In this study, which evaluated endocrine therapy use after ductal carcinoma in situ (DCIS) over a 15-year period, 163 of 727 women with a DCIS diagnosis (22%) initiated endocrine therapy. Age, surgery, and radiation were the primary factors in initiation, but increased estrogen receptor testing has not resulted in corresponding increases in endocrine therapy.
Keywords: Ductal carcinoma in situ, Breast cancer, Tamoxifen, Aromatase inhibitor, Endocrine therapy
Abstract
Background.
Endocrine therapy initiation after ductal carcinoma in situ (DCIS) is highly variable and largely unexplained. National guidelines recommend considering tamoxifen for women with estrogen receptor-positive (ER+) DCIS or who undergo excision alone. We evaluated endocrine therapy use after DCIS over a 15-year period in an integrated health care setting to identify factors related to initiation.
Methods.
Female Group Health Cooperative enrollees ages 18–89 years with a DCIS diagnosis during 1996–2011 were eligible for inclusion. Endocrine therapy was identified through pharmacy records. Tumor and treatment information were from tumor registry reports; demographics and other risk factors were from questionnaires and electronic medical records. Relative risks (RRs) and 95% confidence intervals (CIs) for endocrine therapy initiation were calculated using multivariable generalized linear models.
Results.
We identified 727 women with a DCIS diagnosis, including 163 (22%) who initiated endocrine therapy (149 tamoxifen, 14 aromatase inhibitor). Younger women were more likely to initiate endocrine therapy (RR 1.69; 95% CI 1.16–2.46 for ages 45–54 vs. 65–74 years). Compared with breast-conserving surgery (BCS) with radiation, women who had BCS alone (RR 0.46; 95% CI 0.25–0.84) or mastectomy (RR 0.54; 95% CI 0.39–0.75) were less likely to use endocrine therapy. ER testing increased from 4% of DCIS cases in 2001 to 71% in 2011; however, endocrine therapy initiation decreased from 58% of ER+ DCIS in 2001–2005 to 37% in 2009–2011.
Conclusion.
Increasing ER testing since 2001 has not corresponded to parallel increases in endocrine therapy initiation. Age, surgery, and radiation were the primary factors associated with initiation.
Implications for Practice:
National guidelines recommend considering tamoxifen for women with ductal carcinoma in situ (DCIS) who are estrogen receptor-positive (ER+) or who undergo excision alone. In this study, the rapid increase in ER testing caused by tamoxifen’s approval in 2000 did not lead to increases in endocrine therapy initiation, despite recognition of an increasing number of DCIS tumors as ER+ each year. Contrary to the suggested guidelines, women who had breast-conserving surgery without radiation were less likely to use tamoxifen than those who had radiation. Future Food and Drug Administration approval of new endocrine agents for DCIS (such as aromatase inhibitors) may provide an opportunity to reemphasize benefits by ER and surgery status.
Abstract
摘要
背景. 乳腺导管原位癌 (DCIS) 诊断后内分泌治疗的起始治疗时机变异性很大, 且多数无法解释其原因。国家级指南建议对雌激素受体阳性 (ER+) DCIS 或仅接受切除术的女性患者考虑他莫昔芬治疗。本研究评价了一家综合性卫生健康组织 15 年来 DCIS 确诊后的内分泌治疗使用情况, 旨在鉴别该治疗起始的相关因素。
方法. 在 1996∼2011 年间, 女性健康协作组织的成员中 18∼89 岁且诊断为 DCIS 者符合入选标准。通过处方记录确定内分泌治疗。从肿瘤注册报告中获得肿瘤和治疗信息, 通过问卷和电子病历记录获得人口统计学和其他危险因素。使用多变量广义线性模型计算开始内分泌治疗的相对危险度 (RR) 和 95%可信区间 (CI)。
结果. 共确定 727 例确诊为 DCIS 的女性患者, 其中有 163 例 (22%) 开始内分泌治疗 (149 例接受他莫昔芬治疗, 14 例接受芳香化酶抑制剂治疗)。较年轻的女性开始内分泌治疗的可能性较高 (45∼54 岁vs. 65∼74 岁, RR 1.69; 95%CI 1.16∼2.46)。与保乳术 (BCS) 联合化疗相比, 单行BCS (RR 0.46; 95%CI 0.25∼0.84) 和乳房切除术 (RR 0.54; 95%CI 0.39∼0.75) 较少使用内分泌治疗。ER 检测在 DCIS 病例中的使用比例从 2001 年的 4% 增加到 2011 年的 71%。但 ER+ DCIS 患者接受内分泌治疗的比例从 2001∼2005 年间的 58%下降至 2009∼2011 年间的 37%。
总结. 2001 年以来 ER 检测的增加未导致内分泌治疗的平行增加。与开始内分泌治疗相关的主要因素包括年龄、术式和放疗。The Oncologist 2016;21:134–140
对临床实践的提示: 国家级指南建议雌激素受体阳性 (ER+) 或接受切除术的乳腺导管内原位癌 (DCIS) 患者考虑接受他莫昔芬治疗。本研究中, 由于 2000 年他莫昔芬获批引起的 ER 检测的快速发展虽然使得 ER+ DCIS 的确诊逐年递增, 但并未导致内分泌治疗的增加。与指南建议相反, 单行保乳术不联合放疗的患者接受他莫昔芬治疗的可能性低于接受放疗者。将来食品和药物监督管理局批准用于 DCIS 的内分泌治疗新药 (诸如芳香化酶抑制剂) 可能有望根据不同 ER 和手术状态为患者重新带来获益。
Introduction
Ductal carcinoma in situ (DCIS) is a stage 0 breast cancer that is frequently detected by mammogram and accounts for >20% of all breast cancer diagnoses [1]. The standard of care for DCIS is breast-conserving surgery (BCS) with radiation or mastectomy [2], and 10-year survival exceeds 97% [3]. Mastectomy may be recommended for women with multifocal disease, and approximately 5% of women with a DCIS diagnosis also elect to have a contralateral prophylactic mastectomy [4].
Tamoxifen was approved by the Food and Drug Administration (FDA) as adjuvant endocrine therapy for DCIS in 2000. In the placebo-controlled National Surgical Adjuvant Breast and Bowel Project (NSABP)-B24 trial, tamoxifen reduced the risk of ipsilateral and contralateral second events by 30% and 52%, respectively, when added to BCS and radiation (relative risk [RR] 0.70; 95% confidence interval (CI) 0.50–0.98, and RR 0.48; 95% CI 0.26–0.87, respectively) [5]. In a reanalysis of a subset of 732 participants with estrogen receptor (ER) expression information, this benefit was most apparent among women with ER+ DCIS (hazard ratio [HR] 0.58; 95% CI 0.42–0.81 compared with HR 0.88; 95% CI 0.49–1.59 for women with ER− DCIS) [6]. Current National Comprehensive Cancer Network guidelines recommend ER testing for DCIS patients and consideration of tamoxifen for women with ER+ disease or who undergo BCS without radiation [2].
Providers and women must weigh the benefits of tamoxifen for reducing second breast cancer events and improving bone health (in postmenopausal women) against an increased risk of cataract, endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, and potentially severe vasomotor and gynecologic symptoms [7]. No mortality benefit for tamoxifen after DCIS has been conclusively demonstrated [8, 9], although predictive models estimate a 2- to 6-month survival benefit from adding tamoxifen to BCS and radiation after DCIS and a 2- to 5-month benefit of adding tamoxifen to BCS alone [10].
Striking differences in tamoxifen initiation among women with a DCIS diagnosis—from 30% to 70% across National Comprehensive Cancer Network centers [11]—are largely unexplained. Variation in tamoxifen use may be driven by patterns in ER testing, concurrent treatment decisions, demographics, or other factors. Few studies have had information available on ER testing or personal characteristics that may affect the side-effect profile of tamoxifen, including prior hysterectomy, clotting events, or smoking status, to address characteristics associated with initiation. To identify factors that contribute to tamoxifen initiation, we evaluated tamoxifen use after DCIS diagnosis over a 15-year period in an integrated health care setting with detailed treatment and medical history information.
Methods
We conducted a retrospective cohort study of women with an incident diagnosis of breast carcinoma in situ (American Joint Committee on Cancer stage 0) during 1996–2011 at ages 18–89 years. Among the 1,145 women identified, eligibility criteria required continuous enrollment in Group Health Cooperative for 12 months before and 12 months after diagnosis (n = 789, 69%), except in the case of death. DCIS diagnoses were further limited to histologic codes 8201, 8230, 8500, 8501, 8503, 8507, 8522, 8523, and 8543. The final analytic sample included 727 women. All study procedures, including a waiver of consent to review electronic data and abstract medical records, were approved by the institutional review board at Group Health Cooperative Research Institute.
Data Collection
All women received care at facilities owned and operated by Group Health Cooperative, which serves approximately 600,000 residents of Washington State. Western Washington Surveillance, Epidemiology, and End Results registry data were used to identify diagnosis dates, race/ethnicity, tumor size, comedo status, breast surgery, and radiation therapy. Group Health enrollee records have been linked with the registry annually since 1974. Risk factor information such as education, body mass index, and smoking status were prospectively collected from women before diagnosis as part of the Group Health Breast Cancer Surveillance project [12, 13]. Hysterectomy status was available from an up-to-date clinical database [14] that is maintained to identify women who are not recommended for cervical cancer screening. Osteoporosis and related fractures (733.00–733.03, 733.09, 820–821, 805, 807–807.4, 807.7–808, 810–814, 823–824), deep vein thrombosis (451.1, 451.2, 451.81, 451.9, 453.0, 453.2, 453.4, 453.8, 453.9), pulmonary embolism (415.1, 415.11–415.13, 415.19, 416.2, 453.1, 453.2, 453.9, 673.2, 639.6), and stroke (430, 431, 432.9, 434, 436, 432.0–432.1) were identified from International Classification of Disease, Ninth Revision codes before diagnosis. The Charlson Comorbidity Index [15] was calculated using diagnosis and procedure codes in electronic and administrative databases in the 12 months before diagnosis.
Detailed electronic administrative data provided information on outpatient pharmacy dispensing, including use of osteoporosis medications and adjuvant endocrine therapy. We defined endocrine therapy initiation as the first pharmacy fill record for tamoxifen or an aromatase inhibitor within 12 months after diagnosis [16]. Aromatase inhibitors (AIs) were included to capture potential off-label use. Group Health electronic pharmacy data has been shown to be 97% complete [17, 18]; pharmacy records are captured for all Group Health enrollees who fill prescriptions at Group Health pharmacies and for all Group Health enrollees with a drug benefit who fill prescriptions at outside pharmacies.
Statistical Analysis
We used multivariable generalized linear models with a log link, Poisson distribution, and robust SEs to estimate RRs for endocrine therapy initiation [19]. We assessed race, ER status, breast surgery and radiation, tumor grade, tumor size, comedo status, calendar year of diagnosis, education, body mass index, smoking status, hysterectomy, comorbidity index, and history of osteoporosis, fracture, deep vein thrombosis, pulmonary embolism, and stroke as potential confounders in age-adjusted models. Age adjustment was performed with indicator variables for the following ages at diagnosis: 18–44, 45–54, 55–64, 65–74, and 75–89 years. Final multivariable models included adjustment for the following covariates: age, calendar year of diagnosis (1996–2000, 2001–2005, 2006–2008, and 2009–2011), ER status (positive, negative, and unknown/not tested), surgery and radiation (BCS without radiation, BCS with radiation, and mastectomy), grade (I–II [well to moderately differentiated] and III [poorly differentiated]), tumor size (≤5, 6–14, 15–24, ≥25 mm, and unknown/not documented), comedo status (presence or absence of comedo necrosis), and education (high school or less, some college, and college diploma or more). All analyses were performed with SAS 9.3 (SAS Institute, Cary, NC, https://www.sas.com/en_us/home.html).
Results
Among 727 women with a DCIS diagnosis, the average age at diagnosis was 60.2 years (SD 11.9, range 28–89). Overall, our sample was 87% white, 9% Asian/Pacific Islander, and 4% black and mirrored the racial demographics of Washington State and the Seattle area [20]. Fewer than 2% of women were of Hispanic or Spanish ethnicity. The majority of women had attended at least some college (77%), had a body mass index >25 kg/m2 (58%), and reported never smoking (60%). Approximately one quarter of the women in our sample (26%) had a hysterectomy before diagnosis.
After diagnosis, 149 (21%) women initiated tamoxifen and 14 (2%) an aromatase inhibitor (total n = 163, 22%). Among women who initiated tamoxifen, 91% filled at least 2 tamoxifen prescriptions during the 12 months after diagnosis. The average time to first tamoxifen fill was 130 days from diagnosis (median 129 days; interquartile range 84–168 days). Thirty-one women (4%) had an order record for either tamoxifen or an aromatase inhibitor, but filled neither.
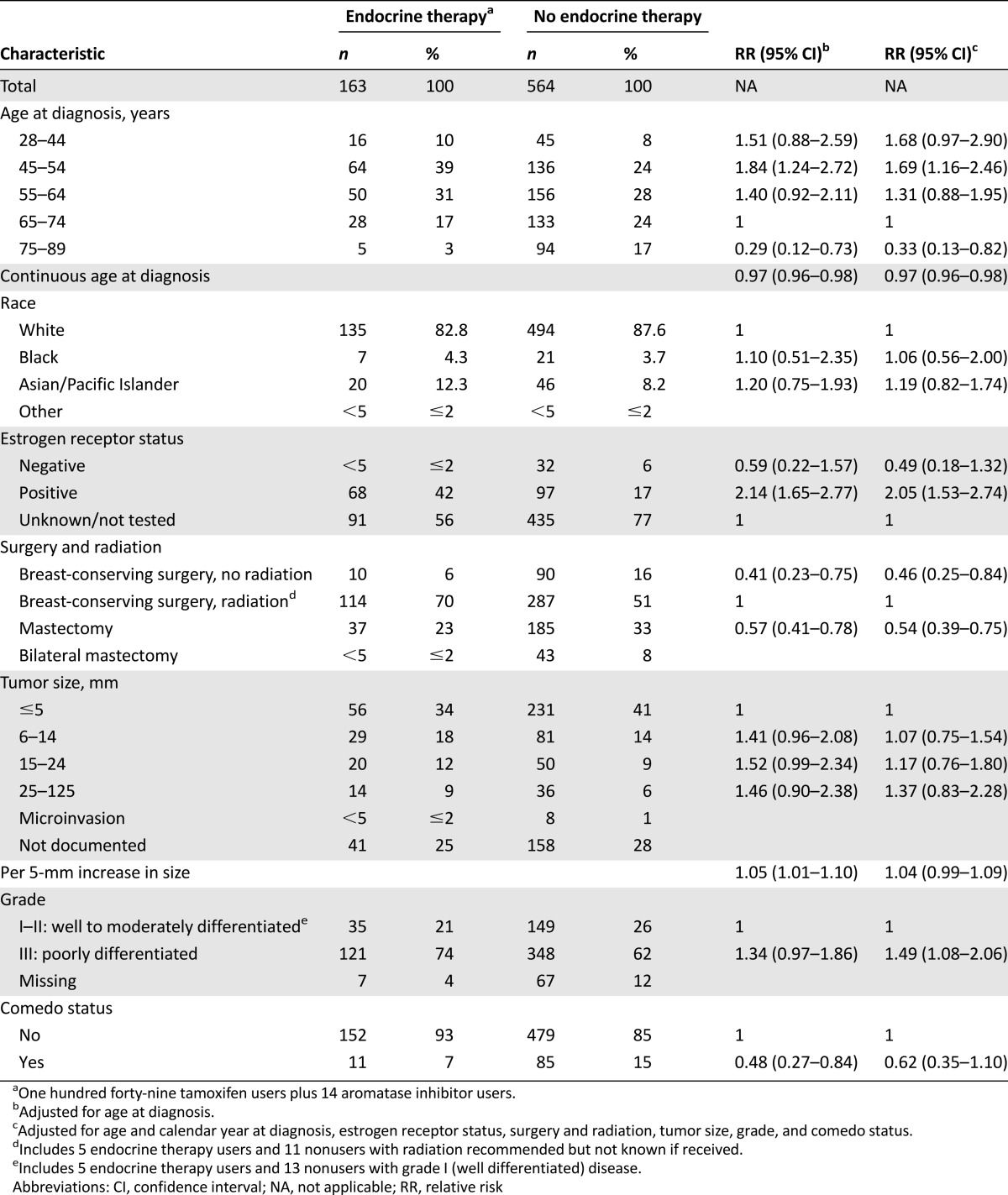
We observed a strong inverse association between endocrine therapy use and age (Table 1). Compared with women ages 65–74 years, those 75 and older were 67% less likely to use endocrine therapy (RR 0.33; 95% CI 0.13–0.82), and those 45–54 were 69% more likely (RR 1.69; 95% CI 1.16–2.46). With each 1-year increase in age at diagnosis, women were 3% less likely to use endocrine therapy (RR 0.97; 95% CI 0.96–0.98) (Table 1).
Table 1.
Tumor registry characteristics among women with a ductal carcinoma in situ diagnosis in relation to use of endocrine therapy, Group Health Cooperative, Seattle, WA, 1996–2011
Endocrine therapy initiation was most common among women who had BCS with radiation. Women with BCS without radiation were 54% less likely to use endocrine therapy (RR 0.46; 95% CI 0.25–0.84), and women who had mastectomy (where radiation is generally not recommended for DCIS) were 46% less likely (RR 0.54; 95% CI 0.39–0.75) compared with women who had BCS and radiation. Overall, 6% of DCIS cases (n = 47) elected to have a contralateral prophylactic mastectomy (Table 1).
We observed a positive trend between increasing tumor size and endocrine therapy initiation that was of borderline statistical significance: each 5-mm increase in tumor size corresponded to a 4% increase in endocrine therapy initiation (RR 1.04; 95% CI 0.99–1.09). Compared with women with grade I–II DCIS, women with grade III disease were 49% more likely to use endocrine therapy (RR 1.49, 95% CI 1.08–2.06). The presence of comedo necrosis appeared to be inversely associated with endocrine therapy use in age-adjusted models; the association was somewhat attenuated and not statistically significant after full multivariable adjustment (RR 0.62; 95% CI 0.35–1.10) (Table 1).
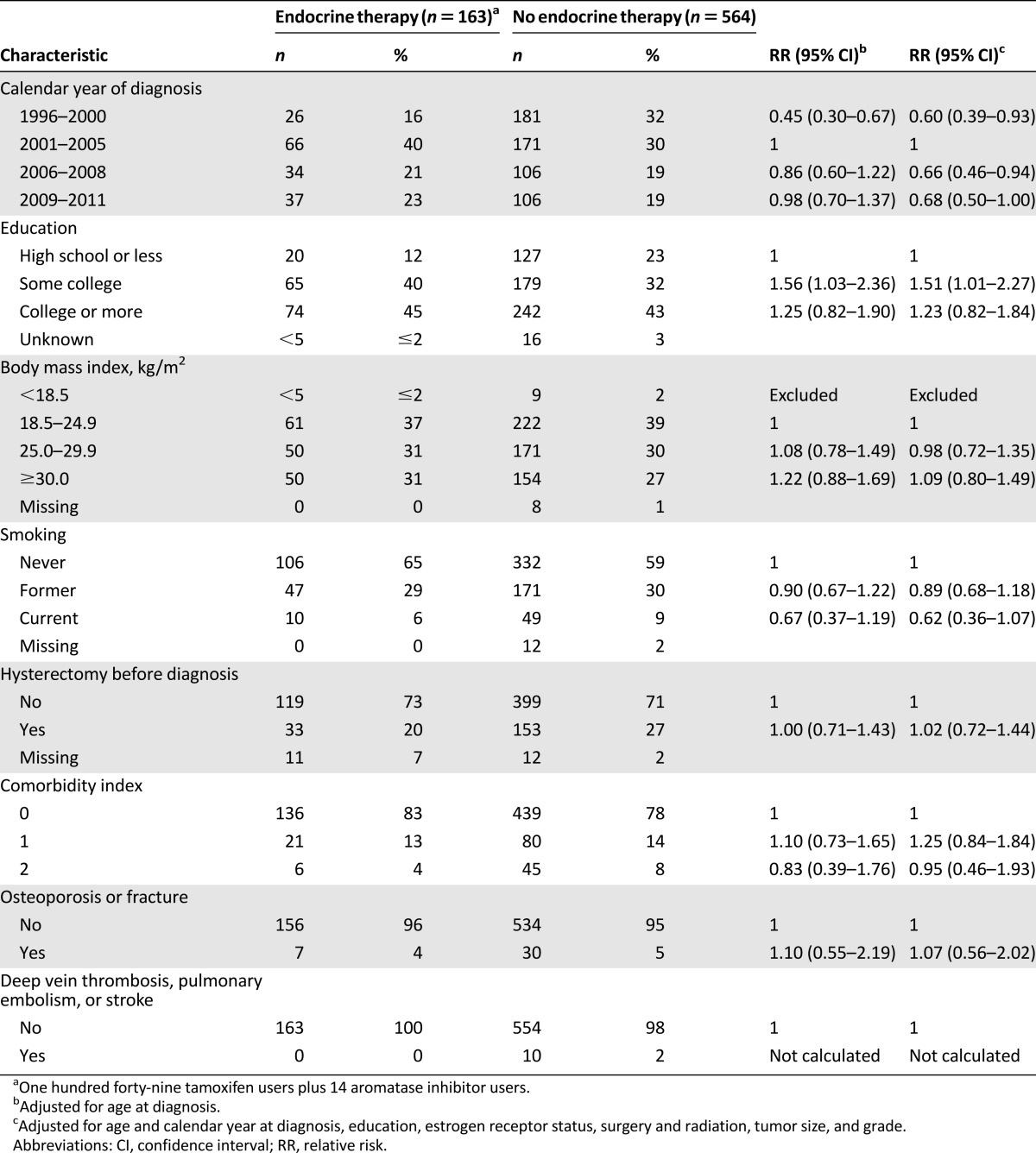
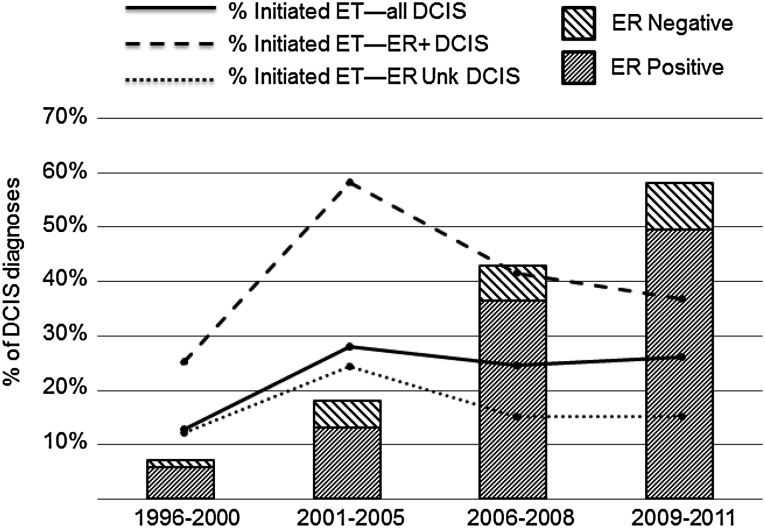
In our sample, ER testing was not commonly performed (<10% of annual cases) before 2001. Over the most recent decade of data (2001–2011), ER testing increased from 4% of cases to 71% (data not shown). Women were less likely to use endocrine therapy before the FDA’s approval of tamoxifen for DCIS in 2000 (Table 2). Endocrine therapy use peaked (27%) in 2001–2005 and then declined in subsequent years (RR 0.66; 95% CI 0.46–0.94 in 2006–2008, and RR 0.68; 95% CI 0.50–1.00 in 2009–2011). Figure 1 demonstrates that the overall proportion of women who initiated endocrine therapy did not correspond to rapid increases in ER testing during this time (with subsequent identification of a higher proportion of patients with ER+ disease). In fact, the steepest declines in endocrine therapy initiation were among women with documented ER+ DCIS: from 58% in 2001–2005 to 37% in 2009–2011 (Fig. 1).
Table 2.
Calendar year of diagnosis and medical history among women with ductal carcinoma in situ in relation to use of endocrine therapy, Group Health Cooperative, Seattle, WA, 1996–2011
Figure 1.
ET initiation and ER testing of DCIS by calendar period, Group Health Cooperative, 1996–2011.
Abbreviations: DCIS, ductal carcinoma in situ; ER, estrogen receptor; ET, endocrine therapy; Unk, unknown.
Women with some college education were more likely to use endocrine therapy compared with women with a high school diploma or less (RR 1.51; 95% CI 1.01–2.27); this increase did not extend to women with formal education beyond college (RR 1.23; 95% CI 0.82–1.84) (Table 2). We did not observe statistically significant associations between body mass index, cigarette smoking, hysterectomy status, or comorbidity index score. However, the point estimate for current smoking compared with never smoking was suggestive of an inverse association with endocrine therapy use (RR 0.62; 95% CI 0.36–1.07) (Table 2).
Few endocrine therapy users (n = 7) had a medical history of osteoporosis or fracture. Tamoxifen can be beneficial for bone in postmenopausal women but detrimental before menopause [21]. Information on menopausal status was not available; however, all women who used endocrine therapy after DCIS who had a medical history of osteoporosis or fracture were aged 50 years and older at DCIS diagnosis. There was no history of deep vein thrombosis, pulmonary embolism, or stroke among endocrine therapy users (Table 2).
AIs are recommended as adjuvant breast cancer therapy only for postmenopausal women; therefore, we repeated all analyses excluding the 14 AI users, and results were very similar. We also varied our definition of endocrine therapy initiation from ≥1 fill of a tamoxifen or aromatase inhibitor prescription within 12 months after diagnosis to ≥2 fills within 12 months of diagnosis; the associations presented here were not substantially changed (data not shown).
Discussion
The rapid increase in ER testing since tamoxifen’s approval in 2000 has not corresponded to increases in endocrine therapy initiation, despite recognition of an increasing number of DCIS tumors as ER+ each year. Furthermore, hysterectomy status, bone health, and vascular clotting conditions at diagnosis [7, 22]—factors that influence the risk profile for tamoxifen use—were not associated with endocrine therapy initiation after DCIS. This may suggest that the factors that prompt women and their providers to accept or prescribe tamoxifen may be more analogous to the chemoprevention context, in which perceived risk of future breast cancer is the most consistently recognized driver of use [23], rather than the invasive disease context, in which specific tumor characteristics most often direct clinical decision making.
Although tamoxifen is currently the only FDA-approved endocrine therapy for DCIS, aromatase inhibitors are currently being evaluated in the NSABP B-35 trial [24, 25]. Results presented at the 2015 American Society of Clinical Oncology meeting showed a 27% decrease in risk of second breast cancer among women with ER+ or progesterone receptor-positive DCIS randomized to anastrozole compared with tamoxifen (HR 0.73; p = .03), with benefits most apparent in women younger than 60 and in the later years of the study [26]. Last year, the IBIS II trial reported an estimated 56% reduction (HR 0.44; 95% CI 0.17–1.15) in future breast cancer risk among postmenopausal women with a DCIS diagnosis randomized to anastrozole compared with placebo [27]. This is similar to the HR of 0.47 for invasive and in situ breast cancer reported in the exemestane arm of the Mammary Prevention.3 trial compared with placebo, which included women with DCIS treated with mastectomy (n = 112) [28]. In our study, we included women who used an aromatase inhibitor after DCIS diagnosis as endocrine therapy users to capture potential off-label use. Aromatase inhibitors made up 9% of endocrine therapy initiation after DCIS. This is similar to the 12% aromatase inhibitor use (anastrozole and letrozole) reported among endocrine therapy users ≥18 years old across California Cancer Registry regions during an overlapping time period [29].
We hypothesized that endocrine therapy initiation would be more common among women who underwent BCS as an alternative to radiation or mastectomy. Instead, we observed that women who had BCS with radiation were most likely to initiate endocrine therapy. Nakhlis et al. reported that a higher proportion of women who had BCS used tamoxifen compared with women who had a mastectomy after DCIS (80% vs. 46%; p = .002) at the Lynn Sage Breast Center in Chicago during 1998–2001; radiation therapy was not evaluated [30]. The association between primary DCIS treatment and endocrine therapy use reported by Livaudais et al. was not statistically significant, but in the same direction as our own: compared with mastectomy, women who reported BCS with radiation were more likely to use endocrine therapy (odds ratio [OR] 1.34; 95% CI 0.77–2.31 across eight California Cancer Registry regions during 2002–2005) [29].
Our study supports an inverse association between endocrine therapy initiation and age at DCIS diagnosis, similar to most [31–33], but not all [29], prior reports. Consistent with our results, a large increase in tamoxifen use, leading to its approval as adjuvant therapy for DCIS in 2000, was reported across three integrated health care delivery systems during 1990–2001 [32, 34] and a population-based cohort of women with DCIS in Wisconsin during 1995–2003 [35]. Also like our findings, tamoxifen use in the Wisconsin cohort subsequently decreased slightly between 2001–2003 and 2004–2006 [35].
Previous studies reported conflicting results on the association between education level and use of endocrine therapy. In our data, women with more education appeared more likely to initiate endocrine therapy. However, in two other studies, educational level was not associated with tamoxifen use after DCIS [29, 32]. In a third report, Kaiser Permanente members residing in census tracts with the highest proportion of college-educated residents appeared less likely to use endocrine therapy (OR 0.81; 95% CI 0.69–0.94) compared with the lowest quintile. Our results for grade and comorbidity index also differ from those reported across six Kaiser Systems where Charlson score ≥2 (compared with 0, OR 0.76; 95% CI 0.64–0.91) and high-grade disease (compared with low/intermediate grade, OR 0.83; 95% CI 0.76–0.92) were associated with lower levels of endocrine therapy use [33].
Across six Kaiser Permanente regions during 2001–2011, African American women were less likely to initiate endocrine therapy (OR 0.82; 95% CI 0.70–0.96), and Asian women were more likely (OR 1.18; 95% CI 1.03–1.34), compared with white women [33]. Our analysis was limited by small sample sizes; however, the estimate for endocrine therapy initiation among Asian women (n = 66) compared with white women (RR 1.19; 95% CI 0.82–1.74) was similar to that reported in the Kaiser sites. Lower levels of endocrine therapy initiation among African American compared with white women with DCIS were observed at the Detroit Medical Center/Karmanos Cancer Institute in Michigan during 1996–2000 [36]. Conversely, in an analysis of women seen at the MD Anderson Cancer Center (Houston, TX) during 1996–2009, white patients diagnosed with DCIS were less likely to receive adjuvant therapy with tamoxifen than Asian/Pacific Islander and African American women [37]. Two other studies reported no association between race and tamoxifen initiation after DCIS [31, 32].
Most previous reports have not had information available on ER status [32, 33, 36], which could influence associations if ER status of DCIS tumors varies according to race or grade [33]. Our statistical models adjusted for ER status; however, in models that did not include ER status as a covariate, the interpretation of race and grade associations was unchanged. One previous report using data from the North Carolina Central Cancer Registry observed no association between ER status and endocrine therapy initiation in 1998–1999; however, ER testing was not performed for 85% of DCIS diagnoses [31]. ER status was not associated with tamoxifen use in an analysis of women treated for DCIS at the Lynn Sage Breast Center in Chicago during 1998–2001 [30].
Strengths of our study included detailed medical history information rarely available in registry-based studies and a 15-year window to evaluate trends in ER testing and endocrine therapy initiation for DCIS. Limitations of our analysis must also be considered. We had no information on physician recommendations or discussion about endocrine therapy to identify women who may have declined offered therapy. Information on BRCA1/2 mutation testing or family history was also not available; however, in prior reports [30, 32], family history of breast cancer was not related to tamoxifen use.
Conclusion
Endocrine therapy initiation was more common among young women and did not appear to be used in place of radiation. Women with higher-grade tumors were also more likely to use endocrine therapy; however, other indicators of potential disease aggressiveness—such as comedo-type DCIS—were not associated with initiation. Despite the widespread adoption of ER testing for DCIS since 2000, uptake of endocrine therapy did not increase during this period. Women with a prior DCIS diagnosis are included in chemoprevention clinical trials as a high-risk population for second breast cancers [27, 28]. Controversy around the best clinical management of women with DCIS [38] suggests that many of the same difficulties may exist for women and providers in determining the best course of therapy after DCIS, including endocrine therapy for secondary chemoprevention, as for high-risk women without a prior breast cancer diagnosis.
Acknowledgments
We thank Drs. Hyman Muss and Dale Sandler for their advice and support during this project. This research was supported in part by the National Center for Advancing Translational Sciences (KL2-TR001109) and the National Cancer Institute (U24 CA171524, HHSN261201100031C, U01CA63731, and P01CA154292).
Author Contributions
Conception/Design: Hazel B. Nichols, Erin J.A. Bowles, Til Stürmer, Diana S.M. Buist
Provision of study material or patients: Erin J.A. Bowles, Diana S.M. Buist
Collection and/or assembly of data: Hazel B. Nichols, Erin J.A. Bowles, Lawrence Madziwa, Diem-Thy Tran, Diana S.M. Buist
Data analysis and interpretation: Hazel B. Nichols, Erin J.A. Bowles, Jessica Islam, Lawrence Madziwa, Til Stürmer, Diem-Thy Tran, Diana S.M. Buist
Manuscript writing: Hazel B. Nichols, Erin J.A. Bowles, Jessica Islam, Lawrence Madziwa, Til Stürmer, Diem-Thy Tran, Diana S.M. Buist
Final approval of manuscript: Hazel B. Nichols, Erin J.A. Bowles, Jessica Islam, Lawrence Madziwa, Til Stürmer, Diem-Thy Tran, Diana S.M. Buist
Disclosures
Til Stürmer: GlaxoSmithKline, UCB BioSciences, Merck (C/A), AstraZeneca, Amgen (RF), Novartis, Roche, BASF, AstraZeneca, Johnson & Johnson (OI). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Available at http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed December 3, 2015.
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, version 1.2015. Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 3, 2015.
- 3.Kerlikowske K. Epidemiology of ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:139–141. doi: 10.1093/jncimonographs/lgq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle TM, Jarosek S, Habermann EB, et al. Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol. 2009;27:1362–1367. doi: 10.1200/JCO.2008.20.1681. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 6.Allred DC, Anderson SJ, Paik S, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: A study based on NSABP protocol B-24. J Clin Oncol. 2012;30:1268–1273. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman AN, Yu B, Gail MH, et al. Benefit/risk assessment for breast cancer chemoprevention with raloxifene or tamoxifen for women age 50 years or older. J Clin Oncol. 2011;29:2327–2333. doi: 10.1200/JCO.2010.33.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eng-Wong J, Costantino JP, Swain SM. The impact of systemic therapy following ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:200–203. doi: 10.1093/jncimonographs/lgq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staley H, McCallum I, Bruce J. Postoperative tamoxifen for ductal carcinoma in situ: Cochrane systematic review and meta-analysis. Breast. 2014;23:546–551. doi: 10.1016/j.breast.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Soeteman DI, Stout NK, Ozanne EM, et al. Modeling the effectiveness of initial management strategies for ductal carcinoma in situ. J Natl Cancer Inst. 2013;105:774–781. doi: 10.1093/jnci/djt096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yen TW, Kuerer HM, Ottesen RA, et al. Impact of randomized clinical trial results in the national comprehensive cancer network on the use of tamoxifen after breast surgery for ductal carcinoma in situ. J Clin Oncol. 2007;25:3251–3258. doi: 10.1200/JCO.2006.10.2699. [DOI] [PubMed] [Google Scholar]
- 12.Group health research institute: Breast cancer surveillance project. Available at http://bcsr.grouphealthresearch.org/. Accessed July 7, 2015.
- 13.Breast Cancer Surveillance Consortium. Available at http://breastscreening.cancer.gov/. Accessed July 7, 2015.
- 14.Phipps AI, Buist DS. Validation of self-reported history of hysterectomy and oophorectomy among women in an integrated group practice setting. Menopause. 2009;16:576–581. doi: 10.1097/gme.0b013e31818ffe28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Bowles EJ, Buist DS, Chubak J, et al. Endocrine therapy initiation from 2001 to 2008 varies by age at breast cancer diagnosis and tumor size. J Oncol Pract. 2012;8:113–120. doi: 10.1200/JOP.2011.000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudreau DM, Doescher MP, Jackson JE, et al. Impact of healthcare delivery system on where HMO-enrolled seniors purchase medications. Ann Pharmacother. 2004;38:1317–1318. doi: 10.1345/aph.1D569. [DOI] [PubMed] [Google Scholar]
- 18.Buist DS, LaCroix AZ, Brenneman SK, et al. A population-based osteoporosis screening program: who does not participate, and what are the consequences? J Am Geriatr Soc. 2004;52:1130–1137. doi: 10.1111/j.1532-5415.2004.52311.x. [DOI] [PubMed] [Google Scholar]
- 19.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 20.United States Census Bureau. Quickfacts. Available at http://quickfacts.census.gov/qfd/states/53000.html. Accessed October 8, 2015.
- 21.Parton M, Smith IE. Controversies in the management of patients with breast cancer: Adjuvant endocrine therapy in premenopausal women. J Clin Oncol. 2008;26:745–752. doi: 10.1200/JCO.2007.14.3016. [DOI] [PubMed] [Google Scholar]
- 22.Gail MH, Costantino JP, Bryant J, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst. 1999;91:1829–1846. doi: 10.1093/jnci/91.21.1829. [DOI] [PubMed] [Google Scholar]
- 23.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: A systematic review and meta-analysis. J Clin Oncol. 2010;28:3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow M. Refining the use of endocrine therapy for ductal carcinoma in situ. J Clin Oncol. 2012;30:1249–1251. doi: 10.1200/JCO.2011.40.5514. [DOI] [PubMed] [Google Scholar]
- 25.NSABP Foundation. Anastrozole or Tamoxifen in Treating Postmenopausal Women with Ductal Carcinoma In Situ Who Are Undergoing Lumpectomy and Radiation Therapy. Available at https://clinicaltrials.gov/show/NCT00053898. Accessed May 17, 2015.
- 26.Margolese RG, Cecchini RS, Julian TB, et al. Primary results, NRG oncology/NSABP B-35: A clinical trial of anastrozole (A) versus tamoxifen (Tam) in postmenopausal patients with dcis undergoing lumpectomy plus radiotherapy. Paper presented at American Society of Clinical Oncology; June 1, 2015; Chicago, IL. [Google Scholar]
- 27.Cuzick J, Sestak I, Forbes JF, et al. IBIS-II investigators Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): An international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383:1041–1048. doi: 10.1016/S0140-6736(13)62292-8. [DOI] [PubMed] [Google Scholar]
- 28.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 29.Livaudais JC, Hwang ES, Karliner L, et al. Adjuvant hormonal therapy use among women with ductal carcinoma in situ. J Womens Health (Larchmt) 2012;21:35–42. doi: 10.1089/jwh.2011.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakhlis F, Lazarus L, Hou N, et al. Tamoxifen use in patients with ductal carcinoma in situ and T1a/b N0 invasive carcinoma. J Am Coll Surg. 2005;201:688–694. doi: 10.1016/j.jamcollsurg.2005.06.195. [DOI] [PubMed] [Google Scholar]
- 31.Jackson LC, Camacho F, Levine EA, et al. Patterns of care analysis among women with ductal carcinoma in situ in North Carolina. Am J Surg. 2008;195:164–169. doi: 10.1016/j.amjsurg.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Haque R, Achacoso NS, Fletcher SW, et al. Treatment of ductal carcinoma in situ among patients cared for in large integrated health plans. Am J Manag Care. 2010;16:351–360. [PMC free article] [PubMed] [Google Scholar]
- 33.Feigelson HS, Carroll NM, Weinmann S, et al. Treatment patterns for ductal carcinoma in situ from 2000-2010 across six integrated health plans. Springerplus. 2015;4:24. doi: 10.1186/s40064-014-0776-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habel LA, Achacoso NS, Haque R, et al. Declining recurrence among ductal carcinoma in situ patients treated with breast-conserving surgery in the community setting. Breast Cancer Res. 2009;11:R85. doi: 10.1186/bcr2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague BL, McLaughlin V, Hampton JM, et al. Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast Cancer Res Treat. 2013;141:145–154. doi: 10.1007/s10549-013-2670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassar H, Sharafaldeen B, Visvanathan K, et al. Ductal carcinoma in situ in African American versus Caucasian American women: Analysis of clinicopathologic features and outcome. Cancer. 2009;115:3181–3188. doi: 10.1002/cncr.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailes AA, Kuerer HM, Lari SA, et al. Impact of race and ethnicity on features and outcome of ductal carcinoma in situ of the breast. Cancer. 2013;119:150–157. doi: 10.1002/cncr.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Punglia RS, Schnitt SJ, Weeks JC. Treatment of ductal carcinoma in situ after excision: Would a prophylactic paradigm be more appropriate? J Natl Cancer Inst. 2013;105:1527–1533. doi: 10.1093/jnci/djt256. [DOI] [PubMed] [Google Scholar]