ABSTRACT
This paper introduces a conceptual framework for the evolution of complex systems based on the integration of regulatory network and niche construction theories. It is designed to apply equally to cases of biological, social and cultural evolution. Within the conceptual framework we focus especially on the transformation of complex networks through the linked processes of externalization and internalization of causal factors between regulatory networks and their corresponding niches and argue that these are an important part of evolutionary explanations. This conceptual framework extends previous evolutionary models and focuses on several challenges, such as the path‐dependent nature of evolutionary change, the dynamics of evolutionary innovation and the expansion of inheritance systems. J. Exp. Zool. (Mol. Dev. Evol.) 324B: 565–577, 2015. © 2015 The Authors. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution published by Wiley Periodicals, Inc.
INTRODUCTION
The debate over the adequacy of current evolutionary theory has again moved center stage (Laland et al., 2014). In essence, this controversy is about how to integrate recent empirical and theoretical advances within evolutionary biology and related fields into the core of evolutionary theory and how to broaden its explanatory scope. These advances include insights from molecular and developmental biology that have led to the concepts of developmental and regulatory evolution and genomic regulatory networks (Davidson, 2001; Davidson, 2006; Materna and Davidson, 2007; Carroll, 2008; Shubin, 2008; Davidson, 2009; Davidson, 2011; Krakauer et al., 2011; Peter and Davidson, 2011; Peter et al., 2012; Ben‐Tabou et al., 2013; Ben‐Tabou de‐Leon et al., 2013) and a deeper integration of ecological and evolutionary theory that has refocused attention on complex phenomena such as phenotypic plasticity or the idea of niche construction with its focus on multiple inheritance systems (Odling‐Smee, 1995; Laland et al., 1999; Laland and Sterelny, 2006; Laland et al., 2008; Jeffares, 2012; Odling‐Smee et al., 2013; Richerson and Christiansen, 2013; Buser et al., 2014). Another challenge has been to expand evolutionary explanations to human psychology, sociality, language, culture, technology, economics and medicine (Piaget, 1970; Carroll, 2004; Boyd and Richerson, 2005; Richerson and Boyd, 2005; Stearns and Koella, 2008; Nesse et al., 2010; Bowles and Gintis, 2011; Gluckman and Bergstrom, 2011; Ruse, 2013; Wimsatt, 2013). Further debates involve patterns of evolutionary change (Grant, 1999; Grant and Grant, 2008; Minelli, 2009; Erwin and Valentine, 2013), the causal mechanisms that generate phenotypic variation (Carroll, 2008; Peter and Davidson, 2015) or the levels of selection (Okasha, 2008). In all cases the question has been whether new data and concepts or new explanatory domains can be accommodated within the existing framework of evolutionary theory, or whether the core of evolutionary theory needs to be re‐conceptualized or, at the very least, expanded (Pigliucci et al., 2010).
Integrating Regulatory Networks with Niche Construction
In this paper we discuss one particular challenge that, we argue, in light of these new insights requires a re‐conceptualization of parts of evolutionary theory—the evolution of innovations within complex systems across scales (Wagner et al., 2000; Muller and Newman, 2005; Davidson and Erwin, 2010; Wagner, 2011; Wagner, 2014). Innovation, the generation of novel characters or behaviors, as opposed to standard patterns of variation and adaptation, involves not only the transformation of regulatory systems, but also the kind of interactions between systems and their environment that have been described as niche construction (Laland et al., 1999; Laland et al., 2000; Odling‐Smee et al., 2003; Erwin and Krakauer, 2004; Erwin, 2008; Laland et al., 2008; Odling‐Smee et al., 2013; Caporael et al., 2014). Explanations of innovations require a detailed understanding of the generation of phenotypic variation that goes beyond referring to mutation as the fundamental variation‐generating mechanism (Khalturin et al., 2009; Jasper et al., 2015) and that includes the specific features of regulatory networks at different scales—including changes to both the structure of these regulatory networks in form of rewiring genomic and other forms of interactions, the transformation of individual elements of these networks by means of mutations in a broad sense and the addition of new elements to the network (for a recent review see (Peter and Davidson, 2015))—as well as a more complex account of the interactions between systems and their various environments than is provided by an aggregate measurement of fitness. Rather we also need to understand how systems actively construct their relevant niches (or how technologies create demand) and how these constructed niches, in turn, affect the possibilities of future transformation of these systems. This last point captures the path‐dependent nature of evolutionary change. Technically this is a question about the structure of search spaces for evolutionary dynamics (Barve and Wagner, 2013). Of the competing views—one that defines a search space abstractly as the sum of all possible combinations at a particular level of the biological hierarchy, such as a sequence space for RNA or DNA molecules of a particular length or sum of all possible metabolic interactions within a particular pathway; the other that argues that in the case of complex systems the search space of future possibilities is actively constructed by the actions and properties of currently existing systems—we clearly argue for the latter. For us, within the current extended landscape of evolutionary biology the challenge of explaining evolutionary innovations thus translates into the need to integrate the complex transformations of regulatory networks and their elements mentioned above with niche construction perspectives.
A focus on regulatory networks, such as gene regulatory networks, helped to discover causal mechanisms that control the development of specific phenotypic characters. Furthermore, comparative studies (of different species and of normal and pathological conditions) have shown how specific transformations of either regulatory network structures or individual elements within those networks are responsible for observed phenotypic variation (Carroll, 2000; Wagner et al., 2000; Davidson, 2006; Carroll, 2008; Peter and Davidson, 2011; Davidson, 2014; Wagner, 2014). While many of these studies have focused on the genome, it has, however, also become clear that many contextual factors interact with the genome‐based control circuits and thus contribute to the regulation of gene expression in a significant way (Linksvayer et al., 2011; Linksvayer et al., 2012; Page, 2013). The specific nature of these interactions can, in principle, be traced outward from the genome and involves intra‐ and extracellular signaling pathways, metabolic and physiological networks, behavior and specific environmental factors that can all contribute to such regulatory cascades. In practice, however, detailed reconstructions of such extended causal networks are still rare and specific contextual effects are generally subsumed under a generalized environmental contribution to the partition of variance and in any case are considered to be a factor that is independent from the genomic, cellular or organismal system.
Niche construction theory (Laland et al., 1999; Lalandet al., 2000; Odling‐Smee et al., 2003; Erwin and Krakauer, 2004; Erwin, 2008; Laland et al., 2008; Odling‐Smee et al., 2013; Caporaelet al., 2014), on the other hand, focuses on the ways systems actively shape or construct their environment. In this view, the niche is not something that exists out there in nature waiting to be discovered or filled by an organism. Furthermore, constructed niches often persist longer than any of their individual inhabitants, which allow these niches to store important hereditary and regulatory information. Niche construction theory thus includes the notion of expanded and multiple inheritance systems (from genomic to ecological, social and cultural). This latter aspect has made the concept of niche construction especially attractive for theories of cultural evolution as it facilitates a more complex notion of inheritance and a closer link between evolutionary dynamics and learning (Odling‐Smee, 1995; Laland et al., 1999; Laland et al., 2000; Boyd and Richerson, 2005; Laland, 2008; Laland et al., 2008; Boyd et al., 2011; Creanza et al., 2012). But most models of niche construction have treated these multiple inheritance systems as quasi‐independent contributions to evolutionarily relevant variation, allowing only limited interactions between them. In part this is a consequence of the formal structure of variance decompositions (the famed Price equation) that is the foundation of much of niche construction theory. But it also reflects a tendency within niche construction theory to focus on multiple broadly defined factors and quantify their relative importance within evolutionary dynamics.
What both of these approaches are missing is a clearly defined conception of how systems at multiple scales interact with each other, where some are defined as internal to the organizational level of study and some are defined as context or environment. A precise definition of the nature of these interactions is, however, a prerequisite for a causal model of the evolution of complex systems and also for understanding innovation across scales. This requires us to clearly define the relevant elements of these systems and their properties. Without conceptual precision it will be impossible to define the measurements and metrics needed to turn integrative conceptual ideas into formal models and to specify the criteria for empirical validation. Another challenge is to trace the consequences of causal interactions at different scales through an iterative sequence of historical stages. The conceptual framework we propose here begins with a conceptual clarification of the properties of extended systems that include both regulatory and niche elements. In our conception, regulatory and niche elements are parts of an extended network of causal interactions. We then apply this conception to a specific problem—innovation—in the context of a specific well‐documented example—the developmental evolution of eusociality. Comparing the extended networks at different stages of this evolutionary trajectory allows us then to reconstruct the co‐evolutionary dynamics between different parts of these extended networks. For the case of genetic systems Linksvayer and Wade (2009) have proposed a model of indirect genetic effects that can be seen as a specific instance of such an extended model. It introduces an expanded conception of genetic effects that includes contributions from different individuals in the context of a behaviorally linked system, such as a colony of social insects or other socially interacting systems. Our framework allows us to go beyond the idea of indirect genetic effects in that it (i) includes a broader range of causal factors, including those that are often subsumed under ecological inheritance (Laland et al., 2008; Odling‐Smee et al., 2013) and (ii) applies to a much broader range of evolutionary phenomena—from genomic to social, cultural and technological.
The Problems of Homology and Innovation
The linked problems of, on the one hand, homology, or sameness of structures and behaviors across a wide range of species, social systems or cultures, and, on the other hand, innovation, i.e., the emergence and successful spread of novel structures, are some of the main challenges for any theory of phenotypic evolution. Homology has traditionally been seen as a consequence of genealogy and inheritance (Laubichler, 2000). Simply put, complex phenotypes are the same because they inherited the same genes (or other types of hereditary information). This historical conception of homology does, however, not account for the observed patterns of sameness and stability as we often see more (gradual) divergence in genes or other parts of the hereditary material than in the resulting phenotypic characters. In response to these challenges a regulatory conception of homology was proposed that explains the stability of phenotypic characters through time as a consequence of conserved structures in regulatory developmental systems or networks (Wagner, 1999; Wagner et al., 2000; Wagner, 2007; Wagner, 2014).
In the context of this developmental view, conserved elements of regulatory networks (referred to in the literature either as kernels or character identity networks) establish the identity or sameness of specific characters or structures while other (more downstream) parts of the network allow for the adaption of these characters to specific functions (Davidson, 2006; Wagner, 2007; Davidson, 2009; Wagner, 2014). These variants of recognizable characters are called character states. The reason for the existence of conserved parts of networks is found in the interdependencies between elements in these complex regulatory networks where changes to certain parts of the network would cause a large number of dramatic and often lethal consequences. The interdependent regulatory network architecture together with the historical accumulation of small changes (adaptations) that all depend on specific core elements of the networks thus account for the observed patterns of stability, path‐dependency and canalization characteristic of all complex biological, social, cultural and technological systems.
This regulatory conception of homology provides an explanation for observed patterns of stability (sameness) across complex systems as well as for more specific features of complex networks, such as their modular and hierarchical architecture and the path‐dependent or canalized nature of change. Any explanation of stability or homology also provides implicitly an explanation of novelty. We define novelty as the emergence of a new character as opposed to the transformation of an existing character into a new character state. There are, of course, several ways how novelties can emerge. These are currently the subject of intense debates within developmental evolution. Most prominently is the argument about the importance of changes in coding vs regulatory regions (Carroll, 2008; Laland et al., 2008; Davidson and Erwin, 2010; Davidson, 2011; Odling‐Smee et al., 2013; Davidson, 2014; Peter and Davidson, 2015). As evidence exists that each type of change can play a role in specific instances of novelty (Khalturin et al., 2009; Jasper et al., 2015), in the context of our framework it is important to note that independent of the kind of mutation all of these, in various ways, ultimately contribute to a rearrangement of the underlying regulatory networks that control the development of characters (Wagner, 2014; Peter and Davidson, 2015). Such a rearrangement can be caused by the addition of new elements (for instance through gene duplication or lateral gene transfer, if we focus on genomic systems) or by the emergence of new links and regulatory relationships among already existing elements. In any case, novelties (or inventions in the context of technological change) are understood as the consequence of a specific type of transformation of regulatory networks. In the context of evolution or history, the eventual fate of these novelties or inventions is determined by the selective conditions of the environment, markets or domains of implementation. Only a successful novelty or invention is then called an innovation sensu Schumpeter (Erwin, 2008; Davidson and Erwin, 2010; Krakauer et al., 2011).
However, this relationship between novel variants and their selective environment can be quite complex, as we have to also account for the role of processes summarized under the general label of niche construction in this process. And most importantly, we need to be able to account for how transitions between regulatory states can actually be viable within specific evolutionary lineages, a problem that has not yet been fully resolved. Our conceptual framework suggests ways how an emphasis on the interactions between these two kinds of processes can contribute to a better understanding of the evolutionary dynamics of homology and innovation.
A Model of Extended Evolution as Transformation of Complex Networks
We, as others before (Erwin, 2008; Laland et al., 2008; Erwin, 2012; Andersson et al., 2014), have identified the integration of regulatory network and niche construction perspectives as one challenge for extending evolutionary theory and suggest that this requires a model that brings together regulatory and niche elements within one network of interacting causal factors. While others have done this for some specific cases and within the conceptual structure of either evolutionary genetics (Linksvayer et al., 2012) or cultural evolution (Laland et al., 2008; Andersson et al., 2014) our proposed perspective aims to bring evolutionary processes at all levels into one conceptual framework. We see this not as an exercise in grandiose theory or abstraction, but rather as a logical consequence of the internal dynamics of such integrated systems. One aspect of this is the role of coarse graining for theory development within biology (Krakauer et al., 2012). In the context of our proposed framework this implies to generalize from individual cases while at the same time provide enough specificity to be able to apply the framework to a number of specific cases. The extensions of the causal networks to include both internal and external (environmental) factors also allows us to focus on those cases that are characterized by multiple kinds of elements, such as cases of social and cultural evolution that include a number of different factors (biological, social, cultural). In this section we provide an abstract formulation of our framework that will be the basis of future modeling.
We begin by defining an internal system as a network of agents capable of persisting through time and reproducing its structure. The agents form the nodes of this network while their causal interactions constitute the links. Such systems may span multiple scales. Therefore an agent or node at one scale can be a network of agents at a different scale. Both persistence and reproduction typically require control and coordination of actions not only within and among the agents constituting the internal system, but also interactions between the system and other systems and their environment.
An extended network includes the environment as part of the network structure. All aspects of the environment that causally affect these interactions form the structured niche of the internal system. Together, system and structured niche constitute the extended regulatory network. The structured niche has itself a network structure induced by the primary network constituted by the internal system. Its nodes are those aspects of the environment that condition, mediate or become the target of actions, in short the environmental resources of the internal system. Its links are causal relations among these resources and between the resources and the internal network structures. Niches and environments are scale‐dependent. The niche for an internal network at one scale can be part of the internal network at another scale. Therefore, from the system's perspective there are no absolute boundaries between an internal network and its environment. These distinctions are thus always process specific and also pragmatic. In modeling a specific type of causal interaction it often makes sense to treat some aspects of these extended causal networks as internal or as context or environment.
Actions are regulated by the structure of the extended regulatory network and may be directed at the environment or at other agents changing their states so as to affect their actions. Actions realize functions related to the possible states of a system and its environment. Actions always involve environmental resources, constituting the material conditions or the targets of their realization. When actions are performed they change the environment in ways that are characteristic for the system and may thus be considered as an “externalization” of the system's internal structures. But actions also do not leave the system itself indifferent and constantly change its internal structure. Therefore also the converse process takes place, an “internalization” of the environment. The internal structures of actors are the result of iterative (through evolutionary and individual time scales) transformations of such action networks.
We can illustrate this abstract notion of network dynamics in the context of models of evolution by natural selection, one prominent explanatory framework within present‐day evolutionary theory. Here the agents correspond to individuals and the networks to populations. The internal structures of these agents include units of inheritance and the system of developmental interactions. The latter turns these agents into units of interaction and establishes the range of behaviors for each agent. Based on the internal complexity of these agents, they can also adapt their behaviors through interaction with the environment. The key regulative structure of interactions at the level of the population network is, however, selective reproduction. It includes the effects of random variation at the level of the internal genetic and developmental system and, as a consequence, heritability across generations. Interactions with the environment take multiple forms. These include the construction of individual niches through interactions between possible internal states and environmental resources and conditions. As a consequence, internal states are externalized into the environment. It also includes selection among the units of inheritance in intergenerational transitions. In this case the interactions between individuals and their environment lead to a corresponding internalization of states in the sense that successful internal‐environmental interactions become the foundation of the next generation.
Recasting the standard dynamics of natural selection this way allows us to see it as a special case of evolutionary network transformation. Regulatory structures in networks other than those directly involved in inheritance may also cross the boundary of individual and population‐level interactions. This is captured by the idea of multiple inheritance systems within niche construction theory. In particular, regulatory network structures may expand by incorporating interactions with other units as well as features of the environment, or the extended network in our model. Due to the process we define as “externalization,” agents shape the range of possible interactions with their environment. They may do so not only by reproduction, but also by constructing niches, as well as by exploring their functional and behavioral potential (“exaptation”) which, in a given environment, is typically larger than the most immediate selected function, making it possible to react to newly emerging challenges. Within our model of extended networks these externalized factors can become an important part of the regulatory structures governing the behavior of agents and, if they are stable enough, also have substantial evolutionary consequences. These are captured by a complementary process we have described as “internalization,”where elements of the transformed environment may, under certain conditions, be integrated into an expanding regulatory structure that, in turn, shapes the interactions within the network. In biological evolution, this extension may at first happen, within a single generation, only at the developmental and behavioral level. Still, it will enlarge the set of regulative networks on which natural selection can act, possibly turning a transient extended regulatory structure into a heritable feature.
The dynamics between externalization and internalization is particularly relevant for explanations of evolutionary innovations. Regulatory evolutionary changes of different kinds (Peter and Davidson, 2015) leading to genuine novelty or innovation can be explained as a consequence of the creation of additional regulatory modules or network transformations. Again, there are many concrete ways how this can actually be realized (Carroll, 2008; Khalturin et al., 2009 Khalturin et al, 2009; Jasper et al., 2015). These often operate upstream of the highly canalized structures that control normal development and organismal function. Furthermore, the complex and hierarchical developmental and cellular systems can either amplify (“facilitate” sensu Kirschner and Gerhart (Kirschner and Gerhart, 2005; Gerhart and Kirschner, 2007)) or suppress the variation induced by changes on the genomic level. One way in which such an additional module may have emerged is from a transient extended regulatory structure. Contingent circumstances and challenges to a network may indeed lead to a specific coordination of actions within the network that can be preserved at the developmental level of the agents within structured niches resulting from externalization and serving as scaffolding for the extended regulative structure sustaining such actions. The idea of scaffolding has been discussed especially in the context of cultural evolution (Caporael et al., 2014) where the effects of constructed niches on evolutionary dynamics are often more straight forward and where the iterative and path‐dependent nature of these processes is also quite visible. Our framework builds on these ideas, but identifies these features more closely as complex systems’ properties, which emphasizes the general dimension of these processes. An internalization of such action control by natural selection would then turn the transient extended regulatory structure into a heritable feature and at the same time decouple it from environmental constraints. In the following section we apply this framework to a number of evolutionary scenarios in the context of social evolution that illustrate these processes in general terms and suggest ways how these dynamics of externalization and internalization could be tested with concrete empirical data.
The Origin of Eusociality as a Case of Extended Evolution
There are many cases of evolutionary innovation that can serve as illustrations for our framework of extended evolution. However, the multiple sequences of evolutionary transitions leading to the emergence of a variety of eusocial systems provide particularly compelling cases. Focusing on evolutionary trajectories rather than a single event better reveals the complex transformations of extended regulatory systems that result from the dynamics of externalization and internalization between regulatory systems and constructed niches that are the core of our model (Wilson, 1971b; Wilson, 1971a; Wilson, 1985; Hölldobler and Wilson, 1990; Stearns, 1992; Page, 1997; Rueppell et al., 2004; Wilson and Holldobler, 2005a; Wilson and Holldobler, 2005b; Gadau et al., 2009; Hölldobler and Wilson, 2009; Amdam and Page, 2010; Nowak et al., 2010; Hölldobler and Wilson, 2011; Page, 2013). While all social systems involve cooperation and frequently division of labor, in the case of eusocial systems this also includes reproductive division of labor. The latter phenomenon has been a major challenge for evolutionary theory since Darwin (Wilson, 1971a; Wilson and Holldobler, 2005a; Hölldobler and Wilson, 2009; Nowak et al., 2010, Page, 2013; West‐Eberhard, 2014). Furthermore, social systems of various degrees of complexity have evolved several times and each time this process involved multiple steps that, we argue, show all the characteristics of the complex co‐evolutionary dynamics that includes both externalization and internalization events.
The multiple evolutionary trajectories from solitary insects to superorganisms with a complete reproductive division of labor contain different types of social systems that can be characterized by the way in which the regulatory mechanisms of development and behavior connect organisms to their niche (Wilson, 1971a; Page, 1997; Fewell, 2003; Amdam et al., 2004; Linksvayer and Wade, 2005; Hughes et al., 2008; Gadau et al., 2009; Hölldobler and Wilson, 2009; Amdam and Page, 2010; Johnson and Linksvayer, 2010; Boomsma et al., 2011; Linksvayer et al., 2012; Linksvayer et al., 2013). These include (i) solitary species which interact with others only during courtship and mating; (ii) subsocial species which care for their offspring; (iii) communal species which share a nest but otherwise do not interact; (iv) quasisocial species which cooperate during brood care; (v) semisocial species with cooperative brood care and reproductive division of labor and (vi) eusocial species which are characterized by cooperative breeding, reproductive division of labor and overlapping generations within the nest. Within eusocial species we distinguish between primitively eusocial species, which do not show morphological caste differentiation and generally have some degree of flexibility in social roles, and highly eusocial species or superorganisms, which show considerable morphological caste differentiation and generally little flexibility in social roles (Wilson, 1971a; Hölldobler and Wilson, 2009). To be clear, we are not saying that these stages represent a single evolutionary trajectory; rather we are using these different types of social systems as examples of different regulatory networks and the transitions between them as illustrations for the dynamics of internalization and externalization, especially as some of these transitions have been well studied and modeled in a way that is consistent with our overarching framework (Heinze et al., 1997; Amdam et al., 2004; Linksvayer, 2006; Patel et al., 2007; Gadau et al., 2009; Linksvayer et al., 2009; Linksvayer and Wade, 2009; Ihle et al., 2010; Johnson and Linksvayer, 2010; Wade et al., 2010; Linksvayer et al., 2011; Linksvayer et al., 2012; Page et al., 2012; Flatt et al., 2013; Linksvayer et al., 2013).
In the case of solitary and subsocial species, the niche does not include constructed structures while communal and quasisocial species live in constructed structures and are distinguished by the degree of social interactions that regulate their behavior. In the case of semisocial and primitively eusocial systems with no morphological caste differentiation, colonies live within constructed nests and division of labor is triggered by behavioral mechanisms such as simple dominance hierarchies (“behavioral regulation”) (Bertram et al., 2003). Highly integrated eusocial systems or superorganisms build the most elaborate nests or stable niches and are characterized by morphological caste differentiation, including reproductive division of labor, controlled by developmental mechanisms that operate through complex nutritional, hormonal and behavioral signaling networks that ultimately control gene expression (“developmental and physiological regulation”) (Beshers and Fewell, 2001; Rueppell et al., 2004; Amdam et al., 2006; Page and Amdam, 2007; Patel et al., 2007; Linksvayer et al., 2009; Amdam and Page, 2010; Ihle et al., 2010; Johnson and Linksvayer, 2010; Leimar et al., 2012; Linksvayer et al., 2012). Each type of social organization is thus characterized by specific interactions of regulatory systems with constructed niches.
These different social systems are marked, on the one hand, by an increasing internalization of the regulatory mechanism at the colony level, from contingent environmental and variable social conditions to stable genomic, developmental and niche‐construction mechanisms and signals inherent to the colony. At the same time, the causes triggering the developmental differentiation of individual organisms are increasingly externalized into the social and ecological niches emitting regulatory signals originating from the state of the colony and the behavior of other individuals in the colony. Taken together, these internalization and externalization mechanisms achieve an ever‐higher integration of the colony. These dynamic transformations of regulatory control mechanisms constitute a conceptual model of possible evolutionary dynamics by which superorganisms could have emerged.
This example also highlights several central features of our model of extended networks of causally interacting agents. First, we see that the behavioral capacities of individuals, together with the environmental resources available to them, enable the construction of social and environmental niches. This leads in turn to an extension of the regulatory control structures governing their behavior. Second, these constructed niches represent a transitional extension of the regulatory control system involved in the emergence of an evolutionary innovation. Third, insofar as these extended systems are favored by natural selection we then observe a gradual internalization of these extended control systems into more stable (from an evolutionary point of view) hereditary and developmental structures. Taken together these steps provide us with a conceptual framework for explanations of evolutionary innovations that combines regulatory networks, niche construction and selection in the context of one model describing the transformations of extended networks.
Our explanation builds on progress reached in recent years in the understanding of social and eusocial systems (West‐Eberhard, 1986; Gadau et al., 2000; Rueppell et al., 2004; Giray et al., 2005; Wilson and Holldobler, 2005b; Wilson and Holldobler, 2005a; Amdam and Seehuus, 2006; Wilson, 2006; Hunt et al., 2007; Nelson et al., 2007; Page and Amdam, 2007; Patel et al., 2007; Linksvayer et al., 2009; Amdam and Page, 2010; Ihle et al., 2010; Abbot et al., 2011; Linksvayer et al., 2011; Strassmann et al., 2011; Dolezal et al., 2012; Kapheim et al., 2012; Linksvayer et al., 2012; Page et al., 2012; Flatt et al., 2013; Wilson and Nowak, 2014). The starting point is the observation that the evolutionary trajectories leading to various social systems are built on the reproductive groundplan of solitary insects (Amdam et al., 2004; West‐Eberhard, 2005a; Amdam et al., 2006; Page and Amdam, 2007; Patel et al., 2007; Linksvayer et al., 2009; Amdam and Page, 2010; Ihle et al., 2010; Linksvayer et al., 2011; Linksvayer et al., 2012; Page, Rueppell and Amdam, 2012). A solitary insect is characterized by a life cycle that goes through different physiological and behavioral stages from an embryonic and developmental to a foraging and finally a reproductive phase. Each of the stages of the reproductive groundplan is characterized by a unique state of genome activation as different gene batteries are expressed, corresponding to, among other things, ovarian activation and different behavioral programs (for a summary see (Page, 2013)). The life cycle of a solitary insect thus corresponds to a sequence of internal network states responding to both external and internal signals. For instance, solitary insects need to have stored enough energy and resources and be able to assess the timing and other relevant environmental clues before they can begin to switch into the reproductive mode, triggering both behavioral and physiological programs that involve mating and egg‐laying.
In case of eusocial insects, the same genomic regulatory circuits that characterize individual stages of the groundplan act as the building blocks for the social life in the colony or the superorganism (Amdam et al., 2004; Amdam and Page, 2010; Johnson and Linksvayer, 2010). Comparative studies have revealed how the transitions from solitary to social life (or from organism to superorganism) involve regulatory changes that transform the underlying reproductive groundplan from a sequential to a parallel mode (Page, 2013). Where solitary insects perform different behavioral tasks sequentially, the colony as a whole performs these tasks simultaneously based on new forms of coordination that regulate and modify the expression of ancestral genomic and behavioral programs. The development and evolution of the superorganism thus involve an expansion and a rearrangement of the regulatory and action‐coordination networks that control the development and behavior of individuals, including differential caste development and the activation of behavioral programs in response to colony level regulatory states.
On the basis of our theory of extended evolution, we can now make use of these insights, first to interpret these changes in terms of internalization and externalization processes, and second, as steps along an evolutionary trajectory. As mentioned above, on the level of the colony or the superorganism as a whole, we observe many regulatory elements that in case of solitary insects are variable external signals from the environment now originating from within the constructed and stabilized niches of the colony and the nest. We claim that these causally relevant elements regulating the actions of the colony have, in the context of evolutionary processes, become internalized into the constructed physical and social structures of these systems.
That this is a plausible perspective becomes clear when one considers the origin of the division of labor, including reproductive division of labor, based on dominance hierarchies. Such dominance hierarchies are a consequence of interactions between individuals within a specific ecological setting—a single nest (Beshers and Fewell, 2001). There are ecological conditions that selectively favor aggregations of originally solitary individuals (West‐Eberhard, 1978; Wilson, 1978; Hölldobler and Wilson, 1990; Wilson and Holldobler, 2005b; Hölldobler and Wilson, 2009; Hölldobler and Wilson, 2011). As a consequence, within this newly constructed social and environmental niche the regulatory network governing the behavior of each individual is made up mainly of input from other individuals in form of social interactions.
In systems with developmental caste determination (which exhibit a close similarity to cellular differentiation within a complex organism (Page, 2013), the amount and the importance of signals internal to the colony increases. The regulatory state of the colony acting through linked behavioral, physiological, metabolic and cellular signaling networks that intersect with the core genomic networks regulating gene expression in the developing larvae controls caste differentiation. The developmental path of each individual is thus regulated by the colony as a whole resulting in a more stable but also more canalized social system. And finally in cases of genetic caste determination, many of those elements originally external to the individual but internal to the colony have been fully incorporated into the system of genomic regulation (Julian et al., 2002).
As we have also mentioned above when analyzing the mechanisms involved in the regulation of colony‐level behavior and function from the point of view of the individual insect inside the colony, we observe that along the evolutionary trajectory towards greater social integration relevant developmental causes are externalized into the social and ecological niches as regulatory signals originating from the state of the colony and the behavior of other individuals in the colony. In the case of developmental caste determination, for example, the signaling cascades that regulate gene expression with the developing larvae extend all the way into the network of social interactions in the colony that influence the feeding behavior of the nurse workers and subsequently the hormonal and physiological signaling cascades within the larvae (Fig 1)
Figure 1.
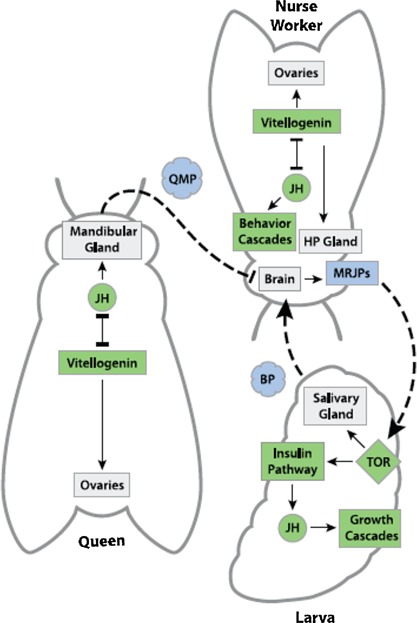
Extended Networks of Regulatory Control: A hypothetical network of developmental control in the superorganism (reprinted with permission from Linksvayer et al., 2012).
On the background of this classification of social systems in terms of internalization and externalization processes leading to an increasing integration of a colony, it now becomes possible to develop plausible evolutionary scenarios for the emergence of this interdependence. Given sufficient plasticity of the developmental and behavioral apparatus of individual organisms, any given niche may act as regulatory input for the individuals as well as for the colony as a whole. The development and reproductive behavior of the colony as a whole is thus governed both by its distributed genome and its extended regulatory system. This potential for integration is also the basis for the emergence of higher levels of selection. Multi‐level selection processes can then, under the right conditions, favor increasingly integrated systems. In this context, mutations favoring stabilization of such initially plastic systems by internalization and externalization processes will be favored until a point of no‐return in the evolutionary trajectory towards a superorganism is reached. As a consequence of increased integration of these extended regulatory systems, colonies become increasingly independent of variable environmental signals, which, in turn, enables them to succeed in a greater number of habitats. This evolutionary dynamics is enabled by internalization processes that encode and stabilize variable environmental conditions within the genome, the developmental apparatus and niche‐construction behavior and externalization processes that create feed‐back mechanisms making sure that individual developmental and behavioral processes can be flexibly adapted within a given niche.
Applying our theory of extended evolution to the case of social evolution thus accomplishes three objectives: We are able to provide an integrative explanatory framework for multiple kinds of empirical data and theoretical perspectives, we can derive the main innovative features of social systems as a consequence of the logic of extended evolution, and we can develop consistent narratives for the emergence of social evolution as an evolutionary innovation.
Conclusion: Innovation, Homology, and the Role of History
Evolutionary and historical change proceeds in different modalities covering a whole spectrum of different types. Gradual change is arguably the predominant type. It involves the kind of “descent with modification” that ever since Darwin has been recognized as a universal principle of nature and culture. Gradual change is, however, not the only kind of historical change. In biological as well as cultural evolution we also observe patterns of discontinuous change, whether in form of major transitions, paradigm shifts, revolutions, or major innovations (Buss, 1987; Maynard Smith and Szathmáry, 1995; Davidson, 2006; Carroll, 2008; Calcott et al., 2011, Wagner, 2014). Complementary to major transitions and innovations are patterns of extreme conservation and canalization that resist change for prolonged periods of time. Both types of phenomena are substantial challenges to a solely gradual and continuous conception of history and evolution.
Our focus on network transformation provides a framework for developing concrete explanations for discontinuous or major changes as well as canalization and can accommodate a wealth of comparative data about genomic and other regulatory networks across a wide range of species and characters (Peter and Davidson, 2015). These data have revealed a spectrum of different types of changes connected with phenotypic evolution that include: (i) slow and gradual divergence, mostly driven by changes in individual genes and (ii) regulatory changes that represent transformations of network structures. In the case of genomes, the latter can be a consequence of genomic rearrangements or of nucleotide substitutions in regulatory regions. We thus often have a good understanding of the endpoints of regulatory network transformations within different evolutionary trajectories, either gradual or discontinuous, at the phenotypic level. What we lack, especially in cases of discontinuous phenotypic evolution, is an understanding of the detailed dynamics of these evolutionary transformations.
The main problem here is to understand how large‐scale rearrangements connected to evolutionary innovations can be viable within populations. Models that propose neutral or hidden genetic variation and gene duplication are among the few that even address this problem (Kimura, 1994; Roughgarden, 1996; Hartl, 2011; Lesk, 2012). Others, such as (Kirschner and Gerhart, 2005) or (Caporael et al., 2014), have also proposed models that can account for the viability of intermediate forms. In contrast to these specific models, our framework of extended evolution provides, from a more systems’ theoretical perspective, a synthetic and dynamic perspective for the explanation of the discontinuity or innovation problem. Two features of our framework are relevant for the first part of the innovation challenge—the origin of novelty. Our framework explicitly recognizes that the horizon of possible states generated by complex extended networks is always larger than what is actually realized. Our framework relies on general insights into the structure and behavior of complex systems (Holland, 2012) that allow us to capture findings about hidden genetic variation and phenotypic plasticity (West‐Eberhard, 2005b). There are more possible phenotypic systems states, either as a consequence of the structure of individual regulatory networks or of the variational properties of populations than are realized at any given time. But these properties alone do not yet explain the actual evolutionarily stable transformations of phenotypes and especially not how those transitioning populations can be stable enough for such a transformation to be successful.
Here our conceptualization of the dynamics of externalization and internalization offers a possible answer. Realizing that elements of the constructed niche are part of the extended regulatory network for any phenotypic trait and also that these niches can provide stable hereditary information suggests how such transformations can happen, and, as we have seen in our example of social insects, actually did happen. The externalization of parts of the regulatory network into the constructed social or environmental niche enables not only the further exploration of phenotypic states, it also stabilizes emerging new characters throughout transitionary phases, as constructed niches can serve as scaffoldings providing stable patterns of heredity. It is important to note here that constructed niches are not only distant structures in the external environment, but that these also include developmental and social contexts that can be as close to the genome as the cytoplasm or maternal behavior. While externalization processes contribute to the transition between network states and therefore also to character transformation, internalization processes then subsequently lead to the increasing stabilization of the new characters and their extended regulatory networks. Selection of favorable variants plays an important role in both cases. But selection and random variation alone do not provide a sufficient explanation for these transitions; the specific structures of regulatory networks and their constructed niches, as well as the dynamics of their transformation through externalization and internalization, are an essential part of the explanation of evolutionary novelties as we have seen in our discussion of the evolution of eusociality.
Cast that way, our framework then presents the evolutionary sequences leading to eusociality as a series of network transformations that pass through a number of steps that, while differing in their individual features, include the following stages: (a) the network of a solitary insect responding to internal states and external cues; (b) a semi‐social phase with reproductive division of labor maintained by behavioral interactions; and (c) a highly eusocial state with reproductive division of labor and morphological caste differentiation generated by developmental regulation.
The main addition of our framework of extended evolution to explanations of phenotypic evolution lies in emphasis of the dynamic sequence of transformations that connect regulatory networks to their respective niches. Focusing on these transformations may yield mechanistic explanations for the dynamics of evolutionary change that include internal—genomic, developmental, organismic, but also, in cases of cultural evolution, cognitive and institutional—factors and environmental elements. All these factors are linked through a causal network generating phenotypes and their variants.
Finally, our framework offers, as we have seen, an explanation for the related challenges of homology and innovation. An extended conception of regulatory networks that involves both internal and niche elements, as well as the dynamics of externalization and internalization explains not only the stability of developmental systems but also the possibility of transitions between stable states. Connecting our framework to population genetics models suggests a possible explanation of how populations can actually cross valleys in Wrightian fitness landscapes.
ACKNOWLEDGMENTS
We would like to thank Jochen Büttner, Lindy Divarci, Doug Erwin, Sascha Freyberg, Christoph Rosol, Matthias Schemmel, Florian Schmaltz, Matteo Valleriani, Sander van der Leeuw, and the members of the Laubichler Lab, especially Guido Caniglia, Erick Peirson, Valerie Racine. MDL acknowledges NSF grants SES 1243575 and SES 1127611. The authors declare no conflict of interest.
Laubichler MD, Renn J. 2015. Extended evolution: A conceptual framework for integrating regulatory networks and niche construction. J. Exp. Zool. (Mol. Dev. Evol.) 324B:565–577.
Conflicts of interest: None.
LITERATURE CITED
- Abbot P, Abe J, Alcock J, et al. 2011. Inclusive fitness theory and eusociality. Nature 471: E1‐4; author reply E9–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE, Jr. 2006. Complex social behaviour derived from maternal reproductive traits. Nature 439:76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Norberg K, Fondrk MK, Page RE, Jr. 2004. Reproductive ground plan may mediate colony‐level selection effects on individual foraging behavior in honey bees. Proceedings of the National Academy of Sciences of the United States of America 101:11350–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Page RE. 2010. The developmental genetics and physiology of honeybee societies. Anim Behav 79:973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Seehuus SC. 2006. Order, disorder, death: Lessons from a superorganism. Adv cancer Res 95:31–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson C, Tornberg A, Tornberg P. 2014. An evolutionary developmental approach to cultural evolution. Curr Anthropol 55:171–154. [PubMed] [Google Scholar]
- Barve A, Wagner A. 2013. A latent capacity for evolutionary innovation through exaptation in metabolic systems. Nature 500:203–206. [DOI] [PubMed] [Google Scholar]
- Ben‐Tabou de‐Leon S, Su YH, Lin KT, Li E, Davidson EH. 2013. Gene regulatory control in the sea urchin aboral ectoderm: Spatial initiation, signaling inputs, and cell fate lockdown. Dev Biol 374:245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram SM, Gorelick R, Fewell JH. 2003. Colony response to graded resource changes: An analytical model of the influence of genotype, environment, and dominance. Theor Popul Biol 64:151–162. [DOI] [PubMed] [Google Scholar]
- Beshers SN, Fewell JH. 2001. Models of division of labor in social insects. Annu Rev Entomol 46:413–440. [DOI] [PubMed] [Google Scholar]
- Boomsma JJ, Beekman M, Cornwallis CK, et al. 2011. Only full‐sibling families evolved eusociality. Nature 471: E4–5; author reply E9‐10. [DOI] [PubMed] [Google Scholar]
- Bowles S, Gintis H. 2011. A cooperative species: Human reciprocity and its evolution. Princeton: Princeton University Press. xii+262pp. [Google Scholar]
- Boyd R, Richerson PJ. 2005. The origin and evolution of cultures. Oxford; New York: Oxford University Press. viii+456pp. [Google Scholar]
- Boyd R, Richerson PJ, Henrich J. 2011. Rapid cultural adaptation can facilitate the evolution of large‐scale cooperation. Behav Ecol Sociobiol 65:431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser CC, Newcomb RD, Gaskett AC, Goddard MR. 2014. Niche construction initiates the evolution of mutualistic interactions. Ecol Lett. doi: 10.1111/ele.12331 [DOI] [PubMed] [Google Scholar]
- Buss LW. 1987. The evolution of individuality. Princeton, N.J: Princeton University Press. xv+201pp. [Google Scholar]
- Calcott B, Sterelny K, Szathmáry Er. 2011. The major transitions in evolution revisited. Cambridge: Mass Press. x+319pp. [Google Scholar]
- Caporael LR, Griesemer JR, Wimsatt WC. 2014. Developing scaffolds in evolution, culture, and cognition. MIT Press. xiv+426pp. [Google Scholar]
- Carroll J. 2004. Literary Darwinism: Evolution, human nature, and literature. New York: Routledge. xxvii+276pp. [Google Scholar]
- Carroll SB. 2000. Endless forms: The evolution of gene regulation and morphological diversity. Cell 101:577–580. [DOI] [PubMed] [Google Scholar]
- Carroll SB. 2008. Evo‐devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell 134:25–36. [DOI] [PubMed] [Google Scholar]
- Creanza N, Fogarty L, Feldman MW. 2012. Models of cultural niche construction with selection and assortative mating. PloS ONE 7:e42744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH. 2001. Genomic regulatory systems : Development and evolution. San Diego: Academic Press. xii+261pp. [Google Scholar]
- Davidson EH. 2006. The regulatory genome : Gene regulatory networks in development and evolution. Burlington, MA ; San Diego: Academic. xi+289pp. [Google Scholar]
- Davidson EH. 2009. Developmental biology at the systems level. Biochimica Et Biophysica Acta 1789:248–249. [DOI] [PubMed] [Google Scholar]
- Davidson EH. 2011. Evolutionary bioscience as regulatory systems biology. Deve Biol 357:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH. 2014. The uncommon roles of common gene regulatory factors in the genomes of differentiating cells. EMBO J 33:1193–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Erwin DH. 2010. Evolutionary innovation and stability in animal gene networks. J Exp zool Part B, Mol Deve Evol 314:182–186. [DOI] [PubMed] [Google Scholar]
- Dolezal AG, Brent CS, Holldobler B, Amdam GV. 2012. Worker division of labor and endocrine physiology are associated in the harvester ant, Pogonomyrmex californicus. J Exp Biol 215:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin DH. 2008. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol Evol 23:304–310. [DOI] [PubMed] [Google Scholar]
- Erwin DH. 2012. Novelties that change carrying capacity. J Exp zool Part B. Mol Dev Evolution 318:460–465. [DOI] [PubMed] [Google Scholar]
- Erwin DH, Krakauer DC. 2004. Evolution. Insights into innovation. Science 304:1117–1119. [DOI] [PubMed] [Google Scholar]
- Erwin DH, Valentine JW. 2013. The Cambrian explosion: The construction of animal biodiversity. Greenwood Village, CO: Roberts and Company; 416pp. [Google Scholar]
- Fewell JH. 2003. Social insect networks. Science 301:1867–1870. [DOI] [PubMed] [Google Scholar]
- Flatt T, Amdam GV, Kirkwood TB, Omholt SW. 2013. Life‐history evolution and the polyphenic regulation of somatic maintenance and survival. Quarterly Rev Biol 88:185–218. [DOI] [PubMed] [Google Scholar]
- Gadau J, Page RE, Jr. , Werren JH, Schmid‐Hempel P. 2000. Genome organization and social evolution in Hymenoptera. Die Naturwissenschaften 87:87–89. [DOI] [PubMed] [Google Scholar]
- Gadau Jr, Fewell J, Wilson EO. 2009. Organization of insect societies : From genome to sociocomplexity. Cambridge Mass: Harvard University Press. x+617pp. [Google Scholar]
- Gerhart J, Kirschner M. 2007. The theory of facilitated variation. Proceedings of the National Academy of Sciences of the United States of America 104:8582–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giray T, Giovanetti M, West‐Eberhard MJ. 2005. Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proceedings of the National Academy of Sciences of the United States of America 102:3330–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Bergstrom CT. 2011. Evolutionary biology within medicine: A perspective of growing value. Bmj 343:d 7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant PR. 1999. Ecology and evolution of Darwin's finches. Princeton, N.J: Princeton University Press. xx+492pp. [Google Scholar]
- Grant PR, Grant BR. 2008. How and why species multiply : The radiation of Darwin's finches. Princeton: Princeton University Press. xix+218pp. [Google Scholar]
- Hartl DL. 2011. Essential genetics : A genomics perspectiv. Sudbury, Mass: Jones and Bartlett Publishers. xxiv+575pp. [Google Scholar]
- Heinze J, Puchinger W, Holldobler B. 1997. Worker reproduction and social hierarchies in Leptothorax ants. Anim Behav 54:849–864. [DOI] [PubMed] [Google Scholar]
- Holland JH. 2012. Signals and boundaries : Building blocks for complex adaptive systems. Cambridge, Mass: MIT Press. viii+308pp. [Google Scholar]
- Hölldobler B, Wilson EO. 1990. The ants. Cambridge, Mass: Belknap Press of Harvard University Press. xii+732pp. [Google Scholar]
- Hölldobler B, Wilson EO. 2009. The superorganism : The beauty, elegance, and strangeness of insect societies. New York: W.W. Norton. xxi+522pp. [Google Scholar]
- Hölldobler B, Wilson EO. 2011. The leafcutter ants : Civilization by instinct. New York: Norton. 160pp. [Google Scholar]
- Hughes WO, Oldroyd BP, Beekman M, Ratnieks FL. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320:1213–1216. [DOI] [PubMed] [Google Scholar]
- Hunt GJ, Amdam GV, Schlipalius D, et al. 2007. Behavioral genomics of honeybee foraging and nest defense. Die Naturwissenschaften 94:247–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle KE, Page RE, Frederick K, Fondrk MK, Amdam GV. 2010. Genotype effect on regulation of behaviour by vitellogenin supports reproductive origin of honeybee foraging bias. Anim Behav 79:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper WC, Linksvayer TA, Atallah J, et al. 2015. Large‐scale coding sequence change underlies the evolution of postdevelopmental novelty in honey bees. Mol Biol Evol 32:334–346. [DOI] [PubMed] [Google Scholar]
- Jeffares B. 2012. Thinking tools: Acquired skills, cultural niche construction, and thinking with things. Behav Brain Sci 35:228–229. [DOI] [PubMed] [Google Scholar]
- Johnson BR, Linksvayer TA. 2010. Deconstructing the superorganism: Social physiology, groundplans, and sociogenomics. Quart Rev Biol 85:57–79. [DOI] [PubMed] [Google Scholar]
- Julian GE, Fewell JH, Gadau J, Johnson RA, Larrabee D. 2002. Genetic determination of the queen caste in an ant hybrid zone. Proceedings of the National Academy of Sciences of the United States of America 99:8157–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapheim KM, Smith AR, Ihle KE, et al. 2012. Physiological variation as a mechanism for developmental caste‐biasing in a facultatively eusocial sweat bee. Proceedings Biological sciences / The Royal Society 279:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalturin K, Hemmrich G, Fraune S, Augustin R, Bosch TC. 2009. More than just orphans: Are taxonomically‐restricted genes important in evolution?. Trends Genet : TIG 25:404–413. [DOI] [PubMed] [Google Scholar]
- Kimura M. 1994. Population genetics, molecular evolution, and the neutral theory : Selected papers. Chicago: University of Chicago Press. xviii+686pp. [Google Scholar]
- Kirschner M, Gerhart J. 2005. The plausibility of life : Resolving Darwin's dilemma. New Haven: Yale University Press. xiii+314pp. [Google Scholar]
- Krakauer DC, Collins JP, Erwin D, et al. 2011. The challenges and scope of theoretical biology. J Theor Biol 276:269–276. [DOI] [PubMed] [Google Scholar]
- Laland K, Uller T, Feldman M. 2014. Does evolutionary theory need a rethink?. Nature 514:161–164. [DOI] [PubMed] [Google Scholar]
- Laland KN. 2008. Exploring gene‐culture interactions: insights from handedness, sexual selection and niche‐construction case studies. Philosophical transactions of the Royal Society of London Series B: Biological sciences; 363 3577–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN, Odling‐Smee FJ, Feldman MW. 1999. Evolutionary consequences of niche construction and their implications for ecology. Proceedings of the National Academy of Sciences of the United States of America 96:10242–10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland KN, Odling‐Smee J, Feldman MW. 2000. Niche construction, biological evolution, and cultural change. Behav Brain Sci 23: 131–146; discussion 146‐175. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling‐Smee J, Gilbert SF. 2008. EvoDevo and niche construction: Building bridges. J Exp zool Part B Mol Dev Evol 310:549–566. [DOI] [PubMed] [Google Scholar]
- Laland KN, Sterelny K. 2006. Perspective: Seven reasons (not) to neglect niche construction. Evol Int J Org Evol 60:1751–1762. [PubMed] [Google Scholar]
- Laubichler MD. 2000. Homology in development and the development of the homology concept. Am Zool 40:777–788. [Google Scholar]
- Leimar O, Hartfelder K, Laubichler MD, Page RE, Jr. 2012. Development and evolution of caste dimorphism in honeybees ‐ a modeling approach. Ecol Evol 2:3098–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesk AM. 2012. Introduction to genomics. Oxford; New York: Oxford University Press. xxii+397pp. [Google Scholar]
- Linksvayer TA. 2006. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evol; Int J Org Evol 60:2552–2561. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Busch JW, Smith CR. 2013. Social supergenes of superorganisms: Do supergenes play important roles in social evolution? Bio Essays : News and reviews in molecular, cellular and developmental biology 35:683–689. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Fewell JH, Gadau J, Laubichler MD. 2012. Developmental evolution in social insects: Regulatory networks from genes to societies. J Exp zool Part B Mol dev Evol 318:159–169. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Fondrk MK, Page RE, Jr. 2009. Honeybee social regulatory networks are shaped by colony‐level selection. Am Nat 173:E99–E107. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Kaftanoglu O, Akyol E, et al. 2011. Larval and nurse worker control of developmental plasticity and the evolution of honey bee queen‐worker dimorphism. J Evol Biol 24:1939–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linksvayer TA, Wade MJ. 2005. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib‐social effects, and heterochrony. Quart Rev Biol 80:317–336. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Wade MJ. 2009. Genes with social effects are expected to harbor more sequence variation within and between species. Evol; Int J Org Evol 63:1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Materna SC, Davidson EH. 2007. Logic of gene regulatory networks. Curr Opin Biotechnol 18:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Szathmáry Er. 1995. The major transitions in evolution. Oxford New Yor: W.H Freeman Spektrum. xiv+346pp. [Google Scholar]
- Minelli A. 2009. Perspectives in animal phylogeny and evolution. Oxford; New York: Oxford University Press. xiii+315pp. [Google Scholar]
- Muller GB, Newman SA. 2005. The innovation triad: An EvoDevo agenda. J Exp Zool Part B, Mol Dev evol 304:487–503. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Ihle KE, Fondrk MK, Page RE, Amdam GV. 2007. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol 5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse RM, Bergstrom CT, Ellison PT, et al. 2010. Evolution in health and medicine Sackler colloquium: Making evolutionary biology a basic science for medicine. Proceedings of the National Academy of Sciences of the United States of America 107:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Tarnita CE, Wilson EO. 2010. The evolution of eusociality. Nature 466:1057–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odling‐Smee FJ. 1995. Niche construction, genetic evolution and cultural change. Behav Process 35:195–205. [DOI] [PubMed] [Google Scholar]
- Odling‐Smee FJ, Laland KN, Feldman MW. 2003. Niche construction : The neglected process in evolution. Princeton: Princeton University Press. xii+472pp. [Google Scholar]
- Odling‐Smee J, Erwin DH, Palkovacs EP, Feldman MW, Laland KN. 2013. Niche construction theory: A practical guide for ecologists. Quart Rev Biol 88:4–28. [DOI] [PubMed] [Google Scholar]
- Okasha S. 2008. Evolution and the levels of selection. Oxford. xi+263pp. [Google Scholar]
- Page RE. 2013. The spirit of the hive : The mechanisms of social evolution. Cambridge, MA: Harvard University Press. xiv+226pp. [Google Scholar]
- Page RE, Jr. 1997. The evolution of insect societies. Endeavour 21:114–120. [DOI] [PubMed] [Google Scholar]
- Page RE, Jr. , Amdam GV. 2007. The making of a social insect: Developmental architectures of social design. BioEssays : News and reviews in molecular, cellular and developmental biology 29:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RE, Jr. , Rueppell O, Amdam GV. 2012. Genetics of reproduction and regulation of honeybee (Apis mellifera L.) social behavior. Ann Rev Genet 46:97–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Fondrk MK, Kaftanoglu O, et al. 2007. The making of a queen: TOR pathway is a key player in diphenic caste development. PloS ONE 2:e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH. 2011. Evolution of gene regulatory networks controlling body plan development. Cell 144:970–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH. 2015. Genomic Control Process: Development and Evolution. Amsterdam: Academic Press. xii+448pp. [Google Scholar]
- Peter IS, Faure E, Davidson EH. 2012. Predictive computation of genomic logic processing functions in embryonic development. Proceedings of the National Academy of Sciences of the United States of America 109:16434–16442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J. 1970. Genetic Epistemology. New York: Columbia University Press. 92pp. [Google Scholar]
- Pigliucci M, Müller G. 2010. Konrad Lorenz Institute for Evolution and Cognition Research Evolution, the extended synthesis. Cambridge Mass: MIT Press. xiii+495pp. [Google Scholar]
- Richerson PJ, Boyd R. 2005. Not by genes alone : How culture transformed human evolution. Chicago: University of Chicago Press. ix+332pp. [Google Scholar]
- Richerson PJ, Christiansen MH. 2013. Cultural evolution : Society, technology, language, and religion. MIT Press. xi+485pp. [Google Scholar]
- Roughgarden J. 1996. Theory of population genetics and evolutionary ecology : An introduction. Upper Saddle River, NJ: Prentice Hall. x+612pp. [Google Scholar]
- Rueppell O, Amdam GV, Page RE, Jr. , Carey JR. 2004. From genes to societies. Sci Aging Knowled Env: SAGE KE 2004 pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruse M. 2013. The Cambridge encyclopedia of Darwin and evolutionary thought. Cambridge England ; New York: Cambridge University Press. xvii+568pp. [Google Scholar]
- Shubin N. 2008. Your inner fish : A journey into the 3.5‐billion‐year history of the human body. New York: Pantheon Books. 229pp. [Google Scholar]
- Stearns SC. 1992. The evolution of life histories. Oxford; New York: Oxford University Press. xii+249pp. [Google Scholar]
- Stearns SC, Koella JC. 2008. Evolution in health and disease. Oxford; New York: Oxford University Press. xxi+374pp. [Google Scholar]
- Strassmann JE, Page RE, Jr. , Robinson GE, Seeley TD. 2011. Kin selection and eusociality. Nature 471: E5–6; author reply E9‐10. [DOI] [PubMed] [Google Scholar]
- Wade MJ, Wilson DS, Goodnight C, et al. 2010. Multilevel and kin selection in a connected world. Nature 463: E8–9; discussion E9‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. 2011. The origins of evolutionary innovations : A theory of transformative change in living systems. Oxford; New York: Oxford University Press. ix+235pp. [Google Scholar]
- Wagner GnP. 2014. Homology, genes, and evolutionary innovation. Princeton: Princeton University Press. xiii+478pp. [Google Scholar]
- Wagner GP. 1999. A research programme for testing the biological homology concept. Novartis Found Symposium 222: 125–134; discussion 134‐140. [PubMed] [Google Scholar]
- Wagner GP. 2007. The developmental genetics of homology. Nat Rev Genet 8:473–479. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Chiu C‐H, Laubichler MD. 2000. Developmental Evolution as a Mechanistic Science: The Inference from Developmental Mechanisms to Evolutionary Processes. Am Zool 40:819–831. [Google Scholar]
- West‐Eberhard MJ. 1978. Temporary queens in metapolybia wasps: Nonreproductive helpers without altruism?. Science 200:441–443. [DOI] [PubMed] [Google Scholar]
- West‐Eberhard MJ. 1986. Animal behavior: Experimental behavioral ecology and sociobiology. Science 231:64–65. [DOI] [PubMed] [Google Scholar]
- West‐Eberhard MJ. 2005a. Developmental plasticity and the origin of species differences. Proceedings of the National Academy of Sciences of the United States of America 102:6543–6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West‐Eberhard MJ. 2005b. Phenotypic accommodation: Adaptive innovation due to developmental plasticity. J Exp zool Part B, Mol Dev evol 304:610–618. [DOI] [PubMed] [Google Scholar]
- West‐Eberhard MJ. 2014. Darwin's forgotten idea: The social essence of sexual selection. Neurosc Biobehav Rev 46P4:501–508. [DOI] [PubMed] [Google Scholar]
- Wilson EO. 1971a. The insect societies. Cambridge, Mas: Belknap Press of Harvard University Press. x+548pp. [Google Scholar]
- Wilson EO. 1971b. Social insects. Science 172:406. [DOI] [PubMed] [Google Scholar]
- Wilson EO. 1978. Ecology of ants and termites. Science 201:337. [DOI] [PubMed] [Google Scholar]
- Wilson EO. 1985. The sociogenesis of insect colonies. Science 228:1489–1495. [DOI] [PubMed] [Google Scholar]
- Wilson EO. 2006. Genomics: How to make a social insect. Nature 443:919–920. [DOI] [PubMed] [Google Scholar]
- Wilson EO, Holldobler B. 2005a. Eusociality: Origin and consequences. Proceedings of the National Academy of Sciences of the United States of America 102:13367–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO, Holldobler B. 2005b. The rise of the ants: A phylogenetic and ecological explanation. Proceedings of the National Academy of Sciences of the United States of America 102:7411–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson EO, Nowak MA. 2014. Natural selection drives the evolution of ant life cycles. Proceedings of the National Academy of Sciences of the United States of America 111:12585–12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimsatt WC. 2013. Articulating babel: An approach to cultural evolution. Studies in history and philosophy of biological and biomedical sciences. Stud Hist Philos Biol Biomed Sci 44:563–571. [DOI] [PubMed] [Google Scholar]


