Abstract
Memory stem T cells (TSCMs) constitute a long-lived, self-renewing lymphocyte population essential for the maintenance of functional immunity. Hallmarks of autoimmune disease pathogenesis are abnormal CD4+ and CD8+ T cell activation. We investigated the TSCM subset in 55, 34, 43, and 5 patients with acquired aplastic anemia (AA), autoimmune uveitis, systemic lupus erythematosus, and sickle cell disease, respectively, as well as in 41 age-matched healthy controls. CD8+ TSCM frequency was significantly increased in AA compared with healthy controls. An increased CD8+ TSCM frequency at diagnosis was associated with responsiveness to immunosuppressive therapy, and an elevated CD8+ TSCM population after immunosuppressive therapy correlated with treatment failure or relapse in AA patients. IFN-γ and IL-2 production was significantly increased in various CD8+ and CD4+ T cell subsets in AA patients, including CD8+ and CD4+ TSCMs. CD8+ TSCM frequency was also increased in patients with autoimmune uveitis or sickle cell disease. A positive correlation between CD4+ and CD8+ TSCM frequencies was found in AA, autoimmune uveitis, and systemic lupus erythematosus. Evaluation of PD-1, CD160, and CD244 expression revealed that TSCMs were less exhausted compared with other types of memory T cells. Our results suggest that the CD8+ TSCM subset is a novel biomarker and a potential therapeutic target for AA.
Keywords: Memory stem T cells, autoimmunity, aplastic anemia, T-cell activation
Introduction
Aplastic anemia (AA), the prototypical bone marrow (BM) failure syndrome, is caused by immune-mediated destruction of hematopoietic stem/progenitor cells (HSPCs) (1). BM transplantation and immunosuppressive therapy (IST) are effective for treatment of AA patients (2). Although no antigen has been convincingly demonstrated in AA, autoimmunity to HSPCs has been considered etiologic, mediated by activated cytotoxic T cells (CTLs) recognizing an HSPC-restricted antigen through their class I or II HLA molecules (3, 4). CD8+ CTLs with restricted TCR diversity (oligoclonal T cells) are expanded in AA, leading to production of proinflammatory cytokines, such as IFN-γ, which induce apoptosis of CD34+ cells (5). Detection of similar clonotypes, as shown by significantly skewed CDR3-size distribution, suggests the presence of common antigen-driven T cell expansion (6). In AA, there is overrepresentation of HLA-DR2 and class I HLA-A*02:01, A*02:06, A*31:01, and B*40:02 (7, 8). Hematologic recovery after IST with anti-thymocyte globulin (ATG) and cyclosporine (CsA) occurs in 60% – 75% of AA patients (2, 9), which correlates to diminution of expanded T cell clones. Relapse appears to be associated with reemergence of the original oligoclonal T cells, and sometimes new clones (6).
Conventionally, memory T cells are divided into central memory (TCM) and effector memory (TEM) subsets which home to secondary lymphoid and peripheral tissues, respectively (10). The percentages of CD4+ and CD8+ TEM subsets are increased in peripheral blood (PB) and BM of AA patients (11). Elevated TEM cells with potent effector capacity may relate to abnormal immunity in AA. Recent studies have identified a new subset of memory T cells with stem cell–like properties, TSCM (12, 13), which are the least differentiated cells of all distinct memory populations; TSCM express multiple naive markers as well as the memory antigen CD95. Functionally, TSCM possess an enhanced capacity for self-renewal and can generate multiple memory T cell populations, and they likely have an important role in controlling immunity (12). In addition, TSCM are endowed with superior immune reconstitution potential in immunodeficient hosts and can mediate antitumor immunity in a humanized mouse model (12).
In autoimmune diseases, there is abnormal CD4+ and CD8+ T cell activation. We hypothesized dysregulation of the TSCM compartment in autoimmunity. In this study, we evaluated TSCM frequency in AA and its association with severity, treatment response, relapse, and changes after IST. Further, to evaluate the TSCM in other autoimmune diseases, we examined CD4+ and CD8+ TSCM frequencies in uveitis, systemic lupus erythematosus (SLE), and sickle cell disease (SCD), as compared with healthy controls. Our results suggest that TSCM may contribute to the pathophysiology not only in cancer immunology but also in autoimmune diseases. Specifically, CD8+ TSCM cells may be a biomarker and a therapeutic target in AA.
Materials and Methods
Patients and Treatment
PB specimens were collected from 55 AA samples and 41 age-matched healthy donor samples after informed consent was obtained in accordance with the Declaration of Helsinki. Among 55 AA samples, 21 samples were analyzed at diagnosis and 34 after IST. Standard criteria were used for diagnosis and disease severity of AA (14, 15). Serial samples before and after IST were collected from 13 cases. Median age of AA patients was 29 years (range: 13 – 69 years). All AA patients received horse ATG + CsA + eltrombopag on a clinical research protocol (clinicaltrials.gov, #NCT01623167). For comparison, blood samples were obtained from 34 uveitis patients (27 inactive or 7 active cases), 43 SLE patients who met the American College of rheumatology (ACR) criteria for the disease (16, 17) [19 inactive SLE (SLE disease activity index-2K (SLEDAI-2K) score < 3; and 24 active SLE (SLEDAI-2K score > 3)], and 5 SCD patients who were receiving frequent transfusions. Demographic and clinical characteristics of patients and healthy controls are summarized in Table 1. There were no significant differences in median ages between each patient group and healthy controls (p > 0.05, respectively; Supplemental Fig. 1A). All human subjects were enrolled on clinical protocols approved by the NHLBI, NEI and NIAMS Institutional Review Boards.
Table I.
Characteristics of patient and healthy control samples
| Features | AA* (n = 55) |
Uveitis (n = 34) |
SLE (n = 43) |
SCD (n = 5) |
Healthy control (n = 41) |
||
|---|---|---|---|---|---|---|---|
| Median Age | 29 | 55 | 48 | 50 | 33 | ||
| Sex (M/F) | 33/22 | 11/23 | 5/38 | 2/3 | 21/20 | ||
| Diagnosis | Idiopathic 55 | Sarcoidosis | 8 | Idiopathic | 43 | SS 5 | NA |
| VKH | 9 | ||||||
| Birdshot | 15 | ||||||
| Idiopathic | 2 | ||||||
| Disease activity or sevirity | Severe 55 | Active | 7 | SLEDAI> 3 | 24 | NA | NA |
| Non-Severe 0 | Quiet | 27 | SLEDAI≤ 3 | 19 | |||
| Timing at sampling Before therapy | 21 | 9 | 1 | 0 | NA | ||
| After therapy | 34 | 25 | 42 | 5 | |||
| Therapy | ATG+CsA+TPO-RA 55 | PSL | 11 | PSL | 33 | Transfusion Program 4 | NA |
| MMF | 14 | HCQ | 33 | Transfusion + HU 1 | |||
| CsA | 4 | MMF | 6 | ||||
| MTX | 6 | AZA | 9 | ||||
| Anti-TNF | 6 | MTX | 10 | ||||
AA, aplastic anemia; SLE, systemic lupus erythematosus; SCD, sickle cell disease; M, male; F, female; NA, not applicable; VKH, Vogt-Koyanagi-Harada disease; SS, sickle cell anemia; SLEDAI, systemic lupus erythematosus disease activity index, IST, immunosuppressive therapy; ATG, anti-thymocyte globulins; CsA, cyclosporine; TPO-RA, thrombopoietin receptor agonist; PSL, prednisolone; MMF, mycophenolate mofetil; MTX, methotrexate; HCQ, hydroxychloroquine; AZA, azathioprine; HU, hydroxyurea.
As serial samples before and after IST were collected from 13 patients (including 3 patients from 3 different time points), the actual patient number is 39.
Peripheral Mononuclear cell (PBMC) separation
PBMCs were separated from PB samples using Lymphocyte Separation Medium (MP Biomedicals LLC, CA) and cryopreserved in RPMI-1640 (Life Technologies, Gaithersburg, MD) supplemented with 20% heat-inactivated fetal bovine serum (Sigma-Aldrich, St Louis, MO) and 10% dimethyl sulfoxide, according to the standard protocol until use.
Antibodies
The following fluorochrome-conjugated monoclonal antibodies (mAbs) were purchased from commercial vendors and used for surface staining: anti-CD4-V500, anti-CD8-APC-H7, anti-CD45RA-PE-Cy7, anti-CD45RO-APC, anti-CCR7-AF700, anti-CD95-PE, anti-CD160-FITC, anti-CD244-FITC, and anti-PD-1-FITC (BD Biosciences, San Jose, CA); anti-CD3-BV605 (Biolegend, San Diego, CA); anti-CD14-Pacific Blue and anti-CD19-Pacific Blue (Life Technologies, Carlsbad, CA); and anti-CD27-PC5 (Beckman Coulter, Indianapolis, IN). The fixable violet amine reactive dye (ViViD; Invitrogen/Molecular Probes, Eugene, OR) was used to eliminate dead cells by flow cytometry. For intracellular cytokine staining, the following mAbs were used: anti-granzyme B (GZMB)-FITC, anti-IL-2-FITC, and anti-IFN-γ-FITC (BD Biosciences).
Immunostaining for surface antigens
Gating strategy for TSCM and experimental protocols were adapted from a previous report (18). Briefly, cryopreserved PBMCs were thawed and subjected to surface staining as follows. PBMCs were incubated with a viability marker at room temperature for 20 min, washed, and then incubated with anti-human CCR7 at 37 °C for 20 min. After washed, cells were further stained with a cocktail of antibodies against CD3, CD4, CD8, CD45RA, CD45RO, CD27, CD95, CD14, and CD19 with or without exhaustion markers (anti-PD-1 antibody, anti-CD160 antibody, and anti-CD244 antibody), respectively, for 30 min on ice. Subsequently, cells were washed and at least 150,000 events gated on CD3+ T cells were acquired with Fortessa flow cytometer (BD Biosciences) for analyzing frequency of each T cell subset accurately. Gating strategy for T cell subsets is summarized in Fig. 1A. Lymphocytes were gated based on their scatter characteristics, and a forward scatter-area (FSC-A) vs. forward scatter-height (FSC-H) profile was used to exclude cell aggregates and to obtain single lymphocytes. Live T cells were separated from dead cells, monocytes, and B cells in a CD3 vs. ViViD/CD14/CD19 bivariate plot. CD4+ and CD8+ T cells were then gated based on characteristic expression patterns of CCR7 and CD45RO, followed by gating based on CD27/CD45RA and CCR7/CD95 expression. Each T cell subset was defined as follows: TCM, ViViD− CD3+ CD4 (CD8)+ CD45RO+ CCR7+; TEM, ViViD− CD3+ CD4 (CD8)+ CD45RO+ CCR7−; terminally-differentiated effector T cells (TE), ViViD− CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7− CD27−; naïve T cells (TN), CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7+ CD27+ CD95−; and TSCM, CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7+ CD27+ CD95+. Data were analyzed using FlowJo software version 9.6 (Tree Star, Ashland, OR). Quantification of inhibitory receptor expression in T cell subsets was previously described in detail. (19)
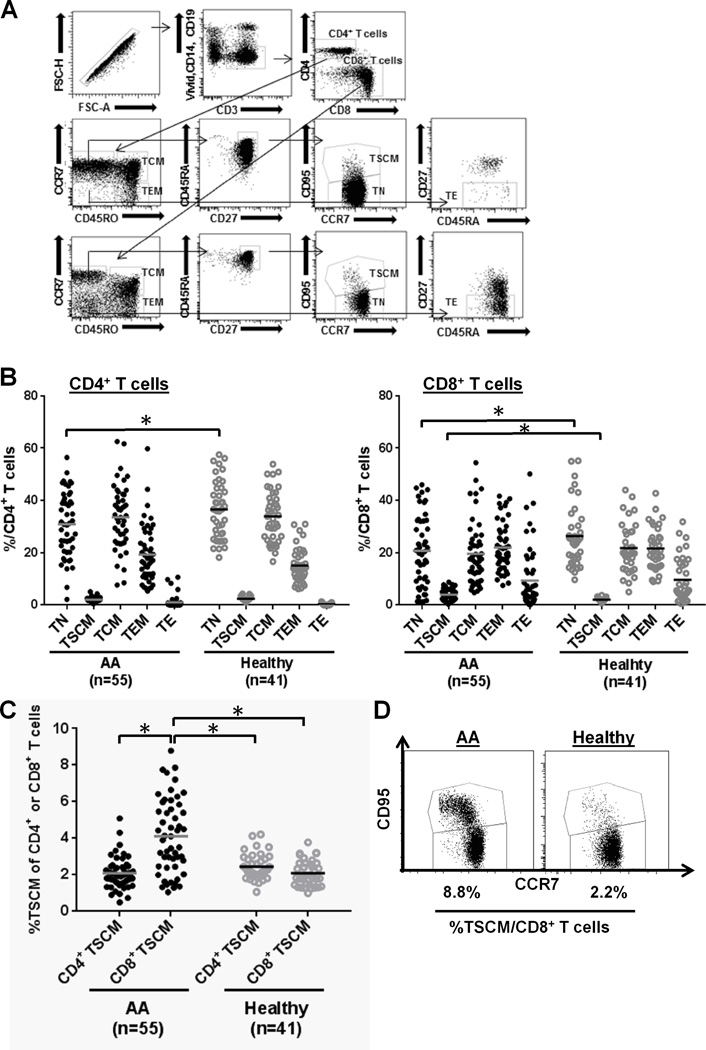
FIGURE 1.
The increased CD8+ TSCM population in AA patients. (A) Gating strategy for T cell subsets. PBMCs were stained with ViViD, anti-CD14-Pacific Blue, anti-CD19-Pacific Blue, anti-CD3-BV605, anti-CD4-V500, anti-CD8-APC-H7, anti-CD45RA-PE-Cy7, anti-CD45RO-APC, anti-CCR7-AF700, anti-CD27-PC5, and anti-CD95-PE. Lymphocytes or single lymphocytes were gated based on their scatter characteristics or forward scatter height vs. forward scatter area, respectively. Live T cells were gated based on positive for CD3 and negative for ViViD, CD14, and CD19 to remove dead cells, monocytes, and B cells. CD4+ and CD8+ T cells were then gated based on the characteristic expression patterns of CCR7 and CD45RO. ViViD− CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7+ CD27+ CD95− TN, ViViD− CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7+ CD27+ CD95+ TSCM, ViViD−CD3+ CD4 (CD8)+ CD45RO+ CCR7+ TCM, ViViD− CD3+ CD4 (CD8)+ CD45RO+ CCR7− TEM, and ViViD− CD3+ CD4 (CD8)+ CD45RO− CD45RA+ CCR7− CD27− TE were identified. (B) Frequency of each CD4+ or CD8+ T cell subset (TN, TSCM, TCM, TEM, or TE) was compared between AA (n = 55) and healthy control (n = 41) groups. *p < .05 (Student's t-test). (C) Frequencies of CD4+ and CD8+ TSCM populations were compared within the same group [AA (n = 55) or healthy control group (n = 41)] or between the two groups. *p < .05 (Student's t-test). (D) Representative flow cytometry dot plots illustrate the increased CD8+ TSCM population in an AA patient (left panel), relative to a healthy individual (right panel).
Immunostaining for intracellular cytokines
Expression levels of GZMB, IL-2, and IFN-γ in CD4+ and CD8+ T cell subsets were analyzed by intracellular cytokine staining 6 h post-stimulation. Briefly, cells were stimulated by addition of Dynabeads® Human T-Activator CD3/CD28 and then 2 h later by further addition of Golgi transport inhibitor (GolgiPlug; BD Biosciences). After another 4-h culture, cells were incubated with the cell surface-staining antibody cocktail as described elsewhere and were fixed/permeabilized using the Cytofix/Cytoperm Fixation/Permeabilization solution kit (BD Biosciences), according to the manufacturer's protocol. Subsequently, intracellular cytokine staining was performed using anti-GZMB-FITC, anti-IL-2-FITC, and anti- IFN-γ-FITC at 4 °C for 30 min.
Statistics
All statistical analyses were performed using GraphPad PRISM version 6.0 (GraphPad Software; La Jolla, CA). Data was represented as Means ± Standard Error of Means (SEM). A Student’s t test was used to calculate statistical significance between two groups. A statistical analysis was performed using one-way or two-way ANOVA with post hoc Tukey's or Dunnett's test for multiple comparisons, when appropriate. The Spearman rank test with linear regression was used for correlation analysis. A two-tailed p value < 0.05 was considered statistically significant.
Results
An increased CD8+ TSCM population in AA
First, we measured five T cell subsets (TN, TSCM, TCM, TEM, and TE) in AA and healthy controls. Within the CD4+ or CD8+ T cell compartments, AA patients showed decreased CD4+ or CD8+ TN frequency (p < 0.05, Fig. 1B), compared to controls, consistent with previous reports (11). CD4+ TE frequency was very low in the CD4+ T cell compartment in both AA and controls, but CD8+ TE frequency was higher among CD8+ T cells in both. In healthy controls, TSCM represented a relatively small percentage of circulating CD4+ or CD8+ T cells (median 2.4% CD4+ TSCM and 2.1% CD8+ TSCM) confirming findings of Gattinoni et al. (12). Samples collected from the same healthy donors but on different dates showed similar results, reassuring of technical and biological reproducibility (Supplemental Fig. 2). A significantly higher CD8+ TSCM frequency was detected in AA patients (4.2% vs. 2.1%, p < 0.05) while there was no difference in the CD4+ TSCM frequency (p > 0.05), compared to controls (Fig. 1C–D). Within the AA group, CD8+ TSCM (4.2%) was more frequent than was CD4+ TSCM (2.1%) (p < 0.05, Fig. 1C), whereas CD4+ and CD8+ TSCM frequencies within the control group showed no differences.
Clinical correlations with TSCM populations in AA
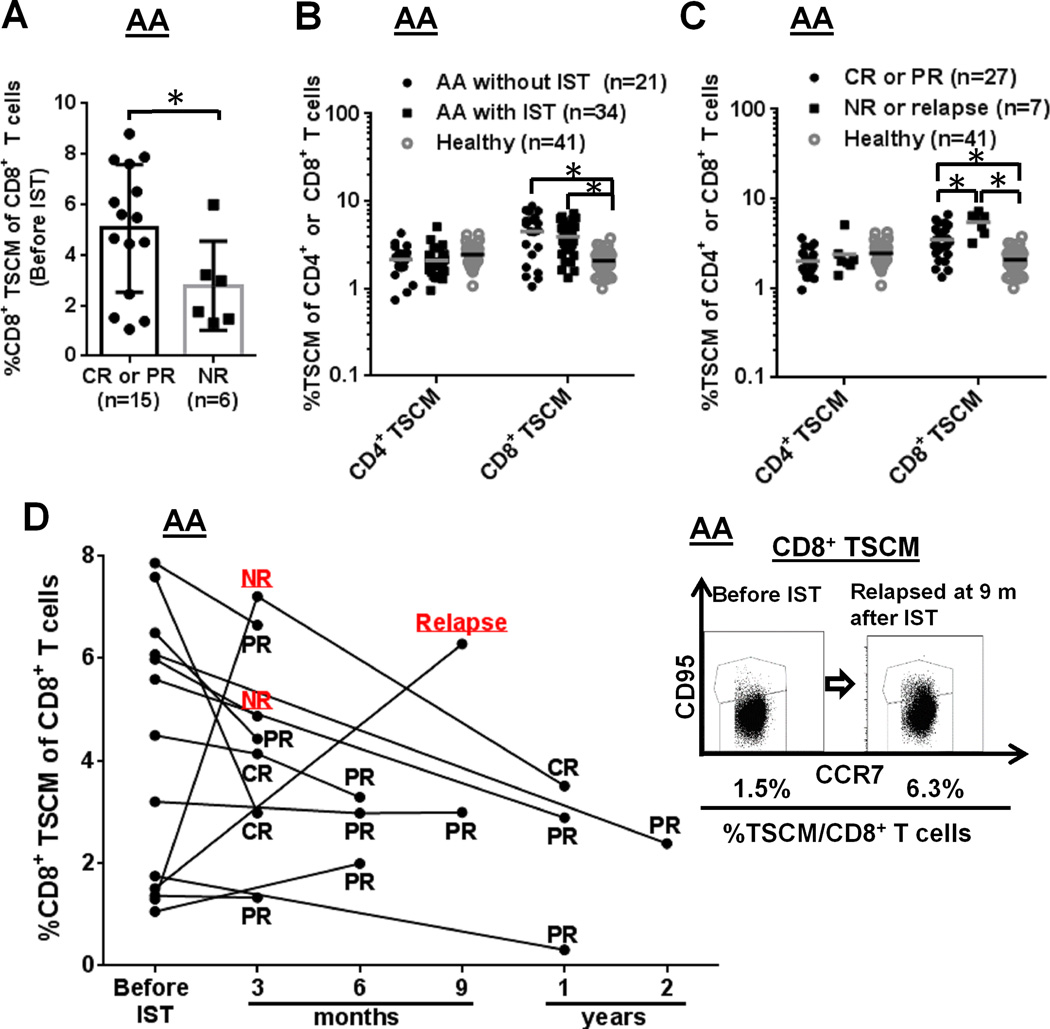
We assessed TSCM subset correlations with clinical manifestations and treatment responses in AA cohort. CD4+ and CD8+ TSCM populations were evaluated in patients by clinical parameter, including IST. Responses to IST were defined according to established criteria (20). In AA (n = 21), CD8+ TSCM frequency was measured at diagnosis and response was assessed at 3 months post-IST (Fig. 2A). In AA, high CD8+ TSCM frequency at diagnosis correlated with complete (CR) or partial response (PR) to IST [5.0 % in CR and PR vs 2.8 % in non-responders (NR), p < 0.05) (Fig. 2A). In AA patients prior to IST (n = 21), CD8+ TSCM frequency was not correlated with age, sex, absolute neutrophil count, platelet count, time from diagnosis to therapy, and serum ferritin levels (p > 0.05, Supplemental Fig. 3A). To elucidate effects of IST on the TSCM populations in AA patients, we next compared CD4+ or CD8+ TSCM frequency among three groups: 21 AA subjects without IST; 34 AA subjects with IST (3 months to 2 years post-IST); and 41 healthy controls. CD8+ TSCM were significantly increased in the two AA cohorts (with or without IST), relative to controls (p < 0.05, respectively), but CD4+ TSCM frequency was not statistically different among the three groups (p > 0.05) (Fig. 2B). We further evaluated CD4+ or CD8+ TSCM frequency among three cohorts [27 responders (CR or PR) and 7 NR or relapsed cases after IST, and 41 healthy donors]. CD4+ TSCM frequency was not significantly different but higher CD8+ TSCM frequency was observed in the 7 NR or relapsed cases after IST, compared with 27 responders (CR or PR) as well as compared to 41 controls. Higher CD8+ TSCM frequency after IST associated with treatment-failure (3.5 % in responders vs 5.5 % in NR or relapse, p < 0.05) (Fig. 2C). In serially collected samples from 13 AA patients, CD8+ TSCM frequency tended to decrease or maintained the same levels after IST, except for two cases (relapse at 9 months and NR at 3 months), who showed marked expanded CD8+ TSCM after IST (Fig. 2D).
FIGURE 2.
Clinical correlations with the TSCM populations in AA patients. (A) CD8+ TSCM frequency in AA patients was measured at diagnosis (Y axis) and then examined IST-response at 3 months post-IST (X axis): CR and PR patients were combined into one group, and NR patients into the other group. *p < .05 (Student's t-test). (B) CD4+ or CD8+ TSCM frequency was compared among three groups [AA patients without IST (n = 21) and with IST (n = 34), and healthy controls (n = 41)]. (C) Frequency comparison of CD4+ or CD8+ TSCM was performed among three groups: CR or PR after IST (n = 27), NR or relapse after IST (n = 7), and healthy controls (n = 41). (D) CD8+ TSCM frequency was measured at diagnosis and then after IST at different time points in the same 13 AA cases. Representative flow cytometry dot plots depict CD8+ TSCM frequency in one AA patient at diagnosis and relapse at 9 months after IST. *p < .05
Cytokine production in TSCM populations in AA
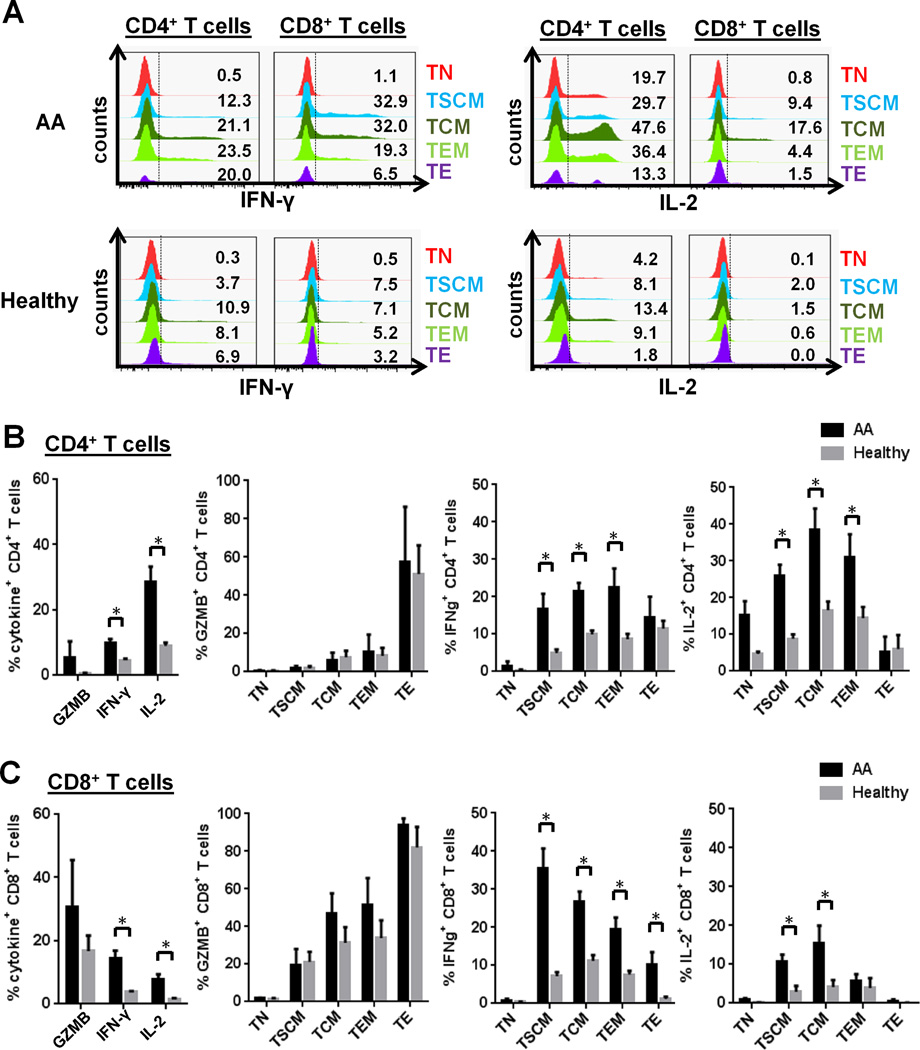
In AA patients, a high CD8+ TSCM frequency at diagnosis correlated with better response to IST while after IST, an increased CD8+ TSCM frequency was observed in non-responders or relapsed cases, suggesting TSCM as a potential biomarker. Memory T cell subsets have often been described by their effector functions: TCM are distinguished by greater proliferative and IL-2-producing capacities, TEM by increased secretion of effector cytokines such as IFN-γ and GZMB. Stimulation with anti-CD3/CD28 beads successfully induced cytokine production in CD4+ and CD8+ T cells from AA and healthy controls. (Fig. 3A) Elevated IFN-γ and IL-2 levels were observed in both bulk CD4+ and CD8+ T cells in AA patients, compared to healthy controls (Fig. 3B–C). We next investigated which CD4+ or CD8+ T cell subsets (TN, TSCM, TCM, TEM, and TE) were responsible for cytokine (GZMB, IFN-γ, and IL-2) production (Fig. 3B–C). Consistent with observations in bulk populations, no significant differences were detected in frequencies of GZMB-producing CD4+ and CD8+ T cell subsets in AA patients, compared to normal controls (Fig. 3B–C). In the CD4+ T cell compartment, both IFN-γ and IL-2 production levels were significantly elevated in the TSCM, TCM, and TEM subsets in AA. T cell subsets in the CD8+ T cell compartment showed greatly increased IFN-γ levels in CD8+ TSCM, TCM, TEM, and TE subsets and moderately elevated IL-2 levels in CD8+ TSCM and TCM subsets. Taken together, elevated IFN-γ and IL-2 levels were seen in CD4+ and CD8+ TSCM in AA compared to healthy controls (Fig. 3B–C).
FIGURE 3.
Cytokine production in CD4+ and CD8+ TSCM populations in AA patients. Cytokine production (GZMB, IFN-γ, and IL-2) was induced by in vitro stimulation with anti-CD3/CD28 beads, followed by immunostaining for intracellular cytokines. (A) Representative histograms showing cytokine production (IFN-γ and IL-2) of CD4+ and CD8+ T cell subsets in AA patients and healthy controls. A percentage of cytokine (GZMB, IFN-γ, or IL-2)-producing CD4+ (B) or CD8+ (C) T cell subset was compared between AA patients at diagnosis (n = 4) and healthy controls (n = 5). * p < 0.05.
TSCM frequencies in various autoimmune diseases
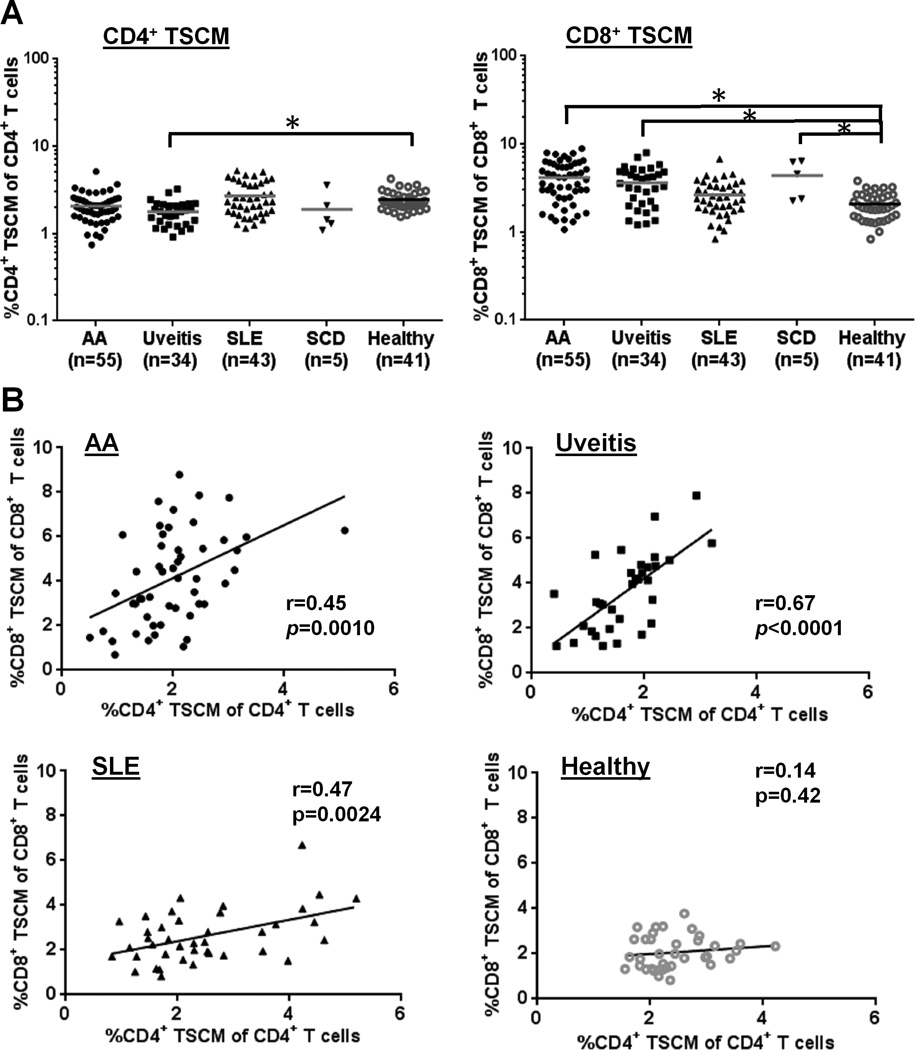
We next compared CD4+ or CD8+ TSCM frequency between each patient group (AA, uveitis, SLE, or SCD) and a healthy control group. We chose uveitis as an organ-specific immune mediated disorder characterized by inflammatory ocular lesions, SLE as systemic autoimmune disease, and SCD as controls for transfusions. Among the four patient groups, the uveitis group alone displayed a reduction in CD4+ TSCM frequency (1.8%) relative to the healthy controls (2.4 %; p < 0.05). An elevated CD8+ TSCM frequency was observed in AA (4.2 %), uveitis (3.6 %), and SCD (4.3 %), but not in SLE, compared to controls (2.1%; p < 0.05) (Fig. 4A). CD4+ and CD8+ TSCM frequencies were positively correlated in AA (r = 0.45, p = 0.0010), uveitis (r = 0.67, p < 0.0001), and SLE (r = 0.47, p = 0.0024) patients but not in healthy controls (r = 0.14, p = 0.42) (Fig. 4B). CD4+ TSCM frequency was inversely correlated with TEM frequency in AA (r = −0.38, p = 0.0045), uveitis (r = −0.58, p = 0.0003), and SLE (r = −0.49, p = 0.0009) (Supplemental Fig. 4A). For CD8+ TSCM and TE frequencies, the same inverse correlation was also observed in AA (r = −0.39, p = 0.0044) and uveitis (r = −0.55, p = 0.0010) (Supplemental Fig. 4B). There were no correlations between TSCM frequency and age (r = 0.03, p = 0.70 for CD4+ TSCM cells; and r = 0.06, p = 0.41 for CD8+ TSCM; Supplemental Fig. 1B–C).
FIGURE 4.
Comparison of CD4+ or CD8+ TSCM populations within AA, uveitis, SLE, or SCD cohort. (A) CD4+ or CD8+ TSCM frequency was compared among AA (n = 55), uveitis (n = 34), SLE (n = 43), SCD (n = 5), and healthy control (n = 41) cohorts. * p < 0.05. (B) results of correlation Spearman rank tests in which CD4+ TSCM frequency was compared with CD8+ TSCM frequency within the same group (AA, uveitis, SLE, or healthy control groups). * p < 0.05; r, a correlation coefficient value.
Inhibitory receptor expression on T cell subsets
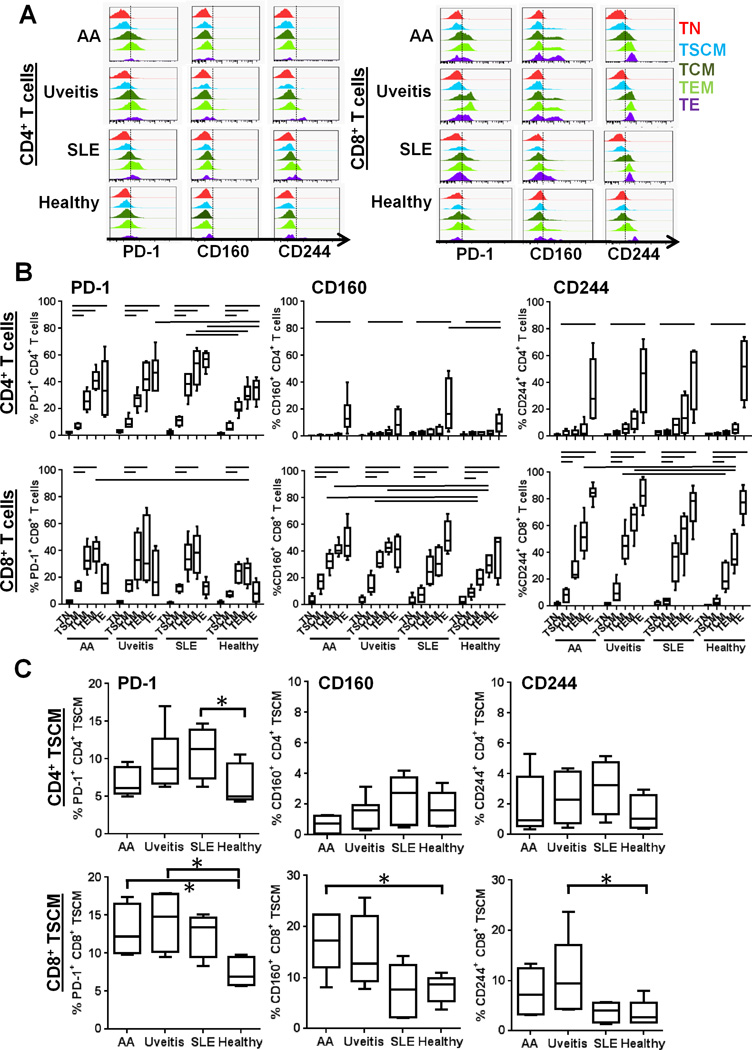
Aberrant overexpression of PD-1 (a cell-surface inhibitory receptor, also known as an exhaustion marker of T cells) is associated with persistent activation of self-reactive T cells in autoimmune diseases (21–23). We measured surface PD-1 expression on individual CD4+ or CD8+ T subsets (focusing on TSCM) in four groups (AA, uveitis, SLE, and healthy control) in order to assess activation in subsets (Fig. 5A). In CD4+ T cell subsets, PD-1 expression was lower on TSCM than on TCM, TEM, and TE within clinical groups (AA, uveitis, SLE, and healthy controls; p < 0.05, respectively), implicating TSCM as the “least exhausted” population among CD4+ T cells. When PD-1 expression levels on individual CD4+ T cell subsets were compared between each patient group and controls, significantly higher PD-1 expression levels were seen on TE in uveitis and TCM, TEM, and TE in SLE (p < 0.05; Fig. 5B). In CD8+ T cell subsets, PD-1 expression was also significantly lower on TSCM than on TCM and TEM, in all four groups (p < 0.05, respectively) (Fig. 5B). Significantly higher PD-1 expression levels were seen on CD8+ TEM in AA. These results were consistent with previous reports demonstrating that PD-1 expression related to differentiation of CD4+ and CD8+ T cells (24, 25). When PD-1 expression on CD4+ or CD8+ TSCM was compared among AA, uveitis, SLE, and controls, it was elevated on CD4+ TSCM in SLE and CD8+ TSCM in AA and uveitis (p < 0.05, respectively; Fig. 5C).
FIGURE 5. Inhibitory receptor expression on T cell subsets.
(A) Representative histograms of inhibitory receptor (PD-1, CD160 and CD244) expression of CD4+ and CD8+ T cell subsets in AA, uveitis, SLE patients, and healthy controls. (B) Surface inhibitory receptor (PD-1, CD160, and CD244) expression levels on CD4+ and CD8+ T cell subsets were analyzed in AA (n = 16), uveitis (n = 15), SLE (n = 22), and healthy control (n = 9) groups and compared within the same groups or between each patient and control groups. Horizontal lines indicate the statistically significant changes. (p < 0.05). (C) PD-1, CD160, and CD244 expression of CD4+ and CD8+ TSCM are plotted using a small scale range to highlight differences of their values. They were compared between each patient and control groups. Boxes represent median and 25th and 75th percentiles; and whiskers represent 10th and 90th percentiles. * p < 0.05.
To verify the results of PD-1 expression in T cell subsets, expression of other inhibitory receptors, CD160 and CD244, was examined in T cell subsets from various autoimmune diseases (19, 26, 27). CD160 and CD244 were mainly expressed in CD8+ T cell subsets among which the CD8+ TSCM population was least exhausted, compared with other types of T cell subsets (Fig. 5A–B). CD160, as well as PD-1, was highly expressed in CD8+ TSCM of AA, compared to those of healthy donors (Fig. 5C).
Clinical correlations with TSCM populations in various autoimmune diseases
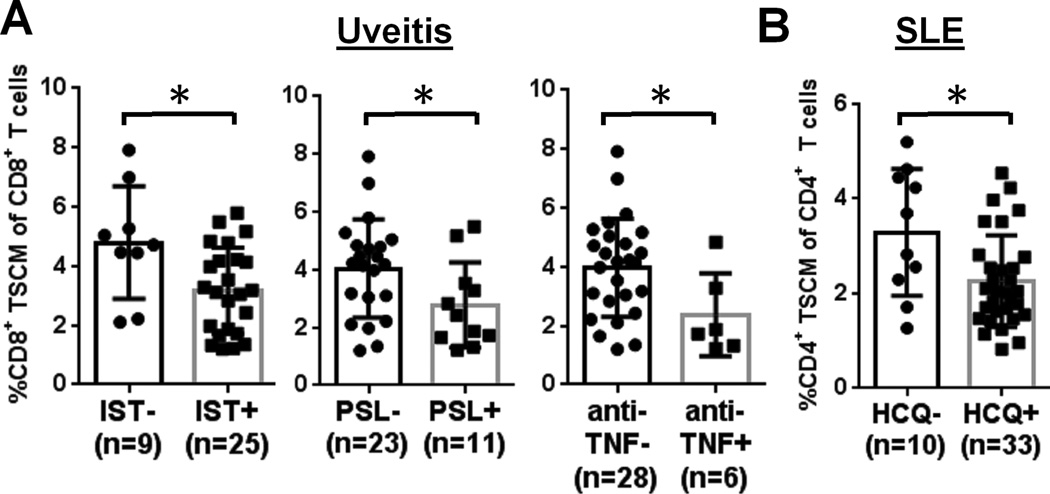
Finally, we assessed TSCM subset correlations with clinical manifestations and treatment responses in uveitis and SLE cohort. CD4+ and CD8+ TSCM populations were evaluated in patients with or without some clinical parameters, including immune therapies specific for individual diseases. In uveitis, patients (n = 34) were classified into two groups, with or without current immunosuppressive therapy, and then compared CD8+ TSCM frequency between the two groups: the group receiving therapy included the patients treated with prednisolone (PSL), mycophenolate mofetil (MMF), CsA, methotrexate (MTX), or anti-TNF therapy, or in combination. A higher CD8+ TSCM frequency (4.8 %) was observed in uveitis patients without any systemic immune therapy, compared to those receiving immune therapies (3.2 %) (p < 0.05, respectively; Fig. 6A). A higher CD8+ TSCM frequency was observed in uveitis patients not receiving PSL (4.1 %) than in those receiving PSL (2.8 %) and in those not receiving the anti-TNF agents infliximab and adalimumab (4.0 %) than in patients receiving these drugs (2.4 %) (p < 0.05, respectively; Fig. 6A). However, there were no statistically significant differences in patients with or without CsA, MTX, or MMF (p > 0.05, Supplemental Fig. 3B). CD8+ TSCM frequency did not correlate with age, sex, diagnosis of underlying disease (sarcoidosis vs. VKH vs. Birdshot), localization of disease, or disease activity (p > 0.05) (Supplemental Fig. 3B).
FIGURE 6.
Clinical correlations with the TSCM populations in uveitis and SLE patients. (A) Frequency of CD8+ TSCM in uveitis (n = 34) are compared between the following two groups: with and without immune therapies (including one drug alone or any combination of drugs); with and without PSL (PSL alone or PSL plus any other drugs); and with and without anti-TNF (anti-TNF alone or anti-TNF plus any other drugs). (B) CD4+ TSCM frequency in SLE (n = 43) was compared between two cohorts: with and without HCQ (HCQ alone or HCQ plus any other drugs). * p < 0.05.
As most SLE patients had received IST before or at the time of sampling, we could not examine therapy effects on the TSCM population. There was a higher frequency of CD4+ TSCM in patients not receiving hydroxychloroquine (HCQ, 3.3 %) than in those receiving HCQ (2.3 %) (p < 0.05, Fig. 6B), but no such relationship with other drugs (Supplemental Fig. 3C). CD4+ TSCM frequency was not correlated with age, disease activity, (p > 0.05; Supplemental Fig. 3C) or with the presence of specific disease associations (including secondary Sjogren syndrome, antiphospholipid syndrome, or immune thrombocytopenic purpura) (data not shown).
Collectively, immune therapies appeared to have negative effects on the TSCM population both in uveitis and SLE patient cohorts, as well as in AA.
Discussion
In our flow cytometry-based study, we retrospectively analyzed CD4+ and CD8+ TSCM populations and their relationship to disease activity, treatment response, and disease severity, by characterizing these subsets in a total of 178 subjects (55 AA, 34 autoimmune uveitis, 43 SLE, 5 SCD, and 41 age-matched healthy controls). An increased CD8+ TSCM population was observed in AA, uveitis, and SCD, and possibly a higher CD4+ TSCM population in SLE. Activation of CD4+ and CD8+ T cells is critical in diseases of uncontrolled inflammation (5, 28, 29), but we believe the current study to be the first report of CD4+ and CD8+ TSCM populations in autoimmune diseases. Consistent with previous studies, we observed approximately 2–4% of the total CD4+ or CD8+ TSCM population in healthy controls (12, 18) and no correlations between CD4+ or CD8+ TSCM frequency and age in all groups (30).
TSCM have been examined in infectious diseases (25, 31, 32), in the development of immunotherapies and vaccines for cancer (33, 34), in allogeneic hematopoietic stem cell transplantation (35, 36), and in adult T-cell leukemia (37). TSCM represent the earliest and longest lasting developmental stage of memory T cells and exhibit a gene expression profile between TN and TCM cells (12, 18). One mechanism for maintenance of long-term T cell memory may be the unique homeostatic properties of TSCM cells (38). In this context, we observed inverse correlation of the CD4+ or CD8+ TSCM population with the CD4+ TEM or CD8+ TE population, respectively, indicating that TSCM differentiation leads to a shift of these cell proportions as a possible mechanism to reestablish the homeostasis of the immune system. TSCM may be progenitors for TEM or TE as these two proportions were inversely correlated. Whether immune activation is attributable to increased TSCM or the increase of this subset is the result of aberrant immune responses is uncertain. Due to their capacities to generate all memory and effector T cell subsets, we hypothesized that the increased frequency of TSCM contributed to progression of the autoimmune diseases.
Indeed, a higher CD8+ TSCM frequency at diagnosis or after IST was associated with better response to IST or treatment failure in AA, respectively, suggesting CD8+ TSCM as a potential biomarker. Longitudinal analysis of 13 AA patients also suggests a potential use of TSCM as disease biomarkers. In uveitis cohort, the CD8+ TSCM population was lower in patients who received PSL and anti-TNF therapy, indicating a potential negative role of IST to this population, although analysis of serial samples would be required to sustain this conclusion. Further, SLE patients receiving HCQ therapy displayed reduced CD4+ TSCM frequency, also suggesting that IST affected this population. HCQ is known to negatively correlate with the percentage of CD45RO+ cells among CD4+ T cells (39). As most SLE samples were collected after IST, IST might have masked the TSCM population in these cases. Additional studies in large patient cohorts are necessary to define whether TSCM are useful as general autoimmune disease biomarkers.
There are similarities and differences of T cell autoimmunity in AA, uveitis, and SLE. Abnormalities of the Tregs and Th17 cells are reported in AA (40–42), uveitis (43, 44), and SLE patients (45–47). In AA, CD8+ CTLs with restricted TCR diversity are expanded in AA and secrete proinflammatory cytokines, which induce apoptosis of CD34+ cells (6). A recent study suggests that the clonally restricted expansion of Th1 cells is most likely to be antigen-driven and induces an inflammatory environment, exacerbating functional impairment of Tregs (40). In uveitis, a CD4+ T cell-driven disease is the dominant paradigm in animal models (28). However, antigen-specific CD8+ T cells can mediate autoimmunity within the eye (48), and CD8+ T cells increase in experimental uveitis (49). SLE is characterized by the production of a wide array of autoantibodies and thus has been traditionally classified as a “B cell disease”. However, evidence has indicated assistance of helper T cells may be required to produce SLE-related inflammation (50).
Upon TCR stimulation in healthy controls, TSCM exhibits effector activity, including TNF-α, IFN-γ, and IL-2 secretion, whereas TN cells remain relatively quiescent (12). In AA patients, CD4 + and CD8+ TSCM produced more IFN-γ and IL-2 upon TCR stimulation, compared to healthy controls, showing effector functions and higher IL-2-producing capacity of these subsets. CD8+ CTLs are expanded in AA and produce proinflammatory cytokines, such as IFN-γ, which induce apoptosis of CD34+ cells (5). Here, we identified TSCM as another source of IFN-γ production by both CD4+ and CD8+ T cells in AA.
PD-1 is one of the immune check point molecules expressed on both activated and exhausted T-cells (51). PD-1 overexpression is detected in CD4+ and CD8+ T cells in AA patients (21). It has been demonstrated in SLE patients that PD-1 expression is upregulated in CD4+ T cells and that PD-1 expression in CD4+ T cells is associated with IFN-γ expression on CD3+ T cells (52). In our current work, PD-1 expression in TSCM implicated this subset as the least exhausted population, relative to other memory T cell subsets. Previous studies have demonstrated PD-1 expression on tumor-infiltrating T-cells (TILs) and its correlation with prognosis (53, 54). The PD-1 pathway exerts inhibitory functions in chronic viral infections and tumors, with special relevance to autoimmunity (23). In addition to well-documented negative regulatory roles, PD-1 expression on CD8+ TILs accurately identified the repertoire of clonally expanded tumor-reactive cells (55). Higher expression of PD-1 in CD8+ TSCM in AA suggests these populations as self-reactive T cells. Recent studies have suggested that PD-1 expression in healthy donors does not correlate with lower functionality but rather with differentiation to an effector memory phenotype (19, 26, 56). We interpret increased expression of PD-1 and increased IFN-γ production in CD8+ TSCM of AA patients as evidence of clonal expansion, as occurs for CD8+ TIL cells (55) and as we have reported previously for CD8+ effector cells in AA (6, 57). Enhanced cytolytic effector activity in CD8+ TSCM of AA was also supported by the observation of higher CD160 expression of this population.
Our study has a number of limitations. We were unable to demonstrate causality. However, it seems likely that increased of TSCM cells may be etiologically associated with immune response to autoantigens and that the mechanism may be related to homeostatic maintenance of other memory T cell subsets. It is also plausible that increased TSCM is a consequence of non-specific inflammation induced by environmental factors, as CD8+ TSCM was also increased in SCD, which is not typically considered as autoimmune disease. Transfusion is chronic immune stimulation, which may lead SCD to active inflammatory state (58, 59). Transfusions administered to AA and SCD patients may affect the CD8+ TSCM population as a result of allogeneic stimulation. However, this seems unlikely because CD8+ TSCM frequency was not correlated with serum ferritin levels in AA (Supplemental Fig. 3A). Although we were unable to characterize the antigen-specific TSCM population because of the limited cell numbers, a nonhuman primate model of SIV infection has demonstrated that SIV-specific TSCM preferentially survive after antigen elimination, compared to other memory subsets, and are fully functional even in chronic infection (38). Thus, antigen-specific TSCM are presumably able to contribute directly to disease progression.
In conclusion, we provide evidence for increased circulating CD8+ TSCM in AA, underscoring the importance of this novel subset in regulation of immune responses and pathogenesis of autoimmunity. Our work described previously unknown potential roles of TSCM in AA, such as cytokine secretion correlated with effector functions. Understanding the CD8+ TSCM population may offer new therapeutic strategies and novel mechanistic insight into the various autoimmune diseases. Longitudinal analysis of AA patients suggests potential use of TSCM as disease biomarkers. Additional studies in large patient cohorts are required for validation of our current data which were obtained from small numbers of patients with autoimmune diseases.
Supplementary Material
Acknowledgments
We thank Marie Desierto, Susan Wong, Pilar Fernandez for technical assistance, Olga Rios, Kinneret Broder, and Carolyne Smith for assistance in obtaining patient and healthy volunteer samples, Barbara Weinstein, Sarfaraz Hasni and Zerai Manna for obtaining patient clinical information. This research was supported by the Intramural Research Program of the NIH, National Heart, Lung, and Blood Institute.
Footnotes
Disclosures
The authors declare no conflict of interest.
Author Contributions
K. Hosokawa, P. Muranski, X. Feng, S. Kajigaya, S. Ito, MJ. Kaplan, RB. Nussenblatt, AJ. Barrett, J O’Shea and NS. Young participated in the design of this analysis. K. Hosokawa conceptualized, and conducted the experiments, analyzed the data, interpreted the results and drafted the manuscript. K. Keyvanfar helped the flow cytometory. X. Feng, DM. Townsley, B. Dumitriu, B. Liu, J. Knickelbein, MJ. Kaplan, and JG. Taylor recruited patient samples to participate in the study and clinical informations. P. Muranski, X. Feng and S. Kajigaya edited the manuscript. NS. Young was involved in the conceptualization, interim discussions, interpretation of results, and editing the paper. All authors critically reviewed the manuscript content and agreed with the submission of the final manuscript.
References
- 1.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185–1196. doi: 10.1182/blood-2011-12-274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Risitano AM, Kook H, Zeng W, Chen G, Young NS, Maciejewski JP. Oligoclonal and polyclonal CD4 and CD8 lymphocytes in aplastic anemia and paroxysmal nocturnal hemoglobinuria measured by V beta CDR3 spectratyping and flow cytometry. Blood. 2002;100:178–183. doi: 10.1182/blood-2002-01-0236. [DOI] [PubMed] [Google Scholar]
- 4.Nakao S, Takami A, Takamatsu H, Zeng W, Sugimori N, Yamazaki H, Miura Y, Ueda M, Shiobara S, Yoshioka T, Kaneshige T, Yasukawa M, Matsuda T. Isolation of a T-cell clone showing HLA-DRB1*0405-restricted cytotoxicity for hematopoietic cells in a patient with aplastic anemia. Blood. 1997;89:3691–3699. [PubMed] [Google Scholar]
- 5.Sloand E, Kim S, Maciejewski JP, Tisdale J, Follmann D, Young NS. Intracellular interferon-gamma in circulating and marrow T cells detected by flow cytometry and the response to immunosuppressive therapy in patients with aplastic anemia. Blood. 2002;100:1185–1191. doi: 10.1182/blood-2002-01-0035. [DOI] [PubMed] [Google Scholar]
- 6.Risitano AM, Maciejewski JP, Green S, Plasilova M, Zeng W, Young NS. In-vivo dominant immune responses in aplastic anaemia: molecular tracking of putatively pathogenetic T-cell clones by TCR beta-CDR3 sequencing. Lancet. 2004;364:355–364. doi: 10.1016/S0140-6736(04)16724-X. [DOI] [PubMed] [Google Scholar]
- 7.Maciejewski JP, Follmann D, Nakamura R, Saunthararajah Y, Rivera CE, Simonis T, Brown KE, Barrett JA, Young NS. Increased frequency of HLA-DR2 in patients with paroxysmal nocturnal hemoglobinuria and the PNH/aplastic anemia syndrome. Blood. 2001;98:3513–3519. doi: 10.1182/blood.v98.13.3513. [DOI] [PubMed] [Google Scholar]
- 8.Katagiri T, Sato-Otsubo A, Kashiwase K, Morishima S, Sato Y, Mori Y, Kato M, Sanada M, Morishima Y, Hosokawa K, Sasaki Y, Ohtake S, Ogawa S, Nakao S. Frequent loss of HLA alleles associated with copy number-neutral 6pLOH in acquired aplastic anemia. Blood. 2011;118:6601–6609. doi: 10.1182/blood-2011-07-365189. [DOI] [PubMed] [Google Scholar]
- 9.Scheinberg P, Nunez O, Weinstein B, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. The New England journal of medicine. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Gu Y, Wang Y, Cong Y, Qu X, Xu C. Increased CD4+ and CD8+ effector memory T cells in patients with aplastic anemia. Haematologica. 2009;94:428–429. doi: 10.3324/haematol.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L. Memory T cells officially join the stem cell club. Immunity. 2014;41:7–9. doi: 10.1016/j.immuni.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718–1721. [PubMed] [Google Scholar]
- 15.Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, Rappeport JM, Storb R. Severe aplastic anemia: a prospective study of the effect of early marrow transplantation on acute mortality. Blood. 1976;48:63–70. [PubMed] [Google Scholar]
- 16.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 17.Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. The Journal of rheumatology. 2002;29:288–291. [PubMed] [Google Scholar]
- 18.Lugli E, Gattinoni L, Roberto A, Mavilio D, Price DA, Restifo NP, Roederer M. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8:33–42. doi: 10.1038/nprot.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory Receptor Expression Depends More Dominantly on Differentiation and Activation than "Exhaustion" of Human CD8 T Cells. Front Immunol. 2013;4:455. doi: 10.3389/fimmu.2013.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camitta BM, Doney K. Immunosuppressive therapy for aplastic anemia: indications, agents, mechanisms, and results. Am J Pediatr Hematol Oncol. 1990;12:411–424. doi: 10.1097/00043426-199024000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Wu H, Miao M, Zhang G, Hu Y, Ming Z, Zhang X. Soluble PD-1 is associated with aberrant regulation of T cells activation in aplastic anemia. Immunol Invest. 2009;38:408–421. doi: 10.1080/08820130902912332. [DOI] [PubMed] [Google Scholar]
- 22.Jiao Q, Liu C, Yang Z, Ding Q, Wang M, Li M, Zhu T, Qian H, Li W, Tu N, Fang F, Ye L, Zhao Z, Qian Q. Upregulated PD-1 Expression Is Associated with the Development of Systemic Lupus Erythematosus, but Not the PD-1.1 Allele of the PDCD1 Gene. Int J Genomics. 2014;2014:950903. doi: 10.1155/2014/950903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12:1091–1100. doi: 10.1016/j.autrev.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One. 2012;7:e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaafoura S, de Goer de Herve MG, Hernandez-Vargas EA, Hendel-Chavez H, Abdoh M, Mateo MC, Krzysiek R, Merad M, Seng R, Tardieu M, Delfraissy JF, Goujard C, Taoufik Y. Progressive contraction of the latent HIV reservoir around a core of less-differentiated CD4(+) memory T Cells. Nature communications. 2014;5:5407. doi: 10.1038/ncomms6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H, Rufer N, Matter M, Michielin O, Speiser DE. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riches JC, Davies JK, McClanahan F, Fatah R, Iqbal S, Agrawal S, Ramsay AG, Gribben JG. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121:1612–1621. doi: 10.1182/blood-2012-09-457531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee RW, Nicholson LB, Sen HN, Chan CC, Wei L, Nussenblatt RB, Dick AD. Autoimmune and autoinflammatory mechanisms in uveitis. Semin Immunopathol. 2014;36:581–594. doi: 10.1007/s00281-014-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gualtierotti R, Biggioggero M, Penatti AE, Meroni PL. Updating on the pathogenesis of systemic lupus erythematosus. Autoimmun Rev. 2010;10:3–7. doi: 10.1016/j.autrev.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Van Epps P, Banks R, Aung H, Betts MR, Canaday DH. Age-related differences in polyfunctional T cell responses. Immun Ageing. 2014;11:14. doi: 10.1186/1742-4933-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mateus J, Lasso P, Pavia P, Rosas F, Roa N, Valencia-Hernandez CA, Gonzalez JM, Puerta CJ, Cuellar A. Low Frequency of Circulating CD8+ T Stem Cell Memory Cells in Chronic Chagasic Patients with Severe Forms of the Disease. PLoS neglected tropical diseases. 2015;9:e3432. doi: 10.1371/journal.pntd.0003432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klatt NR, Bosinger SE, Peck M, Richert-Spuhler LE, Heigele A, Gile JP, Patel N, Taaffe J, Julg B, Camerini D, Torti C, Martin JN, Deeks SG, Sinclair E, Hecht FM, Lederman MM, Paiardini M, Kirchhoff F, Brenchley JM, Hunt PW, Silvestri G. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS pathogens. 2014;10:e1004345. doi: 10.1371/journal.ppat.1004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood. 2013;121:567–568. doi: 10.1182/blood-2012-11-468660. [DOI] [PubMed] [Google Scholar]
- 34.Flynn JK, Gorry PR. Stem memory T cells (TSCM)-their role in cancer and HIV immunotherapies. Clin Transl Immunology. 2014;3:e20. doi: 10.1038/cti.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cieri N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B, Valtolina V, Noviello M, Vago L, Bondanza A, Lunghi F, Marktel S, Bellio L, Bordignon C, Bicciato S, Peccatori J, Ciceri F, Bonini C. Generation of human memory stem T cells after haploidentical T-replete hematopoietic stem cell transplantation. Blood. 2015;125:2865–2874. doi: 10.1182/blood-2014-11-608539. [DOI] [PubMed] [Google Scholar]
- 36.Roberto A, Castagna L, Zanon V, Bramanti S, Crocchiolo R, McLaren JE, Gandolfi S, Tentorio P, Sarina B, Timofeeva I, Santoro A, Carlo-Stella C, Bruno B, Carniti C, Corradini P, Gostick E, Ladell K, Price DA, Roederer M, Mavilio D, Lugli E. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood. 2015;125:2855–2864. doi: 10.1182/blood-2014-11-608406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai Y, Kawahara M, Hishizawa M, Shimazu Y, Sugino N, Fujii S, Kadowaki N, Takaori-Kondo A. T memory stem cells are the hierarchical apex of adult T-cell leukemia. Blood. 2015 doi: 10.1182/blood-2014-10-607465. [DOI] [PubMed] [Google Scholar]
- 38.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M. Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sailler L, Puissant B, Meliani P, Castex JO, Saivin S, Adoue D, Fournie B, Arlet P, Montastruc JL, Lapeyre-Mestre M, Pourrat J, Blancher A. Blood concentrations of hydroxychloroquine and its desethyl derivative correlate negatively with the percentage of CD45RO+ cells among CD4+ lymphocytes in hydroxychloroquine-treated lupus patients. Annals of the New York Academy of Sciences. 2007;1108:41–50. doi: 10.1196/annals.1422.005. [DOI] [PubMed] [Google Scholar]
- 40.Kordasti S, Marsh J, Al-Khan S, Jiang J, Smith A, Mohamedali A, Abellan PP, Veen C, Costantini B, Kulasekararaj AG, Benson-Quarm N, Seidl T, Mian SA, Farzaneh F, Mufti GJ. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119:2033–2043. doi: 10.1182/blood-2011-08-368308. [DOI] [PubMed] [Google Scholar]
- 41.Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, Kajigaya S, Barrett AJ, Young NS. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110:1603–1606. doi: 10.1182/blood-2007-01-066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Latour RP, Visconte V, Takaku T, Wu C, Erie AJ, Sarcon AK, Desierto MJ, Scheinberg P, Keyvanfar K, Nunez O, Chen J, Young NS. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116:4175–4184. doi: 10.1182/blood-2010-01-266098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh S, Li Z, Forooghian F, Hwang FS, Cunningham MA, Pantanelli S, Lew JC, Wroblewski KK, Vitale S, Nussenblatt RB. CD4+Foxp3+ T-regulatory cells in noninfectious uveitis. Arch Ophthalmol. 2009;127:407–413. doi: 10.1001/archophthalmol.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nature medicine. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 45.Martin JC, Baeten DL, Josien R. Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clin Immunol. 2014;154:1–12. doi: 10.1016/j.clim.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Mellor-Pita S, Citores MJ, Castejon R, Tutor-Ureta P, Yebra-Bango M, Andreu JL, Vargas JA. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis. 2006;65:553–554. doi: 10.1136/ard.2005.044974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin MS, Lee N, Kang I. Effector T-cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr Opin Rheumatol. 2011;23:444–448. doi: 10.1097/BOR.0b013e328349a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McPherson SW, Yang J, Chan CC, Dou C, Gregerson DS. Resting CD8 T cells recognize beta-galactosidase expressed in the immune-privileged retina and mediate autoimmune disease when activated. Immunology. 2003;110:386–396. doi: 10.1046/j.1365-2567.2003.01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calder VL, Zhao ZS, Wang Y, Barton K, Lightman SL. Effects of CD8 depletion on retinal soluble antigen induced experimental autoimmune uveoretinitis. Immunology. 1993;79:255–262. [PMC free article] [PubMed] [Google Scholar]
- 50.Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. J Immunol Res. 2014;2014:419029. doi: 10.1155/2014/419029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamphorst AO, Ahmed R. Manipulating the PD-1 pathway to improve immunity. Curr Opin Immunol. 2013;25:381–388. doi: 10.1016/j.coi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolff S, Quandt D, Feldkamp T, Jun C, Mitchell A, Hua F, Specker C, Kribben A, Witzke O, Wilde B. Increased percentages of PD-1 on CD4+ T cells is associated with higher INF-gamma production and altered IL-17 production in patients with systemic lupus erythematosus. Scand J Rheumatol. 2014;43:307–313. doi: 10.3109/03009742.2013.869830. [DOI] [PubMed] [Google Scholar]
- 53.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 54.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 55.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K, Almeida JR, Darko S, Douek DC, Yang JC, Rosenberg SA. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J Clin Invest. 2014;124:2246–2259. doi: 10.1172/JCI73639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duraiswamy J, Ibegbu CC, Masopust D, Miller JD, Araki K, Doho GH, Tata P, Gupta S, Zilliox MJ, Nakaya HI, Pulendran B, Haining WN, Freeman GJ, Ahmed R. Phenotype, function, and gene expression profiles of programmed death-1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186:4200–4212. doi: 10.4049/jimmunol.1001783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kook H, Risitano AM, Zeng W, Wlodarski M, Lottemann C, Nakamura R, Barrett J, Young NS, Maciejewski JP. Changes in T-cell receptor VB repertoire in aplastic anemia: effects of different immunosuppressive regimens. Blood. 2002;99:3668–3675. doi: 10.1182/blood.v99.10.3668. [DOI] [PubMed] [Google Scholar]
- 58.Fasano RM, Booth GS, Miles M, Du L, Koyama T, Meier ER, Luban NL. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 59.Lee SP, Ataga KI, Orringer EP, Phillips DR, Parise LV. Biologically active CD40 ligand is elevated in sickle cell anemia: potential role for platelet-mediated inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1626–1631. doi: 10.1161/01.ATV.0000220374.00602.a2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.