Abstract
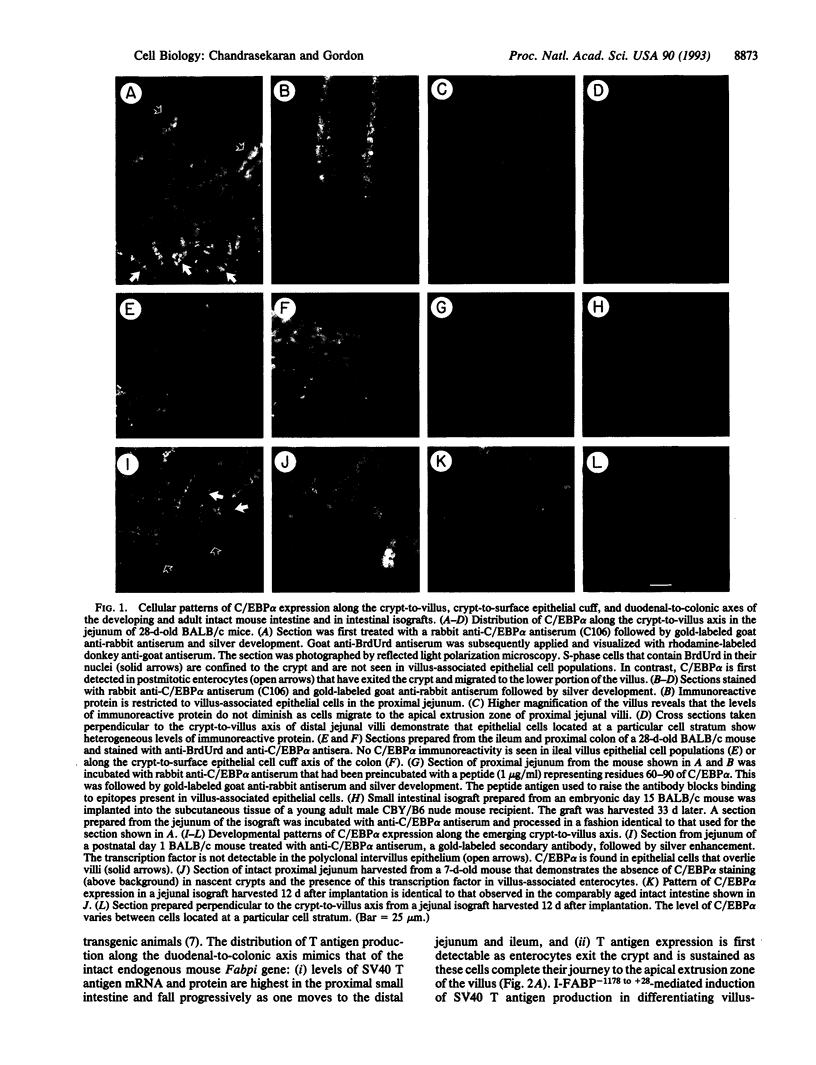
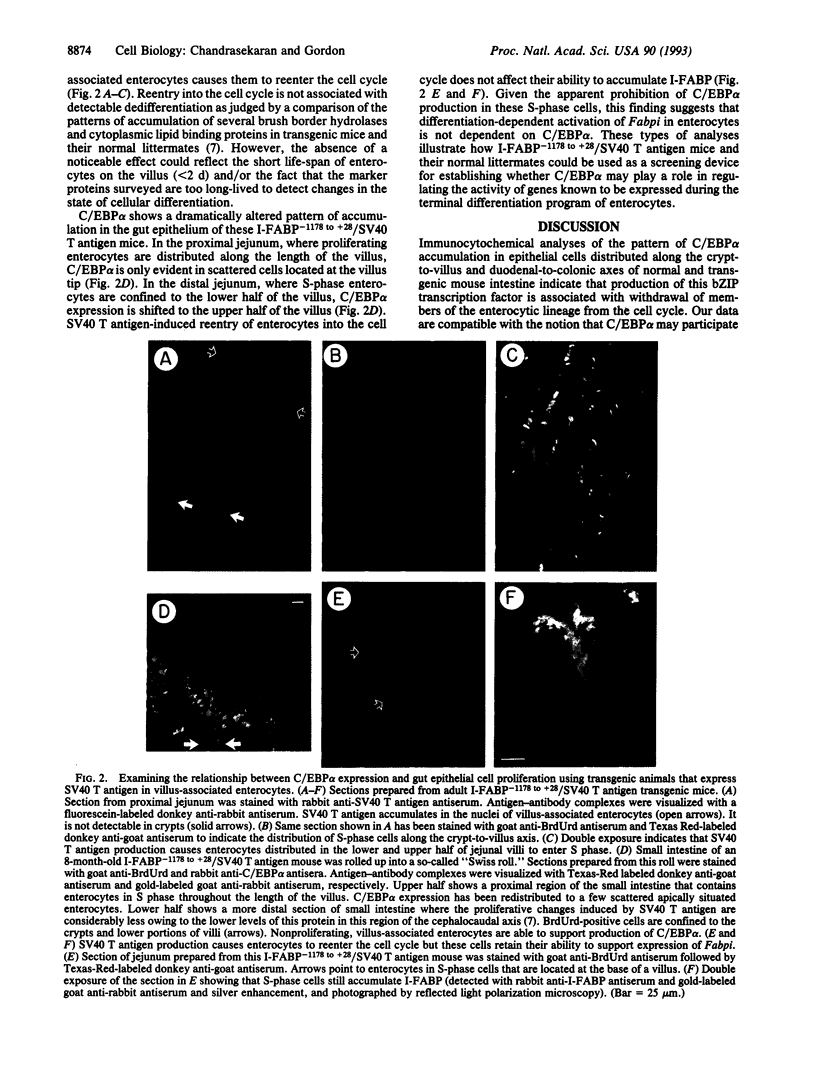
The proliferation and differentiation programs of gut epithelial cells are expressed rapidly and perpetually along an anatomically well defined pathway. The mouse intestine thus provides an excellent in vivo model system to define the contributions of CCAAT enhancer binding protein alpha (C/EBP alpha) and related bZIP proteins to these processes. Immunocytochemical studies revealed that C/EBP alpha is produced in villus-associated enterocytes located in the duodenum and jejunum of adult mice. The protein is located in the cytoplasmic and nuclear compartments of these cells. C/EBP alpha is not detectable in proliferating and nonproliferating epithelial cells situated in small intestinal crypts nor is it evident in any gut epithelial cell lineage located in the ileum and colon. The related C/EBP beta and C/EBP delta proteins are not detectable by sensitive immunocytochemical methods in epithelial cells distributed along the duodenal-to-colonic axis. Developmental surveys indicate that C/EBP alpha is confined to postmitotic, villus-associated epithelial cells during conversion of the polyclonal intervillus epithelium to monoclonal crypts. Analyses of intestinal isografts reveal that these developmental stage-specific, lineage-specific, differentiation-dependent, and regional patterns of C/EBP alpha expression can be established and maintained in the absence of exposure to luminal contents. Transgenic mice containing nucleotides -1178 to +28 of the rat intestinal fatty acid binding protein gene (I-FABP-1178 to +28) linked to the simian virus 40 large tumor antigen (T antigen) gene express T antigen in villus-associated enterocytes. This results in reentry of enterocytes into the cell cycle and a silencing of C/EBP alpha expression without an apparent effect on the accumulation of several markers of this lineage's terminal differentiation program or on gut morphogenesis. These findings indicate that there is a relationship between expression of C/EBP alpha in enterocytes and their exit from the cell cycle and suggest that I-FABP-1178 to +28/simian virus 40 T antigen transgenic mice could provide a screening assay for examining the role of C/EBP alpha in regulating the activity of genes known to be transcribed during differentiation of this gut epithelial cell lineage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Gwynn B., Howard S., Jerry J., Gordon J. I., Landschulz W. H., McKnight S. L. Tissue-specific expression, developmental regulation, and genetic mapping of the gene encoding CCAAT/enhancer binding protein. Genes Dev. 1989 Aug;3(8):1146–1156. doi: 10.1101/gad.3.8.1146. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Simon T. C., Roth K. A., Birkenmeier E. H., Gordon J. I. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992 Oct;119(1):27–44. doi: 10.1083/jcb.119.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R. W., Buchan H. L., Civin C. I., Kastan M. B. Altered cytoplasmic/nuclear distribution of the c-myc protein in differentiating ML-1 human myeloid leukemia cells. Cell Growth Differ. 1993 May;4(5):349–357. [PubMed] [Google Scholar]
- Gordon J. I., Schmidt G. H., Roth K. A. Studies of intestinal stem cells using normal, chimeric, and transgenic mice. FASEB J. 1992 Sep;6(12):3039–3050. doi: 10.1096/fasebj.6.12.1521737. [DOI] [PubMed] [Google Scholar]
- Hauft S. M., Kim S. H., Schmidt G. H., Pease S., Rees S., Harris S., Roth K. A., Hansbrough J. R., Cohn S. M., Ahnen D. J. Expression of SV-40 T antigen in the small intestinal epithelium of transgenic mice results in proliferative changes in the crypt and reentry of villus-associated enterocytes into the cell cycle but has no apparent effect on cellular differentiation programs and does not cause neoplastic transformation. J Cell Biol. 1992 May;117(4):825–839. doi: 10.1083/jcb.117.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Mahoney C. W., Shuman J., McKnight S. L., Chen H. C., Huang K. P. Phosphorylation of CCAAT-enhancer binding protein by protein kinase C attenuates site-selective DNA binding. J Biol Chem. 1992 Sep 25;267(27):19396–19403. [PubMed] [Google Scholar]
- Metz R., Ziff E. cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to trans-locate to the nucleus and induce c-fos transcription. Genes Dev. 1991 Oct;5(10):1754–1766. doi: 10.1101/gad.5.10.1754. [DOI] [PubMed] [Google Scholar]
- Ron D., Habener J. F. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992 Mar;6(3):439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- Roth K. A., Hertz J. M., Gordon J. I. Mapping enteroendocrine cell populations in transgenic mice reveals an unexpected degree of complexity in cellular differentiation within the gastrointestinal tract. J Cell Biol. 1990 May;110(5):1791–1801. doi: 10.1083/jcb.110.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D. C., Swietlicki E., Roth K. A., Gordon J. I. Use of fetal intestinal isografts from normal and transgenic mice to study the programming of positional information along the duodenal-to-colonic axis. J Biol Chem. 1992 Jul 25;267(21):15122–15133. [PubMed] [Google Scholar]
- Schmidt G. H., Winton D. J., Ponder B. A. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988 Aug;103(4):785–790. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- Scott L. M., Civin C. I., Rorth P., Friedman A. D. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992 Oct 1;80(7):1725–1735. [PubMed] [Google Scholar]
- Simon T. C., Roth K. A., Gordon J. I. Use of transgenic mice to map cis-acting elements in the liver fatty acid-binding protein gene (Fabpl) that regulate its cell lineage-specific, differentiation-dependent, and spatial patterns of expression in the gut epithelium and in the liver acinus. J Biol Chem. 1993 Aug 25;268(24):18345–18358. [PubMed] [Google Scholar]
- Traber P. G., Gumucio D. L., Wang W. Isolation of intestinal epithelial cells for the study of differential gene expression along the crypt-villus axis. Am J Physiol. 1991 Jun;260(6 Pt 1):G895–G903. doi: 10.1152/ajpgi.1991.260.6.G895. [DOI] [PubMed] [Google Scholar]
- Umek R. M., Friedman A. D., McKnight S. L. CCAAT-enhancer binding protein: a component of a differentiation switch. Science. 1991 Jan 18;251(4991):288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- Wegner M., Cao Z., Rosenfeld M. G. Calcium-regulated phosphorylation within the leucine zipper of C/EBP beta. Science. 1992 Apr 17;256(5055):370–373. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Irwin M. The kinetics of villus cell populations in the mouse small intestine. I. Normal villi: the steady state requirement. Cell Tissue Kinet. 1982 Nov;15(6):595–609. doi: 10.1111/j.1365-2184.1982.tb01066.x. [DOI] [PubMed] [Google Scholar]




