Abstract
Pancreatic cancer is projected to become the second leading cause of cancer-related death in the United States by 2020. A familial aggregation of pancreatic cancer has been established, but the cause of this aggregation in most families is unknown. To determine the genetic basis of susceptibility in these families, we sequenced the germline genome of 638 familial pancreatic cancer patients. We also sequenced the exomes of 39 familial pancreatic adenocarcinomas. Our analyses support the role of previously identified familial pancreatic cancer susceptibility genes such as BRCA2, CDKN2A and ATM, and identify novel candidate genes harboring rare, deleterious germline variants for further characterization. We also show how somatic point mutations that occur during hematopoiesis can affect the interpretation of genome-wide studies of hereditary traits. Our observations have important implications for the etiology of pancreatic cancer and for the identification of susceptibility genes in other common cancer types.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a devastating disease, with a reported five-year survival rate of seven percent (1). Over 48,000 PDACs are estimated to be diagnosed in the United States in 2015. Of these, up to 10% occur in families with at least two affected first-degree relatives, and these are designated familial pancreatic cancers (FPC) (2). Individuals with a family history of PDAC carry a 2.3 to 32-fold increased risk of developing the disease, depending upon number of affected family members (3). In some FPC kindreds, the aggregation of pancreatic cancer may be due to environmental factors or stochastic events, but many are thought to be caused by inherited genetic susceptibility (4).
Knowledge of the genes responsible for an inherited susceptibility to pancreatic cancer is important for a number of reasons. First, early detection can be targeted to mutation carriers, and pancreatic neoplasms detected at an earlier stage, when therapeutic interventions with curative potential are still available (5). Second, as most previously reported FPC susceptibility genes also increase risk for malignancies other than pancreatic cancer, these extra-pancreatic neoplasms can be screened for as well (6). Third, elucidation of the genetic basis of FPC susceptibility offers opportunities for personalized therapies, as demonstrated by patients whose pancreatic cancers harbor defects in homologous recombination arising from bi-allelic inactivation of BRCA1, BRCA2 or PALB2. In these patients, targeting DNA repair with poly(ADP-ribose) polymerase 1 (PARP-1) inhibitors, platinum compounds, or mitomycin C can result in major therapeutic benefits (7). Finally, identifying causal FPC genes will provide novel insights into PDAC tumorigenesis.
Recent advances in sequencing technology provide an unbiased way to search for the genes underlying disease susceptibility (8). Using this approach, PALB2 and ATM were identified as FPC susceptibility genes, together explaining 3 – 5% of FPC cases (8, 9). In a further 8 – 15% of FPC patients, the increased risk of pancreatic cancer can be attributed to 10 other previously reported FPC susceptibility genes, including BRCA1, BRCA2, CDKN2A, MLH1, MSH2, MSH6, PMS2, PRSS1, STK11, and TP53 (10–16). The genetic basis underlying disease susceptibility in the remaining 80 – 90% of FPC patients is unknown.
To explore the genetic basis of FPC in detail and identify candidate susceptibility genes, we performed whole genome sequencing on the germline DNA of 638 FPC patients from 593 kindreds. This sequencing was supplemented with the whole exome sequencing of surgically resected PDACs from 39 FPC patients. The results identify of novel candidate FPC susceptibility genes and validate the importance of established FPC genes. In addition, our results suggest that somatic mutations in hematologic malignancy driver genes can confound the findings of germline genomic sequencing studies in older populations. Finally, we provide an unprecedentedly large resource of deep, whole genome sequence data that can be used for pancreatic cancer research.
RESULTS
Sample selection and sequencing
638 patients with FPC (Table 1) were selected from 10 registries across North America. FPC patients known to have a deleterious variant in a previously reported FPC susceptibility gene were excluded from the study to maximize the opportunity to discover novel susceptibility genes. Whole genome sequencing generated an average of 135.6Gb of data per patient (range: 102.2 – 253.8Gb) resulting in an average coverage of 39.8-fold (range: 29.8 – 71.1) per genome, with 98.2% (range: 97.9 – 98.6%) and 96.0% (range: 92.8 – 97.2%) of bases covered at least 1x and 10x respectively. An average of 3,742,720 single nucleotide variants (SNVs) were identified per patient (range: 3,623,824 – 4,554,474) with 93.4% (range: 86.9 – 94.1%) of variants present in the database of single nucleotide polymorphisms (dbSNP) (17). The integrity of our pipeline for calling sequence variants was supported by the excellent agreement between whole genome sequencing and Illumina HumanOmni2.5 SNV array (99.2%; range: 99.0 – 99.3%). There were an average of 328,689 (range: 279,767 – 399,378) insertions and 343,418 (range: 305,159 – 421,483) deletions per patient. The insertions averaged 23 bp (range: 1 – 300 bp) and the deletions 11 bp (range: 1 – 300 bp). The genetic ancestry of FPC patients was determined using Local Ancestry in adMixed Populations (LAMP). FPC patients were predominantly of European ancestry (95.9%), but patients of African (2.8%), and Asian (1.3%) ancestry were also represented (Table 1). Identity-by-descent analysis confirmed expected familial relationships.
Table 1.
Characteristics of sequenced FPC patients
| Characteristic | Classificationa | Number |
|---|---|---|
| Cohort | FPC patients | 638 |
| FPC kindred | 593 | |
|
| ||
| Age | Less than 50 | 35 |
| 50–59 | 124 | |
| 60–69 | 214 | |
| 70–79 | 185 | |
| 80+ | 73 | |
| Unknown | 7 | |
|
| ||
| Genetic ancestry | African | 18 |
| Asian | 8 | |
| Caucasian | 612 | |
|
| ||
| Affected relatives | 2 | 358 |
| 3 | 196 | |
| 4 or more | 84 | |
|
| ||
| DNA origin | Blood | 454 |
| LCL | 158 | |
| Tissue | 26 | |
LCL - lymphoblastoid cell line.
Analysis of premature truncating variants
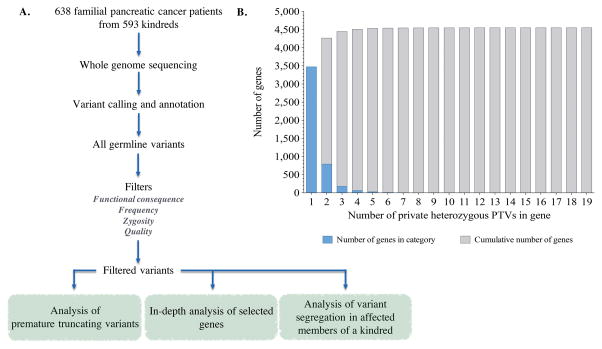
Given that most high-penetrance disease associated variants so far identified are located in coding regions (18), we focused our analyses on genetic variants located in these regions. The functional significance of missense variants is often unclear. We therefore began our analysis with premature truncating variants (PTVs), as these almost always affect protein function. As FPC is a rare disease and common PTVs are less likely confer a high risk of FPC susceptibility due to negative selective pressures, we concentrated our analyses on private heterozygous PTVs. We arbitrarily selected one sequenced member from each of the 593 FPC kindreds and positively-filtered variants using the following criteria (Fig. 1A): i) nonsense variants, splice site variants, and frameshift INDELs, ii) heterozygous in the germline, iii) less than 0.5% minor allele frequency in the 1000 Genomes Project or Exome Variant Server (EVS), iv) and present in only one FPC patient, i.e., “private” (19, 20). Finally, we selected high quality rare heterozygous PTVs by filtering for variants with: i) a mappability score of at least 0.5, and ii) no more than one additional genomic locus as assessed by BLAT (21, 22). Using these filters, we identified 6,114 private heterozygous PTVs, in 4,553 genes.
Figure 1.
A. Overview of filter-based strategy to identify novel candidate familial pancreatic cancer susceptibility genes. 638 familial pancreatic cancer (FPC) patients from 593 kindreds. FPC patients were selected from 10 high-risk family registries in North America with diverse ascertainment screens including: internet recruitments, medical genetics clinics, and tertiary care facilities. Demographic and sample data for the 638 FPC patients is shown in Table 1. FPC patient samples were whole genome sequenced, aligned to the human genome build hg19 before variant calling and annotation. One FPC patient from each kindred was arbitrarily selected for filter-based analyses. All germline variants in selected FPC patients were identified and, depending on analysis, filtered by: 1) functional consequence of variant, 2) frequency of the variant in FPC patients and variant databases (the 1000 Genomes Project and EVS), 3) zygosity of variant, and 4) variant quality. The following analyses were then performed on filtered variants: 1) analysis of premature truncating variants (PTVs), 2) in-depth analysis of selected genes, and 3) analysis of variant segregation in affected members of a kindred. B. Distribution of private heterozygous PTVs in FPC patients selected for filter-based analyses. Blue bars indicate number of genes within each PTV category. Grey bars indictate cumulative number of genes.
In order to identify novel FPC susceptibility genes, we then we ranked 20,049 coding genes by the number of private heterozygous PTVs that they harbored (Supplementary Table S1). Several of the 12 previously reported FPC susceptibility genes were highly ranked, providing support for this general approach. For example, the highest ranked gene was ATM with 19 private heterozygous PTVs. Similarly, PALB2 (five heterozygous PTVs) and CDKN2A (four heterozygous PTVs) were also ranked highly. While most genes harbored only one private heterozygous PTV and presumably do not play a common role in FPC susceptibility, 1,077 genes contained 2 or more private heterozygous PTVs (Fig. 1B). In particular, 16 genes previously identified as a FPC susceptibility gene, cancer driver gene, or DNA repair gene contained 3 or more private heterozygous PTVs and represent the most promising candidates for further study (Table 2) (23, 24).
Table 2.
Genes with three or more private heterozygous PTVs in unrelated FPC patients
| Gene | Number of heterozygous PTVs in FPC kindred | Comment |
|---|---|---|
| ATM | 19 | FPC susceptibility gene; Cancer driver gene; DNA repair gene |
| TET2 | 9 | Cancer driver gene |
| DNMT3A | 7 | Cancer driver gene |
| POLN | 6 | DNA repair gene |
| POLQ | 6 | DNA repair gene |
| ASXL1 | 5 | Cancer driver gene |
| BRCA2 | 5 | Cancer driver gene; DNA repair gene |
| PALB2 | 5 | FPC susceptibility gene; DNA repair gene |
| CDKN2A | 4 | FPC susceptibility gene; Cancer driver gene; DNA repair gene |
| FANCG | 4 | DNA repair gene |
| BUB1B | 3 | DNA repair gene |
| ESCO2 | 3 | DNA repair gene |
| FANCC | 3 | DNA repair gene |
| FANCM | 3 | DNA repair gene |
| MSH4 | 3 | DNA repair gene |
| RAD54L | 3 | DNA repair gene |
We detected private heterozygous PTVs in TET2 (n=9), DNMT3A (n=7), and ASXL1(n=5) (Table 2). Recent evidence has indicated that somatic mutations in genes contributing to hematologic malignancies are detectable in the blood of older individuals, suggesting a potentially pre-leukemic clonal hematopoiesis (25–28). As DNA used for whole genome sequencing was primarily derived from peripheral white blood cells (Table 1), when possible, we sequenced these mutations in DNA from a second non-blood source (two patients, FPC0072 and FPC0083 in Supplementary Table S2). In both cases, the mutation was not found or was found at much lower levels than observed in DNA from blood. Suggesting these mutations may be somatic in nature.
It is possible that rare heterozygous PTVs in our FPC cohort contribute to susceptibility, as would be the case for founder mutations. Allowing the same heterozygous PTV to occur in as many as 10 FPC patients (rather than in only one FPC patient) did not significantly change the outcome our analysis. Specifically, using the same FPC patients, 9,689 heterozygous PTVs across 5,116 genes were observed, and 80% of these were also identified when filtering for only private mutations.
In-depth analysis of selected genes
We conducted an in-depth analysis of 87 genes that included: i) previously reported FPC susceptibility genes, ii) genes associated with hereditary cancers and, iii) genes mutated in hereditary pancreatitis (Supplementary Table S3). As these genes had already been associated with disease, we were able expand our filter beyond just PTV’s to evaluate all variants based on their functional consequences, minor allele frequencies in the 1000 Genomes Project and EVS, and ClinVar classification (19, 20, 29).
We identified SNVs, insertions and deletions (INDELs) less than 300 bp in length, and structural variant deletions (SVDs) greater than 300 bp in length that affected the coding regions of these 87 genes. Variants were classified as either benign, of unknown significance (VUS), or deleterious according to the criteria detailed in Table 3. Among all 638 FPC patients sequenced, 92,933 sequence variants were identified in these 87 genes (Supplementary Table S4). Among the 593 unrelated FPC patients, 86,486 sequence variants were identified, 194 of which were defined as deleterious. In the 12 reported FPC susceptibility genes, there were 62 deleterious variants in 58 FPC kindreds (9.8% of FPC kindreds; 95% confidence interval: 7.6 – 12.4%). In 32 FPC patients, deleterious variants in two or more of the 87 genes analyzed in depth were observed (Supplementary Table S5). Of these patients, four had deleterious variants in two FPC susceptibility genes: one patient had an ATM and a PALB2 deleterious variant, one patient had two deleterious TP53 variants, and two patients had deleterious variants in both BRCA1 and BRCA2. A further, 17 patients had a deleterious variant in an FPC susceptibility gene in addition to a deleterious variant in a hereditary cancer or hereditary pancreatitis gene.
Table 3.
Classification of germline variants
| Classification | Variant type | MAF | ClinVar |
|---|---|---|---|
| Benign | Any | > 0.5% | - |
| Synonymous SNV | ≤0.5% | - | |
|
| |||
| VUS | Missense SNV | ≤0.5% | Not pathogenic or probable-pathogenic |
| In-frame INDEL | ≤0.5% | Not pathogenic or probable-pathogenic | |
|
| |||
| Deleterious | Frameshift INDEL | ≤0.5% | - |
| Nonsense SNV | ≤0.5% | - | |
| Splicing SNV or INDEL | ≤0.5% | - | |
| Missense SNV | ≤0.5% | Pathogenic or probable-pathogenic | |
| In-frame INDEL | ≤0.5% | Pathogenic or probable-pathogenic | |
| SV deletion | - | - | |
It should be noted that FPC patients known to have a deleterious variant in reported FPC susceptibly genes prior to the start of this study were not selected for sequencing. Therefore, our analysis underestimates the true prevalence of previously reported FPC susceptibility genes for which clinical testing is not uncommon, such as BRCA1, BRCA2, CDKN2A, and PALB2. At the time of patient selection, ATM was not commonly tested, and we identified 21 patients from 20 kindreds with deleterious variants in this gene (3.4% of FPC kindreds; 95% confidence interval: 2.2 – 5.2%).
In addition to publicly available data from the 1000 Genomes Project and EVS, we compared our findings in the 87 selected genes to whole exome sequencing data from 967 unrelated participants of European ancestry from the Bipolar Case Control Study (BCCS) (30). In BCCS samples, call rates across the 87 genes averaged 0.889. Structural variant data was not available from the BCCS, therefore analysis was limited to SNVs and INDELs (Supplementary Table S4 and Supplementary Table S6). First, we compared deleterious variants in the 593 FPC kindreds to BCCS samples. Five genes were associated with FPC at a point-wise level of 0.05: ATM (p-value = 1.2×10–7; Benjamini-Hochberg (q-) value = 1.1×10–5), CDKN2A (p-value = 8.8×10–6; q-value = 4.0×10–4), APC (p-value = 0.0174; q-value = 0.3786), PALB2 (p-value = 0.0079; q-value = 0.2290), and BRCA1 (p-value = 0.0317; q-value = 0.5523). In addition, five genes had p-values between 0.05 and 0.10: BUB1B (p-value = 0.0548; q-value = 0.6306), FANCC (p-value = 0.0548; q-value = 0. 6306), BRCA2 (p-value = 0.0671; q-value = 0. 6306), CPA1 (p-value = 0.0725; q-value = 0. 6306), and FANCG (p-value = 0.0725; q-value = 0. 6306). We then limited the analysis to 245 unrelated FPC patients from kindreds with three or more affected relatives (Supplementary Table S7). Five genes had a significant difference in the number of deleterious mutations at a point-wise level of 0.05: ATM (p-value = 4.4×10−6; q-value = 4.0×10−4), CDKN2A (p-value = 1.3×10−5; q-value = 5.0×10−4), APC (p-value = 0.0013; q-value = 0.0376), BUB1B (p-value = 0.0082; q-value = 0.1430), and PALB2 (p-value = 0.0082; q-value = 0.1430) (Supplementary Table S8). These associations remained significant when the analysis was restricted to individuals with greater than 80% European genetic ancestry. (Supplementary Table S8).
Analysis of variant segregation in affected members of a kindred
We hypothesized that a deleterious variant shared among family members with pancreatic cancer was more likely to be associated with pancreatic cancer susceptibility. Therefore, we assessed segregation of: i) private heterozygous PTVs across the exome, and ii) deleterious variants identified from in-depth analysis of hereditary cancer genes, in 38 FPC kindreds (83 FPC patients), where DNA from one affected family member was sequenced.
We identified 904 private heterozygous PTVs in the FPC patients of the 38 kindreds. Of these, 112 private heterozygous PTVs, in 110 genes, were present in all sequenced affected family members of a kindred and therefore segregated with PDAC (Supplementary Table S9). Most of these genes (70 of 110; 63.6%) were found to have private heterozygous PTVs in only a single FPC kindred. Of note, five of the 110 genes were previously associated with DNA repair or are cancer driver genes: ATM, CDKN2A, NUDT1, POLD1, and RECQL. However, only ATM and CDKN2A were found to have private heterozygous PTVs in more than one FPC kindred (23, 24).
Seventeen deleterious variants in one of the 87 genes analyzed in-depth occurred in an FPC patient from a family in which another affected family member had been sequenced. Deleterious variants included six frameshift deletions, two nonsense SNVs, two splice-site SNVs, and nine nonsynonymous SNVs (Supplementary Table S10). In 13 of the 17 cases, the deleterious variant did not perfectly segregate among affected family members. For example, we observed non-segregation of deleterious variants in ATM (one kindred with 1 of 2 affected members carrying a variant), CDKN2A (two kindreds each with 2 of 3 affected members carrying a variant), BRCA1 (one kindred with 1 of 2 members carrying a variant), and PALB2 (one kindred with 1 of 2 members carrying a variant).
Somatic alterations in familial pancreatic cancers
Hereditary cancer susceptibility genes are often tumor suppressors in which a deleterious variant in the germline of an individual is accompanied by a second somatic event resulting in bi-allelic loss of the gene in the tumor (18, 31). To help identify candidate susceptibility genes through the identification of such second somatic “hits”, we sequenced the exomes of 39 pancreatic cancers resected from FPC patients. Whole exome sequencing rather than whole genome sequencing was conducted because PDACs often contain a significant proportion of non-neoplastic cells (even after careful microdissection). Therefore, we could increase coverage to 100X, enhancing sensitivity of somatic mutation detection. Because of the low neoplastic content of these lesions, we did not identify losses of heterozygosity or changes in copy number, and examined only somatic mutations. Exome sequencing revealed 1,409 somatic mutations, with an average of 36 mutations per tumor (Supplementary Table S11). As expected, somatic mutations in KRAS and TP53 were the most common, occurring in 84.6% and 71.8%, respectively (Supplementary Table S12) (32). Other genes somatically mutated in the cancers included SMAD4 (33.3%) and CDKN2A (12.8%). The prevalence of KRAS, TP53, SMAD4, and CDKN2A mutations is similar to previous reports of both sporadic and familial pancreatic cancer (7, 32–34). Hereditary cancer genes were somatically mutated in the 39 PDACs, including FANCM in two tumors, and BRCA2, BUB1B, CREBBP, FLCN, PTCH1, PTEN, RB1, TSC2, and WAS in one tumor each (Supplementary Table S12). FPC patients with a somatic mutation in one of these genes did not have a deleterious germline variant in the same gene. Furthermore, one patient had a deleterious germline variant in a previously reported FPC susceptibility gene (FPC0347; PALB2) but did not have a second somatic mutation in the tumor. Although, loss-of-heterozygosity at this locus could not be ruled out.
Of the 4553 genes that harbored at least one private heterozygous PTV in our genome-wide analysis, 366 (8.0%) also were found to have a somatic mutation in at least one sequenced pancreatic tumor (Supplementary Table S1). Of these 366 genes, 113 had multiple private heterozygous PTVs and for 74 there were more private heterozygous PTVs in the FPC kindreds than similarly analyzed BCCS samples. Of note, five of these 74 genes, BUB1B, CDKN2A, RAD54L, RFC1, and TP53, are associated with DNA repair or known to be a cancer driver gene (Supplementary Table S1) (23, 24).
DISCUSSION
The genetic basis of familial pancreatic cancer is poorly defined. We conducted germline whole genome sequencing of 638 familial pancreatic cancer patients and demonstrate that inherited pancreatic cancer is highly heterogeneous. This heterogeneity has significant implications for the management of patients with a family history of this disease.
Our results provide strong evidence in support of previously reported FPC susceptibility genes, such as ATM, BRCA2, CDKN2A, and PALB2 elevating risk of pancreatic cancer. As well, our study suggests that deleterious variants in the candidate genes BUB1B, CPA1, FANCC, and FANCG, are more frequent in patients with familial pancreatic cancer. (Table 2 and Supplementary Table S8). Interestingly, many of these candidate genes are involved in processes regulating DNA repair or chromosomal stability, just as are the previously identified ATM, BRCA2, and PALB2 genes.
BUB1B encodes a protein involved in spindle-assembly checkpoint and germline mutations in BUB1B are known to predispose to premature chromatid separation syndrome and other cancer types (4, 35). Heterozygous, inactivating mutations in BUB1B were present in three FPC patients. In one FPC patient, a second affected relative was available and in this case, the BUB1B variant was not present (Supplementary Table 10). Still, incomplete segregation of FPC susceptibility genes such as ATM, BRCA1, CDKN2A, and PALB2 is not uncommon in FPC kindreds (Supplementary Table 10) and in comparison samples from BCCS samples, no deleterious variants in BUB1B were identified. Additional support for BUB1B as a candidate pancreatic cancer susceptibility gene can be found in variant databases such as EVS and ExAC where the sum minor allele frequencies of BUB1B PTVs in the general population is 0.00024 and 0.00082 respectively. These frequencies are below the level observed in all FPC kindreds (0.00253) and the most severely affected FPC kindreds with 3 or more affected members (0.00612). Our observation of a somatic BUB1B mutation in one of the 39 pancreatic cancers sequenced provides further evidence in support of BUB1B as a candidate susceptibility gene.
Our results also suggest deleterious germline variants in CPA1 may be more frequent in FPC patients. Four heterozygous nonsense variants in CPA1 were found in FPC patients (three chr7:130020952_C>T; p.R27X variants and one chr7:130021680_C>A; p.Y119X variant). This finding is intriguing given that deleterious variants in this gene have recently been shown to predispose to chronic pancreatitis and that chronic pancreatitis is strongly associated with an increased risk of pancreatic cancer (16, 36). Two of the FPC patients with a deleterious CPA1 variant reported a history of pancreatitis approximately 1 year before diagnosis. Intriguingly, 3.1% of recently diagnosed pancreatic cancer patients report a history of pancreatitis within a year of diagnosis (37). As the p.R27X variant identified in FPC patients has previously been shown to be functionally defective, a history of subclinical chronic pancreatitis cannot be ruled out (36).
Ten patients harbored the same deleterious variant in APC (chr5:112175211_T>A; p.I1307K). As this APC variant is prevalent in Jewish populations and the proportions of FPC patients and BCCS samples of Jewish ancestry are unknown, further studies to validate this association are warranted, especially considering the equivocal role that the APC gene broadly plays in FPC susceptibility (38–40).
There are at least three observations from our study that are likely to have an impact on research involving other hereditary cancers. The first is that FPC appears to be heterogeneous with respect to its genetic underpinnings. Though this statement is not surprising given prior research on FPC, it was possible that a previously undiscovered gene was responsible for the majority of FPC cases. Our data, obtained from a very large number of FPC cases, largely excludes this possibility at least for truncating mutations within the coding regions of the 20,049 recognized protein-coding genes.
Second, and more subtly, we observed that variants in well-recognized FPC susceptibility genes were often not present in other affected individuals from the same family (Supplementary Table S10). Segregation of variants among affected members is the hallmark of susceptibility to any disease, and provides the conceptual foundation for linkage analysis. The extent of phenocopies in our study, though surprising in some respects, is not without precedent. In one of the first reports of a gene conferring susceptibility to cancer, it was noted that a particular TP53 gene mutation was not present in a young patient with breast cancer from a Li-Fraumeni family (41). This patient, in retrospect, was obviously a phenocopy. In our cases, the lack of co-inheritance could either be explained by phenocopies, the possibility that multiple deleterious variants are responsible for the phenotype within that family, or that the variant we classified as deleterious was not responsible for the phenotype. It is of interest to note, that many of the previously reported pancreatic cancer susceptibility genes, such as BRCA1, PALB2, CDKN2A, and ATM, as well as our candidate genes, would be eliminated as susceptibility genes if phenocopies were not considered (Supplementary Table S10).
A third point raised by our results pertains to the nature of the peripheral white blood cell DNA that is used for virtually all large-scale genomic studies. We observed multiple private heterozygous PTVs in ASXL1, DNMT3A, and TET2 in FPC patients, which would suggest they are FPC susceptibility genes. Given these genes have been shown to be somatically mutated in the blood of phenotypically normal individuals (25–28), we attempted to confirm the germline origin of these variants in two FPC patients by sequencing DNA from a second tissue. In both cases, our results indicated that these variants were somatic in nature. These unexpected observations emphasize that DNA derived from peripheral white blood cells cannot always be equated with germline DNA, especially in older individuals. This is of particular importance given that many sequencing studies, including ours use publically available control data where age data are not available and the age distribution of the controls may be different from that of the study population. Thus, somatic mutations in peripheral white blood cells could lead to false positive associations, particularly for diseases strongly related to aging.
Finally, we focused on rare PTVs because these variants alter their encoded proteins in an extreme fashion and are predicted to inactivate them. There are, however, other types of variants that may contribute to FPC susceptibility. Further studies will be necessary to delineate the role of missense and non-coding variants in FPC as current algorithms to discriminate deleterious from benign variants are not accurate. In addition, large INDELs may be poorly detected our sequencing method. As such, alternative approaches may be necessary to determine the contribution of large INDELs to FPC susceptibility. Recognizing the need for long-term research, we chose to employ whole genome rather than exome sequencing. Whole genome analyses provide a more complete resource to the pancreatic cancer research community. As more information about gene regulatory regions become available through projects such as ENCODE, and as more control individuals’ whole genome sequences becomes publicly available, the utility of the resource provided herein will correspondingly increase.
METHODS
Institutional approval and informed consent
This study followed the recommendations of the Declaration of Helsinki. Each study site obtained Institutional Review Board approval for their study protocols. Informed consent was obtained from all study participants at their respective institution.
Familial pancreatic cancer (FPC) patient samples
FPC patients were enrolled into National Familial Pancreatic Tumor Registry (NFPTR) at Johns Hopkins or one of the FPC registries participating at the following sites: Dana-Farber Cancer Institute, Karmanos Cancer Institute, Mayo Clinic, McGill University Health Centre, Memorial Sloan Kettering Cancer Center, Mount Sinai Hospital, University of Michigan, University of Pennsylvania, and University of Pittsburgh. FPC families are defined as kindred with at least one pair of first-degree relatives diagnosed with pancreatic ductal adenocarcinoma (PDAC). When possible, all cancer diagnoses in each kindred were pathologically confirmed. Patients with a previously known deleterious variant in a previously reported FPC susceptibility gene (ATM, BRCA1, BRCA2, CDKN2A, MLH1, MSH2, MSH6, PALB2, PMS2, PRSS1, STK11, and TP53) were excluded from the study. Germline DNA samples were obtained from either blood, Epstein-Barr virus transformed peripheral blood lymphocytes (lymphoblastoid cell line; LCL), or non-tumor tissue.
Whole genome sequencing of germline FPC patient samples
638 FPC patient samples were whole genome sequenced and genotyped with the HumanOmni2.5–8v1 array (Illumina, San Diego, CA) by Personal Genome Diagnostics (Baltimore, MD). Briefly, three micrograms of genomic DNA per patient sample was sequenced using the Illumina Whole Genome Sequencing Service with the Illumina HiSeq 2000 (Illumina, San Diego, CA), generating 200 base pair (bp) (2×100bp reads) per fragment in the final library. Sequence reads were analyzed and aligned to the human reference genome (hg19) using Illumina CASAVA v1.7 and ELAND v.2 software (Illumina, San Diego, CA). Variants were annotated using CRAVAT with: 1) functional consequence in RefSeq gene transcripts, 2) zygosity, 3) minor allele frequency (MAF) using publicly available variant databases (1000 Genomes Project and Exome Variant Server), and 4) presence in ClinVar (19, 20, 29, 42, 43). For each variant, mappability score for a 100bp read, as well as the number of BLAT genomic locations for a 101bp sequence centered about the variant mapping to 80–120bp of the reference genome with at least 90% identity were determined (21, 22).
Identify by descent (IBD) and Local Ancestry in adMixed Populations (LAMP) analysis of FPC patients
IBD sharing analysis was performed on FPC patients using 22,458 independent single nucleotide polymorphisms (SNPs) with an R2 cutoff of 0.0001 and outside regions of high linkage disequilibrium (LD). Reported familial relationships were confirmed.
LAMP analysis was performed using hg19 genomic coordinates and strand alignment was completed with ShapeIT v2 (44, 45). Only SNPs common to both the 1000 Genomes Project reference panel and the FPC patient cohort were analyzed (669,977 SNPs) (19). Ancestral allele frequencies were defined using 1000 Genomes project EUR, AFR, and ASN population groups. LAMP analysis was run using the following parameters: 1) Three populations (EUR, AFR, and ASN); 2) 10 generations of ancestral population mixing; 3) African-American, Asian, and Caucasian proportions in FPC patient cohort of 0.028, 0.012, and 0.960 based on self-reported ancestry; 4) Recombination rate of 1×10−8; and 5) LD cutoff of 0.1. Chromosomes were analyzed separately and then combined to obtain an average proportion of ancestry from each population.
Whole exome sequencing of FPC patient tumor samples
Whole exome captured DNA libraries were prepared from non-tumor tissue and microdissected fresh-frozen, formalin-fixed paraffin-embedded, or cell lines prepared from pancreatic adenocarcinomas from individuals with FPC. Cell line identity in relation to a primary patient sample was confirmed with Identifiler (catalog no. 4322288, Thermo Fisher Scientific, Waltham, MA) prior to sequencing. Library construction, sequencing, and bioinformatic analyses were performed at Personal Genome Diagnostics (Baltimore, MD). In brief, genomic DNA from tumor and normal samples were fragmented and used for Illumina TruSeq library construction (Illumina, San Diego, CA). Exomic regions were captured in solution using the Agilent SureSelect v.4 kit according to the manufacturer’s instructions (Agilent Technologies, Santa Clara, CA). Paired-end sequencing, resulting in 100bp from each end of the fragments, was performed using a HiSeq 2000 Genome Analyzer (Illumina, San Diego, CA). Sequences were aligned to the human genome reference sequence (hg19) using the Eland algorithm of CASAVA 1.7 software (Illumina, San Diego, CA). The chastity filter of the BaseCall software of Illumina was used to select sequence reads for subsequent analysis. The ELANDv2 algorithm of CASAVA 1.7 software (Illumina, San Diego, CA) was then applied to identify point mutations and small insertions and deletions. Known polymorphisms recorded in the database of single nucleotide polymorphisms (dbSNP) were removed from the analysis (17). Potential somatic mutations were filtered and visually inspected as described previously (33). Copy number alterations were identified by comparing normalized average per-base coverage for a particular gene in a tumor sample to the normalized average per-base coverage in a matched normal sample for the patient.
Whole exome sequencing of Bipolar Case-Control Study (BCCS) samples
967 individuals were selected from the BCCS, also known as the Rare BLISS sample, to act as controls (30). DNA libraries were prepared by processing genomic DNA samples into Illumina paired-end libraries using Illumina compatible barcoded DNA adapters. Briefly, 1–3μg purified genomic DNA was initially fragmented using a Covaris S2 instrument (Covaris, Woburn, MA), followed by end-repair and ligation to paired-end adapters. Pre-capture libraries were enriched with an additional eight cycles of high-fidelity PCR, and quality and yield were assessed using the Bioanalyzer DNA 1000 Kit (catalog no. 5067–1504, Agilent Technologies, Santa Clara, CA) and the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Exome capture was performed with the SeqCap EZ Human Exome Library v2.0 (catalog no. 05860504001, Roche Sequencing, Madison, WI). Captured DNA libraries were sequenced with the Illumina HiSeq 2000 (Illumina, San Diego, CA), generating 152bp (2×76bp reads) per fragment in the final library. Sequence reads were processed through a standardized variant calling pipeline at either Cold Spring Harbor Laboratories or the Johns Hopkins University. Sequence reads were aligned to the human reference genome (UCSC hg19) using Burrows-Wheeler Aligner (BWA), allowing for two mismatches in the 30-base seed (46). Picard was used to correct mate pair mismatch, remove duplicate reads, and assess target region coverage (47). Samples with ≥75% of the target region covered at ≥20X sequencing were used for analyses. The Genome Analysis Toolkit (GATK) was used to generate single nucleotide variants (SNV) and small insertion/deletion (INDEL) calls in the target regions, after local realignment around INDELs and base score recalibration with the Unified Genotyper (48). The following GATK filters were used: variant confidence score ≥30, mapping quality ≥40, read depth ≥6, strand bias FSfilter <60. Single nucleotide variant (SNV) clusters, defined as greater than three SNVs per 10 bases, and SNVs falling within a called INDEL region, were masked. Variants were annotated as described for whole genome sequencing of FPC patients.
Confirmation of TET2 and ASXL1 variants
Confirmation of variants was performed on DNA from blood and formalin-fixed paraffin embedded tissues using the Safe-Sequencing System (Safe-SeqS) as previously described (49). Primer sequences used to detect the TET2 (g.chr4:106196537_C>T;p.Q1624X) variant were: cacacaggaaacagctatgaccatgGGGGAGAATAGGAACCCAGA and cgacgtaaaacgacggccagtNNNNNNNNNNNNNNAATCCCATGAACCCTTACCC. Primer sequences used to detect the ASXL1 variant (g.chr20:31022414_T>TA; p.fs) were: cacacaggaaacagctatgaccatgCTCTGCCACCTCCCTCATC and cgacgtaaaacgacggccagtNNNNNNNNNNNNNNGGACCCTCGCAGACATTAAA. Where N’s denote degenerate bases, with an equal representation of A, C, T, and G.
Statistical analyses
Two sided p-values were calculated using a Fisher’s Exact Test. False discovery rate was calculated using Benjamini-Hochberg procedure. A p-value of less than 0.05 was considered significant.
Data availability
Whole genome and exome sequencing data are available (50). Users must obtain Institutional Review Board approval from their institutions and agree to policies that maintain patient privacy prior to use.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
The genetic basis of disease susceptibility in the majority of familial pancreatic cancer patients is unknown. We whole genome sequenced 638 familial pancreatic cancer patients and demonstrate the genetic underpinning of inherited pancreatic cancer is highly heterogeneous. This has significant implications for the management of familial pancreatic cancer patients.
Acknowledgments
FINANCIAL SUPPORT
This work was generously supported by Dennis Troper and Susan Wojcicki, the Lustgarten Foundation for Pancreatic Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Howard Hughes Medical Institute, the Virginia and D.K. Ludwig Fund for Cancer Research, the Stringer Foundation, the Rolfe Foundation for Cancer Research, the Joseph C. Monastra Foundation, the Gerald O. Mann Charitable Foundation, the Ladies Auxiliary to the Veterans of Foreign Wars, the friends and family of Roger L. Kerns Sr., the Weston Garfield Foundation, the NIH Specialized Programs of Research Excellence P50-CA062924 and P50-CA102701, NIH grants K99-CA190889, K01-MH093809, P30-CA006973, R01-CA57345, R01-CA97075, R01-CA154823, R01-DK060694, and R01-MH087979.
We thank all study participants for their generous contribution to this work. We also thank S. Angiuoli, C. Michael, M. Borges, L. Dobbyn, D. Echavarria, C. Harrington, S. Jones, M. Popoli, J. Ptak, R. Romans, J. Schaefer, and N. Silliman for technical assistance.
Footnotes
STATEMENT ON CONFLICT OF INTEREST
Under a licensing agreement between Myriad Genetics, Inc., and the Johns Hopkins University, J.R.E, M.G., R.H.H., K.W.K., A.P.K., and B.V. are entitled to a share of royalty received by the University on sales of products related to PALB2. Under agreements between the Johns Hopkins University, Sysmex-Inostics, Personal Genome Diagnostics, PapGene, Exact Sciences, LabCorp, and QIAGEN, K.W.K., N.P., and B.V. are entitled to a share of the royalties received by the University on sales of products related to genes and technologies described in this manuscript. K.W.K., N.P., and B.V. are co-founders of Inostics and Personal Genome Diagnostics and are members of their Scientific Advisory Boards. They own stock in Sysmex-Inostics, Papgene, and Personal Genome Diagnostics, which is subject to certain restrictions under Johns Hopkins University policy. The terms of these arrangements are managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
Author contributions
Study design: N.J.R., A.N., G.M.P., M.G., N.P., J.R.E., K.W.K., B.V., R.H.H., A.P.K.
Collection and preparation of FPC patient samples: N.J.R., A.N., G.M.P., M.L.B., R.B., S.G., R.C.K., S.O., A.R., A.G.S, E.S., S.S., G.Z., S.A., J.A., M.L.C., J.M.H, C.I-D, A.M-M., A.D.R, A.S., C.L.W., L.W., M.G., J.R.E., R.H.H., A.P.K.
Collection and preparation of BCCS samples: F.S.G, M.K., R.W.M., J.P., M.P., J.B.P., P.P.Z.
Generation of data: N.J.R., A.N., Y-C.C., C.D., F.S.G, M.K., R.W.M., K.M., N.N., J.P., M.P., J.B.P., Y.W., P.P.Z., R.K., N.P., B.V., R.H.H., A.P.K.
Analysis and interpretation of data: N.J.R., A.N., Y.W., M.G., N.P., J.R.E., K.W.K., B.V., R.H.H., A.P.K.
Writing and revision of draft manuscript: N.J.R., B.V., R.H.H, A.P.K.
Approval of final manuscript: all authors.
References
- 1.Cancer Facts & Figures 2015. 2015;2015:1–56. Available from: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/index/ [Google Scholar]
- 2.Hruban RH, Zamboni G. Pancreatic cancer. Special issue--insights and controversies in pancreatic pathology. Archives of pathology & laboratory medicine. 2009;133:347–9. doi: 10.5858/133.3.347. [DOI] [PubMed] [Google Scholar]
- 3.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer research. 2004;64:2634–8. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 4.Yuen RK, Thiruvahindrapuram B, Merico D, Walker S, Tammimies K, Hoang N, et al. Whole-genome sequencing of quartet families with autism spectrum disorder. Nature medicine. 2015;21:185–91. doi: 10.1038/nm.3792. [DOI] [PubMed] [Google Scholar]
- 5.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Brune KA, Visvanathan K, Laheru D, Herman J, Wolfgang C, et al. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2829–34. doi: 10.1158/1055-9965.EPI-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer discovery. 2012;2:41–6. doi: 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy KM, Brune KA, Griffin C, Sollenberger JE, Petersen GM, Bansal R, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer research. 2002;62:3789–93. [PubMed] [Google Scholar]
- 11.Al-Sukhni W, Rothenmund H, Borgida AE, Zogopoulos G, O’Shea AM, Pollett A, et al. Germline BRCA1 mutations predispose to pancreatic adenocarcinoma. Human genetics. 2008;124:271–8. doi: 10.1007/s00439-008-0554-0. [DOI] [PubMed] [Google Scholar]
- 12.McWilliams RR, Wieben ED, Rabe KG, Pedersen KS, Wu Y, Sicotte H, et al. Prevalence of CDKN2A mutations in pancreatic cancer patients: implications for genetic counseling. European journal of human genetics : EJHG. 2011;19:472–8. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. Jama. 2009;302:1790–5. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–53. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 15.Ruijs MW, Verhoef S, Rookus MA, Pruntel R, van der Hout AH, Hogervorst FB, et al. TP53 germline mutation testing in 180 families suspected of Li-Fraumeni syndrome: mutation detection rate and relative frequency of cancers in different familial phenotypes. Journal of medical genetics. 2010;47:421–8. doi: 10.1136/jmg.2009.073429. [DOI] [PubMed] [Google Scholar]
- 16.Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. The New England journal of medicine. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 17.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic acids research. 2001;29:308–11. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahman N. Realizing the promise of cancer predisposition genes. Nature. 2014;505:302–8. doi: 10.1038/nature12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHLBI Exome Sequencing Project (ESP) [Internet] Seattle: University of Washington; [updated 2015 May 14; cited 2015 Nov 18]. Available from: http://evs.gs.washington.edu/EVS/ [Google Scholar]
- 21.Derrien T, Estelle J, Marco Sola S, Knowles DG, Raineri E, Guigo R, et al. Fast computation and applications of genome mappability. PloS one. 2012;7:e30377. doi: 10.1371/journal.pone.0030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kent WJ. BLAT--the BLAST-like alignment tool. Genome research. 2002;12:656–64. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruark E, Snape K, Humburg P, Loveday C, Bajrami I, Brough R, et al. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493:406–10. doi: 10.1038/nature11725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Jr, Kinzler KW. Cancer genome landscapes. Science. 2013;339:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33. doi: 10.1038/nature13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Busque L, Patel JP, Figueroa ME, Vasanthakumar A, Provost S, Hamilou Z, et al. Recurrent somatic TET2 mutations in normal elderly individuals with clonal hematopoiesis. Nature genetics. 2012;44:1179–81. doi: 10.1038/ng.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie M, Lu C, Wang J, McLellan MD, Johnson KJ, Wendl MC, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nature medicine. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. The New England journal of medicine. 2014;371:2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, et al. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic acids research. 2014;42:D980–5. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YC, Carter H, Parla J, Kramer M, Goes FS, Pirooznia M, et al. A hybrid likelihood model for sequence-based disease association studies. PLoS genetics. 2013;9:e1003224. doi: 10.1371/journal.pgen.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:820–3. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris AL, Roberts NJ, Jones S, Wheelan SJ, Papadopoulos N, Vogelstein B, et al. Familial and sporadic pancreatic cancer share the same molecular pathogenesis. Fam Cancer. 2015;14:95–103. doi: 10.1007/s10689-014-9755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rio Frio T, Lavoie J, Hamel N, Geyer FC, Kushner YB, Novak DJ, et al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. The New England journal of medicine. 2010;363:2628–37. doi: 10.1056/NEJMoa1006565. [DOI] [PubMed] [Google Scholar]
- 36.Witt H, Beer S, Rosendahl J, Chen JM, Chandak GR, Masamune A, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nature genetics. 2013;45:1216–20. doi: 10.1038/ng.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2964–70. doi: 10.1093/annonc/mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woodage T, King SM, Wacholder S, Hartge P, Struewing JP, McAdams M, et al. The APCI1307K allele and cancer risk in a community-based study of Ashkenazi Jews. Nature genetics. 1998;20:62–5. doi: 10.1038/1722. [DOI] [PubMed] [Google Scholar]
- 39.Giardiello FM, Offerhaus GJ, Lee DH, Krush AJ, Tersmette AC, Booker SV, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–6. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on familial pancreatic cancer. Advances in surgery. 2010;44:293–311. doi: 10.1016/j.yasu.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava S, Zou ZQ, Pirollo K, Blattner W, Chang EH. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990;348:747–9. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- 42.Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, et al. RefSeq: an update on mammalian reference sequences. Nucleic acids research. 2014;42:D756–63. doi: 10.1093/nar/gkt1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douville C, Carter H, Kim R, Niknafs N, Diekhans M, Stenson PD, et al. CRAVAT: cancer-related analysis of variants toolkit. Bioinformatics. 2013;29:647–8. doi: 10.1093/bioinformatics/btt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasaniuc B, Sankararaman S, Kimmel G, Halperin E. Inference of locus-specific ancestry in closely related populations. Bioinformatics. 2009;25:i213–21. doi: 10.1093/bioinformatics/btp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nature methods. 2012;9:179–81. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 46.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics. 2010;26:589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Picard [Internet] Cambridge, MA: The Broad Institute; c2015. [updated 2015 Nov 16; cited 2015 Nov 18]. Available from: http://broadinstitute.github.io/picard/ [Google Scholar]
- 48.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome research. 2010;20:1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinde I, Bettegowda C, Wang Y, Wu J, Agrawal N, Shih Ie M, et al. Evaluation of DNA from the Papanicolaou test to detect ovarian and endometrial cancers. Science translational medicine. 2013;5:167ra4. doi: 10.1126/scitranslmed.3004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The familial pancreatic cancer genome sequencing project [Internet] Baltimore, MD: The Johns Hopkins University; [updated 2015 Nov 24; cited 2015 Nov 25]. Available from: http://www.familialpancreaticcancer.org/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Whole genome and exome sequencing data are available (50). Users must obtain Institutional Review Board approval from their institutions and agree to policies that maintain patient privacy prior to use.