Abstract
Purpose
To evaluate the impact of curcumin on the disposition of resveratrol phase II metabolites in vivo, and explain the observations by performing in vitro studies in transporter-overexpressed cells.
Methods
Pharmacokinetic studies of resveratrol with and without the co-administration of curcumin were performed in both FVB wild-type and Bcrp1 (−/−) mice. Human UGT1A9-overexpressing HeLa cells and human MRP2-overexpressing MDCK II-UGT1A1 cells were used as in vitro tools to further determine the impact of curcumin as a transporter inhibitor on resveratrol metabolites.
Results
We observed higher exposure of resveratrol conjugates in Bcrp1 (−/−) mice compared to wild-type mice. In wild-type mice, curcumin increased the AUC of resveratrol glucuronide by 4-fold compared to the mice treated without curcumin. The plasma levels of resveratrol and its sulfate conjugate also increased moderately. In Bcrp1 (−/−) mice, there was a further increase (6-fold increase) in AUC of resveratrol glucuronide observed when curcumin was co-administered compared to AUC values obtained in wild-type mice without curcumin treatment. In the presence of 50nM curcumin, the clearance of resveratrol-3-O-glucuronide and resveratrol-3-O-sulfate reduced in both MRP2-overexpressing MDCKII-UGT1A1 cells and Human UGT1A9-overexpressing HeLa cells.
Conclusions
These results suggest that curcumin alters the phase II distribution of resveratrol through inhibiting efflux transporters including MRP2 and BCRP.
Keywords: Resveratrol, Curcumin, MRP2, BCRP, Phase II disposition
INTRODUCTION
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a naturally-occurring polyphenolic phytoalexin found in grapes, berries, red wine, peanuts, and many other plant species (1, 2). In recent decades, resveratrol has received much attention for its potential biological activities. In 2003, the effect of resveratrol in delaying aging in lower organisms was first demonstrated by Howitz and colleagues (3). Shortly thereafter, Sinclair’s group reported a study in Nature in 2006 showing that resveratrol could extend the lifespan of mice (4). Since then, there has been growing research on resveratrol, and resveratrol has become a popular supplement. According to a 2012 Frost & Sullivan report, the global supply market value of resveratrol is USD 50 million, and the U.S. is the world’s largest resveratrol market. A PubMed search for “resveratrol” resulted in more than 7000 published studies as of January 2015. Numerous in vitro studies have demonstrated that resveratrol has a broad range of beneficial effects, such as anticancer, antioxidant, antifungal, antiplatelet, phytoestrogenic, and cardioprotective activities (5–9). However, when studies are conducted using in vivo animal models or humans, significant pharmacological effects are often lacking (10–13)
The major reason for this in vitro/in vivo discrepancy is the low bioavailability of resveratrol caused by extensive phase II metabolism after oral administration (14, 15). In enterocytes and hepatocytes, resveratrol is metabolized to glucuronides and sulfates by UDP-glucuronosyltransferases (UGTs) and sulfotransferases (SULTs). Resveratrol-3-O-glucuronide (RES-3-O-G), resveratrol-3-O-sulfate (RES-3-O-S), and resveratrol-4′-O-glucuronide (RES-4′-O-G) were found as the most abundant metabolites in humans, and their peak plasma levels were 3- to 8-fold higher than that of resveratrol (16).
Although the systemic exposure of resveratrol is very low, therapeutic effects have been observed in some animal models (15, 17). Recent studies, therefore, have been carried out to investigate the beneficial effects of resveratrol metabolites. In one study, RES-3-O-G and RES-4′-O-G were demonstrated to inhibit the cell growth of colon cancer cell lines with comparable IC50 to the parent compound (18). On the other hand, resveratrol sulfates also showed biological activities in vitro (19). Furthermore, the conjugated resveratrol metabolites could release parent compound through deconjugation reaction(20, 21), which indirectly contribute to its pharmacological activities observed in vivo. Considering the importance of the metabolism of resveratrol, a better understanding of the factors involved in phase II disposition of resveratrol and its conjugates would therefore allow new strategies to increase the bioavailability of resveratrol.
Multidrug resistance protein 2/3 (MRP2/3) and breast cancer resistance protein (BCRP) have been shown to mediate the transport of resveratrol phase II metabolites (22–24). All these transporters belong to the ATP-binding cassette (ABC) superfamily of membrane transporters and are highly expressed in gut and liver, which are also major sites of metabolism. In polarized cells (e.g. enterocytes and hepatocytes), BCRP and MRP2 are localized in the apical membrane where they facilitate the efflux of compounds back into the intestinal lumen or bile, while MRP3 is expressed in the basolateral membrane and transports compounds to the circulation (25). Most substances conjugated with glucuronide or sulfate are substrates for various uptake and efflux transporters (26). Because of their hydrophilic properties, these conjugates need efflux transporters to exit cells. Several studies were performed to determine the impact of transporters on phase II disposition and excretion using knockout animals. In these studies, altered pharmacokinetics and phase II disposition were observed in knockout animals for phenolic compounds including resveratrol (27–30). However, to the best of our knowledge, there was no study showing how an efflux transporter inhibitor can modulate the phase II disposition of resveratrol. This is the first study to evaluate the impact of curcumin as an inhibitor of transporters for phase II conjugates.
Curcumin (diferuoyl methane) is the principal curcuminoid found in the rhizomes of turmeric. It is a naturally occurring polyphenol with various beneficial properties such as antitumor, antioxidant, antiarthritic, and anti-inflammatory (31, 32). Curcumin is known to inhibit the function of ABC drug transporters (BCRP, P-gp, and MRP1/2) in vitro and appears to be highly effective in interacting with BCRP (33–36). To demonstrate the in vivo efficacy of curcumin in inhibiting transporter, some studies were performed in mice to assess the effect of curcumin on the oral absorption of sulfasalazine (SASP), which is a BCRP-specific substrate. It was found that the plasma level of SASP was two folds higher if the mice were pretreated with curcumin (37). The advantage of using curcumin as a transporter inhibitor is its lack of toxicity and mild pharmacological activities compared to most of the other transporter inhibitors. In a phase II clinical trial of curcumin performed in patients with advanced pancreatic cancer, it was demonstrated that oral administration of 8,000 mg of curcumin daily up to 18 month was well tolerated and showed some pharmacological activities (38).
The main purpose of this study was to determine the impact of curcumin on the disposition of resveratrol phase II metabolites in vivo, and furthermore, in vitro studies in transporter-overexpressed cells were performed to explain the observations in vivo. Our hypothesis is that, by inhibiting efflux transporters, curcumin treatment can favor the distribution of resveratrol phase II metabolites into the blood and increase systemic concentrations of resveratrol and its metabolites. Our study seeks to derive a better understanding of the interactions between curcumin and resveratrol, especially between curcumin and resveratrol conjugates. Considering the fact that many phase II conjugates are substrates of efflux transporters, our study may also provide a new perspective on using curcumin to modulate and improve pharmacokinetic profile and bioavailability of the conjugated metabolites and their aglycones.
MATERIAL AND METHODS
Chemicals
Resveratrol was purchased from LKT Laboratories (St. Paul, MN). Formononetin and curcumin were bought from LC Laboratories (Woburn, MA). Resveratrol conjugates were biosynthesized by incubating resveratrol with MRP2-overexpressing MDCK II cells. Pooled human liver and intestinal S9 fractions were obtained from XenoTech (Lenexa, KS).β-glucuronidases, sulfatase, 3′-phosphoadenosine 5′-phosphosulfate (PAPS), uridine diphosphoglucuronic acid (UDPGA), alamethicin, magnesium chloride and Hanks’balanced salt solution (powder form) were purchased from Sigma–Aldrich (St. Louis, MO). Ora-Plus oral suspending vehicle was obtained from Paddock Laboratories Inc (Minneapolis, MN). The BCA protein assay kit was from Thermo Fisher Scientific (Waltham, MA).
Cell Culture
Human UGT1A9-overexpressing HeLa cells and human MRP2-overexpressing MDCK II-UGT1A1 cells were engineered and routinely maintained in our lab. The UGT1A9-overexpressing HeLa cell line was successfully developed and used in our previous studies (39). MDCK II cells expressing human MRP2, obtained from NKI-AVL (Netherlands Cancer Institute, Amsterdam, NL), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum at 37°C under 5% CO2. UGT1A1 (NM_000463) human cDNA was cloned into pCMV-A- Hygro vector (Origene, Rochville, MD). Then the transfection of vector expressing human UGT1A1 into MDCK II cells was performed using LipofectAMINE (Invitrogen, Gaithersburg, MD). At 50% confluence, cells in 150-mm dishes were exposed to serum-free DMEM containing plasmid (1 μg/ml) and LipofectAMINE (1 μg/ml). At 5 h after the initiation of transfection, the plasmid-LipofectAMINE complex was removed, and the medium, consisting of DMEM supplemented with 10% fetal bovine serum, was added. After 2–3 weeks of Hygromycin (200ug/mL) selection, single colonies were screened for the expression of UGT1A1 by Western blot analysis. For both cell lines, cells from 3 days post-seeding were used for the excretion experiments described below. The numbers of cells were correlated to protein concentrations of harvested cells, which were determined using a protein assay kit (Bio-Rad, Hercules, CA). To determine the intracellular concentrations based on amount of metabolites present inside cells, cell volume was estimated to be 4μl/mg protein as described previously (39).
Excretion Studies in vitro
The MRP2-overexpressing MDCKII-UGT1A1 cells and HeLa-UGT1A9 cells were washed twice with HBSS (Hank’s Balanced Salt Solution, PH=7.4) and pre-incubated with or without (control experiment) curcumin for 30 min at 37°C. The cells were then incubated with HBSS containing resveratrol with or without (control) curcumin for 4 hours. At pre-determined time points (0, 2, 4 h), 200μl of incubating media from each well was collected and equal volume of loading solutions was added to each well. The collected samples were then mixed with 50μl of Stop Solution (6% acetic acid in (94%) acetonitrile) containing formononetin as internal standard. At the end of experiment, cells were washed twice with ice-cold HBSS buffer and then removed and collected in 200μl of HBSS buffer. The collected cells were lysed by using an Aquasonic 150D sonicator (VWR Scientific, Bristol, CT) for 30min at the maximum power (135 average watts) in ice-cold water bath. After centrifugation at 15,500rpm for 20min, 100μl of supernatants were collected for each sample and mixed with 25μl of Stop Solution. For quantitative analysis, all samples were centrifuged at 15,500rpm for 15min, and 5μl of supernatant were injected into the UPLC-MS/MS system.
After incubating resveratrol with cells, the extent of metabolism was described as fmet value, which was the fraction of dose metabolized (eq.1).
| (1) |
Clearance of efflux transporter (CL) was calculated by dividing the excretion rate of phase II metabolites (J) by the intracellular concentration of metabolites (Ci) (eq.2).
| (2) |
Animals
Male wild-type FVB mice (6–10 weeks) were purchased from Harlan (Indianapolis, IN), and the male Bcrp1 knockout mice with an FVB genetic background (6–10 weeks) were from Taconic (Hudson, NY). All the animals were kept in an environmentally controlled room (temperature: 25±2°C, humidity: 50±5%, 12 h dark-light cycle) for at least 1 week before any experiments.
Pharmacokinetic Studies with Wild-type and Bcrp1 (−/−) Mice
FVB wild-type and Bcrp1 (−/−) mice were used in this study. The animal protocols were approved by the University of Houston’s Institutional Animal Care and Uses Committee. Animals were fasted overnight (approximately 12 hr) with free access to water before the day of experiment. Curcumin dissolved in DMSO/PEG400 (1:4) was given orally at the dose of 1000 mg/kg. After 1 h, a mixture of resveratrol and curcumin dispersed in oral suspending vehicle was given at the dose of 20 mg/kg (for each compound) orally. The second dose of curcumin with resveratrol was made to maintain a high level of curcumin in the gut. For control group, oral dose of resveratrol (20mg/kg) in suspending vehicle was given to mice without curcumin treatment. There were 3 to 4 mice in each group. After being anesthetized by isoflurane, blood samples (about 10μl) from mice were collected at pre-determined time points: 15, 30, 60, 120, 240, 360, 480, 1440min. The blood samples were then spiked with acetonitrile containing formononetin as the internal standard. After centrifugation at 15,500 rpm for 15min, the supernatant was evaporated to dryness and stored at −80°C. Before LC-MS/MS analysis, the residue was reconstituted in 15% acetonitrile solution and centrifuged at 15,500 rpm for 15min. 10μl of supernatant was injected into the LC-MS/MS.
Quantitation of Resveratrol and Its Metabolites Using LC-MS/MS
LC-MS/MS analysis was carried out on an API 5500 Qtrap triple quadrupole mass spectrometer (Applied Biosystem/MDS SCIEX, Foster City, California) with a TurboIonSpray™ source coupled to a Waters Ultra performance liquid chromatography (UPLC) system (Waters, Milford, MA). Quantification was performed by MRM (Multiple Reaction Monitoring) method in the negative ion mode. Data were processed using MultiQuant™ 2.0.2 Software. The instrument dependent parameters for mass spectrum were set as follows: ionspray voltage, −4.5 kV; ion source temperature, 600 °C; gas1, 20 psi; gas2, 30 psi; curtain gas, 10 psi. Unit mass resolution was set in both mass-resolving quadruples Q1 and Q3. Compound-dependent parameters for resveratrol and its metabolites were set as follows: declustering potential (DP), −120V; collision energy (CE), −26V; collision cell exit potential (CXP), −17V. The compound-dependent conditions for formononetin were: DP, −235V; CE, −29V; CXP, −15V. The LC column used was a 1.7μm C18 column (2.1×50mm) (AcQuity, BEH C18, CA). Flow rate was 0.45ml/min, and column temperature was maintained at 45°C. Mobile phase A was 2.5mM ammonium acetate (PH7.4) in water, whereas mobile phase B was acetonitrile. The gradient was: 0–0.5min, 7% B, 0.5–1.5min, 7–15% B, 1.5–3.5min, 15–50% B, 3.5–4.0min, 50–85% B, 4.0–4.5min, 85–7% B, 4.5–6.0min, 7% B.
Pharmacokinetic Calculations
Pharmacokinetic parameters of resveratrol and its metabolites including Cmax (maximum blood concentration), ke (elimination rate constant), t1/2 (half-life), AUC0-t (area under the blood concentration curve from time 0 to t) were obtained by using the noncompartmental model in WinNonlin 3.3 (Parsight, Mountain View, CA). To ensure the pharmacokinetic parameters are accurate and reasonable, we adopted the criteria from our previous study for noncompartmental fitting (29). Briefly, the value of t1/2 was not reported when it was longer than 12 h or the fitting goodness (r2) in the terminal phase was less than 0.8 or the extrapolated AUC was more than 30% of AUCinf. AUC0-t values were presented for the purpose of comparison between groups.
Glucuronidation and Sulfation of Resveratrol in Hepatic and Intestinal S9 Fractions
Pooled S9 fractions from human liver and intestine were bought from XenoTech (Lenexa, KS). Hepatic and intestinal S9 fractions from wild-type and Bcrp1 (−/−) mice were prepared as described before (40). Briefly, mice were fasted overnight and euthanized with ketamine. Intestine and liver were cut out and washed with ice-cold saline containing 1mM dithiothreitol. After segments of intestine were pooled from the same strain, they were washed with the ice-cold washing solution (consisted of 8 mM KH2PO4, 5.6 mM Na2HPO4, 1.5 mM KCl, 96 mM NaCl, 27 mM sodium citrate, and 0.04 mg/ml phenylmethylsulfonyl fluoride or PMSF). Mucosal cells were scraped from intestine and washed with homogenization buffer (consisted of 10 mM pH 7.4 KH2PO4, 250 mM sucrose, 1 mM EDTA, and 0.04 mg/ml PMSF). Mucosal cells and hepatic cells were collected and homogenized in a 4°C cold room. After 15min centrifugation in 9000Xg at 4°C, the fat layer and pellet were discarded and the supernatant was collected, aliquoted, and stored at −80°C until use.
For the glucuronidation reaction, resveratrol in potassium phosphate buffer was added into the reaction mixture with S9 fractions (final concentration: 1mg/ml), saccharolactone (4.4mM), magnesium chloride (0.88 mM), and alamethicin (0.022 mg/ml). UDP-glucuronic acid (UDPGA, 3.5 mM) was added last as the cofactor to make the total volume to 250 μl. For the sulfation reaction, 3′-phosphoadenosine-5′-phosphosufatethe (PAPS) was added (final PAPS concentration=0.1 mM as reaction cofactor instead of UDPGA. For both reactions, the mixture was incubated in a 37°C water bath for 15, 30, 60, 120, 240, and 360min. The reactions were stopped by adding Stop Solution containing 2 μM of formononetin as the internal standard. Each assay was performed in triplicate. After centrifugation, the supernatants of samples were analyzed by Waters ACQUITY UPLC system.
Statistical Analysis
Data were presented as means ± S.D., if not specified otherwise. Analysis of variance or Student’s t test was used to analyze data. The level of significance was set at p<0.05.
RESULTS
Characterization of Resveratrol Metabolites in S9 Fractions and Cell Lines from Different Species
MRM Chromatograms of resveratrol’s glucuronide and sulfate conjugates produced from S9 fractions, whole blood, and cells were shown in Supplemental Fig. S1. Glucuronides and sulfates were first identified by comparing UV absorbance profiles with resveratrol and then confirmed by enzymatic hydrolysis. Treatment of conjugates with β-glucuronidase or sulfatase converted glucuronides or sulfates back into resveratrol (data not shown). The conjugated metabolites were further confirmed by mass spectrometer. Resveratrol glucuronide had an ion [M-H]− at m/z 403, whichwas consistent with a glucuronic acid moiety (176 amu) added to the molecular ion of resveratrol of m/z 227. Resveratrol sulfate had an [M-H]− ion of m/z 307, which was 80 Da higher (addition of one sulfuric acid) than that of parent resveratrol. Resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, and resveratrol-4′-O-sulfate were identified by comparing the order of elution with that of chemically synthesized authentic resveratrol metabolites as were done in a previous study (41).
In pooled human liver S9 fraction, resveratrol-3-O-glucuronide and resveratrol-3-O-sulfate were the major metabolites generated. The amount of resveratrol-4′-O-glucuronide was about one fifth of resveratrol-3-O-glucuronide, and the amount of resveratrol-4′-O-sulfate was negligible when compared to that of resveratrol-3-O-sulfate. In pooled human intestinal S9 fraction, both resveratrol-3-O-glucuronide and resveratrol-4′-O-glucuronide were detected after glucuronidation reaction, whereas resveratrol-3-O-sulfate was the only sulfate detected in sulfation reaction. In mice S9 fractions (from liver and intestine) and whole blood (1 hr after oral dose of 20mg/kg), resveratrol-3-O-glucuronide and resveratrol-3-O-sulfate were found as the main products of phase II metabolism. After incubating resveratrol with human UGT1A9-overexpressing HeLa cells, resveratrol-3-O-glucuronide was found as the major metabolite and only small amount of resveratrol-4′-O-glucuronide and resveratrol-3-O-sulfate were produced. In MRP2-overexpressing MDCK II-UGT1A1 cells, four conjugates were detected, including resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide, resveratrol-3-O-sulfate, and resveratrol-4′-O-sulfate. In this MDCKII-UGT1A1 cell variant, resveratrol-3-O-sulfate was the most abundant metabolite, and the amounts of all the other metabolites were quite small.
Impact of Bcrp1 on Pharmacokinetics of Resveratrol and Its Phase II Metabolites
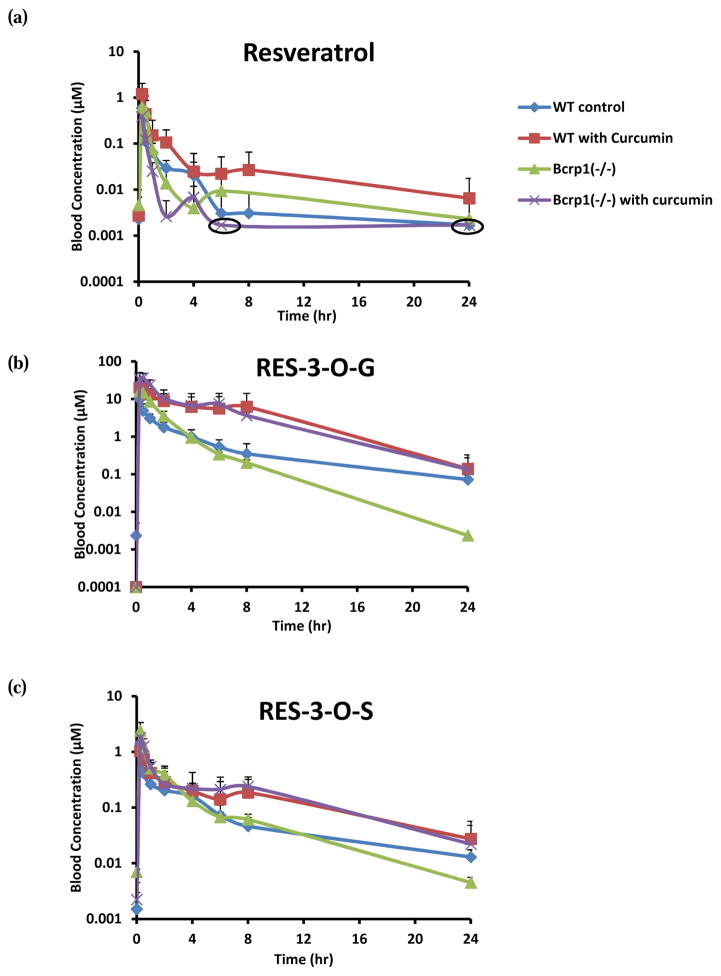
To investigate the role of Bcrp1 in pharmacokinetics and phase II disposition of resveratrol, resveratrol was dosed orally (20mg/kg) in WT and Bcrp1 (−/−) mice. RES-3-O-G and RES-3-O-S were identified as main circulating metabolites in mice. Pharmacokinetic profiles and parameters were shown in Fig. 1, Fig. 3, and Table 1. It was found that the Cmax, Tmax, and AUC of resveratrol were similar in WT and Bcrp1 (−/−) mice, whereas the systemic exposure of resveratrol conjugate metabolites increased moderately in Bcrp1 (−/−) mice. Cmax of resveratrol glucuronide increased from 60% (p>0.05) in Bcrp1 (−/−) mice. AUC of glucuronide conjugate increased 52% (p>0.05) Bcrp1 (−/−) mice. For resveratrol sulfate, our results showed an increase of 82% (p>0.05) in Cmax and 56% (p>0.05) in AUC in Bcrp1 (−/−) mice. Our observations, whereas not always statistically significant, were in agreement with previously published reports indicating an increase of plasma levels of resveratrol conjugates in Bcrp1(−/−) mice (23, 30). However, we did find that the half-life (t1/2) for resveratrol glucuronide decreased significantly in Bcrp1 (−/−) mice (Table 1), consistent with earlier reports of significantly increased plasma levels of resveratrol conjugates in Bcrp1(−/−) mice (23, 30).
FIG. 1.
The blood concentrations of resveratrol (a), RES-3-O-G (b), and RES-3-O-S (c) after oral administration of resveratrol (20mg/kg) with or without curcumin in wild-type (WT) and Bcrp1 (−/−) mice. The circled points represented data points where concentrations for some mouse blood samples fell below the quantitation limit.
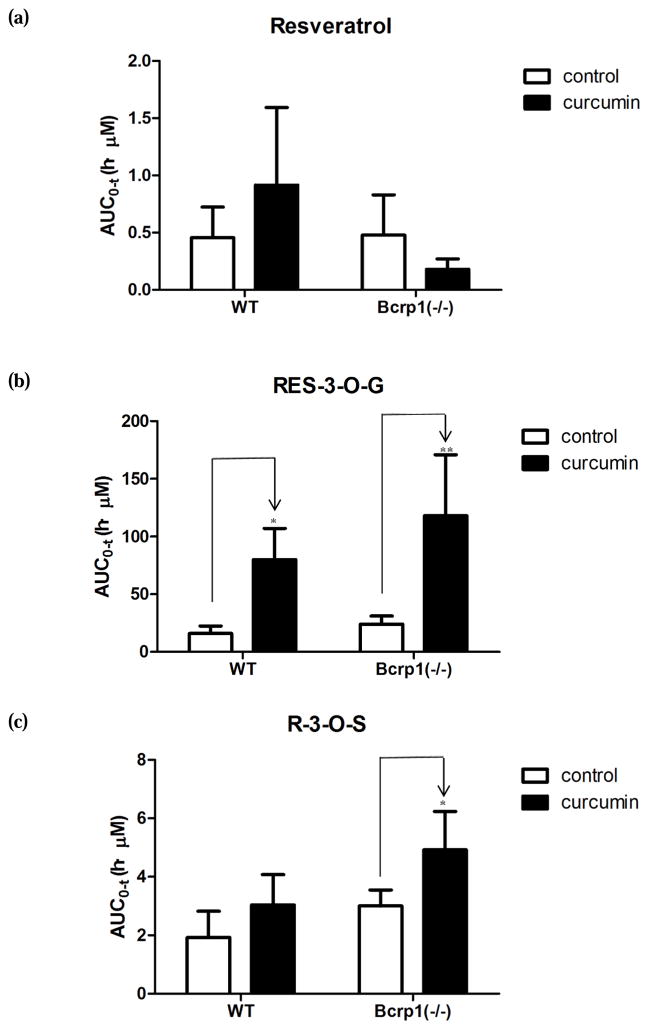
FIG. 3.
The AUC values of resveratrol and its conjugates in wild-type (WT) mice and Bcrp1 (−/−) mice after oral administration of resveratrol at 20mg/kg with or without the treatment of curcumin. Two-way ANOVA followed by post-hoc test was performed. *, p < 0.05; **, p < 0.01.
Table 1.
Pharmacokinetic parameters of resveratrol, RES-3-O-G and RES-3-O-S in WT and Bcrp1 (−/−) mice after oral administration of resveratrol (20mg/kg) with or without the curcumin treatment. Parameters in WT or Bcrp1 (−/−) mice without curcumin treatment were used as control.
| Strain/Treatment | Cmax (μM) | Tmax (h) | t1/2 (h) | |
|---|---|---|---|---|
| Resveratrol | WT control | 0.60 ± 0.26 | 0.25 ± 0.00 | N.A. |
| WT with curcumin | 1.20 ± 0.84 | 0.25 ± 0.00 | N.A. | |
| Bcrp1 (−/−) | 0.79 ± 0.44 | 0.31 ± 0.12 | N.A. | |
| Bcrp1 (−/−) with curcumin | 0.39 ± 0.17 | 0.25 ± 0.00 | N.A. | |
|
| ||||
| R-3-G | WT control | 9.99 ± 5.32 | 0.25 ± 0.00 | 3.69 ± 4.28 |
| WT with curcumin | 26.34 ± 7.16* | 0.37 ± 0.14 | 3.83 ± 1.20 | |
| Bcrp1 (−/−) | 16.09 ± 6.10 | 0.31 ± 0.12 | 1.92 ± 0.06* | |
| Bcrp1 (−/−) with curcumin | 18.46 ± 14.71 | 0.42 ± 0.14 | 3.12 ± 0.95 | |
|
| ||||
| R-3-S | WT control | 1.32 ± 0.71 | 0.25 ± 0.00 | 3.77 ± 1.74 |
| WT with curcumin | 1.21 ± 0.27 | 0.31 ± 0.12 | 9.32 ± 5.64 | |
| Bcrp1 (−/−) | 2.40 ± 0.98 | 0.25 ± 0.00 | 4.41 ± 0.44 | |
| Bcrp1 (−/−) with curcumin | 1.80 ± 0.17 | 0.25 ± 0.00 | 5.82 ± 2.55 | |
p < 0.05;
p < 0.01.
Hepatic and Intestinal Phase II Enzyme Activities toward Resveratrol in WT and Bcrp1 (−/−) Mice
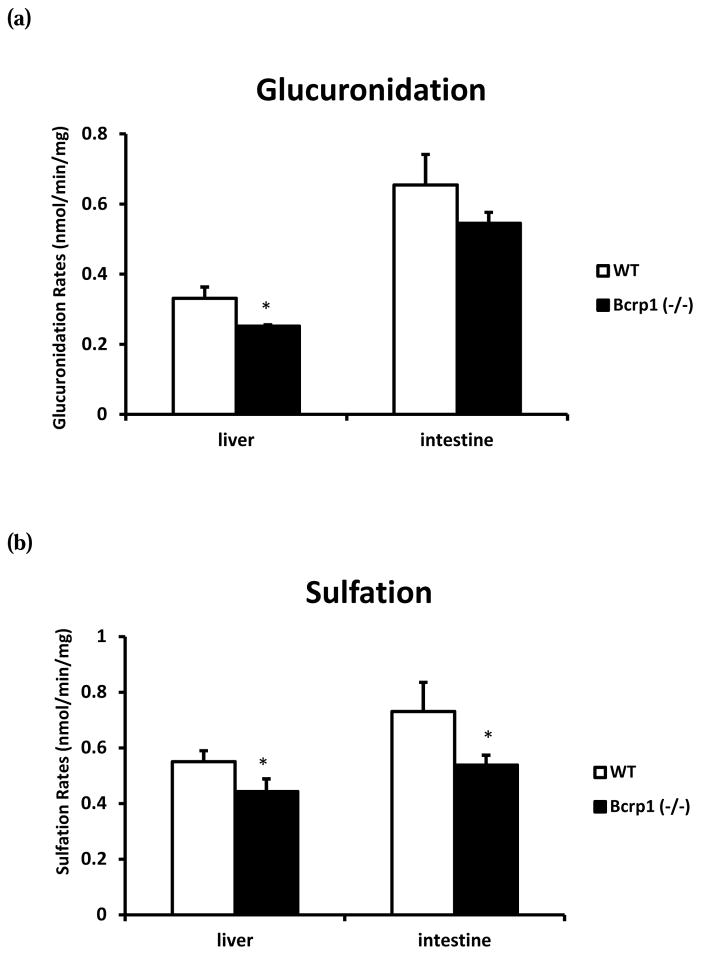
To further investigate the Bcrp1 knockout-related pharmacokinetic changes in mice, we also performed in vitro experiments to determine if the enzyme activities were altered in Bcrp1 (−/−) mice. As shown in Fig. 2, it was found that the rate of resveratrol glucuronidation in small intestinal S9 fraction was about two folds faster than in hepatic S9 fraction. The glucuronidation rates were significantly higher (32%) in the hepatic S9 fraction from WT mice than those from Bcrp1 (−/−) mice. Similarly, the rates of resveratrol glucuronidation were 18% higher in intestinal S9 fraction from WT mice than those from the knockout mice, but the differences were not statistically significant. Additionally, our data showed that the rate of resveratrol sulfation in S9 fraction from Bcrp1 (−/−) mice was significantly slower than in WT mice. In hepatic S9 fraction from Bcrp1 (−/−) mice, resveratrol sulfation decreased by 20%. And in intestinal S9 fraction, the sulfation rate decreased by 26%. The reasons for the mild reduction of glucuronidation and sulfation rates in S9 fractions from the knockout mice remained unknown, but they were not consistent with moderately higher levels of resveratrol conjugates present in the plasma of Bcrp knockout mice shown above.
FIG. 2.
The resveratrol (2.5 μM) glucuronidation rate (a) and sulfation rate (b) in hepatic and intestinal S9 fractions from WT and Bcrp1 (−/−) mice. *, p<0.05.
Impact of Curcumin on Pharmacokinetics of Resveratrol and Its Phase II Metabolites
Impact of curcumin treatment on pharmacokinetics of resveratrol and its conjugates in wild-type mice
As described in Materials and Methods, WT mice were pretreated with 1000mg/kg of curcumin 1 h prior to another dose of 20mg/kg of curcumin and 20mg/kg of resveratrol. As depicted in Fig. 1, Fig. 3, and Table 1, curcumin treatment enhanced the exposure of resveratrol and its metabolites in the systemic circulation of the WT mice. With curcumin, the maximum blood concentration of resveratrol achieved in mice increased nearly 100%. The Cmax values of resveratrol glucuronide significantly increased by ~2.6 folds when mice were treated with curcumin. For resveratrol sulfate, there was no obvious change in Cmax value. The AUC of resveratrol increased by 2 folds in curcumin-treated mice (Fig. 3). For resveratrol glucuronide, the AUC value increased 4.0-fold, and this change was much greater than the change of AUC observed for the parent resveratrol. We also observed 58% elevated AUC value for resveratrol sulfate in curcumin-treated mice. It was noticed that curcumin caused a slight increase (p>0.05) in t1/2 values for resveratrol glucuronide and sulfate, while there was almost no change in Tmax.
Impact of curcumin treatment on pharmacokinetics of resveratrol and its conjugates in Bcrp1 (−/−) mice
To investigate the potential impact of curcumin on transporters other than BCRP, we also determined the influence of curcumin on pharmacokinetics of resveratrol and its phase II metabolites in Bcrp1 (−/−) mice (Fig. 1, Fig. 3, and Table 1). In curcumin-treated mice, the Cmax of resveratrol and resveratrol sulfate decreased by 51% and by 25% compared with curcumin-untreated mice, respectively. The Cmax of resveratrol glucuronide remained similar when curcumin was dosed with resveratrol. Although the change in AUC values of resveratrol was not statistically significant, it was found that the AUC decreased moderately in curcumin-treated mice. As observed in WT mice, the AUC of resveratrol glucuronide increased significantly when the knockout mice were treated with curcumin. There was also a slight increase of AUC observed for resveratrol sulfate in curcumin-treated Bcrp1 (−/−) mice (Fig. 3). As observed in WT mice, we also noticed a slight increase in t1/2 for both resveratrol glucuronide and sulfate when mice were treated with curcumin. In the presence of curcumin, Tmax was slightly delayed (p>0/05) for resveratrol glucuronide.
Effects of Curcumin on MRP2-Mediated Efflux Transport of Resveratrol Metabolites in Human MRP2-overexpressing MDCK II-UGT1A1 Cells
Impact of curcumin on phase II metabolism of resveratrol in MDCK II cells
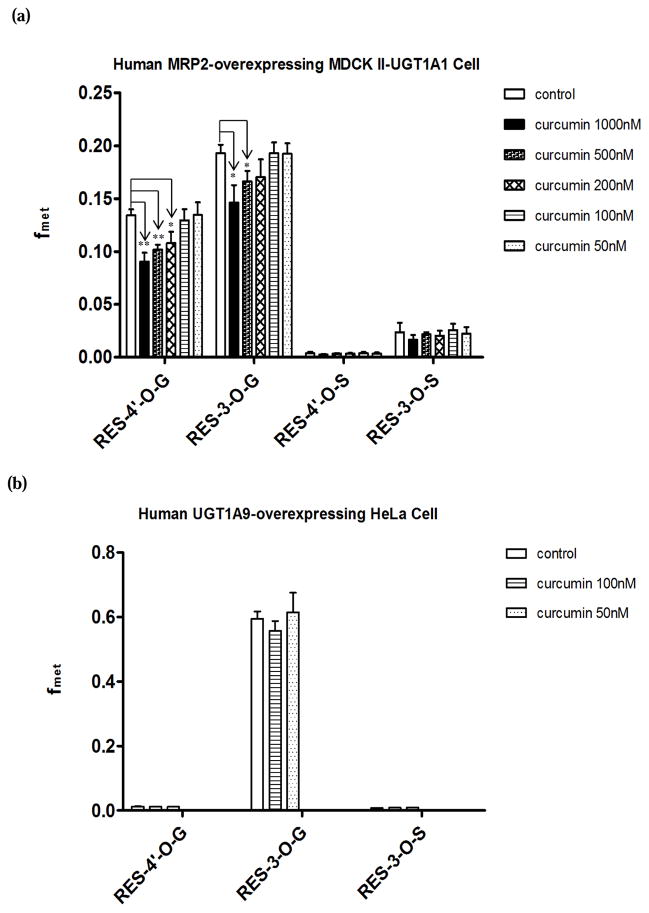
In this study, MRP2-overexpressing MDCK II-UGT1A1 cells were used as an in vitro cell model to further investigate the effects of curcumin on MRP2. Since it was reported that curcumin can also inhibit UGT and SULT mediated glucuronidation and sulfation (42, 43), different concentrations of curcumin (1000nM, 500nM, 200nM, 100nM, 50nM) were used to test the effect of curcumin on phase II metabolism of resveratrol in cells. The extent of glucuronidation and sulfation as measured by fmet reflects cells’ ability to metabolize a substrate. When fmet is not changed in the presence of an inhibitor, it means that the activities of the conjugating enzymes are not affected. As shown in Fig. 4 (a), fmet of glucuronide conjugates decreased significantly when cells were treated with high concentrations of curcumin. For fmet of RES-4′-O-G, the decrease was about 33% (1000nM, p<0.01), 24% (500nM, p<0.01), and 20% (200nM, p<0.05). The reduction in fmet values of RES-3-O-G was approximately 24% (1000nM, p<0.05), 14% (500nM, p<0.05), and 12% (200nM, p>0.05). There was no significant change in fmet values for all metabolites when cells were treated with lower concentrations of curcumin. Therefore, 100nM and 50nM of curcumin was selected to investigate the effects of curcumin on efflux transporters (below).
FIG. 4.
Effects of different concentrations of curcumin on the fmet of resveratrol conjugate metabolites in human MRP2-overexpressing MDCK II-UGT1A1 (a) and human UGT1A9-overexpressing HeLa (b) cells. Cells were treated with 2.5μM of resveratrol (control) or a mixture of 2.5μM of resveratrol and different concentrations of curcumin (1000nM, 500nM, 200nM, 100nM, 50nM) for 4 h, and the fmet values were calculated by eq.1. Student t-test was performed. *, p < 0.05; **, p < 0.01.
Impact of curcumin on MRP2-mediated efflux transport of resveratrol conjugated metabolites
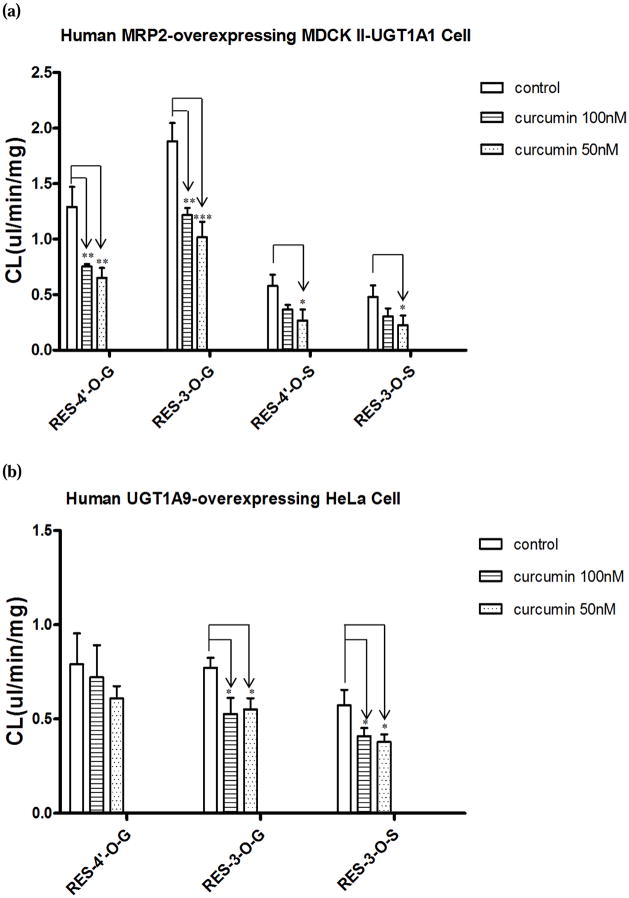
As shown in Fig. 5-a, cellular clearances (CL, eq.2) were inhibited significantly for all metabolites with curcumin treatment. In the presence of 50nM of curcumin, CL of RES-4′-O-G and RES-3-O-G was reduced by 50% (p<0.01) and 46% (p<0.001) respectively, whereas CL of RES-4′-O-S and RES-3-O-S reduced by 54% (p<0.05) and 53% (p<0.05) respectively. In the presence of higher concentration of curcumin (100nM), no greater concentration-dependent inhibitory effect on CL was observed.
FIG. 5.
Effects of curcumin (100nM, 50nM) on cellular clearances (CL) of resveratrol glucuronides and sulfates in human MRP2-overexpressing MDCK II-UGT1A1 (a) and human UGT1A9-overexpressing HeLa (b) cells. Cells were treated with 2.5μM of resveratrol with blank vehicle (control) or a mixture of 2.5μM of resveratrol and curcumin (100nM, 50nM) for 4 h, and the CL values were calculated by eq.2. ANOVA followed by post-hoc test was performed. *, p < 0.05; **, p < 0.01.
Effects of Curcumin on BCRP-mediated Efflux Transport of Resveratrol Metabolites in Human UGT1A9-overexpressing HeLa Cells
In human UGT1A9-overexpressing HeLa cells, RES-4′-O-G, RES-3-O-G, and RES-3-O-S were identified as resveratrol conjugate metabolites. Curcumin (100nM and 50nM) showed no significant inhibition on fmet for all the conjugates (Fig. 4 (b)). As illustrated in Fig. 5-b, curcumin did not show any significant inhibitory effect on CL of RES-4′-G. However, we found that the treatment of 50nM of curcumin resulted in a statistically significant decrease in CL for both RES-3-O-G and RES-3-O-S. In the presence of curcumin (50nM), CL of RES-3-O-G and RES-3-O-S decreased by 28% (p<0.01) and 35% (p<0.05) respectively. When the concentration of curcumin was increased to 100nM, there was no greater extent of inhibitory effect of curcumin on CL observed.
DISCUSSION
In this study, an elevated systemic (i.e., blood) exposure of resveratrol metabolites was achieved in wild-type and Bcrp1 knockout mice when animals were treated with curcumin. Curcumin achieved above-stated effects by inhibiting efflux transporters including MRP2 and BCRP, thereby promoting the distribution of phase II metabolites of resveratrol into the systemic circulation (i.e., increased AUC and Cmax). To our best knowledge, this is the first study showing the impact of curcumin on phase II disposition as an efflux transporter inhibitor. Our study provided a new strategy of using curcumin to increase the systemic exposure of resveratrol in vivo, which could be expanded to other co-administered drugs using the similar disposition mechanisms.
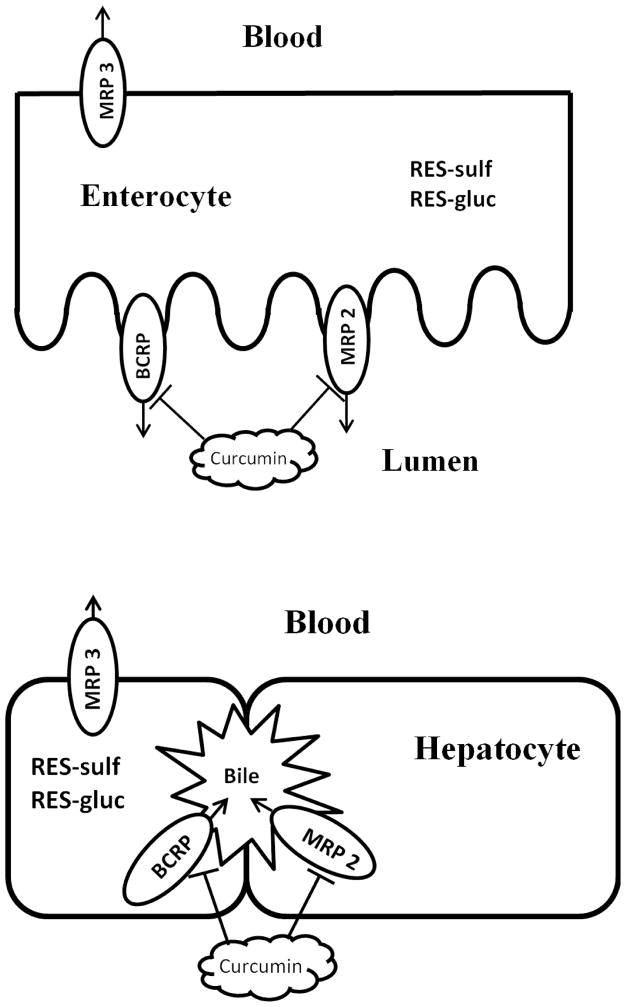
Like most of the phenolic compounds, resveratrol can be absorbed easily because of its lipophilic properties. Once resveratrol is in enterocytes, it can be metabolized to phase II conjugates (glucuronide and sulfate). Because of their hydrophilic properties, the conjugates need to be transported by transporters located on apical or basolateral membrane to exit cells. In enterocytes, curcumin can block efflux transporters on apical side and more conjugates will enter the blood as a result. For parent compound escaped from intestinal metabolism, it has another chance to be metabolized in hepatocytes. In liver, curcumin can also inhibit efflux transporters located on canalicular membrane and driving more intracellular conjugates into the blood. Therefore, as shown in Fig. 6, curcumin has an impact on the polarized distribution of resveratrol conjugates in enterocytes, hepatocytes, intestinal lumen, bile, and the systemic circulation. However, because curcumin concentrations are much higher in the gut than the liver, its impact on gut disposition of resveratrol are likely to be more substantial.
FIG. 6.
An illustration of the impacts of curcumin on polarized distribution of resveratrol glucuronide and sulfate in enterocytes and hepatocytes.
We found that AUC of resveratrol glucuronide increased significantly (~4-fold) when WT mice were treated with curcumin. In addition, there was also a moderate elevation in systemic exposure of resveratrol and resveratrol sulfate (Fig. 1 and Table 1). When Bcrp1 (−/−) mice were treated with curcumin, a further increase of AUC was observed for glucuronide (~6.4-fold increase) and sulfate (~1.56-fold increase) compared to the AUC values obtained in WT mice without curcumin treatment. These pharmacokinetic changes could be explained by the inhibitory effects of curcumin on BCRP and other transporters including MRP2, which was further confirmed in our in vitro findings (Fig. 5) (see later). The fact that the treatment of curcumin had greater impact on pharmacokinetics of resveratrol conjugates suggested that efflux transporters played a major role in the phase II disposition of resveratrol. These results suggest that the inhibitory effect of curcumin on MRP2 or other efflux transporters might make a greater contribution to the alteration in phase II disposition of resveratrol than BCRP. However, BCRP did have significant effects on its phase II disposition as well.
Impact of curcumin on resveratrol’s pharmacokinetics could be explained at least in part by our in vitro findings (Fig. 5), where 50nM and 100 nM curcumin significantly inhibited the glucuronide and sulfate efflux (as measured by CL) without impacting fmet in MRP2-overexpressing MDCK II-UGT1A1 cells (Fig. 5a). The latter overexpresses human MRP2 but also expresses canine BCRP. It is possible that curcumin effects on efflux transporters could be more pronounced, since MRP2-MDCKII cells were capable of metabolizing curcumin (36, 44). In human UGT1A9-overexpressing HeLa cells where only BCRP is significantly expressed, curcumin was also found to decrease the CL of RES-3-O-G and RES-3-O-S significantly (Fig. 4–b, Fig. 5–b), which was consistent with reported mechanism of ABCG2 inhibition; i.e., direct inhibition of ABCG2 (33). Based on our observations, we concluded that both MRP2- and BCRP-mediated transport of resveratrol phase II conjugates could be inhibited by curcumin, and greater impact of curcumin on cellular clearance was observed in MRP2-overexpressing MDCK II-UGT1A1 cells. We expect that curcumin’s impact on the disposition of resveratrol conjugates is mainly through inhibiting MRP2. To further test our hypothesis, however, the expression levels and activities of both efflux transporters in two cells lines need to be determined, and in vivo studies in Mrp2 knockout animals need to be performed.
It had been shown that the knockout of Bcrp1 slightly decrease the activities of relevant UGTs and SULTs in both liver and intestine (Fig. 2). In addition, according to our observations in cells (Fig. 4a) as well as other previous in vitro studies, curcumin was able to inhibit glucuronidation and sulfation (42, 45, 46). Therefore, the elevated systemic exposure of resveratrol metabolites observed in this study could not come from the increases in UGT or SULT enzyme activities. In Bcrp1 knockout and curcumin-treated animals, the distribution of resveratrol metabolites were altered by the inhibition of efflux transporters, which resulted in more metabolites distributed into blood (transported by MRP3 on basolateral membrane), since these conjugates are, at least partially, prevented from excretion from the normal excretion pathway. In fact, similar pharmacokinetic changes for genistein’s phase II metabolites were observed in Bcrp1 (−/−) mice. It was shown that the absent of Bcrp1 did not change the absorption process for genistein. The increased systemic exposures of genistein glucuronides and sulfates were from the impact on the polarized distribution of conjugates across the intestinal membrane and hepatocytes, which resulted in the increased distribution of phase II metabolites into the mouse systemic circulation in Bcrp1 (−/−) mice (29).
In previous studies, a variety of strategies were attempted to increase the bioavailability of resveratrol. For example, piperine was shown to be able to increase the AUC of resveratrol when it was co-administered with resveratrol in mice, and the enhanced systemic exposure of resveratrol was attributed to piperine’s inhibitory effects on UGT (47). Another study focused on using nanotechnological formulations to improve the bioavailability of resveratrol (48). To the best of our knowledge, this is the first study to improve the bioavailability of resveratrol by modulating efflux transporters for its conjugates. According to our observations, curcumin treatment not only improved the systemic exposure of resveratrol, but also increased the AUC of resveratrol glucuronide and sulfate significantly (Fig. 3). Considering the fact that the activity of resveratrol metabolites also contributes to its biological effects (18, 19), our new strategy of using curcumin with resveratrol as a transporter inhibitor may provide a promising approach to improve the pharmacological activities of resveratrol.
Our finding is quite insightful since there have been only scattered studies on the effect of curcumin on efflux transporters in vivo. After Shukla et al. reported that co-administration of curcumin increased the bioavailability of sulfasalazine by inhibiting Bcrp1 using Bcrp1 (−/−) mice (37), Kusuhara, H., et al. performed interaction studies in humans (49). They showed that the plasma concentrations of sulphasalazine also increased in healthy humans when curcumin was administered. Our study is the first one to investigate the impact of curcumin as a transporter inhibitor in the disposition of phase II metabolites, which are often effluxed by transporters such as BCRP and MRPs. Although the bioavailability of curcumin is very low, it has been shown that a plasma level of 0.6 μM of curcumin were achieved after giving 1000mg/kg (the same dose as it was used in our study) of curcumin orally in mice (50). Since curcumin showed inhibitory effects at very low concentrations (as low as 50 nM) in our in vitro studies (Fig. 5), the levels of curcumin in gut and liver can be high enough to affect the distribution of resveratrol conjugates. However, the inhibitory impact of curcumin on transporters in gut is expected to be much higher than in liver because of curcumin’s low bioavailability. The bioavailability of curcumin is very low because of its low solubility and extensive metabolism. Due to its low solubility and the high dose of curcumin we used in our animal studies, curcumin was being absorbed continuously in the gut until it was excreted. In spite of its extensive metabolism, the plasma concentration of curcumin did not decrease rapidly, and a plateau appeared approximately 2-hour after the administration in the concentration-time profile most probably due to the prolonged absorption phase and/or enterohepatic recirculation (50–52). According to previous studies, the liver to plasma exposure ratio for curcumin was reported to be more than 40:1, and the plateau concentration in plasma reached 0.12 μg/ml in mice dosed with 1000mg/kg of curcumin (50). Therefore, curcumin can reach its effective dose to inhibit transporters in liver as long as that there is more than 0.4% of free curcumin (concentration higher than 50 nM) available in liver tissue.
Earlier researches have explored the role of BCRP and MRP2 in the disposition of resveratrol conjugates (23, 24, 30). For example, there were experiments with Mrp2-deficient rats demonstrated that Mrp2 was the major transporter to mediate the canalicular efflux of RES-3-O-G into bile (22). In present study, we believe that curcumin affects the disposition of resveratrol mainly through inhibiting MRP2. We noticed the blood concentrations of both resveratrol glucuronide and sulfate reached a plateau at 3 h after oral gavage when WT or Bcrp1 (−/−) mice were treated with curcumin. This observation could be explained by the delayed clearance of phase II metabolites due to curcumin’s inhibitory effects on MRP2 and other efflux transporters located in intestine and liver. We also observed an unexpected decrease in AUC of resveratrol in curcumin treated Bcrp1 (−/−) mice. However, the decrease was not significant due to large variations among animals. To further demonstrate the impact of curcumin through inhibiting MRP2, we would conduct studies in Mrp2 (−/−) mice for future research. Meanwhile, we also had to take into consideration the fact that other efflux transporters could be overexpressed as a compensation for the knockout of BCRP. In that case, the inhibitory effects on transporters from curcumin treatment could be underestimated. Besides BCRP and MRP2, MRP3 and MRP4 located on basolateral membranes have been shown to be involved in the transport of various phase II conjugates (53–56).
In Bcrp1 (−/−) mice, significantly more resveratrol metabolites have been found in blood. For example, we found that the AUC of RES-3-O-G and RES-3-O-S in Bcrp1 (−/−) mice increased (p<0.05) by 50% and 56%, respectively, even though AUC of resveratrol aglycone was not altered significantly (Fig. 1 and Table 1). Our observation was in agreement with previously published results (23, 30). Our data also showed that resveratrol glucuronide was eliminated at a faster rate and had a shorter t1/2 in Bcrp1 (−/−) mice, and this could be the result of less extent of enterohepatic recycling in Bcrp1 deficient mice. Unlike resveratrol conjugates, the AUC of resveratrol in Bcrp1 (−/−) mice did not increase or even decreased as Alfaras et al. reported earlier, and the reason for this observation was unclear.
In humans, the most abundant conjugated products of resveratrol were identified as RES-3-O-G, RES-3-O-S, and RES-4′-O-G (16), which was consistent with our findings in hepatic and intestinal S9 fractions from human (Supplemental Fig. S1). However, only one glucuronide (RES-3-G) and one sulfate (RES-3-S) were found in S9 fraction or whole blood from mice (Supplemental Fig. S1). As RES-4′-O-G was produced in less amount in human compared with RES-3-O-G, we thought mouse would be considered an appropriate animal model to study the in vivo disposition of phase II resveratrol conjugates. In human UGT1A9-overexpressing HeLa cells, the fmet of resveratrol glucuronide was about 2~3-fold of what was observed in MRP2-overexpressing MDCK II-UGT1A1 cells (Fig. 4), indicating UGT1A9 might be more important for resveratrol glucuronidation than UGT1A1.
Since it has been suggested that MDCK cells have low endogenous expression of transporters, many MDCK cell lines over-expressing different membrane transporters of interest have been employed to study the effect of individual transporter on drug disposition (57). Here we used human MRP2-overexpressing MDCK II-UGT1A1 cells as in vitro tool to generate resveratrol glucuronides and sulfates inside cells and to investigate the impact of curcumin on MRP-2 mediated efflux of resveratrol conjugates. To study the impact of curcumin on BCRP-mediated efflux of resveratrol conjugates, we also used HeLa-UGT1A9 cells as an in vitro tool to determine the clearance of conjugates by BCRP. Our previous studies showed that this simple cell model mainly efflux phenolic glucuronides using BCRP as inhibition of this transporter decreased phenolic glucuronide clearance by more than 95%. Since UGT1A1 and UGT1A9 were demonstrated to be the predominant UGT isoforms responsible for the formation of RES-3-O-G and RES-4′-O-G in human liver and intestine (58), and appropriate amount of sulfates were produced in our engineered MDCK II and HeLa cells, it was reasonable to use these cell lines overexpressing human UGT1A1 or UGT1A9 as in vitro tools to study the efflux behaviors of resveratrol conjugates.
CONCLUSION
In conclusion, curcumin treatment altered the distribution of phase II metabolites of resveratrol, resulting in more metabolites distributed into the systemic circulation and significantly higher AUC values of resveratrol conjugates in WT and Bcrp1 (−/−) mice. And in vitro, curcumin showed inhibitory effects on MRP2- and BCRP- mediated transport of resveratrol conjugates in HeLa and MDCK II cells that overexpress a human UGT and/or an efflux transporter. These results suggested that curcumin modulated the distribution of phase II resveratrol conjugates through inhibiting MRP2- and BCRP-mediated transport of resveratrol conjugates, but not altering UGT or SULT metabolism. Since this is the first study to explore the impact of curcumin on resveratrol phase II disposition, the use of curcumin provided a new approach to increase the bioavailability of resveratrol or other co-administered drugs that undergo similar disposition.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institutes of Health (GM070737) to MH.
ABBREVIATIONS
- RES-3-O-G
resveratrol-3-O-glucuronide
- RES-3-O-S
resveratrol-3-O-sulfate
- RES-4′-O-G
resveratrol-4′-O-glucuronide
- RES-4′-O-S
resveratrol-4′-O-sulfate
- ABC
ATP-binding cassette
- MRP
multidrug resistance-associated protein
- BCRP/Bcrp
breast cancer resistance protein
- WT
wild type
- UGT
UDP-glucuronosyltransferase
- SULT
sulfotransferase
- AUC
area under the plasma concentration curve
- HBSS
Hanks’ balanced salt solution
- PAPS
β-glucuronidases, sulfatase, 3′-Phosphoadenosine 5′-phosphosulfate
- UDPGA
uridine diphosphoglucuronic acid
- UPLC
ultraperformance liquid chromatography
- MS/MS
tandem mass spectrometry
- N.A
not available
References
- 1.Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. Journal of agricultural and food chemistry. 2002;50:431–435. doi: 10.1021/jf010812u. [DOI] [PubMed] [Google Scholar]
- 2.Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- 3.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 4.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 6.Bhatand JM, Pezzuto KP. Cancer chemopreventive activity of resveratrol. Ann N Y Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- 7.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clinica chimica acta; international journal of clinical chemistry. 1995;235:207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 8.Schouten A, Wagemakers L, Stefanato FL, van der Kaaij RM, van Kan JA. Resveratrol acts as a natural profungicide and induces self-intoxication by a specific laccase. Molecular microbiology. 2002;43:883–894. doi: 10.1046/j.1365-2958.2002.02801.x. [DOI] [PubMed] [Google Scholar]
- 9.Luand G, Serrero R. Resveratrol, a natural product derived from grape, exhibits antiestrogenic activity and inhibits the growth of human breast cancer cells. Journal of cellular physiology. 1999;179:297–304. doi: 10.1002/(SICI)1097-4652(199906)179:3<297::AID-JCP7>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Bove K, Lincoln DW, Tsan MF. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2002;291:1001–1005. doi: 10.1006/bbrc.2002.6554. [DOI] [PubMed] [Google Scholar]
- 11.Berge G, Ovrebo S, Eilertsen E, Haugen A, Mollerup S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br J Cancer. 2004;91:1380–1383. doi: 10.1038/sj.bjc.6602125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Xu YX, Divine G, Janakiraman N, Chapman RA, Gautam SC. Disparate in vitro and in vivo antileukemic effects of resveratrol, a natural polyphenolic compound found in grapes. The Journal of nutrition. 2002;132:2076–2081. doi: 10.1093/jn/132.7.2076. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Deeb D, Media J, Divine G, Jiang H, Chapman RA, Gautam SC. Immunomodulatory activity of resveratrol: discrepant in vitro and in vivo immunological effects. Biochemical pharmacology. 2003;66:2427–2435. doi: 10.1016/j.bcp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 15.Baurand DA, Sinclair JA. Therapeutic potential of resveratrol: the in vivo evidence. Nature reviews Drug discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 16.Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Tseng SH, Lai HS, Chen WJ. Resveratrol-induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery. 2004;136:57–66. doi: 10.1016/j.surg.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Polycarpou E, Meira LB, Carrington S, Tyrrell E, Modjtahedi H, Carew MA. Resveratrol 3-O-D-glucuronide and resveratrol 4′-O-D-glucuronide inhibit colon cancer cell growth: evidence for a role of A3 adenosine receptors, cyclin D1 depletion, and G1 cell cycle arrest. Molecular nutrition & food research. 2013;57:1708–1717. doi: 10.1002/mnfr.201200742. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino J, Park EJ, Kondratyuk TP, Marler L, Pezzuto JM, van Breemen RB, Mo S, Li Y, Cushman M. Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. Journal of medicinal chemistry. 2010;53:5033–5043. doi: 10.1021/jm100274c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zunino SJ, Storms DH, Newman JW, Pedersen TL, Keen CL, Ducore JM. Dietary resveratrol does not delay engraftment, sensitize to vincristine or inhibit growth of high-risk acute lymphoblastic leukemia cells in NOD/SCID mice. Int J Oncol. 2012;41:2207–2212. doi: 10.3892/ijo.2012.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Islam MA, Bekele R, Vanden Berg JH, Kuswanti Y, Thapa O, Soltani S, van Leeuwen FX, Rietjens IM, Murk AJ. Deconjugation of soy isoflavone glucuronides needed for estrogenic activity. Toxicology in vitro : an international journal published in association with BIBRA. 2015;29:706–715. doi: 10.1016/j.tiv.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 22.Maier-Salamon A, Hagenauer B, Reznicek G, Szekeres T, Thalhammer T, Jager W. Metabolism and disposition of resveratrol in the isolated perfused rat liver: role of Mrp2 in the biliary excretion of glucuronides. J Pharm Sci. 2008;97:1615–1628. doi: 10.1002/jps.21057. [DOI] [PubMed] [Google Scholar]
- 23.van de Wetering K, Burkon A, Feddema W, Bot A, de Jonge H, Somoza V, Borst P. Intestinal breast cancer resistance protein (BCRP)/Bcrp1 and multidrug resistance protein 3 (MRP3)/Mrp3 are involved in the pharmacokinetics of resveratrol. Mol Pharmacol. 2009;75:876–885. doi: 10.1124/mol.108.052019. [DOI] [PubMed] [Google Scholar]
- 24.Maier-Salamon A, Bohmdorfer M, Riha J, Thalhammer T, Szekeres T, Jaeger W. Interplay between metabolism and transport of resveratrol. Annals of the New York Academy of Sciences. 2013;1290:98–106. doi: 10.1111/nyas.12198. [DOI] [PubMed] [Google Scholar]
- 25.International Transporter C. Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nature reviews Drug discovery. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keppler D. Uptake and efflux transporters for conjugates in human hepatocytes. Methods in enzymology. 2005;400:531–542. doi: 10.1016/S0076-6879(05)00029-7. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno N, Takahashi T, Kusuhara H, Schuetz JD, Niwa T, Sugiyama Y. Evaluation of the role of breast cancer resistance protein (BCRP/ABCG2) and multidrug resistance-associated protein 4 (MRP4/ABCC4) in the urinary excretion of sulfate and glucuronide metabolites of edaravone (MCI-186; 3-methyl-1-phenyl-2-pyrazolin-5-one) Drug Metab Dispos. 2007;35:2045–2052. doi: 10.1124/dmd.107.016352. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez AI, Vallejo F, Barrera B, Merino G, Prieto JG, Tomas-Barberan F, Espin JC. Bioavailability of the glucuronide and sulfate conjugates of genistein and daidzein in breast cancer resistance protein 1 knockout mice. Drug Metab Dispos. 2011;39:2008–2012. doi: 10.1124/dmd.111.040881. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Zhu W, Gao S, Yin T, Jiang W, Hu M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab Dispos. 2012;40:1883–1893. doi: 10.1124/dmd.111.043901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alfaras I, Perez M, Juan ME, Merino G, Prieto JG, Planas JM, Alvarez AI. Involvement of breast cancer resistance protein (BCRP1/ABCG2) in the bioavailability and tissue distribution of trans-resveratrol in knockout mice. Journal of agricultural and food chemistry. 2010;58:4523–4528. doi: 10.1021/jf9042858. [DOI] [PubMed] [Google Scholar]
- 31.Shishodia S, Chaturvedi MM, Aggarwal BB. Role of curcumin in cancer therapy. Current problems in cancer. 2007;31:243–305. doi: 10.1016/j.currproblcancer.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Choi H, Chun YS, Kim SW, Kim MS, Park JW. Curcumin inhibits hypoxia-inducible factor-1 by degrading aryl hydrocarbon receptor nuclear translocator: a mechanism of tumor growth inhibition. Molecular pharmacology. 2006;70:1664–1671. doi: 10.1124/mol.106.025817. [DOI] [PubMed] [Google Scholar]
- 33.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol Cancer Ther. 2006;5:1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 34.Chearwae W, Anuchapreeda S, Nandigama K, Ambudkar SV, Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochemical pharmacology. 2004;68:2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Chearwae W, Wu CP, Chu HY, Lee TR, Ambudkar SV, Limtrakul P. Curcuminoids purified from turmeric powder modulate the function of human multidrug resistance protein 1 (ABCC1) Cancer chemotherapy and pharmacology. 2006;57:376–388. doi: 10.1007/s00280-005-0052-1. [DOI] [PubMed] [Google Scholar]
- 36.Wortelboer HM, Usta M, van Zanden JJ, van Bladeren PJ, Rietjens IM, Cnubben NH. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of alpha, beta-unsaturated carbonyl compounds. Biochemical pharmacology. 2005;69:1879–1890. doi: 10.1016/j.bcp.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Shukla S, Zaher H, Hartz A, Bauer B, Ware JA, Ambudkar SV. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009;26:480–487. doi: 10.1007/s11095-008-9735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–4499. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W, Xu B, Wu B, Yu R, Hu M. UDP-glucuronosyltransferase (UGT) 1A9-overexpressing HeLa cells is an appropriate tool to delineate the kinetic interplay between breast cancer resistance protein (BRCP) and UGT and to rapidly identify the glucuronide substrates of BCRP. Drug Metab Dispos. 2012;40:336–345. doi: 10.1124/dmd.111.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu W, Xu H, Wang SW, Hu M. Breast cancer resistance protein (BCRP) and sulfotransferases contribute significantly to the disposition of genistein in mouse intestine. The AAPS journal. 2010;12:525–536. doi: 10.1208/s12248-010-9209-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel KR, Brown VA, Jones DJ, Britton RG, Hemingway D, Miller AS, West KP, Booth TD, Perloff M, Crowell JA, Brenner DE, Steward WP, Gescher AJ, Brown K. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer research. 2010;70:7392–7399. doi: 10.1158/0008-5472.CAN-10-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volak LP, Ghirmai S, Cashman JR, Court MH. Curcuminoids inhibit multiple human cytochromes P450, UDP-glucuronosyltransferase, and sulfotransferase enzymes, whereas piperine is a relatively selective CYP3A4 inhibitor. Drug Metab Dispos. 2008;36:1594–1605. doi: 10.1124/dmd.108.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vietri M, Pietrabissa A, Mosca F, Spisni R, Pacifici GM. Curcumin is a potent inhibitor of phenol sulfotransferase (SULT1A1) in human liver and extrahepatic tissues. Xenobiotica; the fate of foreign compounds in biological systems. 2003;33:357–363. doi: 10.1080/0049825031000065197. [DOI] [PubMed] [Google Scholar]
- 44.Wortelboer HM, Usta M, van der Velde AE, Boersma MG, Spenkelink B, van Zanden JJ, Rietjens IM, van Bladeren PJ, Cnubben NH. Interplay between MRP inhibition and metabolism of MRP inhibitors: the case of curcumin. Chemical research in toxicology. 2003;16:1642–1651. doi: 10.1021/tx034101x. [DOI] [PubMed] [Google Scholar]
- 45.Abe Y, Fujiwara R, Oda S, Yokoi T, Nakajima M. Interpretation of the effects of protein kinase C inhibitors on human UDP-glucuronosyltransferase 1A (UGT1A) proteins in cellulo. Drug metabolism and pharmacokinetics. 2011;26:256–265. doi: 10.2133/dmpk.DMPK-10-RG-121. [DOI] [PubMed] [Google Scholar]
- 46.Basu NK, Kole L, Basu M, Chakraborty K, Mitra PS, Owens IS. The major chemical-detoxifying system of UDP-glucuronosyltransferases requires regulated phosphorylation supported by protein kinase C. J Biol Chem. 2008;283:23048–23061. doi: 10.1074/jbc.M800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Johnson JJ, Nihal M, Siddiqui IA, Scarlett CO, Bailey HH, Mukhtar H, Ahmad N. Enhancing the bioavailability of resveratrol by combining it with piperine. Molecular nutrition & food research. 2011;55:1169–1176. doi: 10.1002/mnfr.201100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frozza RL, Bernardi A, Paese K, Hoppe JB, da Silva T, Battastini AM, Pohlmann AR, Guterres SS, Salbego C. Characterization of trans-resveratrol-loaded lipid-core nanocapsules and tissue distribution studies in rats. Journal of biomedical nanotechnology. 2010;6:694–703. doi: 10.1166/jbn.2010.1161. [DOI] [PubMed] [Google Scholar]
- 49.Kusuhara H, Furuie H, Inano A, Sunagawa A, Yamada S, Wu C, Fukizawa S, Morimoto N, Ieiri I, Morishita M, Sumita K, Mayahara H, Fujita T, Maeda K, Sugiyama Y. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. British journal of pharmacology. 2012;166:1793–1803. doi: 10.1111/j.1476-5381.2012.01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pan MH, Huang TM, Lin JK. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab Dispos. 1999;27:486–494. [PubMed] [Google Scholar]
- 51.Maiti K, Mukherjee K, Gantait A, Saha BP, Mukherjee PK. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. International journal of pharmaceutics. 2007;330:155–163. doi: 10.1016/j.ijpharm.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 52.Yang KY, Lin LC, Tseng TY, Wang SC, Tsai TH. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. Journal of chromatography B, Analytical technologies in the biomedical and life sciences. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Brand W, Oosterhuis B, Krajcsi P, Barron D, Dionisi F, van Bladeren PJ, Rietjens IM, Williamson G. Interaction of hesperetin glucuronide conjugates with human BCRP, MRP2 and MRP3 as detected in membrane vesicles of overexpressing baculovirus-infected Sf9 cells. Biopharmaceutics & drug disposition. 2011;32:530–535. doi: 10.1002/bdd.780. [DOI] [PubMed] [Google Scholar]
- 54.Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J Pharmacol Exp Ther. 2004;309:156–164. doi: 10.1124/jpet.103.062091. [DOI] [PubMed] [Google Scholar]
- 55.Kitamura Y, Kusuhara H, Sugiyama Y. Functional characterization of multidrug resistance-associated protein 3 (mrp3/abcc3) in the basolateral efflux of glucuronide conjugates in the mouse small intestine. J Pharmacol Exp Ther. 2010;332:659–666. doi: 10.1124/jpet.109.156943. [DOI] [PubMed] [Google Scholar]
- 56.Zamek-Gliszczynski MJ, Nezasa K, Tian X, Bridges AS, Lee K, Belinsky MG, Kruh GD, Brouwer KL. Evaluation of the role of multidrug resistance-associated protein (Mrp) 3 and Mrp4 in hepatic basolateral excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in Abcc3−/− and Abcc4−/− mice. J Pharmacol Exp Ther. 2006;319:1485–1491. doi: 10.1124/jpet.106.110106. [DOI] [PubMed] [Google Scholar]
- 57.Guo A, Marinaro W, Hu P, Sinko PJ. Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT) Drug Metab Dispos. 2002;30:457–463. doi: 10.1124/dmd.30.4.457. [DOI] [PubMed] [Google Scholar]
- 58.Brill SS, Furimsky AM, Ho MN, Furniss MJ, Li Y, Green AG, Bradford WW, Green CE, Kapetanovic IM, Iyer LV. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. The Journal of pharmacy and pharmacology. 2006;58:469–479. doi: 10.1211/jpp.58.4.0006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.