, Pitting = 1, Loss of Contour = 1, Randall's plaque = a [PPLA 4a]). (B) This papilla has several foci of yellow plaque (black arrows) and dilated ducts (asterisk), moderate pitting (black circle), and complete loss of contour. There is minimal Randall's plaque (arrowhead) (Plugging = 2, Pitting = 1, Loss of Contour = 2, Randall's plaque = a [PPLA 5a]).
Discussion
Review of endoscopic videos has made a tremendous contribution to our understanding of papillary disease and injury. There is currently no system to our knowledge that characterizes papillary disease in a measurable and reproducible manner, however. We have created a papillary grading system that quantifies the degree of visible papillary injury based on recurring abnormal features commonly encountered in patients with stone disease. These features are particularly important to identify and characterize given their known association with cellular injury, fibrosis, and damage to the papilla and kidney.11,13
We have also chosen to include a subscore within our grading scale delineating the relative amount of surface Randall's plaque. Randall's plaque is the most proven pathway for stone formation, serving as an anchor for calcium oxalate stone overgrowth in idiopathic calcium stone formers.13,17,18 To date, it has not been shown to be a direct cause of renal injury; however, this does not mean that it should be considered a normal or inert finding.
Randall's plaque has been demonstrated to be far more common in patients with a history of stone disease compared with those without.19 Furthermore, the percent coverage of the papilla by Randall's plaque has been shown to correspond directly to an increasing number of stones.20 It is possible that as our collective knowledge regarding mechanisms of stone pathogenesis continues to evolve, our understanding regarding the role and importance of Randall's plaque may grow as well. There is also a possibility that the presence and degree of Randall's plaque may have previously overlooked phenotypic associations with other papillary findings in certain types of stone-forming diseases. As such, we believe that it is an important component of our proposed scale.
Establishing a standardized system for grading papillary injury is necessary from both a clinical and academic perspective. On a clinical level, it would allow physicians who care for patients with nephrolithiasis to more accurately and consistently document intraoperative findings and follow changes in their patients over time. Furthermore, it has the potential to create a reference standard of normal and abnormal through which it might help distinguish patients with more pressing needs of metabolic evaluations, medical therapy, and frequent surveillance imaging.
As a research tool, a grading system has the potential to allow surgeons to better correlate endoscopic findings to pathologic findings and clinical outcomes such as stone analysis, associated metabolic diseases, risk of progressive renal injury, and stone recurrence. It also has the potential to simplify the process of endoscopic characterization of the kidney. Currently, there are only two ongoing institutional efforts to endoscopically characterize stone formers.10,13,21 One likely reason for this relative lack of interest is the intensely demanding nature of the characterization process whereby video editing and image processing is used to precisely calculate surface plaque percentages after the procedure is completed. With the proposed grading scale, characterization can be performed much more efficiently, even at the same time as stone removal rather than necessitating video review at a later time.
Currently, the majority of research in the field of nephrolithiasis focuses on the relationship between radiographic findings and clinical outcomes. Radiologic imaging, while useful, does not provide nearly the same degree of detail as renal endoscopy, however. It cannot reliably predict the precise location of stones within the collecting system, whether they are attached to plaque, located within a duct, or free floating. Most notably, radiographic imaging is nearly entirely unable to deliver detailed information regarding the status or health of the papillae, the predominant point of origin of the majority of stones.
It is our belief that radiographic imaging in stone formers is best used in adjunct to renal endoscopy because each methodology offers unique information. We are hopeful that in creating a standardized method of describing papillary appearance, we help shift the perception of renal endoscopy as solely a tool for treatment to one of diagnosis as well.
There are several important future aims of our currently proposed grading scale. Next, we must validate the instrument among the wider urologic community, ensuring that accurate grading is widely reproducible. Data from our own center suggest that this is feasible, at least among those most familiar with the system. Among 90 papillae independently graded in a blinded fashion by the senior and junior surgeon involved in the creation of this system (JEL and MSB), correlation of pathology was high (Table 2) with a minimum 72% agreement for each of the four measured domains. The papillary injury sum was also able to be reliably scored with agreement within one degree in 86% of cases. Further validation efforts among a larger number of graders familiar with renal endoscopy are currently ongoing.
Table 2.
Comparison of Grades in 90 Papillae Based on Senior Level Versus Junior Level Surgeon
| PPLA | Assigned same grade | 1 degree difference in assigned grade | >1 degree difference in assigned grade |
|---|---|---|---|
| Plugging | 80% | 20% | N/A |
| Pitting | 76% | 21% | 2% |
| Loss of contour | 72% | 28% | N/A |
| Amount of Randall's plaque | 84% | 16% | N/A |
| Sum | 58% | 28% | 13% |
PPLA = plugging, pitting, loss of contour, amount of Randall's plaque.
In addition, we hope to study potential associations between papillary scoring and clinical outcomes. To date, the foundational studies establishing that abnormal papillary appearance on endoscopy correlates to cellular injury on a microscopic level have been performed based on biopsy specimens from small cohorts of patients representing pure phenotypes of stone-forming diseases.6 Given the small sample sizes in these cohorts, however, determining the overall clinical significance of these findings has been a challenge. The establishment of a grading scale would be a great asset in this regard, because it would enable researchers to study links between papillary pathology and other important clinical outcomes such as stone analysis, renal function, stone recurrence, and links to metabolic pathophysiologies on a much larger scale.
Finally, we must determine whether accurate and reproducible scoring is dependent on the type of endoscope used. Digital endoscopes are able to provide a more detailed image during endoscopy and are our preferred instrument for such procedures; however, we believe that the information necessary for papillary grading is readily apparent using a fiberoptic scope as well. Studies will be needed to determine whether the type of instrument used significantly affects the ability to assign a proper grade, although this may become a moot point as digital technology supplants older fiberoptic instruments over time.
Conclusions
We have established a papillary grading system that characterizes abnormal papillary appearance and visible injury. Ultimately, we are hopeful that such a system proves useful as a powerful clinical and research tool. Furthermore, we are hopeful that it might help raise awareness regarding the importance of examining the papillae at the time of endoscopy, because such observations can have profound implications in providing optimal patient care.
Abbreviation Used
- PPLA
plugging, pitting, loss of contour, amount of Randall's plaque.
Acknowledgments
The authors appreciate the assistance of Philip Blomgren for assistance in the preparation of digital images.
Research was funded in part by NIH grant #P01 DK56788.
Author Disclosure Statement
Dr. Lingeman is a consultant/advisor, investor, meeting participate/lecturer, scientific study trial participant for Boston Scientific Corp.; owner, medical director for Beck Analytical; Dr. Coe is a consultant for Labcorp; Dr. Worcester is a consultant for Litholink. For the remaining authors, no competing financial interests exist.’
References
- 1.Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int 2007;71:795–801 [DOI] [PubMed] [Google Scholar]
- 2.Matlaga BR, Williams JC, Jr, Kim SC, et al. Endoscopic evidence of calculus attachment to Randall's plaque. J Urol 2006;175:1720–1724 [DOI] [PubMed] [Google Scholar]
- 3.Miller NL, Williams JC, Jr, Evan AP, et al. In idiopathic calcium oxalate stone-formers, unattached stones show evidence of having originated as attached stones on Randalls plaque. BJU Int 2010;105:242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coe FL, Evan AP, Worcester EM, Lingeman JE. Three pathways for human kidney stone formation. Urol Res 2010;38:147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evan AE, Lingeman JE, Coe FL, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int 2008;74:223–229 [DOI] [PubMed] [Google Scholar]
- 6.Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 2005;67:576–591 [DOI] [PubMed] [Google Scholar]
- 7.Matlaga BR, Coe FL, Evan AP, Lingeman JE. The role of Randall's plaques in the pathogenesis of calcium stones. J Urol 2007;177:31–38 [DOI] [PubMed] [Google Scholar]
- 8.Evan AP, Lingeman JE, Worcester EM, et al. Contrasting histopathology and crystal deposits in kidneys of idiopathic stone formers who produce hydroxy apatite, brushite, or calcium oxalate stones. Anat Rec (Hoboken) 2014;297:731–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberlin DT, Flum AS. Bachrach L, et al. Contemporary surgical trends in the management of upper tract calculi. J Urol 2015;193:880–884 [DOI] [PubMed] [Google Scholar]
- 10.Kuo RL, Lingeman JE, Evan AP, et al. Endoscopic renal papillary biopsies: A tissue retrieval technique for histological studies in patients with nephrolithiasis. J Urol 2003;170:2186–2189 [DOI] [PubMed] [Google Scholar]
- 11.Evan AP, Worcester EM, Coe FL, et al. Mechanisms of human kidney stone formation. Urolithiasis 2015;43(suppl 1:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coe FL, Evan AP, Lingeman JE, Worcester EM. Plaque and deposits in nine human stone diseases. Urol Res 2010;38:239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evan AP, Lingeman JE, Coe FL, et al. Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003;111:607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan AP, Coe FL, Gillen D, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec (Hoboken), 2008;291:325–334 [DOI] [PubMed] [Google Scholar]
- 15.Evan AP, Worcester EM, Williams JC Jr, et al. Biopsy proven medullary sponge kidney: Clinical findings, histopathology, and role of osteogenesis in stone and plaque formation. Anat Rec (Hoboken), 2015;298:865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cifuentes Delatte L, Minon-Cifuentes JL, Medina JA. Papillary stones: Calcified renal tubules in Randall's plaques. J Urol 1985;133:490–494 [DOI] [PubMed] [Google Scholar]
- 17.Evan A, Lingeman J, Coe F, Worcester E. Randall's plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 2006;69:1313–1318 [DOI] [PubMed] [Google Scholar]
- 18.Williams JC, Jr, Matlaga BR, Kim SC, et al. Calcium oxalate calculi found attached to the renal papilla: Preliminary evidence for early mechanisms in stone formation. J Endourol 2006;20:885–890 [DOI] [PubMed] [Google Scholar]
- 19.Kuo RL, Lingeman JE, Evan AP, et al. Urine calcium and volume predict coverage of renal papilla by Randall's plaque. Kidney Int 2003;64:2150–2154 [DOI] [PubMed] [Google Scholar]
- 20.Kim SC, Coe FL, Tinmouth WW, et al. Stone formation is proportional to papillary surface coverage by Randall's plaque. J Urol 2005;173:117–119 [DOI] [PubMed] [Google Scholar]
- 21.Linnes MP, Krambeck AE, Cornell L, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int 2013;84:818–825 [DOI] [PMC free article] [PubMed] [Google Scholar]