Abstract
Extensive studies have demonstrated that infant immune responses are distinct from those of adults. Despite these differences, infant immunization can elicit protective immune responses at levels comparable to or, in some cases, higher than adult immune responses to many vaccines. To date, only a few HIV vaccine candidates have been tested in infant populations, and none of them evaluated vaccine efficacy. Recent exciting studies showing that HIV-infected infants can develop broad neutralizing antibody responses and that some HIV vaccine regimens can elicit high levels of potentially protective antibodies in infants provide support for the development and testing of HIV vaccines in pediatric populations. In this review, we discuss the differences in adult and infant immune responses in the setting of HIV infection and vaccination.
INTRODUCTION
Infancy is a critical window for the generation of protective immunity via vaccination due to the vulnerability of infants to infections. Vaccination early in life can establish immunity that persists throughout adulthood or that can be boosted prior to periods of potential increased pathogen exposure. In addition, the frequent interactions of young infants and their families with health care providers provide an opportune time to achieve high vaccine coverage. In fact, the majority of vaccines used for the prevention of human infections are administered in early childhood, and they typically offer long-lived protection. In the case of an HIV vaccine, effective immunization in infancy might both protect against HIV acquisition via breastfeeding and provide mature anti-HIV immunity prior to sexual debut, potentially contributing to protection against sexually acquired infections from early adolescence through adulthood. Thus, the use of infant vaccination, perhaps followed by later boosting in preadolescence, might be a highly desirable tool in the quest for an HIV-free generation.
Despite the success of many vaccines in the youngest age groups, our understanding of vaccine-generated immune responses in infants and how they differ from those of adults remains limited. Important factors that distinguish the infant immune system from that of adults include differences in effector cell subsets, immunoregulatory mechanisms of fetal development, passive acquisition of maternal antibodies, and limited preexposure to environmental immune stimuli. These immunologic differences may result in distinct immune responses following infant and adult vaccination. An understanding of the infant immune landscape is therefore critical for the design of vaccines that will elicit optimal immune responses in infants and target long-term immunity.
EARLY LIFE AND ADULT IMMUNE RESPONSES
The immune system undergoes changes throughout early age due to the abrupt transition from a sterile environment in the womb to an environment with repeated immune stimuli (1). Substantial evidence demonstrates that the neonatal immune system is not unresponsive but instead is adapted for early life. In contrast, immunologically mature adults have acclimated to persistent antigen exposure, including a host of commensal bacteria and viruses that reside in the gut and skin, and as a result orchestrate immune responses differently than infants. In this section, we will use selected examples to demonstrate that although infants and adults respond differently to antigenic stimulation, infants are capable of mounting robust immune responses.
Phenotypic and qualitative differences in immune responses between infants and adults.
Analysis of immune cell populations has demonstrated substantial phenotypic and functional differences between human infants and adults (Table 1). For example, neonatal neutrophils have lower chemotactic responses (2) and reduced phagocytic capacities (3) compared to adult neutrophils. Moreover, cord blood displays a higher ratio of plasmacytoid to conventional dendritic cells than adult blood, but cord blood dendritic cells express lower levels of major histocompatibility complex (MHC) class II, CD80, and CD86 (4). Interestingly, although infant plasmacytoid dendritic cells have a lower ability to respond to stimulation by bacterial DNA CpG motifs than adult dendritic cells (5), they can secrete higher levels of interleukin-1 beta (IL-1β), IL-6, and IL-10 (6), demonstrating that they are not deficient in cytokine production. Cord blood also contains higher proportions of NK cells than adult blood, but they have distinct expression levels of activating and inhibitory markers (7). Although infant and adult NK cells express similar levels of CD16 (FcγRIII), cord blood cells have a reduced capacity to respond to stimuli and lower cytotoxic capabilities than adult cells (8). Nevertheless, the expression of activating markers and function of cord blood NK cells can be enhanced in vitro in the presence of IL-2, IL-12, and IL-15 (9–11). Thus, under certain conditions, neonatal innate immune cells can be as functionally potent as adult cells.
TABLE 1.
Examples of immune parameters that differ between infants and adults
| Immune parameter | Infants | Adults | Reference(s) |
|---|---|---|---|
| NK cell activity | CD16 expression similar to adult cells but lower cytotoxic capacity | Lower no. of NK cells than infants | 8, 9 |
| Dendritic cells | Higher ratio of plasmacytoid to monocyte-derived DCs than that in adults | Higher response to stimulation by CPG motifs than infant DCs | 4, 5 |
| CD4+ T cell responses | Th2 bias | Th2 and Th1 responses | 15 |
| Trega | High levels of Treg compared to adults | Normal Treg levels | 12 |
| Memory B cell formation | Lower markers of memory B cells, impaired bone marrow homing markers | High expression of memory B cell markers | 20, 21 |
| IgG subclass | IgG1 and IgG3 attain adult levels earlier than IgG2 and IgG4; low levels of IgG2 to polysaccharide antigens | IgG2 responses to polysaccharide antigens | 18, 19 |
| Maternal antibodies | Interference with development of B cell but not T cell responses in infants | Not present | 34–38 |
Treg, T regulatory cells.
It was previously thought that infants have deficient CD4+ T cell responses. However, recent discoveries indicate that infant hyporesponsiveness is largely modulated by T regulatory cells. In fact, infants have a higher proportion of T regulatory cells but a lower proportion of T follicular helper cells than adults (12). Infants have lower numbers of circulating CD4+ CCR5+ T cells, the main target for HIV (13), although a high proportion of CD4+ CCR5+ T cells was recently reported in the infant gut (14). Infants also exhibit a bias toward Th2 responses (15). This Th2 polarization is likely due to the high levels of Th2-promoting cytokines, such as IL-10, and prostaglandin E2 in early life (16, 17). In addition, infants have impaired humoral responses. They develop poor antibody responses to bacterial capsular antigens and are deficient in the production of antibodies of the IgG2 subclass (18, 19). Moreover, studies in mouse models have demonstrated that neonatal B cells have an impaired ability to become long-lasting memory B cells, which may be related to their lack of bone marrow homing receptors and survival markers or to impaired survival signals in the bone marrow stromal environment (20, 21). Interestingly, recent studies have indicated that infant B cell responses can be improved by adjuvants (22). For example, cord blood B cells are able to produce polysaccharide-specific antibodies following CpG engagement of Toll-like receptor 9 (23). Moreover, the oil-in-water adjuvant MF-59 can induce robust germinal center formation in mice as young as 3 weeks of age (24), and immunization of infant mice using the adjuvant IC31 can enhance IgG1, IgG2a, and IgG2b subclass antibodies to polysaccharide antigens (25). These findings indicate that under certain circumstances, infants can develop robust responses, highlighting the need to tailor immunization strategies to pediatric populations.
Infants are able to develop robust immune responses to non-HIV vaccines.
Several studies have demonstrated that infant immunization can generate high-magnitude immune responses. For example, 3-day-old neonates immunized with the oral poliomyelitis vaccine (OPV) and boosted throughout the first year of life developed long-lived antibody responses, and close to 100% of infants achieved seroconversion after the fourth immunization dose (26). Similarly, a recent vaccine efficacy study against enterovirus 71 (EV71) conducted in a total of 10,077 Chinese children age 6 months to 3 years showed a remarkable 94.8% efficacy rate (27). Interestingly, newborns immunized with the hepatitis B vaccine can produce higher anti-hepatitis B antibody levels than vaccinated adults (28). Furthermore, the gamma interferon (IFN-γ) CD4+ T cell response in infants immunized with the bacillus Calmette-Guérin vaccine is comparable to that in vaccinated adults (29). These infant immunization studies provide a proof of principle that infants can mount comparable or higher immune responses than adults following vaccination.
Importantly, vaccine boosting schedules and the vaccine adjuvant can significantly affect the quality and magnitude of immune responses in infants. For example, infants vaccinated against diphtheria, tetanus, and pertussis at 2, 3, and 4 months of age generate lower-magnitude antibody responses than infants immunized at 3, 5, and 9 months (30). The only adjuvants approved by the FDA for use in commercial pediatric vaccines in the United States are aluminum salts, but in other areas of the world, additional adjuvants, such as the oil-in-water adjuvant MF-59, are also used. A recent study compared the vaccine efficacies of unadjuvanted and MF-59-adjuvanted trivalent influenza vaccines in 4,707 German and Finnish children between 6 months and 36 months of age. The vaccine efficacy against the vaccine-matched strain was 45% for the unadjuvanted vaccine and 89% for the MF-59-adjuvanted vaccine. Similarly, the MF-59-adjuvanted vaccine was more protective against all virus strains (86% efficacy rate) than the unadjuvanted vaccine (43% efficacy rate) (31). Thus, the choice of the vaccine adjuvant is critical in the setting of pediatric immunization.
Maternal antibodies can interfere with infant response to vaccination.
Maternal IgG antibodies are transferred to infants across the placenta (32), and they persist throughout the first year of life. These maternal passively acquired antibodies are critical for protecting infants against common pathogens (33, 34), but they can interfere with infant immune responses to vaccines (35). In fact, infants immunized against measles at 6 months of age generate weaker vaccine-induced antibody responses than infants immunized at 9 months of age. Moreover, there is an inverse correlation between maternal antibody titers and infant responses against the measles vaccine (36). Interestingly, maternal antibodies do not always affect infant vaccine responses. For example, a recent study reported an inverse correlation between birth levels of antibodies and infant vaccine-elicited responses against tetanus and pneumococcus but not against pertussis and Haemophilus influenzae B (37). Similarly, maternal levels of anti-hepatitis B surface antibodies do not appear to interfere with the long-term immunogenicity of the hepatitis B vaccine (38). Several hypotheses have been proposed to explain the mechanism by which maternal antibodies interfere with infant vaccine responses. These include inhibition of B cell responses through masking of important epitopes, inhibition of B cell responses by binding of maternal passively acquired IgG to FcγRIIB expressed in infant naive B cells, neutralization of live-attenuated vaccines, and antigen removal by infant macrophages (reviewed in reference 39). Importantly, as this interference appears to vary from one vaccine to another, the potential impact of maternal antibodies should be investigated when developing novel infant vaccines.
IMMUNE RESPONSES TO HIV INFECTION IN INFANTS AND ADULTS
HIV disease progression differs significantly between adults and infants. In the absence of treatment, progression to AIDS or death in adults usually occurs at a median time of 10 years after infection. In contrast, 20 to 30% of HIV-infected infants die within the first year of life (40). The kinetics of plasma virus load is also strikingly different between adults and infants. During adult infection, the initial plasma virus load peak is rapidly followed by a 100- to 1,000-fold decrease in viral copies, reaching a relative stable “set point” within weeks of infection. In contrast, in infants, plasma viral load levels slowly decline and do not reach a set point until around 5 years of age. The difference in disease progression between adults and infants might be related to the differences between the infant and adult immune systems. These may include the ongoing development of the infant immune system, with a rapid expansion of CD4+ T cells, and the distinct HIV-specific immune responses in adults and infants (Table 2).
TABLE 2.
Comparison of anti-HIV responses between infected adults and infants
| Immune parameter | Infants | Adults | References |
|---|---|---|---|
| Env-specific antibody responses | gp160-specific antibodies develop first, followed by gp120- and gp41-specific responses | gp41-specific antibodies develop first, followed by gp120 responses | 41, 44 |
| Broadly neutralizing responses | Can develop broad neutralization as early as 2 yr postinfection | Develop after several yr of infection | 54, 55 |
| ADCC responses | Delayed development of ADCC-mediating antibodies in infants infected in utero and intrapartum but not in infants infected after 6 wk of age | Peak ADCC responses as early as 4 mo postinfection | 47–49 |
| T cell responses | Low CTL responses; possible association between noncytotoxic T cell responses and virus controla | CTL responses associated with virus set point | 56–62 |
| Target predominantly variable proteins | Target conserved and variable proteins |
CTL, cytotoxic T lymphocytes.
Ontogeny of HIV envelope-specific antibody responses in HIV-infected adults and infants.
The initial envelope (Env)-specific antibody response in HIV-infected adults arises approximately 14 days after infection and targets the glycoprotein 41 (gp41) envelope (41). Recent reports have demonstrated cross-reactivity between gp41 and gut flora antigens (42, 43), leading to the hypothesis that the early gp41 response is a secondary antibody response driven by a preexisting pool of memory B cells raised against commensal bacteria. As newborns are unlikely to have this pool of memory B cells, it is possible that the specificity of the early antibody response to HIV infection differs between adults and infants. Because the presence of maternally acquired antibodies clouds the investigation of infant antibody responses, Pollack et al. (44) assessed the ability of lymphocytes isolated from 46 HIV-infected infants age 0 to 12 months to produce HIV-specific antibodies in vitro. Their results indicated that in the neonatal period, infants produced only low levels of antibodies against a restricted number of HIV antigens, but by 6 months of age, the majority of infants had detectable levels of antibodies against two or more HIV antigens. Antibodies against gp160 appeared first, followed by anti-gp120 and anti-gp41 antibodies, indicating possible differences in the ontogeny of HIV-specific antibodies between adults and infants. The use of advanced methods to produce monoclonal antibodies from antigen-specific memory B cells might provide a deeper understanding of potential differences in the development of HIV-specific antibodies in infected adults and infants.
The lack of correlation between the magnitude of adult early HIV-specific antibody responses and the viral load set point suggests that antibody responses may not play a major role in the suppression of viral load during adult acute HIV infection. To investigate the association between infant antibody responses and disease progression in infants, Henrard et al. (45) measured HIV-specific antibody responses in a prospective cohort of 32 HIV-exposed infants. While HIV-specific maternal antibodies decreased over time in HIV-exposed uninfected infants, 9 of the 12 HIV-infected infants showed an increase in HIV-specific antibodies at a median time of 6 months. Importantly, the 8 infants who remained asymptomatic at 1 year of age developed de novo antibody responses, whereas 3 of the 4 infants with rapid disease progression showed no significant rise in antibodies. These results suggest that HIV-specific antibody responses might contribute to disease control in infants. It will be important to confirm these results in large cohorts of infected infants and to identify the specificities of antibodies associated with disease control.
HIV-infected infants develop neutralization breadth early in life, but antibody-dependent cell-mediated cytotoxicity responses are delayed in infants.
The presence of antibodies capable of mediating neutralization or antibody-dependent cell-mediated cytotoxicity (ADCC) in infants has been associated with better clinical outcome (46, 47); however, infants infected in utero rarely develop ADCC antibodies before 12 months of age (48). Interestingly, while a delay in the development of ADCC-mediating responses is also observed in infants infected during the first 6 weeks of life, ADCC antibodies can be detected as early as 2 months postinfection when infants are infected later in life (46). The development of ADCC activity in older HIV-infected infants is comparable to that in adults in whom ADCC responses can be detected within the first months of infection (49). Interestingly, whereas in adults, the ADCC response peaks around 4 months postinfection and decreases thereafter (49), in some infants, ADCC antibody levels continue to increase for more than a year after infection (46). Importantly, until now, investigations of ADCC responses have only been conducted on a restricted number of infected infants. Moreover, most studies measured ADCC responses in children using adult effector cells. Future studies should therefore investigate the development of functional antibody responses in large cohorts of infants infected by different routes to fully decipher the potential differences between adults and infants.
A small proportion (10 to 25%) of HIV-infected adults develops antibodies able to neutralize many primary virus isolates (50–53). These broadly neutralizing antibodies are usually directed against a few epitopes, including the CD4 binding site, the membrane proximal external region in gp41, the gp120-gp41 interface, and glycan-dependent sites of the Env variable loops (V1V2 or V3) (54), and they arise after several years of infection. Until recently, the ability of infants to develop broad neutralizing antibody responses was unknown. In an elegant study by Goo et al. (55), it was reported that infants can develop neutralization breadth. Remarkably, two and a half years after infection, some infants showed a neutralization breadth comparable to the breadth observed in the top 1% adult neutralizers several years after infection. As neutralizing antibody responses are thought to be important for an effective HIV vaccine, investigation of the developmental pathway of broadly neutralizing responses in infants might have important implications for HIV vaccine development.
Cellular immune responses to HIV in adults and infants.
Previous studies in infants and adults have attempted to define the role of cellular immune responses during HIV infection and the ability of these responses to suppress viremia. In HIV-infected adults, virus-specific CD8+ T cells appear in blood just before peak viremia, and cytotoxic T cell responses have been associated with a decrease in viral load (56–58). Early studies have indicated that while cytotoxic responses are rarely detected in infants before 6 months of age (59, 60), noncytolytic CD8 viral suppressive activity can be detected early and is associated with lower viral load in infected infants (61). A more recent study also reported that HIV-specific CD8+ T cells are frequently detected in 6- to 10-week-old perinatally HIV-infected infants (62). Interestingly, the CD8+ T cell response in infants was shown to predominantly target variable proteins (Nef, Reg, and Env), whereas the response in their chronically infected mothers was equally spread between variable and conserved (Gag and Pol) proteins. In contrast to the high frequency of HIV-specific CD8+ T cells detected in infants, only 5 out of 15 tested infants had detectable HIV-specific CD4+ T cells responses at 6 to 10 weeks of age. Thus, these studies suggest potential differences in the specificities and virus suppression mechanisms of CD8+ T cells in adults and infants.
HIV VACCINE RESPONSES IN INFANTS AND ADULTS
Only a few infant pediatric phase I/IIa vaccine trials have been conducted to date, and their results have consistently demonstrated that HIV vaccines are safe in infants. In the Pediatric AIDS Clinical Trial Group (PACTG) protocol 230, the safety and immunogenicity of two recombinant gp120 vaccines were evaluated in infants born to HIV-infected women from the United States (63). Infants were immunized with 4 vaccine doses between 0 and 20 weeks of age. Both vaccines were shown to be safe and to induce humoral and cellular immune responses (64, 65). In the initial phase of the study, infants were immunized with escalating doses of vaccines to identify an “optimal vaccine dose” (63). Interestingly, infants who received the lowest vaccine dose had a higher frequency of T cell lymphoproliferative responses than infants immunized with higher vaccine doses (65), suggesting that low vaccine doses may be required in early life.
HIV vaccine safety in infants was also demonstrated in PACTG 326 (66). In the first part of that trial, the immunogenicity and safety of ALVAC-HIV vCP205 (containing HIV-1 Gag, Env, and protease inserts [67]) were investigated in U.S. infants born to HIV-infected mothers, and in the second phase of the trial, ALVAC-HIV vCP1452 (containing MNgp120, LAIgp41, p55 LAIgag, pol, and pol and nef HLA-A2-restricted T cell epitope inserts [68]) was evaluated when administered either alone or with an AIDSVAX recombinant gp120 (rgp120) boost. Interestingly, while all vaccine regimens induced cellular responses, only infants immunized with the ALVAC-AIDSVAX regimen developed Env-specific antibody responses (69). Finally, in HPTN027, Ugandan infants born to HIV-infected mothers were immunized with ALVAC-HIV vCP1521 (containing HIV-1 clade E env, clade B gag, and protease [70]). No severe or life-threatening events were observed in vaccinated infants, and adverse events were equally distributed in the placebo and vaccine arms. As in PACTG 326, this ALVAC-only regimen did not elicit significant humoral immune responses. Overall, these trials demonstrated that HIV-exposed infants can develop humoral immune responses when immunized with protein-based HIV vaccines.
Immune correlates of protection in the moderately effective adult RV144 vaccine trial.
The adult RV144 HIV vaccine trial conducted in Thailand evaluated the efficacy of an ALVAC-HIV (vCP1521) prime expressing clade E env and clade B gag and pol, followed by an AIDSVAX clade B/E gp120 protein boost. The vaccine strains were similar to viruses commonly circulating in Thailand at the time of the trial. Interestingly, in the first year postimmunization, the vaccine efficacy was 60%, with a decline to 31% efficacy after 3 years (71, 72). To identify immune responses associated with vaccine efficacy, a case-control study was performed on 41 infected vaccine recipients, 205 uninfected vaccine recipients, and 40 placebo recipients (20 infected and 20 uninfected) (73). Two distinct antibody responses were found to be associated with a risk of HIV acquisition: a high magnitude of IgG antibodies that bound to a scaffold recombinant protein of the HIV Env variable loop 1 and 2 (gp70 V1V2) was associated with a decreased risk of HIV acquisition, whereas high-magnitude plasma Env-specific IgA correlated with an increased risk of infection. Importantly, neither low levels of V1V2 IgG nor high levels of Env-specific IgA were associated with higher rates of infection in vaccine recipients than in the placebo group.
Infant vaccination can induce high-magnitude potentially protective anti-V1V2 IgG responses.
Because of the differences in the adult and infant immune systems, whether HIV vaccine discoveries in adults are applicable to infant settings remains unknown. To date, no HIV vaccine efficacy trial has been conducted in infants; the identification of immune correlates of HIV acquisition in the RV144 adult trial provided a unique opportunity to determine if HIV vaccination can elicit potentially protective responses in infants. We therefore reevaluated vaccine-elicited antibody responses in PACTG 230 and in PACTG 326. In PACTG 230, infants were vaccinated with four doses of Chiron rgp120 (SF-2 strain) with the adjuvant MF-59, VaxGen rgp120 (MN-strain) with aluminum hydroxide, or placebo between 0 and 20 weeks of age (63). In PACTG 326, infants received four doses of ALVAC vCP1452 alone, ALVAC vCP1452 with AIDSVAX B/B with the adjuvant alum, or placebo between 0 and 12 weeks of age (69). Reanalysis of the antibody responses elicited by infant Env vaccination revealed that infants developed robust Env-specific antibody responses to these first-generation HIV Env vaccines. At 1 year of age, the majority of the maternally acquired antibodies had waned, and vaccine Env-specific IgG responses were significantly higher in vaccinees than in placebo recipients (74). All vaccine regimens induced a frequency of V1V2 response in vaccinees similar to or higher than that in RV144 vaccinees (Fig. 1). Remarkably, at peak immunogenicity, 98% of the infants immunized with the Chiron vaccine had detectable levels of anti-V1V2 IgG, a response associated with a reduced risk of HIV acquisition in the RV144 adult vaccine trial (Fig. 1). Moreover, the anti-V1V2 IgG concentration was 22-fold higher in infants who received the Chiron vaccine than in RV144 adult vaccinees. Importantly, there was no correlation between anti-V1V2 IgG levels at birth and levels either at peak immunogenicity or at 1 year of age, indicating that maternal antibodies did not interfere with the development of robust anti-V1V2 IgG responses in infants. Moreover, vaccine-elicited IgA responses, which were associated with an increased risk of HIV acquisition in RV144 adult vaccinees, were rarely detected in PACTG 230 vaccinated infants. These results clearly demonstrate that infants can develop robust antibody responses following HIV vaccination and suggest that adult immunogenicity data may not be transposable to pediatric settings. However, it is important to keep in mind that the adult RV144 trial and the infant PACTG 230 trial did not evaluate the same vaccines. Thus, a comparison of adult and infant immune responses following immunization with the same HIV vaccines is warranted to gain more understanding regarding the potential differences between adults and infants. In addition, the immune correlates of HIV acquisition in infants are not known, and it is unclear if the same mechanisms are important for blocking HIV infection through breastfeeding and sexual transmission. In fact, it is possible that protecting infants against breast milk HIV transmission is easier than protecting adolescents/adults against sexual HIV transmission, because infants are born with maternal antibodies raised against viruses to which they are exposed and their period of exposure to the virus is relatively short and well defined.
FIG 1.
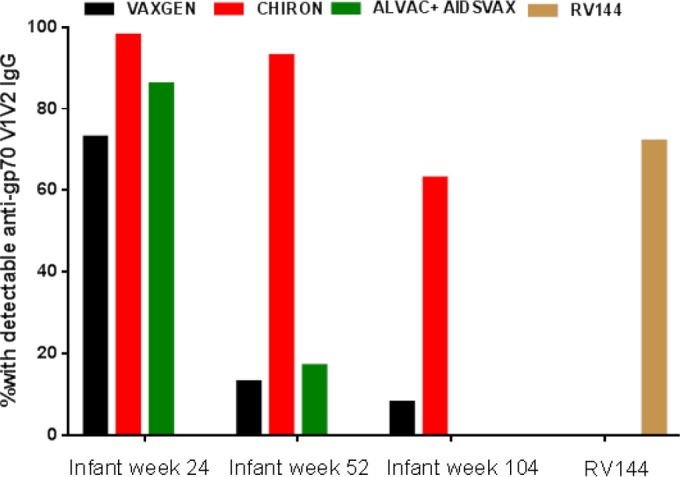
Infant HIV vaccination elicits high frequency of potentially protective anti-V1V2 IgG responses. Shown are the proportions of infants vaccinated with the Chiron vaccine (n = 43), the VaxGen vaccine (n = 44), and the ALVAC + AIDSVAX regimen (n = 7) with detectable IgG antibodies against gp70 B case A V1V2 at peak immunogenicity and subsequent time points. The reported proportion of adult RV144 vaccine recipients with a detectable IgG response against the gp70 B case A V1V2 construct (101) is presented for comparison.
Can infant HIV vaccination elicit polyfunctional antibodies?
The mechanism of protection in the RV144 adult vaccine trial is not completely understood, but it is generally thought that nonneutralizing effector immune responses contributed to the observed protection. In support of this hypothesis, subsequent analysis of samples from RV144 vaccinees and from the nonprotective VAX003 trial revealed that the RV144 vaccine induced a polyfunctional IgG response, whereas the VAX003 vaccine elicited a monofunctional response (75). The polyfunctionality of RV144 vaccine-elicited responses was driven by highly functional IgG3 antibodies, whereas the monofunctional response in VAX003 was influenced by the production of poorly functional IgG4. Importantly, a strong correlation was observed between IgG3 antibodies against the V1V2 loop and a reduced risk of HIV acquisition in RV144 vaccinees (76). We therefore sought to determine if infant vaccination could induce IgG3 antibodies against gp70 B case A2 V1V2, a murine leukemia glycoprotein containing a V1V2 clade B-scaffolded construct that mimics the native V1V2 structure on gp120 (77). The IgG subclass distribution of V1V2-IgG antibodies was assessed among PACTG 230 participants immunized with the Chiron vaccine and in placebo recipients. The majority of infants had IgG1 antibodies against gp70 B case A2 V1V2, but 42% of them also had V1V2-specific IgG3 antibodies at peak immunogenicity (74). However, this response was short-lived, as 8 months after vaccination, V1V2-specific IgG3 antibodies were no longer detected in infants. A similarly short duration of IgG3 antibody response was also observed in adult RV144 vaccine recipients (76). V1V2-specific IgG2 and IgG4 responses were rarely observed in vaccinated infants (our unpublished data). Thus, infant HIV vaccination can induce potentially highly functional IgG3 antibodies, but whether infant vaccine-elicited antibodies are capable of mediating nonneutralizing effector functions remains to be determined.
Modulation of infant and adult HIV vaccine-elicited antibody response by adjuvants.
The role of adjuvants in modulating antibody responses to HIV vaccines was recently addressed by comparing antibody responses in adults from the RV132 and RV135 phase I/II vaccine trials (78). In RV132, healthy Thai volunteers were immunized with ALVAC-HIV (vCP1521), followed by a bivalent clade B/AE HIV gp120 protein boost (SF-2/CM235) absorbed into MF-59, whereas RV135 participants were immunized with vCP1521, followed by a bivalent B/AE gp120 protein boost (MN gp120/A244 gp120) using alum as an adjuvant. Most vaccine recipients in both trials developed Env-specific antibodies after the first and second protein boosts. Interestingly, while there was no difference in the binding to a clade-matched V2 cyclic peptide between RV135 and RV132 vaccine recipients, at peak immunogenicity, the response against gp70 B case A V1V2 (RV144 immune correlate) was significantly higher in RV135 vaccine recipients in whom alum was used as an adjuvant. Thus, in HIV-vaccinated adults, alum might be more effective than MF-59 in directing the immune response toward potentially protective antibodies. These results contrast with those observed in PACTG 230 infant vaccine recipients, in whom the MF-59-adjuvanted vaccine (Chiron) elicited higher-magnitude and higher-frequency IgG responses against gp70 B case A V1V2 (Table 3). In fact, at peak immunogenicity, the percentage of infants with detectable IgG responses against gp70 B case A V1V2 was 98% among the Chiron vaccinees and 73% among the VaxGen vaccine recipients. More impressively, 8 months after the administration of the last vaccine dose, 93% of the Chiron vaccine recipients but only 13% of the VaxGen vaccine recipients had a detectable anti-gp70 B case A V1V2 IgG response.
TABLE 3.
Adjuvants may differently modulate adult and infant antibody responses to HIV vaccines
| Patient group | Trial | Vaccine(s) | Adjuvant | Protein dose (μg) | V1V2-specific IgG response |
|---|---|---|---|---|---|
| Adults | RV135 | ALVAC (vCP1521), rgp120 MN/rgp120 A244 | Alum | 300/300 | Higher with alum than with MF-59 |
| RV132 | ALVAC (vCP1521), rgp120 SF2/gp120 CM235 | MF-59 | 100/50 | Short-lived antibody responses with MF-59 and alum | |
| Infants | PACTG 230 | VaxGen, rgp120 MN | Alum | 30, 100, or 300 | Higher with MF-59 than with alum |
| PACTG 230 | Chiron, rgp120 SF2 | MF-59 | 5, 15, or 50 | Long-lived antibody responses with MF-59 but not with alum |
In contrast, adults from the RV135 and RV132 trials had short-lived anti-gp70 B case A V1V2-specific antibody responses. This might indicate that MF-59-adjuvanted HIV vaccines can elicit more durable antibody responses in infants than in adults. It is also possible that the difference between adult and infant responses results from the distinct vaccine regimens used in the trials. Nevertheless, in both the adult and infant trials, the dose used in the alum-adjuvanted vaccine was higher than that used in the MF-59-adjuvanted vaccine. Moreover, the same clade B vaccine strains were used with the same adjuvants in the adult and infant trials (MN with alum and SF-2 with MF-59). This strongly suggests that alum and MF-59 differentially modulate infant and adult responses to HIV vaccines.
Characterization of Env-specific monoclonal antibodies isolated from vaccinated infants might provide deeper understanding of vaccine-elicited antibodies.
Vaccine-elicited responses are evaluated by comparing immune responses after vaccination to those measured prior to vaccination or to those of a placebo control group. Because HIV-exposed infants are born with high levels of maternal passively acquired Env-specific antibodies, an assessment of vaccine-elicited responses relies on a comparison between vaccine and placebo recipients. Importantly, it is possible that this approach masks the detection of low levels of vaccine-elicited antibodies in infants. The isolation of Env-specific antibodies from vaccinated infants can circumvent this limitation, providing a deeper understanding of vaccine-induced antibody responses in infants. Recent advances in antigen-specific B cell sorting and antibody gene cloning have provided insights into the immune mechanisms required for the development of neutralization breadth (79, 80). In addition, the characterization of monoclonal antibodies from HIV-vaccinated adults has uncovered novel specificities and functional interactions that could not be measured in whole plasma. For example, analysis of monoclonal antibodies from RV144 vaccinees has demonstrated competition between IgG and IgA antibodies directed against the same epitope (81). These findings led to the hypothesis that in RV144, vaccine-elicited IgA antibodies interfered with the effector functions of potentially protective IgG antibodies, explaining why high-magnitude IgA responses were associated with an increased risk of HIV acquisition in this trial. To date, no studies have characterized antigen-specific B cells in infants. Aside from providing insights into the specificity and function of vaccine-elicited antibody responses, such investigations will determine if differences in affinity maturation exist between infants and adults.
Infant vaccination might be beneficial for the prevention of sexual HIV transmission.
Studies in nonhuman primates have demonstrated that broadly neutralizing antibodies can protect against simian-human immunodeficiency virus acquisition (82–85); yet, all HIV vaccines tested to date in humans have failed to induce broadly neutralizing antibody responses (86, 87). The fact that the RV144 vaccine achieved moderate protection while eliciting antibodies capable of neutralizing only tier 1 (easy-to-neutralize) viruses suggests that nonneutralizing antibodies mediated the protective effect of the vaccine. It is therefore likely that a vaccine capable of eliciting broad neutralizing responses is more efficacious than RV144. Recent studies demonstrating that broadly neutralizing antibodies coevolve with virus through multiple rounds of virus escape to immune pressure (88, 89) have led to the hypothesis that the induction of broad Env-specific neutralizing responses through immunization will require sequential vaccinations with a series of related antigens (88–90). Thus, it may be difficult to raise protective antibody responses prior to sexual debut if vaccination is initiated in adolescence or adulthood. The initiation of HIV immunity early in life via infant vaccination has the possibility of inducing long-term affinity maturation of the antibody response and generating the broad HIV immunity needed to protect against sexually transmitted HIV. Moreover, the infant immune responses will potentially be more flexible than those of adults, due to the more limited exposure to environmental antigens that have predefined the immune repertoire (42, 91). Thus, a pediatric HIV vaccine might have benefits beyond the prevention of transmission through breast milk.
Therapeutic HIV vaccines in children.
Current antiretroviral regimens can lead to sustained viral suppression, but they cannot cure HIV even after several years of treatment, because of the persistence of a reservoir of latently infected cells (92). The eradication of this latent virus reservoir will surely require a combination of pharmacologic and immunologic approaches. Several immunologic strategies have been proposed to achieve viral remission. These include passive immunization with monoclonal antibodies and therapeutic vaccines.
Two HIV vaccine trials have been conducted in HIV-infected children to date. ACTG 218 evaluated the safety and immunogenicity of three HIV Env protein vaccines in HIV-infected infants and children ages 1 month to 18 years (93). The vaccines were administered at entry and then 1, 2, 3, 4, and 6 months later. The vaccine was well tolerated, and there was no significant deterioration in clinical status, no occurrence of serious opportunistic infections, no evidence of adverse effects on the immune system, and no increase in viral replication in vaccinees. Up to 56% of the vaccinated children developed lymphoproliferative responses against the vaccine antigens and 65% of the vaccinated children exhibited moderate to strong antibody responses to the vaccine antigens, but none of the placebo recipients had moderate to strong HIV-specific antibody responses. Similarly, the administration of an HIV DNA vaccine construct expressing HIV-1 subtypes A, B, and C, Env, Rev, Gag, and reverse transcriptase (RT) to 10 HIV-infected children (6 to 16 years of age) on antiretroviral therapy was well tolerated in the PEDVAC trial (94). Interestingly, vaccinated children developed higher lymphoproliferative responses to gag than did adults immunized with the same vaccine. These two studies provide a proof of principle that HIV vaccines can be safely administered to HIV-infected children, and they pave the way for future larger trials. Importantly, it was recently argued by Klein et al. (95) that children are ideal candidates for immunotherapeutic interventions to achieve viral remission. The arguments presented by the authors are based on the fact that perinatally infected children for whom antiretroviral (ARV) treatment is initiated early on have a small latent virus reservoir (96, 97) and normal development of the memory B cell and T cell compartments (98, 99). The potential for viral eradication in children was recently illustrated by the “Mississippi baby.” This perinatally infected baby received triple-ARV treatment 30 h after birth, but the treatment was interrupted by the mother after 18 months. For >20 months after treatment interruption, the baby had undetectable virus levels in peripheral blood. However, at 46 months of age, the plasma viral load rebounded (100), indicating that very early treatment is not enough to prevent the establishment of the latent reservoir. In future studies, it will be critical to determine if a combination of early antiretroviral treatment and immunotherapeutic interventions can prolong virus remission in children.
CONCLUSION
Ridding the world of HIV will require strategies that impede all modes of transmission, including vertical and sexual transmission. A vaccine administered early in life to elicit broad and mature antibody responses in infants and preadolescents prior to sexual debut might therefore be a valuable resource for the global elimination of HIV. Recent works have indicated that the neonatal immune system is equipped to respond to HIV antigen exposure in a way that induces broad neutralizing antibody responses (55). In addition, infants can develop robust antibody responses that are equal to or higher in magnitude than adult vaccine responses that were associated with a reduced risk of sexual HIV infection (74). Importantly, because immune pathways that lead to protective immune responses may differ between infants and adults, HIV vaccine strategies may need to be tailored to the infant immune landscape in order to direct the development of protective HIV immunity in early life. Preclinical vaccine studies in animal models and clinical vaccine studies in HIV-exposed infants will increase our current understanding of how infants respond to HIV antigens and guide the development of an effective global HIV vaccine strategy.
ACKNOWLEDGMENTS
We thank the PACTG 230 and 326 study participants and the IMPAACT staff involved in these studies.
Overall support for the IMPAACT group was provided by the NIAID, NIH (grant U01 AI068632), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Siegrist CA, Aspinall R. 2009. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol 9:185–194. doi: 10.1038/nri2508. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger B, Laskin DL, Mariano TM, Sunil VR, DeCoste CJ, Heck DE, Gardner CR, Laskin JD. 2001. Mechanisms underlying reduced responsiveness of neonatal neutrophils to distinct chemoattractants. J Leukoc Biol 70:969–976. [PMC free article] [PubMed] [Google Scholar]
- 3.Miller ME. 1979. Phagocyte function in the neonate: selected aspects. Pediatrics 64:709–712. [PubMed] [Google Scholar]
- 4.Willems F, Vollstedt S, Suter M. 2009. Phenotype and function of neonatal DC. Eur J Immunol 39:26–35. doi: 10.1002/eji.200838391. [DOI] [PubMed] [Google Scholar]
- 5.De Wit D, Olislagers V, Goriely S, Vermeulen F, Wagner H, Goldman M, Willems F. 2004. Blood plasmacytoid dendritic cell responses to CpG oligodeoxynucleotides are impaired in human newborns. Blood 103:1030–1032. [DOI] [PubMed] [Google Scholar]
- 6.Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES III, Hajjar AM, Hawkins NR, Self SG, Wilson CB. 2009. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 183:7150–7160. doi: 10.4049/jimmunol.0901481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundström Y, Nilsson C, Lilja G, Kärre K, Troye-Blomberg M, Berg L. 2007. The expression of human natural killer cell receptors in early life. Scand J Immunol 66:335–344. doi: 10.1111/j.1365-3083.2007.01980.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalle JH, Menezes J, Wagner E, Blagdon M, Champagne J, Champagne MA, Duval M. 2005. Characterization of cord blood natural killer cells: implications for transplantation and neonatal infections. Pediatr Res 57:649–655. doi: 10.1203/01.PDR.0000156501.55431.20. [DOI] [PubMed] [Google Scholar]
- 9.Guilmot A, Hermann E, Braud VM, Carlier Y, Truyens C. 2011. Natural killer cell responses to infections in early life. J Innate Immun 3:280–288. doi: 10.1159/000323934. [DOI] [PubMed] [Google Scholar]
- 10.La Pine TR, Joyner JL, Augustine NH, Kwak SD, Hill HR. 2003. Defective production of IL-18 and IL-12 by cord blood mononuclear cells influences the T helper-1 interferon gamma response to group B streptococci. Pediatr Res 54:276–281. doi: 10.1203/01.PDR.0000072515.10652.87. [DOI] [PubMed] [Google Scholar]
- 11.Gaddy J, Broxmeyer HE. 1997. Cord blood CD16+56− cells with low lytic activity are possible precursors of mature natural killer cells. Cell Immunol 180:132–142. doi: 10.1006/cimm.1997.1175. [DOI] [PubMed] [Google Scholar]
- 12.Rabe H, Lundell AC, Andersson K, Adlerberth I, Wold AE, Rudin A. 2011. Higher proportions of circulating FOXP3+ and CTLA-4+ regulatory T cells are associated with lower fractions of memory CD4+ T cells in infants. J Leukoc Biol 90:1133–1140. doi: 10.1189/jlb.0511244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalekoff S, Gray GE, Tiemessen CT. 2004. Age-related changes in expression of CXCR4 and CCR5 on peripheral blood leukocytes from uninfected infants born to human immunodeficiency virus type 1-infected mothers. Clin Diagn Lab Immunol 11:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunders MJ, van der Loos CM, Klarenbeek PL, van Hamme JL, Boer K, Wilde JC, de Vries N, van Lier RA, Kootstra N, Pals ST, Kuijpers TW. 2012. Memory CD4(+)CCR5(+) T cells are abundantly present in the gut of newborn infants to facilitate mother-to-child transmission of HIV-1. Blood 120:4383–4390. doi: 10.1182/blood-2012-06-437566. [DOI] [PubMed] [Google Scholar]
- 15.Zaghouani H, Hoeman CM, Adkins B. 2009. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol 30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. 1996. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med 184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilkens CM, Vermeulen H, van Neerven RJ, Snijdewint FG, Wierenga EA, Kapsenberg ML. 1995. Differential modulation of T helper type 1 (Th1) and T helper type 2 (Th2) cytokine secretion by prostaglandin E2 critically depends on interleukin-2. Eur J Immunol 25:59–63. doi: 10.1002/eji.1830250112. [DOI] [PubMed] [Google Scholar]
- 18.Umetsu DT, Ambrosino DM, Quinti I, Siber GR, Geha RS. 1985. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N Engl J Med 313:1247–1251. doi: 10.1056/NEJM198511143132002. [DOI] [PubMed] [Google Scholar]
- 19.Aucouturier P, Berthier M, Bonneau D, Preud'homme JL. 1988. Serum levels of IgG subclasses in the normal child. Evaluation by an immunoenzymatic method using monoclonal antibodies. Arch Fr Pediatr 45:255–258. (In French.) [PubMed] [Google Scholar]
- 20.Belnoue E, Pihlgren M, McGaha TL, Tougne C, Rochat AF, Bossen C, Schneider P, Huard B, Lambert PH, Siegrist CA. 2008. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood 111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 21.Pihlgren M, Schallert N, Tougne C, Bozzotti P, Kovarik J, Fulurija A, Kosco-Vilbois M, Lambert PH, Siegrist CA. 2001. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur J Immunol 31:939–946. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Levy O, Goriely S, Kollmann TR. 2013. Immune response to vaccine adjuvants during the first year of life. Vaccine 31:2500–2505. doi: 10.1016/j.vaccine.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, Seganti G, Zuntini R, Ferrari S, Cagliuso M, Quinti I, Carsetti R. 2008. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol 180:800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 24.Mastelic Gavillet B, Eberhardt CS, Auderset F, Castellino F, Seubert A, Tregoning JS, Lambert PH, de Gregorio E, Del Giudice G, Siegrist CA. 2015. MF59 mediates its B cell adjuvanticity by promoting T follicular helper cells and thus germinal center responses in adult and early life. J Immunol 194:4836–4845. doi: 10.4049/jimmunol.1402071. [DOI] [PubMed] [Google Scholar]
- 25.Kamath AT, Rochat AF, Valenti MP, Agger EM, Lingnau K, Andersen P, Lambert PH, Siegrist CA. 2008. Adult-like anti-mycobacterial T cell and in vivo dendritic cell responses following neonatal immunization with Ag85B-ESAT-6 in the IC31 adjuvant. PLoS One 3:e3683. doi: 10.1371/journal.pone.0003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong D, Hu X, Liu W, Li J, Jin Y, Tan S, Chen T, Fu J, Niu B, Yu H, Zhou Y. 1986. Immunization of neonates with trivalent oral poliomyelitis vaccine (Sabin). Bull World Health Organ 64:853–860. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, Hu Y, Ji T, Song L, Liang Q, Zhang B, Gao Q, Li J, Wang S, Hu Y, Gu S, Zhang J, Yao G, Gu J, Wang X, Zhou Y, Chen C, Zhang M, Cao M, Wang J, Wang H, Wang N. 2014. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 370:818–828. doi: 10.1056/NEJMoa1304923. [DOI] [PubMed] [Google Scholar]
- 28.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Whittle H, Lambert PH, McAdam KP, Siegrist CA, Marchant A. 2004. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 22:511–519. doi: 10.1016/j.vaccine.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Vekemans J, Amedei A, Ota MO, D'Elios MM, Goetghebuer T, Ismaili J, Newport MJ, Del Prete G, Goldman M, McAdam KP, Marchant A. 2001. Neonatal bacillus Calmette-Guérin vaccination induces adult-like IFN-gamma production by CD4+ T lymphocytes. Eur J Immunol 31:1531–1535. doi:. [DOI] [PubMed] [Google Scholar]
- 30.Booy R, Aitken SJ, Taylor S, Tudor-Williams G, Macfarlane JA, Moxon ER, Ashworth LA, Mayon-White RT, Griffiths H, Chapel HM. 1992. Immunogenicity of combined diphtheria, tetanus, and pertussis vaccine given at 2, 3, and 4 months versus 3, 5, and 9 months of age. Lancet 339:507–510. doi: 10.1016/0140-6736(92)90336-2. [DOI] [PubMed] [Google Scholar]
- 31.Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. 2011. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 365:1406–1416. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 32.Shen C, Xu H, Liu D, Veazey RS, Wang X. 2014. Development of serum antibodies during early infancy in rhesus macaques: implications for humoral immune responses to vaccination at birth. Vaccine 32:5337–5342. doi: 10.1016/j.vaccine.2014.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benowitz I, Esposito DB, Gracey KD, Shapiro ED, Vazquez M. 2010. Influenza vaccine given to pregnant women reduces hospitalization due to influenza in their infants. Clin Infect Dis 51:1355–1361. doi: 10.1086/657309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochola R, Sande C, Fegan G, Scott PD, Medley GF, Cane PA, Nokes DJ. 2009. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 4:e8088. doi: 10.1371/journal.pone.0008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siegrist CA. 2003. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 21:3406–3412. doi: 10.1016/S0264-410X(03)00342-6. [DOI] [PubMed] [Google Scholar]
- 36.Gans HA, Yasukawa LL, Alderson A, Rinki M, Dehovitz R, Maldonado Y, Arvin AM. 2004. T cell immunity to measles viral proteins in infants and adults after measles immunization. Viral Immunol 17:298–307. doi: 10.1089/0882824041310522. [DOI] [PubMed] [Google Scholar]
- 37.Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. 2014. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine 32:996–1002. doi: 10.1016/j.vaccine.2013.11.104. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Zhang S, Luo C, Wu Q, Liu Q, Zhou YH, Hu Y. 2011. Transplacentally acquired maternal antibody against hepatitis B surface antigen in infants and its influence on the response to hepatitis B vaccine. PLoS One 6:e25130. doi: 10.1371/journal.pone.0025130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niewiesk S. 2014. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 5:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunn D, HIV Paediatric Prognostic Markers Collaborative Study Group. 2003. Short-term risk of disease progression in HIV-1-infected children receiving no antiretroviral therapy or zidovudine monotherapy: a meta-analysis. Lancet 362:1605–1611. doi: 10.1016/S0140-6736(03)14793-9. [DOI] [PubMed] [Google Scholar]
- 41.Tomaras GD, Yates NL, Liu P, Qin L, Fouda GG, Chavez LL, Decamp AC, Parks RJ, Ashley VC, Lucas JT, Cohen M, Eron J, Hicks CB, Liao HX, Self SG, Landucci G, Forthal DN, Weinhold KJ, Keele BF, Hahn BH, Greenberg ML, Morris L, Karim SS, Blattner WA, Montefiori DC, Shaw GM, Perelson AS, Haynes BF. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol 82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trama AM, Moody MA, Alam SM, Jaeger FH, Lockwood B, Parks R, Lloyd KE, Stolarchuk C, Scearce R, Foulger A, Marshall DJ, Whitesides JF, Jeffries TL Jr, Wiehe K, Morris L, Lambson B, Soderberg K, Hwang KK, Tomaras GD, Vandergrift N, Jackson KJ, Roskin KM, Boyd SD, Kepler TB, Liao HX, Haynes BF. 2014. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe 16:215–226. doi: 10.1016/j.chom.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF. 2011. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med 208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollack H, Zhan MX, Ilmet-Moore T, Ajuang-Simbiri K, Krasinski K, Borkowsky W. 1993. Ontogeny of anti-human immunodeficiency virus (HIV) antibody production in HIV-1-infected infants. Proc Natl Acad Sci U S A 90:2340–2344. doi: 10.1073/pnas.90.6.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henrard D, Fauvel M, Samson J, Delage G, Boucher M, Hankins C, Stephens J, Lapointe N. 1993. Ontogeny of the humoral immune response to human immunodeficiency virus type 1 in infants. J Infect Dis 168:288–291. doi: 10.1093/infdis/168.2.288. [DOI] [PubMed] [Google Scholar]
- 46.Milligan C, Richardson BA, John-Stewart G, Nduati R, Overbaugh J. 2015. Passively acquired antibody-dependent cellular cytotoxicity (ADCC) activity in HIV-infected infants is associated with reduced mortality. Cell Host Microbe 17:500–506. doi: 10.1016/j.chom.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broliden K, Sievers E, Tovo PA, Moschese V, Scarlatti G, Broliden PA, Fundaro C, Rossi P. 1993. Antibody-dependent cellular cytotoxicity and neutralizing activity in sera of HIV-1-infected mothers and their children. Clin Exp Immunol 93:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pugatch D, Sullivan JL, Pikora CA, Luzuriaga K. 1997. Delayed generation of antibodies mediating human immunodeficiency virus type 1-specific antibody-dependent cellular cytotoxicity in vertically infected infants. WITS Study Group. Women and Infants Transmission Study. J Infect Dis 176:643–648. [DOI] [PubMed] [Google Scholar]
- 49.Dugast AS, Stamatatos L, Tonelli A, Suscovich TJ, Licht AF, Mikell I, Ackerman ME, Streeck H, Klasse PJ, Moore JP, Alter G. 2014. Independent evolution of Fc- and Fab-mediated HIV-1-specific antiviral antibody activity following acute infection. Eur J Immunol 44:2925–2937. doi: 10.1002/eji.201344305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol 82:11651–11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wibmer CK, Moore PL, Morris L. 2015. HIV broadly neutralizing antibody targets. Curr Opin HIV AIDS 10:135–143. doi: 10.1097/COH.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goo L, Chohan V, Nduati R, Overbaugh J. 2014. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat Med 20:655–658. doi: 10.1038/nm.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68:6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68:4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, Ferrari G, Giorgi E, Ganusov VV, Keele BF, Learn GH, Turnbull EL, Salazar MG, Weinhold KJ, Moore S, CHAVI Clinical Core B, Letvin N, Haynes BF, Cohen MS, Hraber P, Bhattacharya T, Borrow P, Perelson AS, Hahn BH, Shaw GM, Korber BT, McMichael AJ. 2009. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luzuriaga K, Koup RA, Pikora CA, Brettler DB, Sullivan JL. 1991. Deficient human immunodeficiency virus type 1-specific cytotoxic T cell responses in vertically infected children. J Pediatr 119:230–236. doi: 10.1016/S0022-3476(05)80732-2. [DOI] [PubMed] [Google Scholar]
- 60.Buseyne F, Blanche S, Schmitt D, Griscelli C, Riviere Y. 1993. Detection of HIV-specific cell-mediated cytotoxicity in the peripheral blood from infected children. J Immunol 150:3569–3581. [PubMed] [Google Scholar]
- 61.Pollack H, Zhan MX, Safrit JT, Chen SH, Rochford G, Tao PZ, Koup R, Krasinski K, Borkowsky W. 1997. CD8+ T-cell-mediated suppression of HIV replication in the first year of life: association with lower viral load and favorable early survival. AIDS 11:F9–F13. doi: 10.1097/00002030-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Shalekoff S, Meddows-Taylor S, Gray GE, Sherman GG, Coovadia AH, Kuhn L, Tiemessen CT. 2009. Identification of human immunodeficiency virus-1 specific CD8+ and CD4+ T cell responses in perinatally-infected infants and their mothers. AIDS 23:789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham CK, Wara DW, Kang M, Fenton T, Hawkins E, McNamara J, Mofenson L, Duliege AM, Francis D, McFarland EJ, Borkowsky W, Pediatric AIDS Clinical Trials Group 230 Collaborators . 2001. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin Infect Dis 32:801–807. doi: 10.1086/319215. [DOI] [PubMed] [Google Scholar]
- 64.McFarland EJ, Borkowsky W, Fenton T, Wara D, McNamara J, Samson P, Kang M, Mofenson L, Cunningham C, Duliege AM, Sinangil F, Spector SA, Jimenez E, Bryson Y, Burchett S, Frenkel LM, Yogev R, Gigliotti F, Luzuriaga K, Livingston RA, AIDS Clinical Trials Group 230 Collaborators. 2001. Human immunodeficiency virus type 1 (HIV-1) gp120-specific antibodies in neonates receiving an HIV-1 recombinant gp120 vaccine. J Infect Dis 184:1331–1335. doi: 10.1086/323994. [DOI] [PubMed] [Google Scholar]
- 65.Borkowsky W, Wara D, Fenton T, McNamara J, Kang M, Mofenson L, McFarland E, Cunningham C, Duliege AM, Francis D, Bryson Y, Burchett S, Spector SA, Frenkel LM, Starr S, Van Dyke R, Jimenez E. 2000. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J Infect Dis 181:890–896. [DOI] [PubMed] [Google Scholar]
- 66.Johnson DC, McFarland EJ, Muresan P, Fenton T, McNamara J, Read JS, Hawkins E, Bouquin PL, Estep SG, Tomaras GD, Vincent CA, Rathore M, Melvin AJ, Gurunathan S, Lambert J. 2005. Safety and immunogenicity of an HIV-1 recombinant canarypox vaccine in newborns and infants of HIV-1-infected women. J Infect Dis 192:2129–2133. doi: 10.1086/498163. [DOI] [PubMed] [Google Scholar]
- 67.Fang ZY, Kuli-Zade I, Spearman P. 1999. Efficient human immunodeficiency virus (HIV)-1 Gag-Env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J Infect Dis 180:1122–1132. doi: 10.1086/315028. [DOI] [PubMed] [Google Scholar]
- 68.Jin X, Ramanathan M Jr, Barsoum S, Deschenes GR, Ba L, Binley J, Schiller D, Bauer DE, Chen DC, Hurley A, Gebuhrer L, El Habib R, Caudrelier P, Klein M, Zhang L, Ho DD, Markowitz M. 2002. Safety and immunogenicity of ALVAC vCP1452 and recombinant gp160 in newly human immunodeficiency virus type 1-infected patients treated with prolonged highly active antiretroviral therapy. J Virol 76:2206–2216. doi: 10.1128/jvi.76.5.2206-2216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McFarland EJ, Johnson DC, Muresan P, Fenton T, Tomaras GD, McNamara J, Read JS, Douglas SD, Deville J, Gurwith M, Gurunathan S, Lambert JS. 2006. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS 20:1481–1489. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 70.Kintu K, Andrew P, Musoke P, Richardson P, Asiimwe-Kateera B, Nakyanzi T, Wang L, Fowler MG, Emel L, Ou SS, Baglyos L, Gurunathan S, Zwerski S, Jackson JB, Guay L. 2013. Feasibility and safety of ALVAC-HIV vCP1521 vaccine in HIV-exposed infants in Uganda: results from the first HIV vaccine trial in infants in Africa. J Acquir Immune Defic Syndr 63:1–8. doi: 10.1097/QAI.0b013e31827f1c2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Robb ML, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Kunasol P, Khamboonruang C, Thongcharoen P, Morgan P, Benenson M, Paris RM, Chiu J, Adams E, Francis D, Gurunathan S, Tartaglia J, Gilbert P, Stablein D, Michael NL, Kim JH. 2012. Risk behaviour and time as covariates for efficacy of the HIV vaccine regimen ALVAC-HIV (vCP1521) and AIDSVAX B/E: a post-hoc analysis of the Thai phase 3 efficacy trial RV 144. Lancet Infect Dis 12:531–537. doi: 10.1016/S1473-3099(12)70088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators . 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 73.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fouda GG, Cunningham CK, McFarland EJ, Borkowsky W, Muresan P, Pollara J, Song LY, Liebl BE, Whitaker K, Shen X, Vandergrift NA, Overman RG, Yates NL, Moody MA, Fry C, Kim JH, Michael NL, Robb M, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Liao HX, Haynes BF, Montefiori DC, Ferrari G, Tomaras GD, Permar SR. 2015. Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses. J Infect Dis 211:508–517. doi: 10.1093/infdis/jiu444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 6:228ra238. [DOI] [PubMed] [Google Scholar]
- 76.Yates NL, Liao HX, Fong Y, deCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O'Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. 2014. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 6:228ra239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pinter A, Honnen WJ, Kayman SC, Trochev O, Wu Z. 1998. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 16:1803–1811. doi: 10.1016/S0264-410X(98)00182-0. [DOI] [PubMed] [Google Scholar]
- 78.Karasavvas N, Karnasuta C, Savadsuk H, Madnote S, Inthawong D, Chantakulkij S, Rittiroongrad S, Nitayaphan S, Pitisuttithum P, Thongcharoen P, Siriyanon V, Andrews CA, Barnett SW, Tartaglia J, Sinangil F, Francis DP, Robb ML, Michael NL, Ngauy V, de Souza MS, Paris RM, Excler JL, Kim JH, O'Connell RJ. 2015. IgG antibody responses to recombinant gp120 proteins, gp70V1/V2 scaffolds, and a cyclic V2 peptide in Thai phase I/II vaccine trials using different vaccine regimens. AIDS Res Hum Retroviruses 31:1178–1186. doi: 10.1089/aid.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao HX, Levesque MC, Nagel A, Dixon A, Zhang R, Walter E, Parks R, Whitesides J, Marshall DJ, Hwang KK, Yang Y, Chen X, Gao F, Munshaw S, Kepler TB, Denny T, Moody MA, Haynes BF. 2009. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods 158:171–179. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 81.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 83.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 84.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voronin Y, Mofenson LM, Cunningham CK, Fowler MG, Kaleebu P, McFarland EJ, Safrit JT, Graham BS, Snow W. 2014. HIV monoclonal antibodies: a new opportunity to further reduce mother-to-child HIV transmission. PLoS Med 11:e1001616. doi: 10.1371/journal.pmed.1001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vaine M, Wang S, Liu Q, Arthos J, Montefiori D, Goepfert P, McElrath MJ, Lu S. 2010. Profiles of human serum antibody responses elicited by three leading HIV vaccines focusing on the induction of Env-specific antibodies. PLoS One 5:e13916. doi: 10.1371/journal.pone.0013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O'Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program, Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams WB, Liao HX, Moody MA, Kepler TB, Alam SM, Gao F, Wiehe K, Trama AM, Jones K, Zhang R, Song H, Marshall DJ, Whitesides JF, Sawatzki K, Hua A, Liu P, Tay MZ, Seaton KE, Shen X, Foulger A, Lloyd KE, Parks R, Pollara J, Ferrari G, Yu JS, Vandergrift N, Montefiori DC, Sobieszczyk ME, Hammer S, Karuna S, Gilbert P, Grove D, Grunenberg N, McElrath MJ, Mascola JR, Koup RA, Corey L, Nabel GJ, Morgan C, Churchyard G, Maenza J, Keefer M, Graham BS, Baden LR, Tomaras GD, Haynes BF. 2015. HIV-1 Vaccines. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science 349:aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 93.Lambert JS, McNamara J, Katz SL, Fenton T, Kang M, VanCott TC, Livingston R, Hawkins E, Moye J Jr, Borkowsky W, Johnson D, Yogev R, Duliege AM, Francis D, Gershon A, Wara D, Martin N, Levin M, McSherry G, Smith G. 1998. Safety and immunogenicity of HIV recombinant envelope vaccines in HIV-infected infants and children. National Institutes of Health-sponsored Pediatric AIDS Clinical Trials Group (ACTG-218). J Acquir Immune Defic Syndr Hum Retrovirol 19:451–461. [DOI] [PubMed] [Google Scholar]
- 94.Palma P, Romiti ML, Montesano C, Santilli V, Mora N, Aquilani A, Dispinseri S, Tchidjou HK, Montano M, Eriksson LE, Baldassari S, Bernardi S, Scarlatti G, Wahren B, Rossi P. 2013. Therapeutic DNA vaccination of vertically HIV-infected children: report of the first pediatric randomised trial (PEDVAC). PLoS One 8:e79957. doi: 10.1371/journal.pone.0079957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klein N, Palma P, Luzuriaga K, Pahwa S, Nastouli E, Gibb DM, Rojo P, Borkowsky W, Bernardi S, Zangari P, Calvez V, Compagnucci A, Wahren B, Foster C, Munoz-Fernandez MA, De Rossi A, Ananworanich J, Pillay D, Giaquinto C, Rossi P. 2015. Early antiretroviral therapy in children perinatally infected with HIV: a unique opportunity to implement immunotherapeutic approaches to prolong viral remission. Lancet Infect Dis 15:1108–1114. doi: 10.1016/S1473-3099(15)00052-3. [DOI] [PubMed] [Google Scholar]
- 96.Martínez-Bonet M, Puertas MC, Fortuny C, Ouchi D, Mellado MJ, Rojo P, Noguera-Julian A, Munoz-Fernandez MA, Martinez-Picado J. 2015. Establishment and replenishment of the viral reservoir in perinatally HIV-1-infected children initiating very early antiretroviral therapy. Clin Infect Dis 61:1169–1178. doi: 10.1093/cid/civ456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Persaud D, Patel K, Karalius B, Rainwater-Lovett K, Ziemniak C, Ellis A, Chen YH, Richman D, Siberry GK, Van Dyke RB, Burchett S, Seage GR III, Luzuriaga K, Pediatric HIV/AIDS Cohort Study. 2014. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 168:1138–1146. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cagigi A, Rinaldi S, Cotugno N, Manno EC, Santilli V, Mora N, Zangari P, Aquilani A, Tchidjou KH, Giaquinto C, Bernardi S, Rossi P, Palma P. 2014. Early highly active antiretroviral therapy enhances B-cell longevity: a 5 year follow up. Pediatr Infect Dis J 33:e126–e131. doi: 10.1097/INF.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 99.Simani OE, Izu A, Violari A, Cotton MF, van Niekerk N, Adrian PV, Madhi SA. 2014. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS 28:531–541. doi: 10.1097/QAD.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 100.Luzuriaga K, Gay H, Ziemniak C, Sanborn KB, Somasundaran M, Rainwater-Lovett K, Mellors JW, Rosenbloom D, Persaud D. 2015. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 372:786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zolla-Pazner S, deCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O'Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. 2014. Vaccine-induced IgG antibodies to V1V2 regions of multiple HIV-1 subtypes correlate with decreased risk of HIV-1 infection. PLoS One 9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]


