Abstract
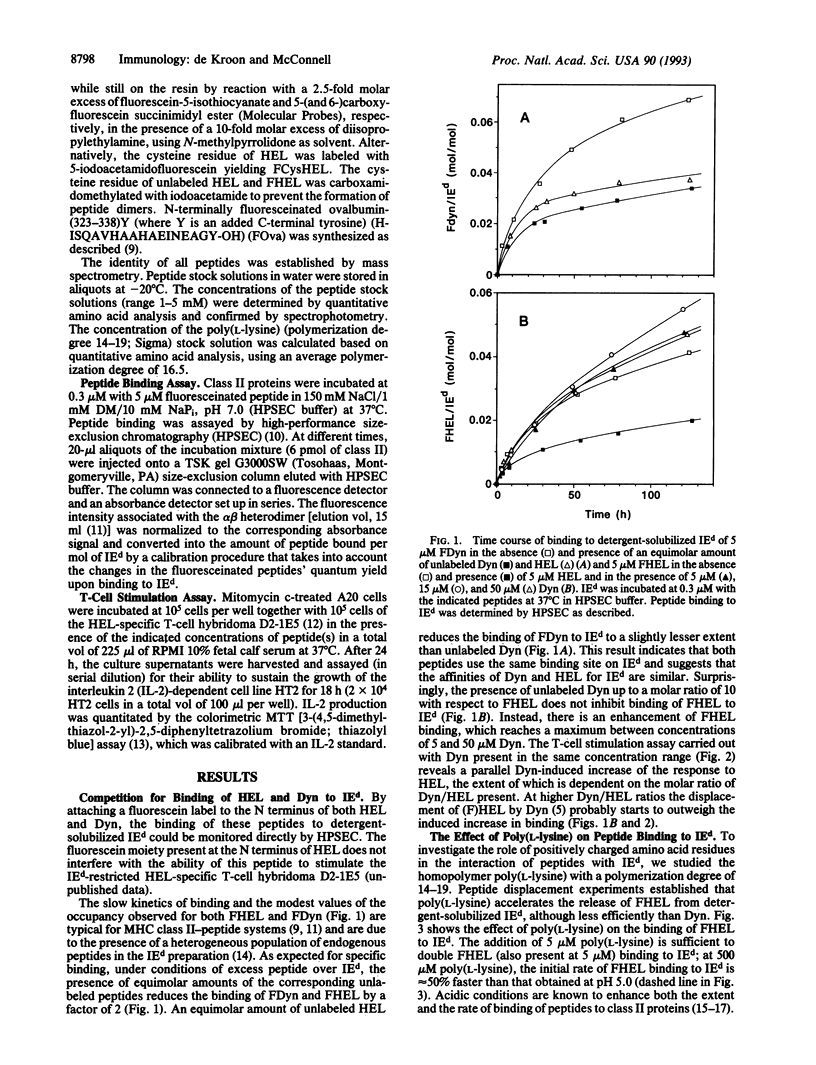
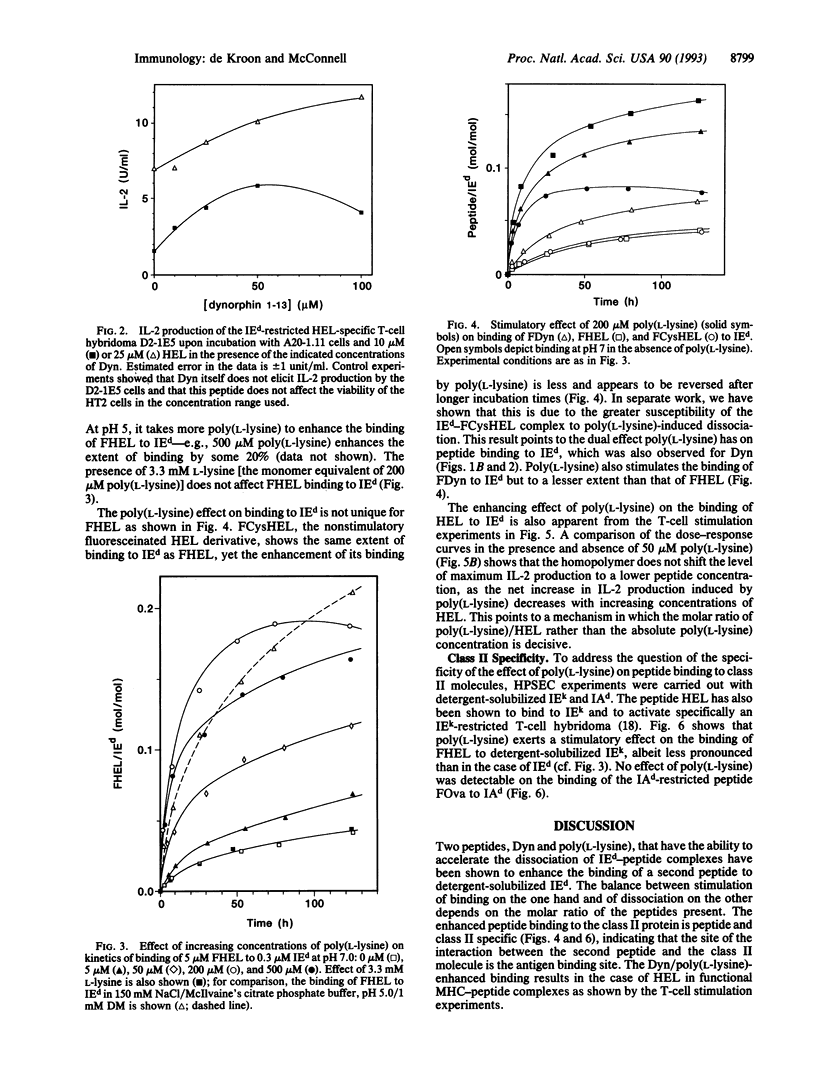
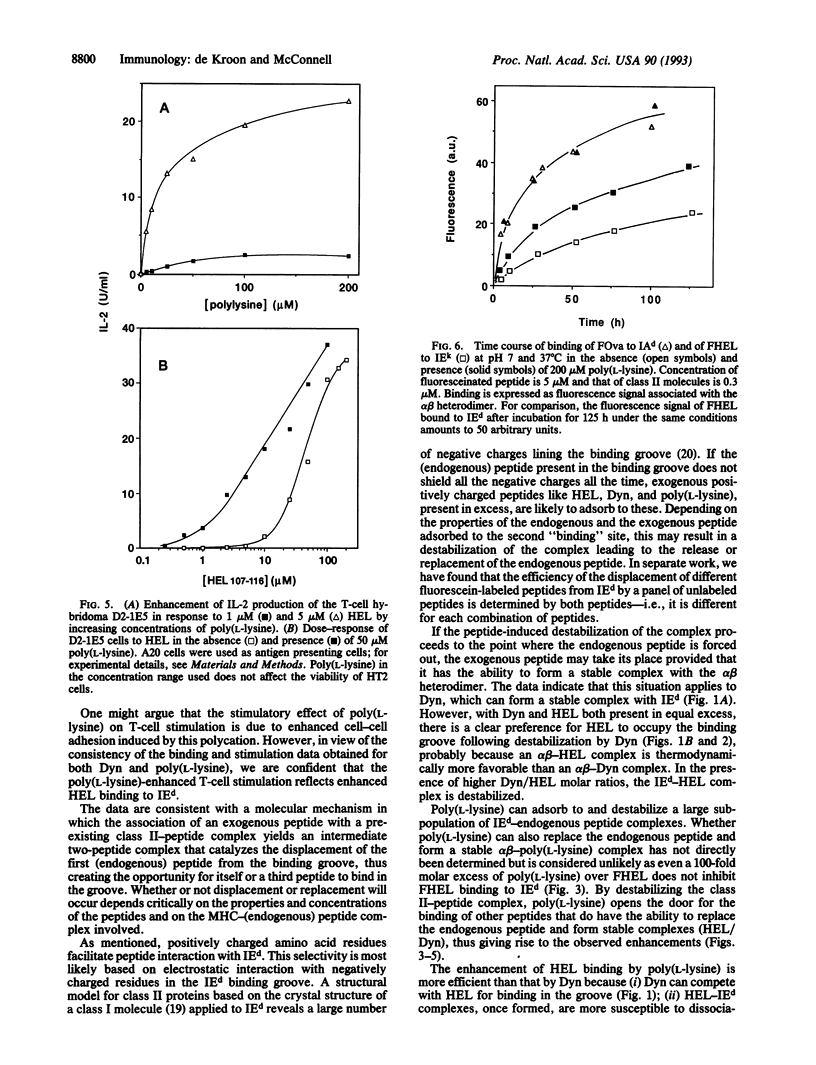
The influence of nonstimulatory "competitor" peptides on the binding of an antigenic peptide to a major histocompatibility complex (MHC) class II molecule was investigated. Using high-performance size-exclusion chromatography and fluorescein-labeled peptides, we show that the presence of the peptides dynorphin A-(1-13) and poly(L-lysine) results in enhancement rather than inhibition of the binding of hen egg lysozyme peptide-(107-116) [HEL-(107-116)] to the detergent-solubilized mouse class II molecule IEd. In parallel, dynorphin A-(1-13) and poly(L-lysine) were found to enhance the specific activation of an IEd-restricted T-cell hybridoma by HEL-(107-116). A molecular mechanism involving an intermediate two peptide-MHC class II protein complex is proposed to explain the enhancement of peptide binding to class II molecules by an irrelevant second peptide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Sette A., Buus S., Grey H. M., Darsley M., Lehmann P. V., Doria G., Nagy Z. A., Appella E. Interaction of an immunodominant epitope with Ia molecules in T-cell activation. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5181–5185. doi: 10.1073/pnas.85.14.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhayani H., Paterson Y. Analysis of peptide binding patterns in different major histocompatibility complex/T cell receptor complexes using pigeon cytochrome c-specific T cell hybridomas. Evidence that a single peptide binds major histocompatibility complex in different conformations. J Exp Med. 1989 Nov 1;170(5):1609–1625. doi: 10.1084/jem.170.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen B., Lambris J. D. Minimum length of an idiotypic peptide and a model for its binding to a major histocompatibility complex class II molecule. EMBO J. 1989 Jul;8(7):1947–1952. doi: 10.1002/j.1460-2075.1989.tb03599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Grey H. M. Autologous peptides constitutively occupy the antigen binding site on Ia. Science. 1988 Nov 18;242(4881):1045–1047. doi: 10.1126/science.3194755. [DOI] [PubMed] [Google Scholar]
- Choppin J., Martinon F., Gomard E., Bahraoui E., Connan F., Bouillot M., Lévy J. P. Analysis of physical interactions between peptides and HLA molecules and application to the detection of human immunodeficiency virus 1 antigenic peptides. J Exp Med. 1990 Sep 1;172(3):889–899. doi: 10.1084/jem.172.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Enhanced binding of peptide antigen to purified class II major histocompatibility glycoproteins at acidic pH. J Exp Med. 1991 Nov 1;174(5):1111–1120. doi: 10.1084/jem.174.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Regulation of antigen presentation by acidic pH. J Exp Med. 1990 May 1;171(5):1779–1784. doi: 10.1084/jem.171.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton J., Sette A., Sidney J., Appella E., Ehrhardt C., Fuchs S., Adorini L. Comparison of structural requirements for interaction of the same peptide with I-Ek and I-Ed molecules in the activation of MHC class II-restricted T cells. J Immunol. 1991 Jul 1;147(1):198–204. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Ploegh H. L. Intracellular transport of MHC class II molecules. Immunol Today. 1992 May;13(5):179–184. doi: 10.1016/0167-5699(92)90123-O. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Sette A., Albertson M., Grey H. M. Free ligand-induced dissociation of MHC-antigen complexes. J Immunol. 1991 May 15;146(10):3496–3501. [PubMed] [Google Scholar]
- Reay P. A., Wettstein D. A., Davis M. M. pH dependence and exchange of high and low responder peptides binding to a class II MHC molecule. EMBO J. 1992 Aug;11(8):2829–2839. doi: 10.1002/j.1460-2075.1992.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberdy J. M., Newcomb J. R., Surman M. J., Barbosa J. A., Cresswell P. HLA-DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature. 1992 Dec 3;360(6403):474–477. doi: 10.1038/360474a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Sadegh-Nasseri S., McConnell H. M. A kinetic intermediate in the reaction of an antigenic peptide and I-Ek. Nature. 1989 Jan 19;337(6204):274–276. doi: 10.1038/337274a0. [DOI] [PubMed] [Google Scholar]
- Sette A., Adorini L., Appella E., Colón S. M., Miles C., Tanaka S., Ehrhardt C., Doria G., Nagy Z. A., Buus S. Structural requirements for the interaction between peptide antigens and I-Ed molecules. J Immunol. 1989 Nov 15;143(10):3289–3294. [PubMed] [Google Scholar]
- Sette A., Buus S., Appella E., Smith J. A., Chesnut R., Miles C., Colon S. M., Grey H. M. Prediction of major histocompatibility complex binding regions of protein antigens by sequence pattern analysis. Proc Natl Acad Sci U S A. 1989 May;86(9):3296–3300. doi: 10.1073/pnas.86.9.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette A., Ceman S., Kubo R. T., Sakaguchi K., Appella E., Hunt D. F., Davis T. A., Michel H., Shabanowitz J., Rudersdorf R. Invariant chain peptides in most HLA-DR molecules of an antigen-processing mutant. Science. 1992 Dec 11;258(5089):1801–1804. doi: 10.1126/science.1465617. [DOI] [PubMed] [Google Scholar]
- Sette A., Sidney J., Albertson M., Miles C., Colón S. M., Pedrazzini T., Lamont A. G., Grey H. M. A novel approach to the generation of high affinity class II-binding peptides. J Immunol. 1990 Sep 15;145(6):1809–1813. [PubMed] [Google Scholar]
- Sette A., Southwood S., O'Sullivan D., Gaeta F. C., Sidney J., Grey H. M. Effect of pH on MHC class II-peptide interactions. J Immunol. 1992 Feb 1;148(3):844–851. [PubMed] [Google Scholar]
- Stern L. J., Wiley D. C. The human class II MHC protein HLA-DR1 assembles as empty alpha beta heterodimers in the absence of antigenic peptide. Cell. 1992 Feb 7;68(3):465–477. doi: 10.1016/0092-8674(92)90184-e. [DOI] [PubMed] [Google Scholar]
- Tampé R., Clark B. R., McConnell H. M. Energy transfer between two peptides bound to one MHC class II molecule. Science. 1991 Oct 4;254(5028):87–89. doi: 10.1126/science.1656526. [DOI] [PubMed] [Google Scholar]
- Tampé R., McConnell H. M. Kinetics of antigenic peptide binding to the class II major histocompatibility molecule I-Ad. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4661–4665. doi: 10.1073/pnas.88.11.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S. N., McConnell H. M. A first-order reaction controls the binding of antigenic peptides to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8164–8168. doi: 10.1073/pnas.88.18.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]


