Abstract
Levels of Evidence
NA (animal study)
Objective
Age-related changes in laryngeal muscle structure and function may contribute to deficits in voice and swallowing observed in elderly people. We hypothesized that treadmill running, an exercise that increases respiratory drive to upper airway muscles, would induce changes in thyroarytenoid muscle myosin heavy chain (MHC) isoforms consistent with a fast-slow transformation in muscle fiber type.
Study Design
Randomized parallel group controlled trial.
Methods
Fifteen young adult and 14 old Fischer 344/Brown Norway rats received either treadmill running or no exercise (5 days/week/8 weeks). Myosin heavy chain isoform composition in the thyroarytenoid muscle was examined at the end of 8 weeks.
Results
Significant age and treatment effects were found. The young adult group had the greatest proportion of superfast contracting MHCIIL. The treadmill running group had the lowest proportion of MHCIIL and the greatest proportion of MHCIIx.
Conclusion
Thyroarytenoid muscle structure was affected both by age and treadmill running in a fast-slow transition that is characteristic of exercise manipulations in other skeletal muscles.
Keywords: Voice, Aging, Exercise, Larynx, Thyroarytenoid, Voice Disorders, Respiration, Swallowing, Swallowing Disorders
Introduction
Age-related changes in intrinsic laryngeal muscle structure may contribute to vocal function deficits, dysphagia, and aspiration observed in elderly people. These functional deficits may be caused by mechanisms associated with age-related reductions in skeletal muscle mass and strength, termed sarcopenia 1. Because voice and swallowing disorders are so prevalent in elderly people 2,3, the development of effective treatments that target underlying changes within aging cranial muscles active in these critical functions is a clinical priority.
The thyroarytenoid (TA) muscle is a paired intrinsic laryngeal muscle that is active during voice production, the pharyngeal phase of the swallow, and during respiration 4-7. The right and left TA muscles when contracted provide tension to the vocal folds and are active during vocal fold adduction, which is critical to voice production and airway protection. During respiration, the TA is phasically active during expiratory actions and tonically active during inspiratory actions, suggesting active adduction of the vocal folds, a laryngeal breaking mechanism during exhalation, and slight movement of the vocal folds toward midline during inspiration 5,8,9. The specific mechanical properties that govern actions of the TA muscle, such as maximum shortening velocity, power, and ATP consumption, are defined by the isoform composition, or molecular signature, of muscle fibers 10.
The rat TA is composed primarily of myosin heavy chain (MHC) type II isoforms isoforms: MHCIIx, IIb, and IIL11-14. Fibers of this phenotype have the highest force generation capacities and the most rapid twitch response (IIx < IIb < IIL). The superfast MHCIIL isoform is unique to the laryngeal and extraocular musculature, and fibers of this composition have the fastest contraction times (<10ms)13,15. Because of the fast contraction times characteristic of the TA and its cross-system role in voice, swallow, respiration, and airway protection, age-related alterations in TA muscle structure may have implications for performance of these critical functions.
Age-related declines in TA muscle structure and physiology have been observed in both human and animal models. With increasing age, the degeneration of the musculoskeletal system may induce muscle atrophy and denervation-like changes at the neuromuscular junction 16-18, cause a transition from fast-to-slow contracting muscle fiber types 19-21, and affect laryngeal-respiratory kinematics 22,23, resulting in an overall decline in muscle function 24. It has also been shown that with increasing age, the microvascular geometry within the TA is altered 25, and may suggest impaired blood flow within this muscle. In addition, the regenerative capacity of the TA muscle may be impaired with age 26. A decrease in the regenerative potential of TA muscle satellite cells may contribute to a reduction in muscle regeneration after injury in elderly people 26. It is clear that these age-related alterations in TA muscle structure and physiology may contribute to deficits in laryngeal function and thus to dysfunction in phonation, deglutition, and/or respiration.
Current clinical treatments that target the intrinsic laryngeal muscles with the goals of strengthening or increasing the activity of the associated musculature have done so directly, either with voice and/or respiratory therapy. Voice and respiratory therapies have been associated with functional improvements in humans 27-36 and in animal models, but very few studies have examined the underlying effects of therapy on intrinsic laryngeal muscle structure and physiology. In an aging rat model, it was demonstrated that behavioral vocal training increased vocalization rate, reduced age-related effects on vocal acoustics, and reduced neuromuscular junction motor endplate dispersion in the TA muscle 18. However, it is not known if exercise affects TA muscle biochemistry in a manner similar to exercise of other skeletal muscles, with a transformation reflecting a fast-to-slow transformation in muscle fiber type 37-40. In addition, a targeted vocal training approach may not be the only exercise-based treatment feasible for age-related changes and disorders that affect the intrinsic laryngeal muscles. Non-specific exercises, such as treadmill running, that serve to increase TA muscle activity through increased respiratory drive41,42 may also induce changes in TA muscle structure, capable of improving overall function.
The purpose of this study was to determine whether increased respiratory-system-based activation of the TA muscle through treadmill running would affect its biochemical composition in young adult and old rats. To test the hypothesis that treadmill running would induce alterations in TA muscle biochemistry consistent with a fast-to-slow muscle fiber type transformation observed in previous studies of skeletal muscle exercise, assays of myosin heavy chain (MHC) composition of the TA muscle were performed in young adult and old rats following 8 weeks of treadmill running compared with non-running age-matched controls.
Materials and Methods
Animals
This study was performed in compliance with the NIH Guide for Care and Use of Laboratory Animals Eight Edition, and approved by the Animal Care and Use Committee of the University of Wisconsin School of Medicine and Public Health.
Male Fischer 344/Brown Norway rats (n=29) were obtained from the National Institute on Aging colony (Harlan Laboratories, Indianapolis, Indiana, USA; Charles River Laboratories, Kingston New York, USA and Raleigh, North Carolina, USA) at the ages of 6 and 29 months old. Upon the completion of the study, rats were 9 months old (young adult, n=15) and 32 months old (old, n=14). The median life expectancy of the Fischer 344/Brown Norway rat is approximately 36 months 43.
Rats were obtained 2-3 weeks prior to the start of the experiment to allow for acclimation to the vivarium, handling, and the treadmill equipment and were housed in pairs in standard polycarbonate cages on a 12:12 hour light-dark reversed light cycle. Rats in the treadmill running group (n=15) were a subset randomly selected from larger group of rats that contributed treadmill running, physiological, and histological data to a prior report concerning lingual muscle plasticity with exercise 44. Animals in the treadmill running group were water restricted to 3 hours per day 45. Body weights were recorded weekly at the same time of day to monitor the effects of the water restriction and training paradigms. Rats within the control group did not receive any exercise (n=14).
Treadmill Running
Experimental methods for rats in the treadmill running group have been detailed previously 44. Briefly, rats were trained to run on a treadmill at a 20° incline. Each treadmill running session lasted 10 minutes, and was performed 5 days per week for a total of 8 weeks. Before and following the 8 weeks of exercise, each rat completed an endurance test (ET) and a progressive running test (PRT). The times for the ET and maximal speeds from the PRT were recorded for each rat. Following the baseline PRT, rats were matched into treadmill running groups based on their performance. Throughout the 8 weeks of exercise, treadmill speeds were increased as rats achieved performance goals. Rats were trained at 50% of their PRT speed (weeks 1 and 2), 60% PRT (weeks 3 and 4), 70% PRT (weeks 5 and 6), and 80% PRT (weeks 7 and 8).
No Exercise
Rats were handled identically to those in the treadmill running group, but did not participate in the exercise treatment for the 8-week duration of the experiment.
Myosin Heavy Chain Isoforms
The protein concentration of each TA muscle was optimized so that 0.4 μg of protein was added to each gel channel in duplicate. SDS-PAGE was performed on each concentrate with a 0.75 mm thick 6% acrylamide/30% glycerol separating gel (18 × 16 cm) and a 4% acrylamide/30% glycerol stacking gel. A silver staining kit (SilverQuest Silver Staining Kit, Life Technologies, New York, USA) was used to stain the gel for visualization of protein bands. Each silver-stained gel was imaged digitally. An investigator masked to rat age and treatment determined the optical density of each band using computer-assisted image analysis and densiometry (UN-Scan-IT gel Version 6.1, Silk Scientific, Inc, Utah, USA). The ratio of the density of an individual MHC isoform band to the total density within a column was used to determine the percentage of each MHC isoform per lane 19,44,46-51. Good to excellent reliability using these procedures has been demonstrated in previous studies 50.
Statistical Analysis
Paired t-tests were used to compare maximal endurance test time (sec) and progressive exercise test speed (cm/s) within each age group prior to and following the treadmill running treatment. Analysis of variance (ANOVA) was used to compare age groups on endurance test time and progressive running test speed measures.
Two-way analysis of variance (ANOVA) was used to examine age effects, treatment effects, and interactions for MHC isoform composition of the TA. Pair-wise comparisons were made between groups using Fisher's protected least significant difference tests (LSD). SigmaPlot 12 was used for all analyses (Systat Software Inc., San Jose, California, USA). The critical value for obtaining statistical significance was set at α = 0.05.
Results
Treadmill running
Eight weeks of treadmill running was completed by all but one old rat. The old rat was classified as a non-runner, based on predetermined criteria, and removed from the study. Treadmill running data for a larger cohort of 35 rats was published previously 44. Running data from the subset of 15 rats randomly chosen for this study from the larger group is reported below.
When the pre- and post-endurance test (ET) times (s) were examined between age groups, a significant increase in the duration of time spent on the treadmill was observed following 8 weeks of exercise using paired t-tests (t[5] = 3.35, P = 0.005). There was a 67.33% increase in time after the 8-week exercise program, indicating improved endurance for running after treadmill exercise. Paired t-tests within each age group showed a significant increase in time spent on the treadmill following the 8 weeks of treadmill running within young adult rats (t[8] = 6.34, p < 0.001). There were not significant time increases following the exercise training in the old group (t[7] = -0.31, p = 0.77; Fig. 1). The young adult group had the greatest percent gain in performance on the ET, with an average time increase of 126.39% compared with the old group (-0.16%). The percent change in ET time between age groups was significantly different (F[1,14] = 11.94, p = 0.004).
Figure 1.
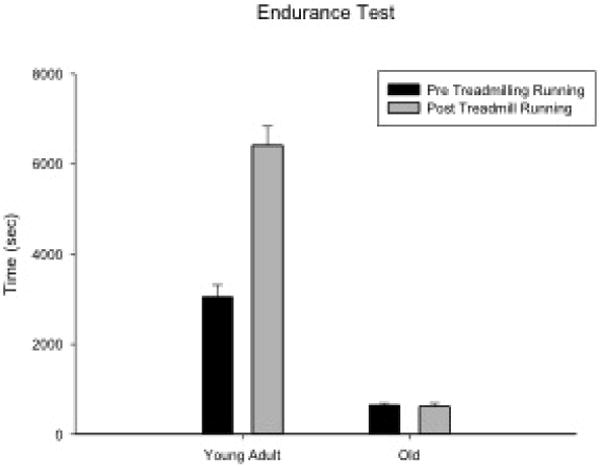
Endurance test times were significantly increased following 8 weeks of treadmill running within the young adult rats (P < 0.001). There were not significant increases following the exercise period in the old group.
A significant increase in speed (cm/s) was observed using paired t-tests (t[15], = 7.14, p < 0.001) when pre- and post-progressive running tests (PRT) were examined. There was an average overall 46.57% increase in speed after the 8 week exercise program, demonstrating that treadmill running increased the speed at which the rats were capable of running. Paired t-tests within each age group showed a significant speed increase following the 8 weeks of treadmill running within young adult (t[8] = 11.88, p < 0.001) and old rats (t[7] = 5.44, P = 0.002; Fig. 2). In addition, significant age effects were found for the PRT (F [1,14] = 13.66, p = 0.003). The young adult group had greatest percent gain in speed (60.3%) compared to the old group (30.87%).
Figure 2.
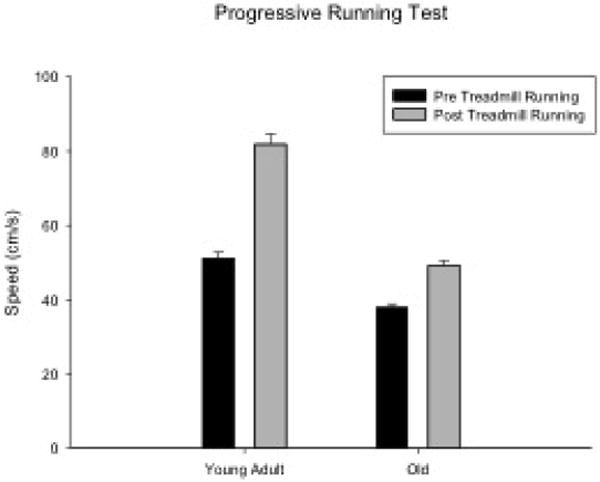
Progressive running test speeds significantly increased following 8 weeks of treadmill running for both age groups (P < 0.001).
MHC isoform composition
Descriptive data for each measure by age and experimental group are shown in Table 1. Representative silver-stained SDS-PAGE gels from the TA muscles of a young adult and old rat in the treadmill running and no exercise groups are presented in Figure 3A,B.
Table 1.
Data are expressed as average percentages (SE) of myosin heavy chain (MHC) isoforms in the thyroarytenoid (TA) muscle for each age group and exercise treatment.
| Rat Group | I | IIX | IIB | IIL |
|---|---|---|---|---|
| Young adult | ||||
| Treadmill Running | 0 (0) | 21.5 (2.7) | 43.0 (0.8) | 35.5 (3.0) |
| No Exercise | 0 (0) | 13.9 (1.8) | 42.6 (6.3) | 43.6 (2.1) |
| Old | ||||
| Treadmill Running | 0 (0) | 25.6 (1.2) | 43.3 (1.7) | 31.1 (1.3) |
| No Exercise | 1.1 (1.1) | 16.5 (2.9) | 48.2 (3.4) | 34.2 (3.2) |
Figure 3.
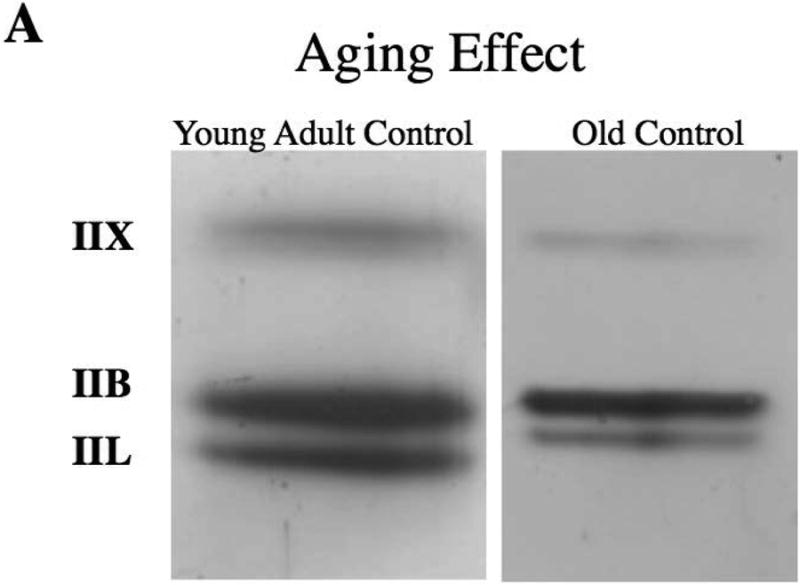
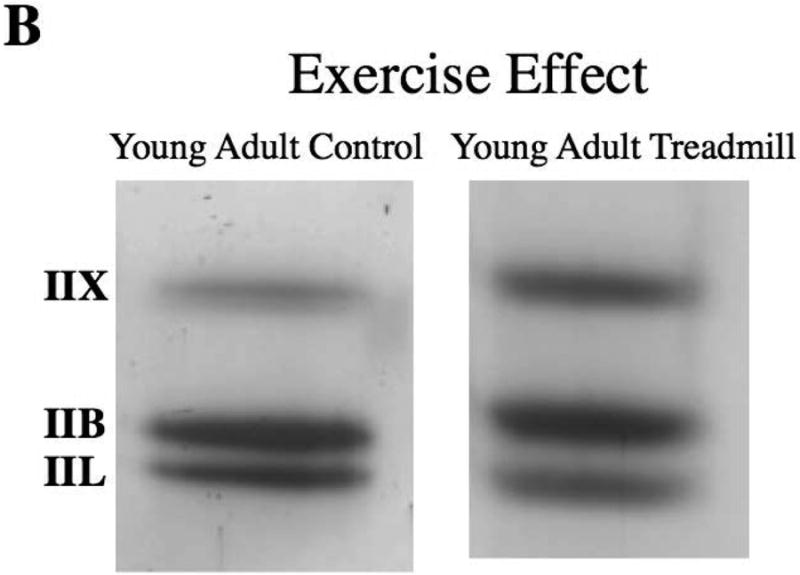
A,B. Representative silver stained SDS-PAGE gels from the thyroarytenoid (TA) muscles that show aging (A) and exercise (B) effects.
On two-way ANOVAs, significant main effects for age were found for MHCIIL in the absence of significant interaction effects with experimental group. The young adult group had a significantly greater proportion of MHCIIL than the old group (F[1,25]=7.247, p=0.012; Fig. 4).
Figure 4.
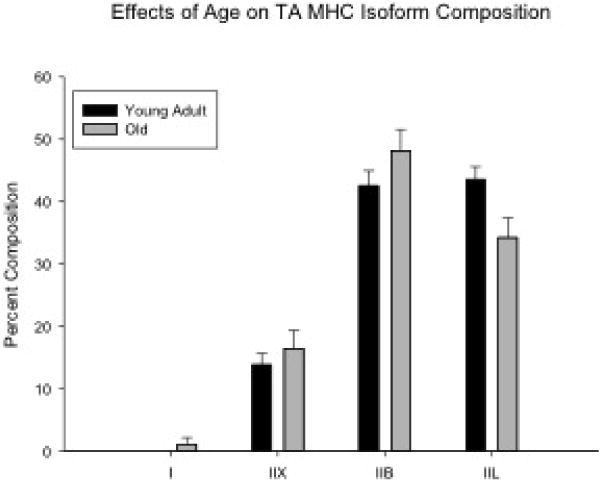
Significant main effects for age were found for the myosin heavy chain isoform, MHCIIL. The young adult group (n = 15) had a significantly greater proportion of MHCIIL than the old group (n = 14) (P = 0.012).
Significant main effects for experimental group were found for MHCIIL and MHCIIx in the absence of significant interaction effects with age. The treadmill running group had a significantly lower proportion of MHCIIL (F[1,25]=4.794, p=0.038) and significantly greater proportion of MHCIIx (F[1,25]=13.129, p=0.001; Fig. 5) than the no exercise group. No other significant age, experimental, or interaction effects were found.
Figure 5.
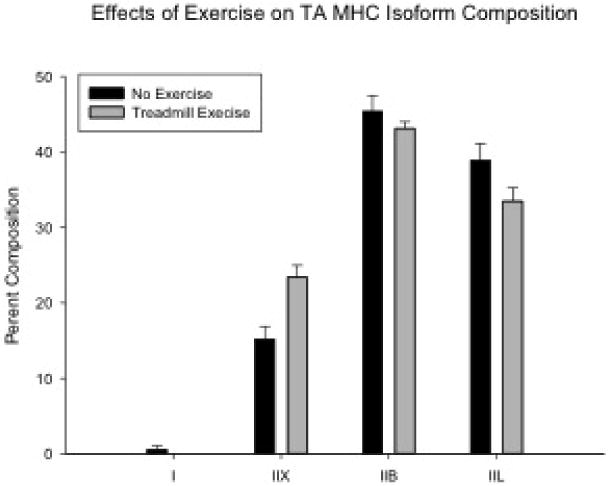
Significant main effects for experimental group were found for MHCIIx and MHCIIL isoforms. The treadmill running group (n = 15) had a significantly lower proportion of MHCIIL (P = 0.038) and significantly greater proportion of MHCIIx (P = 0.035) than the no exercise group (n = 14).
Discussion
The hypothesis of this study was that treadmill running would induce changes in the structural properties of the TA muscle in young adult and old rats consistent with a fast-slow transformation observed in previous studies of exercise training in skeletal muscle 37-40. Our results supported this hypothesis, and are indicative of musculoplasticity within the TA as a function of age and exercise. With both age and exercise, there was a shift towards more slowly contracting MHC isoforms, as evidenced by a reduction in the MHCIIL with increasing age and a reduction in the MHCIIL isoform and increase in the MHCIIx isoform following 8 weeks of treadmill running.
Biochemical alterations in TA muscle composition have structural, physiological, and functional implications, as demonstrated in this and previous studies using an aging rat model. Age-related changes in TA muscle structure are evidenced by an overall reduction in the number of TA muscle fibers, muscle fiber atrophy, and an increase in the abundance of intramuscular connective and adipose tissues 20,26,52. The reduction in the proportion of fast contracting isoforms, specifically a reduction in the superfast MHCIIL isoform, we observed in the present study is consistent with previously reported findings, and may indicate the transformation or replacement to a slower contracting muscle fiber type 19,21. A reduction in the MHCIIL isoform, may reduce the overall contraction speed of the TA muscle and may contribute to functional deficits in voice and swallowing. Aging has also been shown to affect the neuromuscular system, inducing changes in neuromuscular junction morphology 16-18,20 consistent to those observed after denervation, and motoneuron loss in brain stem nuclei that innervate the laryngeal muscles 53. Changes in muscle structure and NMJ morphology may have physiological implications, and may contribute to reduced force production, increased contraction times, and increased fatigue observed in aged rats 20. These age-related changes in the laryngeal neuromuscular system may contribute to discoordinated laryngeal and respiratory motor actions 22,23, and subsequently affect vocal function. For example, in a recent study, rat ultrasonic vocalizations (USV) showed diminished acoustic complexity with increasing age with reduced amplitude, bandwidth, and intensity, reduced mean frequency for vocalizations classified as “simple” and complex”, and longer duration 18. Thus, it is evident that age-related alterations in the rat TA have structural, physiological, and functional implications.
This study was the first to investigate MHC isoform composition of the TA muscle following nonspecific laryngeal muscle exercise using treadmill running and to report that this form of exercise altered TA muscle biochemical structure. The treadmill running exercise paradigm aimed to increase running speeds over the 8-week training period, while also increasing respiratory drive and TA activation to maintain a patent airway. Running performance improved in both age groups throughout the training program as evidenced by the ability to run at faster speeds, but running endurance only improved in the young adult group. Because we did not measure muscle contractile properties, MHC isoform composition, or the cross-sectional area of the limb muscles following the training paradigm, we cannot determine underlying biochemical or physiological mechanisms for lack of endurance improvement in the old rats. It is well known that aging affects exercise endurance54,55 and this 8-week program may not have been sufficient to improve endurance levels in our old subjects.
Other work has shown that a hypercapnic state also increases TA activation during expiration to reduce airflow and maintain alveolar expansion, and can result from exercise that induces an exaggerated respiratory response 41,42. These findings, in combination with other results in which vocalization training in the aging rat altered both USV acoustics and NMJ morphology in the TA 18 indicate that exercise training can take several forms. Because the TA is involved in respiratory actions, a promising translational pathway for laryngeal muscle exercise may be through a route that upregulates breathing by increasing respiratory drive, such as treadmill running.
With endurance and strength training exercise in the limb and tongue musculature, fiber types generally shift to a more slowly contracting fatigue-resistant fiber type 37-40. We observed a similar transition in our treadmill running group, a decrease in the proportion of the MHCIIL isoform and an increase in the proportion of the MHCIIx isoform. This transition in MHC isoform composition may contribute functionally to an overall reduction in TA muscle fatigue and possibly vocal fatigue, if it occurs in humans. However, we cannot confirm this potential explanation in our rat subjects because we did not measure the contractile properties of the TA muscle in this study nor did we have a functional measurement of rat vocalization following the 8 weeks of treadmill running.
There are some limitations to consider when translating the findings of our study to phonation, deglutition, or respiration. First, there were no functional voice, swallowing, or respiratory measures. Because we did not include any behavioral (videofluoroscopy or ultrasonic vocalizations), contractile, or respiratory measures, it is difficult to attribute possible functional improvements to the changes we observed in the biochemical properties of the TA muscle. Second, there are additional intrinsic laryngeal muscles that have key roles in voice production, deglutition, and respiration that we did not include in the study. It would be advantageous in the future to study the effects of exercise on the lateral cricoarytenoid, interarytenoid, posterior cricoarytenoid, and the cricothyroid. Third, we did not perform any immunohistochemical assays to measure muscle fiber cross-sectional area to assess age- or exercise-related muscle hyper/atrophy. Fourth, human and rat vocalizations are produced by different mechanisms and generalization of our findings to human phonation must be made with caution. However, there are more similarities than differences in rat/human TA muscle structure, making the rat a useful model for our specific research questions.
Conclusion
Following treadmill running in the rat, we observed a shift towards a more fatigue resistant phenotype in the TA muscle; that is, a reduction in MHCIIL and increase in MHCIIx, presumably due to the increased activity of the TA resulting from increased respiratory demands. The clinical implication of these findings is that these transitions in MHC composition may have physiological correlates that prove important for airway protection during the pharyngeal phase of the swallow or lead to reduced vocal fatigue in elderly people. Further studies are needed to determine the physiological, behavioral, and functional changes of the intrinsic laryngeal musculature after exercise treatments.
Acknowledgments
We acknowledge the contributions of Ben Becker, Dr. Tiffany Glass, Heather Mosley, Fatima Salem, and Allison Schaser in the completion of this work. This work was funded by grants from the National Institutes of Health, the National Institute on Deafness and other Communication Disorders [T32DC-009401, R01DC-008149].
Funding: This work was funded by grants from the National Institute on Deafness and other Communication Disorders [T32DC009401, R01DC008149, R01DC005935].
Abbreviations
- TA
thyroarytenoid
- MHC
myosin heavy chain
- ET
endurance test
- PRT
progressive running test
- ANOVA
analysis of variance
- LSD
least significant difference
- SE
standard error
Footnotes
We presented this study at the 2014 American Speech-Language-Hearing Association Convention Annual Meeting on November 21, 2014 in Orlando, FL.
We have no conflicts of interest or financial disclosures to report.
References
- 1.Rosenberg IH. Epidemiologic and Methodologic Problems in Determining Nutritional-Status of Older Persons - Proceedings of a Conference Held in Albuquerque, New Mexico, October 19-21, 1988 - Summary Comments. American Journal of Clinical Nutrition. 1989;50:1231–1233. [PubMed] [Google Scholar]
- 2.Roy N, Stemple J, Merrill RM, Thomas L. Dysphagia in the elderly: Preliminary evidence of prevalence, risk factors, and socioemotional effects. Annals of Otology Rhinology and Laryngology. 2007;116:858–865. doi: 10.1177/000348940711601112. [DOI] [PubMed] [Google Scholar]
- 3.Roy N, Stemple J, Merrill RM, Thomas L. Epidemiology of voice disorders in the elderly: Preliminary findings. Laryngoscope. 2007;117:628–633. doi: 10.1097/MLG.0b013e3180306da1. [DOI] [PubMed] [Google Scholar]
- 4.McCulloch TM, Perlman AL, Palmer PM, VanDaele DJ. Laryngeal activity during swallow phonation, and the valsalva maneuver: An electromyographic analysis. Laryngoscope. 1996;106:1351–1358. doi: 10.1097/00005537-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid Muscle-Activity During Wakefulness and Sleep in Normal Adults. Journal of Applied Physiology. 1988;65:1332–1339. doi: 10.1152/jappl.1988.65.3.1332. [DOI] [PubMed] [Google Scholar]
- 6.Hiroto I, Hirano M, Toyozumi Y, Shin T. Electromyographic Investigation of Intrinsic Laryngeal Muscles Related to Speech Sounds. Annals of Otology Rhinology and Laryngology. 1967;76:861–&. doi: 10.1177/000348946707600415. [DOI] [PubMed] [Google Scholar]
- 7.Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. Journal of Applied Physiology. 2014;116:291–301. doi: 10.1152/japplphysiol.00670.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett DJ, Remmers JE, Gautier H. Laryngeal Regulation of Respiratory Air Flow. Respiration Physiology. 1973;18:194–204. doi: 10.1016/0034-5687(73)90050-9. [DOI] [PubMed] [Google Scholar]
- 9.Chanaud CM, Ludlow CL. Single Motor Unit-Activity of Human Intrinsic Laryngeal Muscles During Respiration. Annals of Otology Rhinology and Laryngology. 1992;101:832–840. doi: 10.1177/000348949210101006. [DOI] [PubMed] [Google Scholar]
- 10.Wells L, Edwards KA, Bernstein SI. Myosin heavy chain isoforms regulate muscle function but not myofibril assembly. Embo Journal. 1996;15:4454–4459. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YZ, Baker MJ, Crumley RL, Caiozzo VJ. Single-fiber myosin heavy-chain isoform composition of rodent laryngeal muscle - Modulation by thyroid hormone. Archives of Otolaryngology-Head & Neck Surgery. 2000;126:874–880. doi: 10.1001/archotol.126.7.874. [DOI] [PubMed] [Google Scholar]
- 12.Alli O, Berzofsky C, Sharma S, Pitman MJ. Development of the Rat Larynx: A Histological Study. Laryngoscope. 2013;123:3093–3098. doi: 10.1002/lary.24145. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A, Jones RM, Flint PW. Postnatal development of myosin heavy chain isoforms in rat laryngeal muscles. Annals of Otology Rhinology and Laryngology. 1999;108:509–515. doi: 10.1177/000348949910800517. [DOI] [PubMed] [Google Scholar]
- 14.Hoh JFY. Laryngeal muscle fibre types. Acta Physiologica Scandinavica. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 15.Briggs MM, Schachat F. Early specialization of the superfast myosin in extraocular and laryngeal muscles. Journal of Experimental Biology. 2000;203:2485–2494. doi: 10.1242/jeb.203.16.2485. [DOI] [PubMed] [Google Scholar]
- 16.Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Annals of Otology Rhinology and Laryngology. 2002;111:579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- 17.McMullen CA, Andrade FH. Functional and Morphological Evidence of Age-Related Denervation in Rat Laryngeal Muscles. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2009;64:435–442. doi: 10.1093/gerona/gln074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson AM, Ciucci MR, Connor NP. Vocal Training Mitigates Age-Related Changes Within the Vocal Mechanism in Old Rats. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2013;68:1458–1468. doi: 10.1093/gerona/glt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki T, Bless DM, Connor NP, Ford CN, Lee K, Inagi K. Age-related alterations in myosin heavy chain isoforms in rat intrinsic laryngeal muscles. Annals of Otology Rhinology and Laryngology. 2002;111:962–967. doi: 10.1177/000348940211101102. [DOI] [PubMed] [Google Scholar]
- 20.McMullen CA, Andrade FH. Contractile dysfunction and altered metabolic profile of the aging rat thyroarytenoid muscle. Journal of Applied Physiology. 2006;100:602–608. doi: 10.1152/japplphysiol.01066.2005. [DOI] [PubMed] [Google Scholar]
- 21.Nagai H, Ota F, Connor NP. Effect of deficits in laryngeal sensation on laryngeal muscle biochemistry. Annals of Otology Rhinology and Laryngology. 2005;114:352–360. doi: 10.1177/000348940511400504. [DOI] [PubMed] [Google Scholar]
- 22.Nagai H, Ota F, Konopacki R, Connor NP. Discoordination of laryngeal and respiratory movements in aged rats. American Journal of Otolaryngology. 2005;26:377–382. doi: 10.1016/j.amjoto.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Connor NP, Lee K, Leverson G, Ford CN. Laryngeal-respiratory kinematics are impaired in aged rats. Annals of Otology Rhinology and Laryngology. 2002;111:684–689. doi: 10.1177/000348940211100805. [DOI] [PubMed] [Google Scholar]
- 24.Thomas LB, Harrison AL, Stemple JC. Aging thyroarytenoid and limb skeletal muscle: Lessons in contrast. Journal of Voice. 2008;22:430–450. doi: 10.1016/j.jvoice.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Russell JA, Nagai H, Connor NP. Effect of Aging on Blood Flow in Rat Larynx. Laryngoscope. 2008;118:559–563. doi: 10.1097/MLG.0b013e31815bac17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, Kletzien H, Connor NP, Schultz E, Chamberlain CS, Bless DM. Effects of aging on thyroarytenoid muscle regeneration. Laryngoscope. 2012;122:2800–2807. doi: 10.1002/lary.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas L, Stemple J. Voice therapy: Does science support the art? Communicative Disorders Review. 1997:49–77. [Google Scholar]
- 28.Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of 2 Forms of Intensive Speech Treatment for Parkinsons-Disease. Journal of Speech and Hearing Research. 1995;38:1232–1251. doi: 10.1044/jshr.3806.1232. [DOI] [PubMed] [Google Scholar]
- 29.Ramig LO, Sapir S, Fox C, Countryman S. Changes in vocal loudness following intensive voice treatment (LSVT (R)) in individuals with Parkinson's disease: A comparison with untreated patients and normal age-matched controls. Movement Disorders. 2001;16:79–83. doi: 10.1002/1531-8257(200101)16:1<79::aid-mds1013>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 30.Sapir S, Ramig LO, Fox CM. Intensive voice treatment in Parkinson's disease: Lee Silverman Voice Treatment. Expert Review of Neurotherapeutics. 2011;11:815–830. doi: 10.1586/ern.11.43. [DOI] [PubMed] [Google Scholar]
- 31.Stemple JC, Lee L, Damico B, Pickup B. Efficacy of Vocal Function Exercises as a Method of Improving Voice Production. Journal of Voice. 1994;8:271–278. doi: 10.1016/s0892-1997(05)80299-1. [DOI] [PubMed] [Google Scholar]
- 32.Roy N, Weinrich B, Gray SD, Tanner K, Stemple JC, Sapienza CM. Three treatments for teachers with voice disorders: A randomized clinical trial. Journal of Speech Language and Hearing Research. 2003;46:670–688. doi: 10.1044/1092-4388(2003/053). [DOI] [PubMed] [Google Scholar]
- 33.Sabol JW, Lee L, Stemple JC. The Value of Vocal Function Exercises in the Practice Regimen of Singers. Journal of Voice. 1995;9:27–36. doi: 10.1016/s0892-1997(05)80220-6. [DOI] [PubMed] [Google Scholar]
- 34.Pitts T, Bolser D, Rosenbek J, Troche M, Okun MS, Sapienza C. Impact of Expiratory Muscle Strength Training on Voluntary Cough and Swallow Function in Parkinson Disease. Chest. 2009;135:1301–1308. doi: 10.1378/chest.08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapienza C, Troche M, Pitts T, Davenport P. Respiratory strength training: concept and intervention outcomes. Seminars in speech and language. 2011;32 doi: 10.1055/s-0031-1271972. [DOI] [PubMed] [Google Scholar]
- 36.Troche MS, Okun MS, Rosenbek JC, et al. Aspiration and swallowing in Parkinson disease and rehabilitation with EMST A randomized trial. Neurology. 2010;75:1912–1919. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haddad F, Qin AX, Zeng M, McCue SA, Baldwin KM. Effects of isometric training on skeletal myosin heavy chain expression. Journal of applied physiology. 1998;84:2036–2041. doi: 10.1152/jappl.1998.84.6.2036. [DOI] [PubMed] [Google Scholar]
- 38.Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microscopy research and technique. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 39.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochemistry and cell biology. 2001;115:359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 40.Scott W, Stevens J, Binder-Macleod SA. Human skeletal muscle fiber type classifications. Physical Therapy. 2001;81:1810–1816. [PubMed] [Google Scholar]
- 41.Adachi T, Umezaki T, Matsuse T, Shin T. Changes in laryngeal muscle activities during hypercapnia in the cat. Otolaryngology-Head and Neck Surgery. 1998;118:537–544. doi: 10.1177/019459989811800418. [DOI] [PubMed] [Google Scholar]
- 42.Insalaco G, Kuna ST, Cibella F, Villeponteaux RD. Thyroarytenoid Muscle-Activity During Hypoxia, Hypercapnia, and Voluntary Hyperventilation in Humans. Journal of Applied Physiology. 1990;69:268–273. doi: 10.1152/jappl.1990.69.1.268. [DOI] [PubMed] [Google Scholar]
- 43.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. Journals of Gerontology Series A-Biological Sciences and Medical Sciences. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 44.Kletzien H, Russell JA, Leverson GE, Connor NP. Differential effects of targeted tongue exercise and treadmill running on aging tongue muscle structure and contractile properties. Journal of Applied Physiology. 2013;114:472–481. doi: 10.1152/japplphysiol.01370.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toth LA, Gardiner TW. Food and water restriction protocols: Physiological and behavioral considerations. Contemporary Topics in Laboratory Animal Science. 2000;39:9–17. [PubMed] [Google Scholar]
- 46.D'Antona G, Megighian A, Bortolotto S, et al. Contractile properties and myosin heavy chain isoform composition in single fibre of human laryngeal muscles. Journal of muscle research and cell motility. 2002;23:187–195. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- 47.Harris LR, Churchward MA, Butt RH, Coorssen JR. Assessing detection methods for gel-based proteomic analyses. Journal of Proteome Research. 2007;6:1418–1425. doi: 10.1021/pr0700246. [DOI] [PubMed] [Google Scholar]
- 48.Humphries BJ, Newton RU, Abernethy PJ, Blake KD. Reliability of an electrophoretic and image processing analysis of human skeletal muscle taken from m vastus lateralis. European journal of applied physiology and occupational physiology. 1997;75:532–536. doi: 10.1007/s004210050200. [DOI] [PubMed] [Google Scholar]
- 49.Kingham PJ, Birchall MA, Burt R, Jones A, Terenghi G. Reinnervation of laryngeal muscles: A study of changes in myosin heavy chain expression. Muscle & nerve. 2005;32:761–766. doi: 10.1002/mus.20409. [DOI] [PubMed] [Google Scholar]
- 50.Schaser AJ, Wang H, Volz LM, Connor NP. Biochemistry of the Anterior, Medial, and Posterior Genioglossus in the Aged Rat. Dysphagia. 2011;26:256–263. doi: 10.1007/s00455-010-9297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volz LM, Mann LB, Russell JA, Jackson MA, Leverson GE, Connor NP. Biochemistry of anterior, medial, and posterior genioglossus muscle in the rat. Dysphagia. 2007;22:210–214. doi: 10.1007/s00455-006-9075-y. [DOI] [PubMed] [Google Scholar]
- 52.Nishida N, Taguchi A, Motoyoshi K, Hyodo M, Gyo K, Desaki J. Age-related changes in rat intrinsic laryngeal muscles: analysis of muscle fibers, muscle fiber proteins, and subneural apparatuses. European Archives of Oto-Rhino-Laryngology. 2013;270:975–984. doi: 10.1007/s00405-012-2231-0. [DOI] [PubMed] [Google Scholar]
- 53.Basken JN, Connor NP, Ciucci MR. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Experimental Brain Research. 2012;219:351–361. doi: 10.1007/s00221-012-3096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiatarone MA, Oneill EF, Ryan ND, et al. Exercise Training and Nutritional Supplementation for Physical Frailty Ln Very Elderly People. New England Journal of Medicine. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 55.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]


