Abstract
The aim of cardiovascular regeneration is to mimic the biological and mechanical functioning of tissues. For this it is crucial to recapitulate the in vivo cellular organization, which is the result of controlled cellular orientation. Cellular orientation response stems from the interaction between the cell and its complex biophysical environment. Environmental biophysical cues are continuously detected and transduced to the nucleus through entwined mechanotransduction pathways. Next to the biochemical cascades invoked by the mechanical stimuli, the structural mechanotransduction pathway made of focal adhesions and the actin cytoskeleton can quickly transduce the biophysical signals directly to the nucleus. Observations linking cellular orientation response to biophysical cues have pointed out that the anisotropy and cyclic straining of the substrate influence cellular orientation. Yet, little is known about the mechanisms governing cellular orientation responses in case of cues applied separately and in combination. This review provides the state-of-the-art knowledge on the structural mechanotransduction pathway of adhesive cells, followed by an overview of the current understanding of cellular orientation responses to substrate anisotropy and uniaxial cyclic strain. Finally, we argue that comprehensive understanding of cellular orientation in complex biophysical environments requires systematic approaches based on the dissection of (sub)cellular responses to the individual cues composing the biophysical niche.
Keywords: Mechanotransduction, Actin cytoskeleton, Focal adhesion, Strain avoidance, Contact guidance, Structural pathway
Introduction
Cardiovascular regenerative medicine has emerged as a promising approach to replace or regenerate damaged or diseased cardiovascular tissues. This interdisciplinary field, at the cross-section of engineering and life sciences, has the potential to restore normal cardiovascular function by using (the properties of) living cells in combination with biomaterials, genes, or drugs. Novel in situ tissue engineering approaches rely on the regenerative potential of the body itself by guiding and controlling cell behavior inside the human body with tailored biomaterials.
The premise of this approach is that, to recapitulate tissue function, an in-depth understanding of native cell behavior under physiological conditions and in response to a biomaterial is needed. Only then, strategies for controlling cell behavior can be designed towards the restoration of tissue functionality and mechanical integrity.52
One crucial, but often overlooked, aspect of mimicking native tissue functioning is obtaining and retaining cellular organization. The importance of cellular organization is demonstrated by the fact that biological and mechanical functioning of most tissues is dictated by the cellular arrangement.42
The tissues of the cardiovascular system are highly organized. For instance, the myocardial wall,118 heart valves120 and larger arteries134 are characterized by a layered structure with a well-defined cellular arrangement conferring the tissues their native unique anisotropic mechanical behavior needed to perform their function. Given the correlation between structural organization and function, it becomes clear that the loss of cellular organization is indicative of tissue malfunctioning, which can eventually lead to pathophysiological conditions. The disorganized arrangement of cardiac cells, for example, is a histological hallmark of cardiac dysfunction in hypertrophic cardiomyopathy.23,58,61,102
Cellular organization in cardiovascular tissues depends on the complex interactions between cells, the properties of the microenvironment and the cyclic strains resulting from the hemodynamic environment. Living adherent cells actively interact, respond, and adapt to biochemical and biophysical perturbations. These perturbations trigger intracellular signaling events leading to specific cellular mechanoresponses capable of directing biological relevant processes such as cell differentiation, proliferation and contractility. The mechanisms employed by cells to respond and adapt to the biochemical and biophysical cues of the micro-environment consist of a myriad of distinct but interconnected pathways whose details remain to be unraveled. The outside-in and inside-out feedback loop, referred to as mechanotransduction, is traditionally regarded as the process of converting mechanical stimuli into biochemical signals. Recently, it has been suggested that the structural pathway connecting the extracellular environment to the nucleus,149 here defined as “the structural mechanotransduction pathway”, might be as important as the biochemical transduction pathway for conducting biophysical signal to the nuclear interior. This new concept is supported by the fact that the long-range force propagation into the cell, resulting in deformations deep inside the cytoskeleton and nucleus, occurs 40 times faster than biochemical signaling.97 The structural mechanotransduction pathway consists of structural load bearing elements, such as integrins and focal adhesion complexes at the cellular membrane, and actin cytoskeleton stress fibres connected to the nucleus via so-called LINC (Linkers of the Nucleoskeleton and Cytoskeleton) complexes. Experimental evidence for this direct interconnection arises from studies where forces were applied directly to a small spot on the cell surface and consequently induced deformations and movements in the cellular interior.91,93 Clearly, defects in the complex and delicate interplay between the cell and its micro-environment resulting, for instance, from aberrations of the structural mechanotransduction pathway, may result in altered cellular mechanoresponse, in case no compensatory signaling mechanisms arise.
The recent development of micro-fabricated devices capable of effectively mimicking controlled biophysical cues has triggered numerous studies aiming at unraveling cellular responses to the properties of the micro-environment. It has become clear that cell orientation is actively determined by the actin stress fibres.132 Stress fiber orientation and, consequently, cellular alignment can be induced by two important biophysical cues of the cellular environment, such as those occurring during hemodynamic loading: (1) the anisotropy of the environment, e.g., the substrate on which cells are cultured and (2) uniaxial cyclic strain.7,88 These cues induce rapid and specific orientation of the intracellular elements of the structural mechanotransduction pathway, i.e., the focal adhesions, the actin cytoskeleton and the nucleus, suggesting that the direct structural mechanotransduction pathway plays a fundamental role in the cellular orientation response.30,73
Although a wealth of information has been obtained by recent in vitro mechanotransduction studies at the tissue-level, single cell observations provide detailed mechanistic insights towards a comprehensive understanding of cellular mechanotransduction. Yet, integrating the results of different investigations is a difficult task because of the complexity of the cellular response, which is not only highly dependent on the choice of the physical and mechanical experimental parameters, but also dependent on the cell-type. Moreover, the effects of combined biophysical cues on the cellular orientation response have just begun to be explored.
Here, we present a state-of-the-art review on the complex interplay between cells, topographical and cyclic strains cues of the extracellular environment, with a focus on cells of the cardiovascular system. Focusing on single cell observations, we first introduce the structural mechanotransduction pathway, i.e., the connected cellular components forming the physical link between the extracellular environment and the nuclear genome. Then, we continue our discussion with a review of experimental observations regarding cellular orientation response to anisotropy of the substrate and uniaxial cyclic strain in two-dimensional (2D) environments. We conclude with a brief outlook on future research directions for improving our current knowledge of cellular mechanoresponse to complex biophysical environments.
The Structural Mechanotransduction Pathway: A Physical Connection Between the Extracellular Environment and the Genome
In this section we provide background information on the cellular structural components forming the structural mechanotransduction pathway, i.e., the physical connection between the extracellular matrix (ECM) and the genome contained by the nucleus.
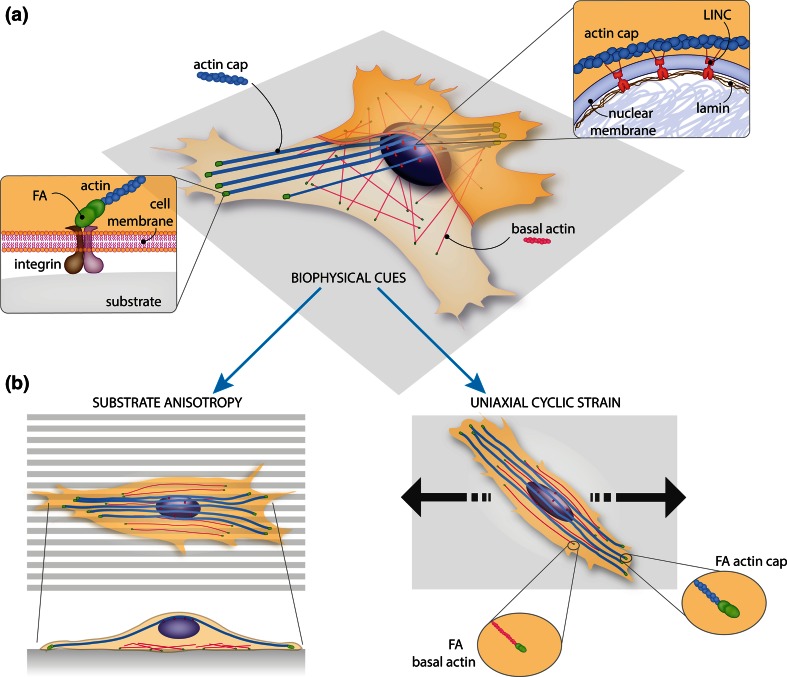
The structural components are represented by the focal adhesions situated at the cell membrane, the cytoskeletal filaments and, at last, the nucleus (Fig. 1). Among the cytoskeletal elements we concentrate on the actin filaments, since these structures are directly connected to the focal adhesions and play an important role in determining cell orientation.131,154 Moreover their behavior is relatively easy to analyze and quantify from microscopy imaging as they form anisotropic networks when cells are aligned.10,154 In this section also the relevance of the nucleo-cytoskeletal connections for correct mechanotransduction is elucidated.
Figure 1.
The structural mechanotransduction pathway and cellular orientation response to anisotropy of the substrate and uniaxial cyclic strain. (a) Schematic illustration highlighting the (protein) structural elements forming the structural mechanotransduction pathway. Integrins at the plasma membrane connect the extracellular environment (substrate) to the actin cytoskeleton. The connection is realized, in the cellular interior, by the focal adhesion complex. Within the actin cytoskeleton filaments, two kinds of fibers can be distinguished. The basal actin fibers (pink) that can be found underneath the nucleus and the actin cap fibers running on top of the nucleus (cyan). Actin cap fibers are connected to the nuclear interior via the LINC complex and lamins, a group of proteins underlying the nuclear membrane. This network of components forms a direct connection between the extracellular environment and the nuclear interior and function as a fast passing system for the biophysical stimuli. (b) Schematic illustration of cellular response to substrate anisotropy and uniaxial cyclic strain. When plated on an anisotropic substrate (left), the cell tends to align in the direction of the anisotropy. Focal adhesions as well as the actin cytoskeleton align accordingly. The side view shows the arrangements of the actin cap and basal actin fibers. Upon uniaxial cyclic strain (right), the cell responds by strain avoidance. The focal adhesions and the actin cytoskeleton align at an angle with respect to the straining direction. Overall cell orientation coincides with the actin cytoskeleton orientation. Note that the focal adhesions associated with the actin cap fibers are bigger than those associated with the basal actin fibers. Figure by Anthal Smits.
Interconnection Between the Extracellular Environment and the Actin Cytoskeleton
In vivo adhesive cells are embedded in a filamentous network called extracellular matrix (ECM). The integrins are the first components that physically link the ECM (outside of a cell) with the actin cytoskeleton (inside of the cell). Integrins are transmembrane αβ heterodimeric receptors that mediate cell adhesion to various ECM ligands such as collagen, fibronectin and laminin. The integrin family consists of about 25 members which are composed of combinations of α and β subunits, where the α subunit determines the ligand specificity for cell adhesion to the ECM.68 During cell adhesion, conformational changes in the integrins are induced by bidirectional (inside-out and outside-in) signaling of mechanical and biochemical signals across the cell membrane.4,113,114 Ligand binding to the integrins leads to clustering of integrin molecules at the cell membrane and recruitment of actin filaments inside the cell. The result of this process is the formation of the so-called nascent focal adhesion complexes (Fig. 1a, left inset), multi-molecular complexes that consist of a large number of different proteins, including talin, vinculin, paxillin and tensin.
Focal adhesion complex formation initially starts with immature, small structures (approximately 100 nm in diameter45). These structures reside at the leading edge in protrusions of the cells and provide the structural links between the ECM and the actin cytoskeleton. Strikingly, the maturation of the small focal adhesion complexes into bigger, mature focal adhesions is dependent on actin cytoskeleton bundling and generation of mechanical force. The actin cytoskeleton spans the whole cytoplasm of eukaryotic cells, continuously remodels and reorganizes to perform specific cellular functions.95,140 It is made of globular actin (G-actin), which continuously polymerizes into semi-flexible actin filaments, the filamentous actin (F-actin). F-actin assembles into bundles of fibres interconnected by actin crosslinkers (such as alpha-actinin and filamin) and motor proteins such as myosin II.103 These bundles of F-actin fibers are referred to as stress fibers. The presence of myosin II within the stress fibers is responsible for their contractility. The newly formed focal adhesions (FAs) reside in both central and peripheral regions of the cell. During this process the morphology of the FAs changes from a dot-like structure to a bigger and more elongated structure (2–10 μm).22,44 This happens also as a consequence of the recruitment at the adhesion complex of several other proteins, for instance zyxin and alpha-actinin.163 A critical molecule for both maturation of FAs and mechanosensing is focal adhesion kinase (FAK). This molecule is involved in the transmission of external signals to the cytoskeleton by phosphorylation.50
The maturation of FAs provides stable adhesive interconnections between the stress fibers and the ECM. This allows the cell to probe its complex biophysical environment in various directions and over large temporal and spatial scales.121 Focal adhesions do not actively generate forces, but rather serve to regulate force transmission between the cytoskeleton and ECM.104 The actin cytoskeleton is the intracellular structure able to impose increasing forces when facing growing resistance. This confers the actin cytoskeleton intrinsic mechanosensing and ability to adapt to developing mechanical cues of the cellular environment. However, to which extent stress fibers participate in sensing and transducing environmental signals has not been fully elucidated yet.
Diseases Associated with Dysfunctional ECM-Actin Connections
The relevance of correct functioning of all components within the mechanotransduction route between ECM and the actin cytoskeleton has become clear in numerous studies in the past few years.
Starting at the ECM, its composition appears to have major impact on cellular behavior. Either weakening or increased ECM stiffness due to decreased or increased amount of collagen evokes a cellular response, which, upon disturbed mechanotransduction, can lead to an even more deregulated ECM. For example, cardiac tissue damage that normally causes controlled levels of enhanced myofibroblast proliferation and collagen production, will, without proper feedback due to disturbed mechanosensing, lead to cardiac fibrosis and stiffening of the cardiac muscle.92 For a recent review on the interplay between ECM and mechanotransduction, see Ref. 43
Not only amounts of ECM components but also abnormalities in the molecular composition of its components lead to disturbed mechanosignaling. For instance, mutations in the ECM protein fibrillin-1 as seen in the Marfan syndrome, cause the development of cardiomyopathies in affected mice due to disturbed mechanosignaling. Normalizing the extracellular matrix composition in these mice resulted in disappearance of these symptoms.25
Proteins, assembled in the FA complex, including the transmembrane integrins as well as the other binding partners, mostly localized at the cytoplasmic side of the cellular membrane, all appear to be critical for the transmission of extracellular forces. Especially, any structural abnormalities in integrins have a devastating effect on mechanotransduction. Even minor modifications of the β1-integrin gene resulted in multiple defects in mechanotransduction signaling in adult cardiomyocytes.86 An overwhelming number of diseases now have been assigned to failure in any of the other components of the FAs, based on mouse models.90 While not all of these diseases are caused by disturbed mechanotransduction, it has become clear that especially improper functioning of the key enzyme FAK leads to severe abnormalities in heart development and heart failure.117
While this brief overview suggests that most mechanotransduction diseases are associated with heart (muscle) tissue there is growing evidence that impaired or sustained mechanotransduction at the cellular boundaries leads to several other diseases. Abnormal mechanical stimulation can switch on signaling pathways such as beta-catenin signaling in adenomatous polyposis coli (APC) deficient colon tissue, stimulating the development of colon cancer.156 Moreover ECM stiffness can drive epithelial to mesenchymal transition of cancer cells,153 increasing the malignant behavior of these cells.
Interconnection Between the Actin Cytoskeleton and the Nucleus
In the surrounding of the nucleus, a subset of actin stress fibers have been found to organize in thick parallel and well-ordered bundles of fibers, physically anchored to the apical surface of the nucleus.75,76 Wirtz and co-workers have made an effort to characterize these fibers (actin cap) which are strikingly terminated by wide, long and dynamic focal adhesions (Fig. 1a).17,64,75 First, they have demonstrated that the actin cap stress fibers differ from the conventional stress fibers found below the nucleus (basal actin layer, Fig. 1b). By containing more myosin II and the actin bounding protein alpha-actinin, actin cap stress fibers are very contractile and highly dynamic.98 Furthermore, these fibers not only play a major role in shaping and positioning the nucleus,19,64,75,77,98,139 but they are also involved in mechanosensing of substrate elasticity. For instance, cells without an actin cap were observed to be less responsive to changes in matrix elasticity. Finally, fast mechanotransduction also seems to be enabled by this subset of stress fibers. In their study, Chambliss et al. showed that, in response to shear stress stimulation, cells without the actin cap build up thick stress fibers in a shorter time span as compared to in response to biochemical stimulation.18 From these findings it has become clear that the perinuclear actin cap is a key component of the physical pathway from the ECM to the nuclear interior for mechanosensing and mechanotransduction.
The coupling between the perinuclear actin cap and the nucleus (nucleo-cytoskeletal connection) is mediated by a group of recently discovered proteins, referred to as the LINC complex (Linker of Nucleoskeleton and Cytoskeleton, Fig. 1a right inset).26,108,127 Hooking at the cytoplasmic side of the nucleus, on the outer nuclear membrane (ONM), we find the nesprins (KASH domains proteins), which are connected to the various cytoskeletal filaments.125,166 Among the four variants of nesprins, nesprin-1 and -2 bind to actin filaments.54 Nesprins, in turn, bind to SUN domain proteins spanning the whole nuclear envelope reaching the nuclear interior. SUN proteins then bind to lamins, a family of type V intermediate filaments underlying the inner nuclear membrane (INM).56 Lamins in turn physically connect to chromatin. Thus, in this way a physical bridge is formed from the cellular exterior via focal adhesion complexes, actin, the LINC complex, and lamins to chromatin.
Lamins form an elastic meshwork called nuclear lamina (Fig. 1a, right inset).29,109 Lamins consist of two main subtypes, A- and B-type lamins (encoded by the gene LMNA, or LMNB1 and LMNB2 respectively).53 While B-type lamins are essential for cell survival, A-type lamins are thought to contribute significantly to the maintenance of mechanical integrity of the nucleus.12,63,80,138 The nuclear lamina interacts also with the chromatin of the nucleoplasm, and therefore plays a major role in gene expression, DNA replication and repair, chromatin organization and transcriptional response.35,49,122,168
The role of the nucleo-cytoskeletal connection in force transmission has been examined recently by many groups. The results of various experimental approaches based on two- and three-dimensional substrates or application of mechanical load, have shown that the structural integrity of this connection is indeed needed for propagation of forces to the nucleus. Indirect demonstration has come from studies employing LMNA-depleted cells. By using this model, it has been shown that nuclear deformations in response to local cellular membrane stretch are completely abolished.91 In addition, the studies by Poh et al.111 and Zweger et al.169 have provided direct evidence that forces are not transmitted to the nucleus when LMNA is depleted from cells, thus when the nucleo-cytoskeletal connection is lost. Recently, it has emerged as well that the tension exerted by the actin on the nucleus directly mediates the spatial polarization of nuclear lamina and the intranuclear architecture.78 In cells lacking A-type lamins, the formation of a nuclear actin cap is partially abolished.75 Also, the impaired activation of mechanosensitive genes has been reported in studies with cells lacking A-type lamins.62,81
A number of other studies in which either the LINC complex was disrupted or a loss of lamins was induced, support these findings adding that also other cellular functions such as migration, polarization and developmental processes become affected.14,83
Although the role of the LINC complex in force propagation to the nucleus has been clarified, controversy remains about its impact on the activation of mechanotransduction pathways. Clues to understand these mechanisms might come from studying diseases arsing form mutations in any of the components connecting actin to the nucleus.
Diseases Associated with Defective Actin-Nucleus Coupling
Mutations in the LMNA gene encoding for A-type lamins in the nuclear lamina cause a broad spectrum of genetic diseases, collectively referred to as laminopathies.13 Several hundred mutations in the gene have been discovered and most of them have tissue-specific phenotypes. Twelve different diseases are included into this group: those affecting striated muscle (ranging from Emery/Dreifuss muscular dystrophy (EDMD) to dilated cardiomiopathy with conduction system defects (DCM-CD) and Limb-girdle muscular dystrophy (LGDM), those affecting the adipose tissue (partial lipodystrophy of Dunningan type (FPLD) and those affecting the nervous system (Autosomal recessive Charcot-Marie-Tooth type 2 and Autosomal dominant axonal Charcot-Marie-Tooth disease). However, primary laminopathies can also affect tissues in a systemic fashion and cause premature-aging syndromes like Restrictive Dermopathy (RD) and Hutchinson-Gilford progeria syndrome (HGPS).159 The mechanisms underlying tissue-specific effects observed in laminopathies are still largely unknown. Especially in the most diffuse laminopathies, the muscular dystrophies and cardiomyopathies,16 it might well be that the lack of structural integrity, thus the susceptibility to mechanical stress could result in altered chromatin organization which, on its turn, results in altered gene expression.
Recently it was observed that also mutations in other components of the LINC complex (e.g., emerin, nesprin-1, nesprin-2, etc.) can give rise to the same disease pathology as seen in EDMD due to LMNA mutations.8,87,94,164 Next to this, combinations of mutations in the nucleo-cytoskeletal system have been shown to lead to more severe diseases than the individual component mutations.85,96,129 At the same time, it has been suggested that the biochemical signals coming from the cytoplasm might take over or compensate for the lack of the physical nucleo-cytoskeletal interconnection.14,91
Altogether, the examples above demonstrate that several structural components of the mechanotransduction pathway connecting the cellular micro-environment and the nuclear interior have been identified. While we do not know the degree of completeness of our understanding, we can confidently state that the structural interconnection is crucial for determining the cellular mechanoresponse.
Cellular Orientation Response to Substrate Anisotropy and Cyclic Strain
In the previous section, in order to appreciate the inside-in part of the cellular mechanotransduction, i.e., how environmental signals are transmitted to the nucleus, we introduced the components of the structural mechanotransduction pathway interconnecting the extracellular environment and the nucleus. To get a comprehensive understanding of the interplay between cellular responses and complex biophysical environments, it is also necessary to have a deep understanding of the inside-out signaling used for cellular mechanoresponse, i.e., how cells respond to environmental cues and which mechanisms are employed by cells for mechanoresponse. In this section we discuss the cellular orientation response to substrate anisotropy and uniaxial cyclic strain (Fig. 1b), focussing on the main components of the structural mechanotransduction pathway, i.e., the focal adhesions, the actin cytoskeleton and the nucleus.
Cellular Orientation Response to Substrate Anisotropy
Various biophysical cues such as topography, cyclic strain and the mechanical properties of the extracellular environment can induce the alignment of adherent cells by promoting an anisotropic arrangement of structural components at the subcellular level. In 1912, Harrison reported for the first time that the topography of a substrate could influence cell behavior.57 Weiss confirmed this in 1945, with the observation that cells preferentially orient and migrate along fibers, an organization principle he named contact guidance.155 Today the connotation of this term is slightly different. Contact guidance is now regarded as the ability of cells to sense and align with the anisotropy of the surrounding micro-environment.
Recent developments in microfabrication technologies have led to the manufacturing and application of a variety of substrates with different geometries and length scales, from which several substrates can be used to study contact guidance. Observations obtained using microfabricated substrates engineered to induce contact guidance, have confirmed that a variety of tissue cells, ranging from endothelial cells,38,135,136 to fibroblasts,37,39,105,141,142 and smooth muscle cells116 orients along the direction of the anisotropy of the substrate. A summary of illustrative studies showing the response of cells of the cardiovascular system to anisotropic features of the culture substrate in the sub-micrometer to micrometer scale is reported in Table 1.
Table 1.
Experimental investigations on cell orientation response induced by anisotropy of the substrate.
| Cell type | Method | Parameters | Main observed results | Source |
|---|---|---|---|---|
| Fibroblasts (REF52 cells) |
Parallel microcontact printed fibronectin lines Glass |
Lines: 2 μm wide separated by 4, 6, 10 or 12 μm wide stripes | Focal adhesions are formed at the adhesive lines If spacing is larger than 6 μm, focal adhesions orient either perpendicular to the lines or orient in the direction of the lines Actin stress fibres can cross several stripes when the non-adhesive spacing is smaller than 6 μm With wider spacing stress fibres form between adjacent adhesive stripes or along single stripes |
Zimerman et al. 167 |
| Chick heart fibroblasts | Parallel grooves Quartz |
Ridge width from 1.65 to 8.96 μm Groove width from 3 to 32 μm Constant depth: 0.69 μm |
Focal adhesions observed on the floor of the grooves and at the ridges Actin bundles associated with focal contact on the floor of grooves are parallel to the groove axis and hardly ever nearly perpendicular to this axis. No such restriction is found on the ridges |
Dunn and Brown39 |
| Human gingival fibroblasts | Parallel grooves Silicon Titanium coating |
Ridge and groove width of 15 μm Constant depth: 3 μm |
Microtubules located at the bottom of the grooves are the first component to align along the grooves. Subsequently, focal adhesions and actin microfilaments align At a single groove or ridge, focal adhesions are oriented both parallel and perpendicular to the groove direction |
Oakley and Brunette105 |
| Rat dermal fibroblasts | Parallel grooves PDMS RFGD treatment |
Ridge and groove width from 1 to 10 μm Depth: 0.45 or 1 μm |
Cells at surfaces with a ridge width smaller than 10 μm elongate along the surface grooves. If the ridge width is larger than 4 μm, cellular orientation was random and the shape of the cells became more circular. The ridge width is the most important parameter, since varying the groove width and groove depth does not affect cell size, shape, nor the angle of cellular orientation | den Braber et al. 37 |
| Ridge and groove width from 1 to 20 μm Depth: 0.5, 1, 1.5, 1.8, 5.4 μm |
The cells always elongate in the groove direction without any significant difference in behavior between a 2–20 μm wide grooves. However, groove depth affects the cellular orientation | Walboomers et al. 142 | ||
| Ridge and groove width from 1 to 10 μm Constant depth: 0.5 μm |
Actin fibers orient in direction of the grooves. This happens more rapidly on the narrow grooves The addition of cytochalasin-B only causes delay in cell attachment and spreading, thus a well-formed cellular actin cytoskeleton is no prerequisite for the occurrence of contact guidance |
Walboomers et al. 141 | ||
| Myofibroblasts (HVSCs) |
Elastomeric microposts PDMS microcontact printed Human Plasma Fibronectin on top of microposts |
Elliptical microposts: a = 1.5 μm, b = 0.87 μm Circular microposts: d = 2 μm |
Elliptical microposts: orientation in the direction of the major axis of the ellipse, even for very stiff microposts. Topographical cues induce cellular alignment | Tamiello et al. 128 |
| Vascular smooth muscle cells | Parallel grooves PDMS Fibronectin coating |
Ridge width: 12 µm Groove width: 20, 50, and 80 µm Constant depth: 5 µm |
For all groove widths investigated, cells align in the direction of the microgrooves The actin filaments are highly aligned and parallel to the grooves on the smallest groove widths As the groove width increases to 50 and 80 µm, there is a clear decrease in the number of highly aligned fibers |
Sarkar et al. 119 |
| Parallel microcontact printed fibronectin or laminin lines PDMS |
Lines width from 20 to 100 μm, separated by 100 μm wide stripes | Actin cytoskeleton aligns along all patterns | Alford et al. 3 | |
| Aortic smooth muscle cells | Parallel grooves PDMS Gelatine, fibronectin, vitronectin or poly-d-lysine coating |
A PDMS sheet was stretched uniaxially using a custom-made stretcher to produce a fixed amount of prestretch The stretched sheet was treated with oxygen plasma for a fixed period of time. This resulted in substrates with or without parallel grooves. |
Focal adhesions are more prone to become mature when they run along microgrooves, causing mature focal adhesions to align in the direction of the microgrooves. These adhesions have straighter actin bundles oriented parallel to the microgrooves | Saito et al. 116 |
| Bovine aortic endothelial cells | Parallel grooves PDMS Fibronectin coating |
Ridge width from 3 to 5.5 μm Groove width from 3 to 4 μm Depth: 200 nm, 500 nm, 1 μm, 5 μm |
Majority of the focal adhesions and actin fibers orient in direction of the ridges Focal adhesions localized at the ridge edges and along the sidewalls of 1 μm deep microgrooves No focal adhesions on the bottom of the 5 μm deep microgrooves |
Uttayarat et al. 135 |
| Endothelial cells (HUVECs) |
Parallel grooves Silicone Human Plasma Fibronectin coating |
Ridge and groove width of 2, 5, and 10 μm Constant depth: 5 μm |
On 2 μm surfaces the vast majority of focal contacts are deposited on the ridges On the 5 μm and 10 μm surfaces, cells are increasingly able to form these contacts also in the grooves |
van Kooten and von Recum136 |
| Parallel grooves PDMS Serum-free medium and without protein coating |
Ridge and groove width from 200 to 2000 nm Constant depth: 300 nm |
HUVECs orient parallel to the long axis of underlying ridges, even in the absence of added protein The actin stress fibers align parallel to the ridges and grooves on patterned substrates |
Dreier et al. 38 | |
| Epithelial (MDCK) cells | Elastomeric microposts PDMS Microcontact printed fibronectin on top of microposts and microcontact printed fibronectin elliptical islands glass |
Elliptical microposts: a = 0.95 μm, b = 0.55 μm Elliptical adhesive islands: a = 0.95 μm, b = 0.55 μm |
Elliptical posts: orientation of the focal adhesions and actin cytoskeleton in the direction of the major axis of the ellipse Elliptical islands: no preferential orientation of the focal adhesions and actin cytoskeleton. Alignment is induced by differential stiffness of elliptical microposts |
Saez et al. 115 |
HUVECs human umbilical vein endothelial cells, MDCK Madin–Darby canine kidney, PDMS polydimethylsiloxane, RFGD radio frequency glow discharge
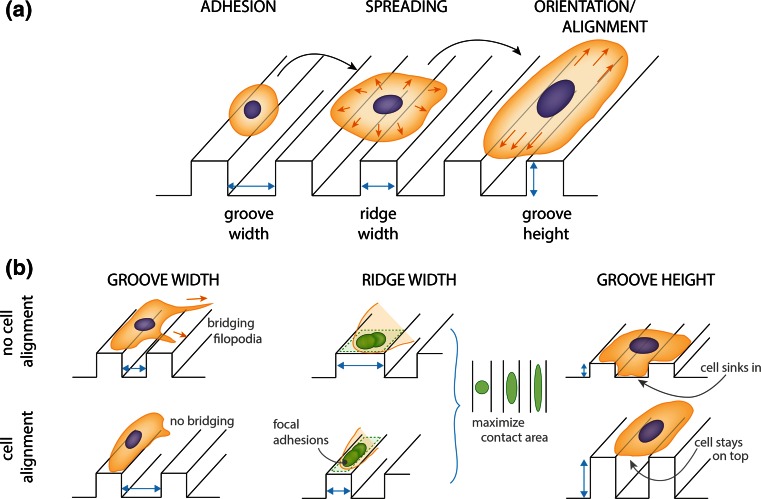
The most used substrates for studying contact guidance are microgrooves, i.e., microengineered arrays of parallel micrometer-sized grooves and ridges. When culturing adherent cells on these substrates it is observed that, at the subcellular level, the focal adhesions and actin fibers follow cellular orientation (Fig. 1b, left). However, the specific response of these structural cellular components depends on many parameters such as groove width,38,39,119,136,142 ridge width,11,37–39,130,136 groove height11,24,130,135,142 and surface treatment.38 The general trend is that when either the groove width or groove height increases, the cell forms focal adhesions on the ridges and consequently orients in their direction. Next to these observations, several theoretical frameworks have been elaborated for explaining cell alignment in relation to the microgroove’s parameters. The schematic representation of these theories is shown in Fig. 2.
The mechanical restriction theory by Dunn and Heath focuses on the relative inflexibility of cytoskeletal structures as a primary regulator of cellular alignment.40 The shape of the substratum is demonstrated to impose mechanical restrictions for the formation of cytoskeletal protrusions, called filopodia, as recently shown also by Zimerman et al.167 and Ventre et al.137. According to this theory, the distance between the anisotropic features, either the groove width (Fig. 2b, left), on microgrooved substrates or the distance between adhesive lines on flat substrates, is the crucial factor for cell alignment. If this distance cannot be bridged by the formation of any filopodia, cells become highly polarized and elongate in the direction of the substrate anisotropy. When cells align because of this mechanism, actin filaments as well as long focal adhesions are observed in the direction of the anisotropy. These focal adhesions are usually anchored to thick stress fibres and, therefore, are presumably the focal adhesions of the actin cap stress fibers.
The focal adhesion theory by Ohara and Buck proposes that the orientation of cells is caused by the tendency of focal adhesions to maximize their contact area.107 According to this theory, on a microgrooved substrate, focal adhesion maturation and, consequently, cell alignment occur along the ridge only if ridge width (Fig. 2b, center) is comparable to the size of a focal adhesion. An argument against this theory is the observation of focal adhesions oriented both perpendicular and parallel to ridges of the microgrooved substrate.105,137,167 However, as pointed out by Ventre et al.,137 focal adhesions observed perpendicular to the ridge direction are unstable and connected to isolated actin fibers, while the focal adhesions parallel to the ridge are mature and connected to stress fibers. This ultimately guides cellular orientation in the ridge direction.
Discontinuity theory: more recently Curtis and Clark proposed the idea that sharp discontinuities in the substrates, e.g., edges of microgrooves, induce cell alignment by triggering, first, actin condensations in these locations and, consequently, promoting focal adhesion formation at the same place.27 Despite the fact that this theory includes both the involvement of focal adhesions and actin filaments in cell alignment, it raises the question of how cells sense discontinuity, as already discussed by Curtis et al.28 Clark et al.24 observed that by increasing the groove height (Fig. 2b, right), more cells orient in the direction of the microgrooves. Based on these observations, it is proposed that for sufficiently high microgrooves, cells are more exposed to substrate discontinuity and, as a result, align along the microgrooves.
Figure 2.
Cellular orientation response to microgrooves. (a) Schematic illustration showing the overall cellular orientation response from cell adhesion to alignment on a microgrooved substrate. At the moment the cell adheres to the microgrooved substrate, the cell undergoes spreading followed by cell alignment, i.e., orientation along the direction of the microgrooves, a phenomenon called contact guidance. The parameters characterizing the microgrooved substrate are pointed out with light blue arrows: groove width, ridge width and groove height. (b) Schematic representation of the proposed mechanisms explaining contact guidance in relation to the microgroove’s parameters. (Top) No cell alignment and (bottom) cell alignment. (Left) groove width—mechanical restriction theory. When the microgrooves are too narrow, cell’s filopodia succeed in bridging the space between two consecutives ridges. Therefore, the cell does not align (top). When the width of the microgrooves increases, filopodia are not able to bridge two consecutive ridges, giving the signal for cell alignment in the direction of the microgrooves (bottom). (Center) ridge width—focal adhesion theory. Ridge width influences the orientation and maturation of focal adhesions. Wide ridges do not impose geometrical confinement on the focal adhesion (green). Therefore, the maturation of the focal adhesions can occur in both directions, preventing any cell alignment (top). Narrower ridges impose geometrical confinement to the focal adhesions, which tend to maximize their contact area with the substrate. As a result, focal adhesions align and mature in the direction of the ridges, i.e., the direction of the microgrooves (bottom). (Right) groove height—discontinuity theory. For low microgrooves, the cell sinks into the microgrooves and, consequently, it does not align in direction of the microgrooves (top). For sufficiently high microgrooves, the cell senses the discontinuities of the microgrooves represented by their edges and forms focal adhesions only on the ridges. Consequently, the cell aligns in the direction of the microgrooves (bottom). Figure by Anthal Smits.
Although these theories have shed light on the possible mechanisms behind contact guidance, the role of each individual structural component has not been fully elucidated yet. A straightforward approach to investigate the influence of the actin cytoskeleton in cellular alignment to microgrooves consists by inhibiting the actin cytoskeleton via disrupting agents, such as performed by Walboomers et al.141 and Gerecht et al.46 On one hand, Walboomers et al. observed that fibroblasts can still align along the microgrooves even if the polymerization of actin is inhibited with the use of cytochalasin-B.141 Contrarily, Gerecht et al. found that by adding actin disrupting agents to human embryo stem cells on sub-micrometer sized grooves, the morphology of the cells gets rounder.46 These results illustrate that there is no consensus yet on the role played by the actin cytoskeleton in the cellular response to contact guidance.
To unravel the relevance of each of the cellular components in the contact guidance phenomenon, a systematic approach is, in our view, needed. The various substrate features creating anisotropy need to be dissected (e.g., height, edges, biochemical patterning) and it is necessary to distinguish between substrate anisotropy by biochemical features (e.g., geometrical features given by printing of extracellular matrix proteins), i.e., a purely two-dimensional (2D) environment, and substrate anisotropy by topographical features (e.g., pillars, posts, microgrooves, fibers), here named two-and-a-half-dimensional (2.5D) environment. The first step towards this systematic approach, is neglecting the influence of the height of topographic features (e.g., discontinuity). Thus, as a first step, it is suggested to study contact guidance in 2D environments. Pure biochemical anisotropic features can be produced for instance by microcontact printing. According to this methodology, an elastomeric stamp of polydimethylsiloxane (PDMS) incubated with an extracellular matrix protein (e.g., fibronectin) can be used to create adhesive patterns on flat surfaces, such as glass or PDMS. The bare regions are then backfilled with a non-adhesive protein or polymer, to avoid non-specific cell adhesion. Microcontact printing has proven to be a useful technique to adhere cells to single or multiple islands.20,21 In this way one can geometrically control cell adhesion to regulate cell functions. However, there are only limited studies where this technique has been used to induce cellular alignment via printed lines whose width is in the order of micrometers.3,158,167 In our view, this kind of studies will elucidate the precise mechanisms behind cellular alignment (Fig. 3).
Figure 3.
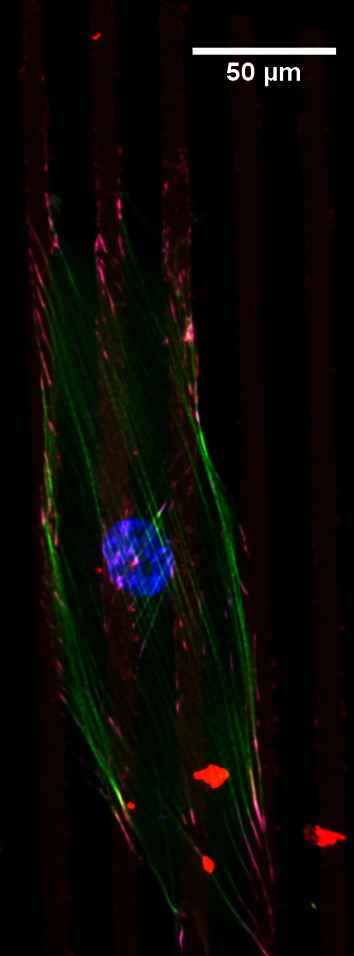
Cellular orientation response to a two-dimensional anisotropic environment. Representative microscopy image of a myofibroblast (Human Vena Saphena Cell) cultured on top of microcontact printed fibronectin (red) lines (10 μm width and 10 μm spacing) on polydimethylsiloxane (PDMS). The focal adhesions are stained in magenta, the actin stress fibers in green, and the nucleus in blue. The cell orients in direction of the lines. The focal adhesions and the actin stress fibers follow cellular orientation.
Cellular Orientation Response to Uniaxial Cyclic Strain
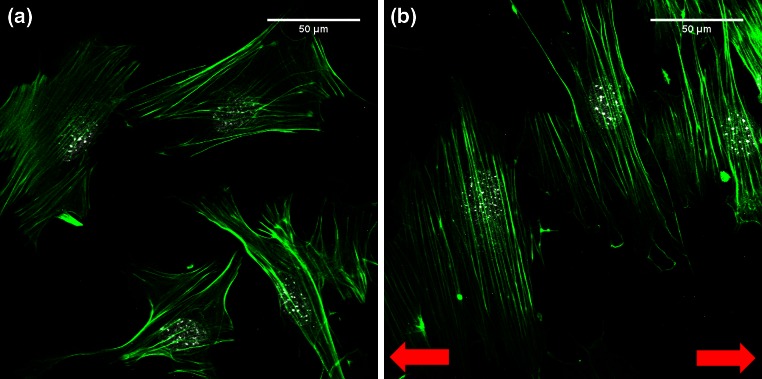
Cellular response to uniaxial cyclic strain is demonstrated by the dynamic reorganization and reorientation of cells and stress fibers. It has become clear that stress fibers play a crucial role in cell ability to remodel and respond appropriately to cyclic strain. Indeed, stress fiber disruption causes inhibition of cellular reorientation.47,59,145 In the 80s, the response of tissue cell to strain was for the first time observed and interpreted as an avoidance response to the strain of the substrate on which the cells were cultured, the so called strain avoidance response (Fig. 1b, right, Fig. 4).15 Since then, further studies have highlighted that, on 2D substrates, cell reorientation occurs at angles (nearly) perpendicular to the stretch direction, i.e., the direction of minimal substrate deformation (Fig. 4). In the last decades, several studies have been carried out in order to quantify and unravel the mechanisms of this phenomenon. Stretch avoidance appears to be a behavior belonging to many kinds of tissue cells, ranging from endothelial cells,6,31,66,73,74,82,101,144,145,157,161 to fibroblasts9,70,100 and smooth muscle cells.32,71,126 However, the dependence of such response on the spatiotemporal parameters of the cyclic stimulation, such as frequency,66,70,88 magnitude,9,32,66,71,73,144,157 strain rate,67,82,100,133 duration,160,161,165 or even the combination of some of those,148 makes any attempt to correlate the effects of these factors with cellular response unsuccessful. Moreover stretch avoidance response seems to be cell type dependent and a minimal strain amplitude,6,32,100 frequency66,67,70 or cell contractile status41 may be required for the response to occur. Summary of studies about cells of the cardiovascular system (fibroblasts, tissue cells, endothelial and progenitor cells) and stress fiber response to uniaxial cyclic stretch are reported in Table 2.
Figure 4.
Cellular orientation response to uniaxial cyclic strain. (a) Mouse embryonic fibroblasts (MEFs) cultured in static conditions for 6 h on a homogenously fibronectin-coated silicone membrane and stained for actin stress fibers (green) and nucleus (white). Cells and actin stress fibers are oriented randomly. (b) MEFs after 24 h of uniaxial cyclic strain (7%, 0.5 Hz). Cells and stress fiber are oriented almost perpendicularly to the strain direction (red arrows). This response is called strain avoidance.
Table 2.
Experimental investigations on cell and stress fiber orientation response upon uniaxial cyclic strain
| Cell type | Method | Parameters | Main results | Source |
|---|---|---|---|---|
| Human Aortic Endothelial Cells |
Custom-built device Silicone membranes ProNectin-F coating148 |
10% at 0.5 Hz for 3 h | Reorientation is inhibited by stress fiber disruption | Wang et al. 145 |
| 5 and 10% at 0.5 and 1 Hz for 3.5 h | Reorientation depends on stretch magnitude | Wang et al. 144 | ||
| 5 and 10% at 0.5 to 2 Hz | Reorientation depends on stretch magnitude Cell reorientation is independent of SFs presence |
Wille et al. 157 | ||
| Custom-built device Silicone membranes Fibronectin coating |
10% at 10%/s for 3 h For time lapse: 5% at a constant rate of 5%/s for 6 h |
Cytoskeletal reorganization within few seconds from onset of stretching SFs break and disassemble FAs loosen and cells become nearly round |
Ngu et al. 101 | |
| Human Umbilical Vein Endothelial Cells |
Custom-built device Silicon membranes Plasma treated |
10% at 1 Hz for 3 h | Threshold for reorientation 1.8% strain magnitude | Barron et al. 6 |
| Custom-built device Silicon membranes Collagen type I coating |
10% at 0.5 Hz for 0 to 20 h | The variance of actin fiber orientation became smaller after 2 h of stretch Actin fiber density increased within 30 min of stretching and decreased after 10 h of stretching |
Yoshigi et al. 161 | |
| Gottinger Minipigs Aorta Endothelial Cells |
Custom-built device Silicon membranes Collagen type I coating32 |
15% at 1 Hz for 3 days | Strain avoidance observed both in SFs and cells | Dartsch and Betz31 |
| Bovine Aortic Endothelial Cells (BAECs) | Custom-built device Silicon membranes Fibronectin coating |
10% at 1 Hz for 6 h | SFs reorientation depends on interplay between Rho pathway activity and the stretch magnitude | Kaunas et al. 73 |
| 10% at 1 Hz for 8 h | Stretch-induced remodeling of the actin cytoskeleton modulates JNK signaling in response to cyclic stretch | Kaunas et al. 74 | ||
| 0 to 20% at 0.01 to 1 Hz for 4 h | Reorientation depends on frequency (optimal at 1 Hz) | Hsu et al. 66 | ||
| 10% at 0.01 to 1 Hz for 4 h | SF reorientation depends on strain rate Threshold 1 Hz for reorientation |
Hsu et al. 67 | ||
| BAECs and U2OS Osteosarcoma cells Stably expressing GFP-actin |
Custom-built device Silicon membranes Fibronectin coating |
10% at 0.1 and 1 Hz for 2 h | Reorientation depends on strain rate FAs and SFs distal ends do not disassemble SFs exhibit heterogeneous behavior within the cell, but also along the length of individual SFs |
Lee et al. 82 |
| Rabbit Aortic Smooth Muscle Cells |
Custom-built device Silicon membranes Collagen type I coating |
2 to 20% at 1 Hz for 3 to 12 h | Threshold 2% for reorientation Reorientation depends on stretch amplitude SF reorient prior to the reorientation of the cell bodies |
Dartsch and Hammerle32 |
| 2 to 10% at 1.2 Hz for 14 days | Reorientation depends on stretch amplitude | Dartsch et al. 33 | ||
| a7r5 Rat Aortic Smooth Muscle Cells |
FlexCell Collagen I-coated plates |
100 to 124% of cell resting length at 1 Hz for 48 h | Cellular reorientation is independent of stretch-activated calcium channels Alignment is reversible after 48 h from stretch cessation Reorientation depends on stretch amplitude |
Standley et al. 126 |
| Rat Aortic Smooth Muscle Cells |
10% at 0.5 to 2.0 Hz for 24 h | SFs are needed for reorientation 1.25 Hz, the most effective frequency for reorientation |
Liu et al. 88 | |
| A10 Rat aortic Smooth Muscle Cells |
Custom-built device Silicone membranes Collagen type I coating |
20% at 1 Hz for 3 h | SFs reorient within 15 min after the onset of stretching Cells reorient within 1–3 h |
Hayakawa et al. 59 |
| 1.2 times cell original length at 1 Hz for 3 h | Cell orientation but not SF reorientation depends on stretch-activated calcium channels Rapid withdrawal of the cell periphery located in the direction of stretching and gradual extension toward the direction oblique to the stretching axis |
Hayakawa et al. 60 | ||
|
A10 Rat Aortic
Smooth Muscle Cells Transfected EGFP-tagged moesin (fragments with actin-binding ability) |
SFs aligned along the stretching direction are torn into pieces soon after stretching, and then reorient obliquely to the direction of stretching | Hayakawa et al. 60 | ||
| Earle’s Fibroblast | Custom-built device Silicon membrane |
Elongation and recoil at 15 s intervals for 18 to 24 h |
Pioneering study: Strain avoidance is observed | Buck15 |
| Human Dermal Fibroblasts | Custom-built device Silicone membranes ProNectin-F coating |
4 to 12% at 1 Hz for 24 h | Reorientation depends on combination of strain rate and amplitude Threshold for reorientation is 4.2% for fibroblasts |
Neidlinger-Wilke et al. 100 |
| 8% at 1 Hz for 24 h | Reorientation starts within 2-3 h from stretch onset and is complete at 24 h | Neidlinger-Wilke et al. 99 | ||
| MRC5 Lung Human Fibroblasts | Instron 5564 testing Instrument Silicone sheets Fibronectin coating |
1 to 25% at 0.5 Hz and 2% at 0.25 to 3 Hz for 3 h | Reorientation depends on strain amplitude Rapid response of cytoskeleton to strain |
Boccafoschi et al. 9 |
| Primary Human Umbilical Cord Fibroblasts |
Custom-built device PDMS membranes of 1, 3, 11 and 50 kPa Fibronectin coating |
4.9 to 32% at 9 to 52 mHz for 16 h | Threshold in amplitude On very soft substrates no reorientation occurs, even for high strain Changes in cell shape follow cytoskeletal reorientation with a significant temporal delay |
Faust et al. 41 |
| REF-52 Rat Embryonic Fibroblasts and Human Dermal Fibroblasts |
Custom-built device Silicon membranes Fibronectin coating |
1 to 15% at 0.0001 to 20 s−1 for 8 h | Reorientation depends on strain frequency (from 1 to 5 h) | Jungbauer et al. 70 |
|
NIH3T3 Fibroblasts
Transfected with GFP-Vinculin |
Custom-built device
Silicon membranes Fibronectin coating |
8% at 1 Hz for 3 h |
FAs reorient by sliding, without turnover and reassembly
Reorientation is independent of microtubules but dependent of actin stress fiber presence |
Goldyn et al. 48 |
|
Monkey Kidney Fibroblasts
Transfected with GFP-Actin |
Custom-built device
PDMS membranes Fibronectin coating |
16 and 28% for 3 h ca. |
Reorientation occurs by dynamic rotation of intact actin stress fibers in fibroblasts
Subcellular reorganization begins within minutes from strain application SFs at cell center region rotate, while SFs at cell periphery remain stable |
Ahmed et al. 1 |
|
NIH3T3 Fibroblasts
Transfected with EGFP-actin |
Custom-built device
Silicon membranes Fibronectin coating |
6 to 32% in 2 s and kept stretched for 10 m, relaxation membrane
within 2 s |
SFs disassembly during stretching
New filament bundling occurs after stretch |
Wang et al. 150 |
|
REF-52 Rat Embryonic Fibroblasts
Transfected with Life Act |
Custom-built device
Silicon membranes Fibronectin coating 70 |
8% at 4 Hz for 90 m |
Cell reorient by realigning pre-existing SFs
GFP-actin fusion proteins influence the mechanical behavior of cells |
Deibler et al. 36 |
|
NIH3T3 Fibroblasts
Transfected with GFP-LifeAct and mCherry-Vinculin |
8% at 0.1 to 3 Hz |
Increasing frequency induces less spreading
Above 1 Hz level of perpendicular cell reorientation is not further increased Disruption of contractility affects cells reorientation SFs rather form de novo in the perpendicular direction where low mechanical forces are acting on the cell |
Greiner et al. 51 | |
|
REF-52 Fibroblasts
Stably expressing YFP-paxillin |
4 to 24% at 1.2 Hz wide range of stretch configurations | Cell and SF orientation deviate from the zero strain and zero stress prediction | Livne et al. 89 | |
| U2OS Osteosarcoma cells Stably expressing GFP-Actin |
STREX Silicon chambers Fibronectin coating |
Different waves for 10.5 h | Reorientation depends on strain rate | Tondon et al. 133 |
| Rat Bone Marrow Mesenchymal Stem Cells (BMSCs) | Custom-built device Silicon membranes Gelatine coating |
10% at 1 Hz for 0 to 36 h | Cell reorientation depends on strain duration Cell reorganization depends on the duration of the stretching |
Zhang et al. 165 |
Live imaging and study of SFs reorientation dynamics are emphasized in italics
SF stress fiber, FA focal adhesion, PDMS polydimethylsiloxane, JNK c-Jun N-terminal kinase
Most of these studies are carried out with custom-built devices for which an accurate and rigorous strain characterization is needed, but often overlooked. These devices are made of motorized stages capable to stretch silicone membranes coated with extracellular matrix proteins such as fibronectin or collagen. Given the mechanical properties of the elastomeric materials, once the membrane is stretched along one direction, it contracts in the perpendicular direction (Poisson’s effect). New commercially available devices have been designed to avoid this drawback (FlexCell5,88,126 and STREX133). Nevertheless, the use of such diverse instrumentations cannot help to distinguish between the impacts of the different factors. Moreover, the interference of signaling mechanisms cannot be excluded when different coatings are employed. Altogether, controlled experimental conditions are needed towards a comprehensive understanding of cell reorientation.
Efforts to unravel the spatiotemporal dynamics of cellular adaptations especially at the level of stress fibers are still limited. Most of the observations come from a state-to-state like manner, making use of fixed cells that do not allow observations of subcellular dynamics. The technological challenges that must be overcome include the use of actin stress fiber probes that do not interfere with the dynamics of actin polymerization and the mechanical properties.36 Moreover, the timescale of actin reorganization pushes further the experimental limits.
From time-lapse studies, it has been established that cells become nearly round in the first phases of reorientation and, subsequently elongate along the strain avoidance direction.59,70,101 During this second phase, a process of reinforcement and repair of the stress fiber strain sites occurs. Zyxin is recruited at strain-induced damage sites of stress fibers and subsequently activates actin cytoskeleton repair and reinforcement.55,84,123,124,162 In terms of temporal dynamics, stress fibers significantly anticipate cell overall reorientation. Stress fiber reorganization response occurs within the first minutes from the onset of the cyclic strain stimulation, while complete cell reorientation is seen in the time range of hours.32,59 In 2001, Hayakawa et al. observed in rat smooth muscle cells the breakdown of stress fibers aligned along the stretching direction soon after the start of the mechanical stimulation, followed by stress fiber reorientation at an oblique angle with respect to the axis of stretching.60 Similar observations were reported by Ngu et al. in bovine endothelial aortic cells.101 Also, the investigation of Lee et al. pointed out that bovine aortic endothelial cell reorientation involved the disassembly of the stress fiber proximal section (far from the focal adhesions) and de-novo formation of stress fiber at a reoriented angle with comparatively little focal adhesion turnover.82 These studies suggest that reorientation of stress fiber takes place through stress fiber turnover and re-assembly. However, there is also another line of evidence which suggests that stress fiber turnover might occur via focal adhesion sliding and consequent stress fiber rotation. Deibler et al.36 demonstrated that rat embryonic fibroblasts reorient by realigning pre-existing stress fiber while Goldyn et al.48 tracked the dramatical sliding of focal adhesions induced by uniaxial cyclic strain in NIH3T3 fibroblasts. Most probably, the aforementioned mechanisms are not mutually exclusive. Still, the challenge for the future remains to uncover the precise mechanisms of stress fiber and cell reorientation, by focusing on the heterogeneity observed not only on subcellular locations but also along the same stress fibers.1,82
A number of theoretical models have been elaborated in the endeavor to describe the relationship between the actin cytoskeleton reorganization and the uniaxial cyclic strain acting on cells. In 2000, Wang et al. proposed that stress fibers tend to orient in the direction of minimal normal strain, where the unperturbed state is maintained.143 Other models, mostly based on the molecular aspects of stress fiber assembly,72,106,152 were developed based on the same approach. Instead, the work of De et al. predicts stress fiber orientation in the minimal matrix stress direction using a coarse-grained model of cells approximated as single force dipoles.34 While consistency between the predictions of these models and experimental results was proven in many studies, recently, Livne et al. have found significant deviation between their results and the theoretical predictions proposed by the existing models.89 By investigating strain avoidance response over a wide range of stretch configurations, they demonstrated that stress fiber reorganization does not coincide with the direction of minimal strain or stress of the substrate. Therefore, they developed a new theoretical approach based on the molecular and physical properties of the stress fiber-focal adhesion system. Yet, it remains to be tested whether this model is cell-type independent.
Cellular Orientation Response to Combined Substrate Anisotropy and Uniaxial Cyclic Strain
From the previous paragraphs it appears that cell and stress fiber orientation can be influenced by anisotropic cues or by imposing uniaxial cyclic strain on cell growth substrates. This legitimates to ask what the influence of anisotropic cues and cyclic strain is when these cues are applied in combination and along the same direction. This simultaneous stimulation, theoretically, would lead to competing stimuli for cell reorientation. An overview of the studies conducted applying anisotropic cues and uniaxial cyclic strain are reported in Table 3. We have considered all cell types, as the number of these studies is limited.
Table 3.
Experimental studies about cell and stress fiber orientation response to combined topography and uniaxial cyclic strain
| Cell type | Anisotropic cues | Stretching method and parameters | Main results | Source |
|---|---|---|---|---|
| MC3T3-E1 osteoblasts | Parallel microgrooves: 1.6 μm depth and varying groove/ridge widths from 1 to 6 μm ProNectin-F coating Parallel to stretching direction |
Custom-built device148
Silicon membranes 4% at 1 Hz for 20 days |
Cells align with grooves independently of topographic features SFs highly aligned and elongated after stretch. |
Wang et al. 147 |
| Human Skin Fibroblasts | Parallel microgrooves: 1.6 μm depth, with widths from 1 to 6 μm (2 to 6 μm wide ridge) ProNectin-F coating Parallel to stretching direction |
Custom-built device148
Silicon membranes 4 to 12% at 1 Hz for 24 days |
Until 8% stretch, cells maintain the alignment imposed by the microgrooves, regardless of their dimensions For high strain (12%) and small microgrooves (1 μm width and 2 ridge width) cells change orientations Dimension of the microgrooves and the strain magnitude are two important factors in determining cell alignment |
Wang et al. 146 |
| Human Patellar Tendon Fibroblasts | Parallel microgrooves: 3 μm depth and 10 μm width ProNectin-F coating Parallel to stretching direction |
Custom-built device148
Silicon membranes 8% at 0.5 Hz for 72 h |
Cells do not change alignment, regardless of the alignment to stretching direction | Wang et al. 151 |
| Mesenchymal Stem Cells | Parallel microgrooves: 10 μm width and 3 μm height Parallel and perpendicular to strain direction |
Custom-built device Silicone membranes Rat tail collagen I coating 5% at 1 Hz for 2–4 days |
Cells and SFs remain well aligned with the microgrooves in both parallel and perpendicular microgrooves Distinct effects on gene expression in parallel and perpendicular microgrooves |
Kurpinski et al. 79 |
| Bovine Vascular Smooth Muscle Cells | Parallel microgrooves of varying widths Human plasma fibronectin coating Parallel or perpendicular to the direction of strain |
Custom-built device Elastomeric membranes 10% at 1 Hz for 24 h |
Strain parallel to microgrooves limit cell orientation response, small (15 μm) and large (70 μm) grooves are more favorable for reorientation than average size (40 μm) Strain perpendicular to microgrooves: enhanced cellular alignment |
Houtchens et al. 65 |
| C2C12 Skeletal Myoblasts | Parallel microcontact printed fibronectin lines: 30 μm width, 40 um spacing on hydrogels Arranged parallel, horizontal and 45° to strain direction |
Custom-built device70
Elastomeric membranes 7% at 0.5 Hz for 4 days |
SFs reorient while cell are geometrically constrained to the lines Parallel lines: SFs reorient at 48°, with a large scatter Perpendicular lines: SFs reorient at 91° Lines at 45°: SFs reorient at 52° |
Ahmed et al. 2 |
| Rat Bone Marrow Mesenchymal Stem Cells | Parallel nano- and micro-grooves: 300 nm width and 60 nm depth (600 nm pitch) and 1 μm wide, and 500 nm deep (pitch 2 μm) RFGD treatment Parallel to strain direction |
Custom-built device100
Elastomeric membranes 1 to 8% at 1 Hz with intermittent stretch duration (15 min stretch/15 min rest for 16 h, followed by 8 h of rest) |
Nanodimensions induce less alignment than microdimensions in static conditions Cells have strain avoidance response on nanotextured surface but not on micrometer-sized textures |
Prodanov et al. 112 |
| Human Vena Saphena Cells (Myofibroblasts) |
Elastomeric microposts Elliptical cross-section (major semi-axis minor semi-axis) 1, 3 and 6 μm height Human Plasma Fibronectin microcontact printed coating on top of microposts |
FlexCell 7% at 0.5 Hz for 19 h |
Competition between contact guidance and strain avoidance results from distinct behavior of actin cap and basal actin layer | Tamiello et al. 128 |
SF stress fiber, RFGD radio frequency glow discharge
By using microgrooves integrated in a custom-built stretching device, Wang and Grood demonstrated that micro-topography generally overrules strain avoidance, i.e., adherent cells maintain the original orientation imposed by the microgrooves, even if strain stimulation occurs along the same direction.146,147,151 Prodanov et al. have added to this evidence that cellular response might be influenced by the dimension of the anisotropic textures.112 They showed that osteoblasts plated on nanogrooves and subjected to cyclic strain responded by strain avoidance, while on micro-sized features, they remained aligned with the anisotropy of the substrate. Next to this, it has been observed by Ahmed et al. that topographical cues combined with cyclic strain can have distinct impact on stress fibers as compared to cell body reorientation response.2 In their study, myoblasts were confined on substrates patterned with parallel fibronectin lines (widths comparable to cell size) and exposed to uniaxial cyclic stretch. It appeared that, while cell bodies remained confined on the micropatterned lines, stress fiber succeeded in reorganizing perpendicular to the strain direction. This points to different mechanisms involved in strain and anisotropy sensing.
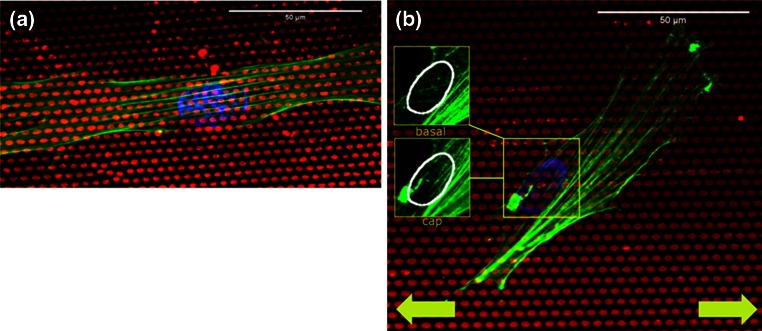
Recently, a study from our group has provided further insight on the mechanisms underlying stress fiber response to combined cyclic strain and anisotropic cues. It was observed that distinct responses occur at the actin cap and basal layer (Fig. 5). The actin cap stress fibers clearly tend to neglect the topographical cues and respond to strain, while the basal actin fibers remain aligned with the topography of the substrate.128 These findings provided evidence that cellular response to anisotropy of the substrate and cyclic strain is the complex integration of subcellular structural responses. Nevertheless, most of the mentioned studies reported on cell and stress fiber orientation but neglected the response of crucial structures such as focal adhesions.
Figure 5.
Cellular orientation response to combined substrate anisotropy and uniaxial cyclic strain. (a) Myofibroblasts (Human Vena Saphena Cells) cultured on top of fibronectin-coated elliptical microposts (red) in static conditions for 6 h. The stress fibers, colored in green, orient along the substrate anisotropy, i.e., along the micropost major axis. The nucleus is shown in blue. (b) The system made of elliptical microposts can be stretched along the micropost major axis (horizontal direction, yellow arrows). The use of this model system revealed that, the orientation response of myofibroblasts exposed to substrate anisotropy and strain (19 h, 7%, 0.5 Hz) along the same direction is determined by the distinct response of the actin stress fibers running on top of the nucleus (inset cap) and the ones present underneath the nucleus and connected to the microposts (inset basal). While the cap actin fibers respond by strain avoidance, the basal stress fibers tend to follow the direction on the micropost major axis.118
Altogether, the examples reported above show that, although general indication exists that anisotropic cues modulate cell and stress fiber orientation response to uniaxial cyclic strain, a deeper understanding of the phenomenon is still needed. Detailed quantification of stress fiber reorientation dynamics at the subcellular level upon presentation of simultaneous anisotropic and cyclic strain cues would be of great benefit for unraveling the temporal dynamics of the processes involved in cellular adaptation.
Summary and Outlook
A deep understanding of the mechanisms by which biophysical cues regulate cellular orientation is fundamental for cardiovascular regeneration strategies, such as biomaterial-based in situ engineering approaches that need to guide and control cell and tissue organization for proper tissue functioning. In particular, cell alignment is a primary aim of regeneration of cardiovascular tissues, as controlled cellular organization is essential for matching native tissue micro architecture and functionality.110
In this work, we provide an overview of the knowledge obtained in the last decades about the components of the structural mechanotransduction pathway, an interconnected chain of proteins implicated in force propagation from the extracellular environment to downstream targets such as the nucleus and gene expression regulation. We also report on the current understanding of cellular orientation responses induced by the application of anisotropic cues and uniaxial cyclic strain focusing on the experimental evidence obtained with in vitro studies using single cell observations. The development of in vivo-like micro-devices has enabled researchers to perform experimental studies under controlled conditions, in the effort to uncover the link between applied biophysical cues and cellular response. Still, the large body of knowledge generated by using such diverse approaches and the cell-type dependence of the results complicate the attempt of unifying the knowledge.
All in all, the above examples demonstrate that an intact structural mechanotransduction pathway plays a crucial role in the control of normal cellular functionality. Nevertheless, in order to move forward towards understanding the complex interplay between cellular mechanoresponses and biophysical properties of the micro-environment, it is important to identify the main scientific challenges.
Firstly, further research is required to achieve an in-depth understanding of the role of the structural mechanotransduction pathway. It is necessary to determine the completeness of our current understanding of such a pathway. More importantly, it is crucial to identify the relevance of individual and combined components of this pathway for controlling cell orientation. An interesting strategy within consists in considering cells with defected structural connections or knock-out cellular models as tools for novel investigations. A first attempt has been conducted by Tamiello et al. (unpublished) by using actin cap-lacking fibroblasts. In this study the relevance of the actin cap in the response to anisotropic cues and strain stimulation was studied by exposing the cells to both cues, applied separately and in combination. Interestingly, since these knock-out cells have been obtained by elimination A-type lamins, they represent also a useful model for studying the development of the family of mechanotransduction diseases named laminopathies. In general, mechanotransduction studies on cells from diseased patients will not only advance our understanding of the relevance of the structural connection in the cellular mechanoresponse, but also elucidate whether diseases/disorders of mechanotransduction primarily result from structural defects, impaired biochemical signaling or a synergy between the two mechanotransduction processes. Progress in this field will eventually lead to the design of effective regenerative strategies for a variety of diseases arising for mechanotransduction defects.69
Secondly, future studies should focus on designing a unified systematic approach for studying cellular responses to individual biophysical cues. This is needed in order to get quantifiable measurements of the effects of the various parameters of the micro-environment on cell and stress fiber orientation, for instance. Such a simplified approach will enable the integration of the overwhelming amount of information obtained using an array of diverse devices and, consequently, enhance our knowledge about the influence of biophysical cues on cellular alignment. Once cellular responses to individual cues are established, the next step we envision is to develop integrative approaches to study cellular response to combined cues, in a more tissue-like context. This is necessary in order to unravel whether a signaling hierarchy coming from distinct cues exists. Another suggestion is to develop high-throughput systems based on the simplified systematic approach in order to screen the effects of individual and combined biophysical cues on relevant cell outputs (orientation, force, proliferation etc.). Such a systematic approach can give reliable inputs for computational models in cell mechanics to interpret experimental observations and elucidate main governing processes of cellular mechanoresponse.
Finally, more investigations are necessary to obtain an in-depth understanding of the mechanisms underlying cell mechanoresponse. To address issues on how cells integrate and transduce physical signals, it is crucial to develop live imaging techniques to analyze the structural responses at subcellular level with higher spatial and temporal resolutions. The next big technological challenge will in the application of these tools to more complex environments, such as three-dimensional (3D) substrates. These substrates are of special interest because they mimic more closely the physiological environment of tissue cells. Recent evidences suggest that cellular behaviors in 3D environments differ from observations obtained employing two-dimensional (2D) environments.
As the intricate aspects about the cellular mechanoresponse become to be better characterized, it may become possible to open new avenues for controlling the way in which cell interact and respond with their physical micro-environment. We envision this is the way forward to effectively elaborate targeted strategies for tissue regeneration and therapeutical approaches.
Conflict of interest
Chiara Tamiello, Antonetta Buskermolen, Frank Baaijens, Jos Broers and Carlijn Bouten declare that they have no conflict of interest.
Human and Animal Rights
No human and animal studies were carried out by the authors of this article.
Footnotes
Chiara Tamiello and Antonetta B. C. Buskermolen have contributed equally to this work.
References
- 1.Ahmed WW, Kural MH, Saif TA. A novel platform for in situ investigation of cells and tissues under mechanical strain. Acta Biomater. 2010;6:2979–2990. doi: 10.1016/j.actbio.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed WW, Wolfram T, Goldyn AM, Bruellhoff K, Rioja BA, Moller M, Spatz JP, Saif TA, Groll J, Kemkemer R. Myoblast morphology and organization on biochemically micro-patterned hydrogel coatings under cyclic mechanical strain. Biomaterials. 2010;31:250–258. doi: 10.1016/j.biomaterials.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 3.Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr. Biol. (Camb.) 2011;3:1063–1070. doi: 10.1039/c1ib00061f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J. Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- 6.Barron V, Brougham C, Coghlan K, McLucas E, O’Mahoney D, Stenson-Cox C, McHugh PE. The effect of physiological cyclic stretch on the cell morphology, cell orientation and protein expression of endothelial cells. J. Mater. Sci. Mater. Med. 2007;18:1973–1981. doi: 10.1007/s10856-007-3125-3. [DOI] [PubMed] [Google Scholar]
- 7.Bettinger CJ, Langer R, Borenstein JT. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed Engl. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D. Identification of a novel x-linked gene responsible for emery-dreifuss muscular-dystrophy. Nat. Genet. 1994;8:323–327. doi: 10.1038/ng1294-323. [DOI] [PubMed] [Google Scholar]
- 9.Boccafoschi F, Bosetti M, Gatti S, Cannas M. Dynamic fibroblast cultures: response to mechanical stretching. Cell Adh. Migr. 2007;1:124–128. doi: 10.4161/cam.1.3.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borejdo J, Burlacu S. Measuring orientation of actin-filaments within a cell—orientation of actin in intestinal microvilli. Biophysical Journal. 1993;65:300–309. doi: 10.1016/S0006-3495(93)81060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Britland S, Morgan H, Wojiak-Stodart B, Riehle M, Curtis A, Wilkinson C. Synergistic and hierarchical adhesive and topographic guidance of BHK cells. Exp. Cell Res. 1996;228:313–325. doi: 10.1006/excr.1996.0331. [DOI] [PubMed] [Google Scholar]
- 12.Broers JL, Peeters EA, Kuijpers HJ, Endert J, Bouten CV, Oomens CW, Baaijens FP, Ramaekers FC. Decreased mechanical stiffness in LMNA-/- cells is caused by defective nucleo-cytoskeletal integrity: implications for the development of laminopathies. Hum. Mol. Genet. 2004;13:2567–2580. doi: 10.1093/hmg/ddh295. [DOI] [PubMed] [Google Scholar]
- 13.Broers JL, Ramaekers FC, Bonne G, Yaou RB, Hutchison CJ. Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 2006;86:967–1008. doi: 10.1152/physrev.00047.2005. [DOI] [PubMed] [Google Scholar]
- 14.Brosig M, Ferralli J, Gelman L, Chiquet M, Chiquet-Ehrismann R. Interfering with the connection between the nucleus and the cytoskeleton affects nuclear rotation, mechanotransduction and myogenesis. Int. J. Biochem. Cell Biol. 2010;42:1717–1728. doi: 10.1016/j.biocel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Buck RC. Reorientation response of cells to repeated stretch and recoil of the substratum. Exp. Cell Res. 1980;127:470–474. doi: 10.1016/0014-4827(80)90456-5. [DOI] [PubMed] [Google Scholar]
- 16.Burke B, Stewart CL. The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu. Rev. Genom. Hum. Genet. 2006;7:369–405. doi: 10.1146/annurev.genom.7.080505.115732. [DOI] [PubMed] [Google Scholar]
- 17.Burridge K, Wittchen ES. The tension mounts: stress fibers as force-generating mechanotransducers. J. Cell Biol. 2013;200:9–19. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambliss AB, Khatau SB, Erdenberger N, Robinson DK, Hodzic D, Longmore GD, Wirtz D. The LINC-anchored actin cap connects the extracellular milieu to the nucleus for ultrafast mechanotransduction. Sci. Rep. 2013;3:1087. doi: 10.1038/srep01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chancellor TJ, Lee J, Thodeti CK, Lele T. Actomyosin tension exerted on the nucleus through nesprin-1 connections influences endothelial cell adhesion, migration, and cyclic strain-induced reorientation. Biophys. J. 2010;99:115–123. doi: 10.1016/j.bpj.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem. Biophys. Res. Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 21.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 22.Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat. Cell Biol. 2008;10:1039–1050. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 24.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108:635–644. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- 25.Cook JR, Carta L, Benard L, Chemaly ER, Chiu E, Rao SK, Hampton TG, Yurchenco P, Costa KD, Hajjar RJ, Ramirez F. Abnormal muscle mechanosignaling triggers cardiomyopathy in mice with Marfan syndrome. J. Clin. Invest. 2014;124:1329–1339. doi: 10.1172/JCI71059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis AS, Clark P. The effects of topographic and mechanical properties of materials on cell behavior. CRC Rev. Biocompatibility. 1990;5:343–362. [Google Scholar]
- 28.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18:1573–1583. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 29.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalby MJ. Topographically induced direct cell mechanotransduction. Med. Eng Phys. 2005;27:730–742. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Dartsch PC, Betz E. Response of cultured endothelial cells to mechanical stimulation. Basic Res. Cardiol. 1989;84:268–281. doi: 10.1007/BF01907974. [DOI] [PubMed] [Google Scholar]
- 32.Dartsch PC, Hammerle H. Orientation response of arterial smooth muscle cells to mechanical stimulation. Eur. J. Cell Biol. 1986;41:339–346. [PubMed] [Google Scholar]
- 33.Dartsch PC, Hammerle H, Betz E. Orientation of cultured arterial smooth muscle cells growing on cyclically stretched substrates. Acta Anat. (Basel) 1986;125:108–113. doi: 10.1159/000146146. [DOI] [PubMed] [Google Scholar]
- 34.De R, Safran SA. Dynamical theory of active cellular response to external stress. Phys. Rev. E. Stat. Nonlinear Soft. Matter Phys. 2008;78:031923. doi: 10.1103/PhysRevE.78.031923. [DOI] [PubMed] [Google Scholar]
- 35.Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD. Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008;22:832–853. doi: 10.1101/gad.1652708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deibler M, Spatz JP, Kemkemer R. Actin fusion proteins alter the dynamics of mechanically induced cytoskeleton rearrangement. PLoS One. 2011;6:e22941. doi: 10.1371/journal.pone.0022941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.den Braber ET, de Ruijter JE, Ginsel LA, von Recum AF, Jansen JA. Quantitative analysis of fibroblast morphology on microgrooved surfaces with various groove and ridge dimensions. Biomaterials. 1996;17:2037–2044. doi: 10.1016/0142-9612(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 38.Dreier B, Gasiorowski JZ, Morgan JT, Nealey PF, Russell P, Murphy CJ. Early responses of vascular endothelial cells to topographic cues. Am. J. Physiol Cell Physiol. 2013;305:C290–C298. doi: 10.1152/ajpcell.00264.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dunn GA, Brown AF. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J. Cell Sci. 1986;83:313–340. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- 40.Dunn GA, Heath JP. A new hypothesis of contact guidance in tissue cells. Exp. Cell Res. 1976;101:1–14. doi: 10.1016/0014-4827(76)90405-5. [DOI] [PubMed] [Google Scholar]
- 41.Faust U, Hampe N, Rubner W, Kirchgessner N, Safran S, Hoffmann B, Merkel R. Cyclic stress at mHz frequencies aligns fibroblasts in direction of zero strain. PLoS One. 2011;6:e28963. doi: 10.1371/journal.pone.0028963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feinberg AW, Alford PW, Jin H, Ripplinger CM, Werdich AA, Sheehy SP, Grosberg A, Parker KK. Controlling the contractile strength of engineered cardiac muscle by hierarchal tissue architecture. Biomaterials. 2012;33:5732–5741. doi: 10.1016/j.biomaterials.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman BR, Bade ND, Riggin CN, Zhang S, Haines PG, Ong KL, Janmey PA. The (dys)functional extracellular matrix. Biochim. Biophys. Acta. 2015;1853:3153–3164. doi: 10.1016/j.bbamcr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- 45.Geiger B, Yamada KM. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 2011;3:a005033. doi: 10.1101/cshperspect.a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldyn AM, Kaiser P, Spatz JP, Ballestrem C, Kemkemer R. The kinetics of force-induced cell reorganization depend on microtubules and actin. Cytoskeleton (Hoboken.) 2010;67:241–250. doi: 10.1002/cm.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldyn AM, Rioja BA, Spatz JP, Ballestrem C, Kemkemer R. Force-induced cell polarisation is linked to RhoA-driven microtubule-independent focal-adhesion sliding. J. Cell Sci. 2009;122:3644–3651. doi: 10.1242/jcs.054866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez-Suarez I, Redwood AB, Gonzalo S. Loss of A-type lamins and genomic instability. Cell Cycle. 2009;8:3860–3865. doi: 10.4161/cc.8.23.10092. [DOI] [PubMed] [Google Scholar]
- 50.Gregor M, Osmanagic-Myers S, Burgstaller G, Wolfram M, Fischer I, Walko G, Resch GP, Jorgl A, Herrmann H, Wiche G. Mechanosensing through focal adhesion-anchored intermediate filaments. FASEB J. 2014;28:715–729. doi: 10.1096/fj.13-231829. [DOI] [PubMed] [Google Scholar]
- 51.Greiner AM, Chen H, Spatz JP, Kemkemer R. Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells. PLoS One. 2013;8:e77328. doi: 10.1371/journal.pone.0077328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grenier G, Remy-Zolghadri M, Larouche D, Gauvin R, Baker K, Bergeron F, Dupuis D, Langelier E, Rancourt D, Auger FA, Germain L. Tissue reorganization in response to mechanical load increases functionality. Tissue Eng. 2005;11:90–100. doi: 10.1089/ten.2005.11.90. [DOI] [PubMed] [Google Scholar]
- 53.Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 2005;6:21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- 54.Gundersen GG, Worman HJ. Nuclear positioning. Cell. 2013;152:1376–1389. doi: 10.1016/j.cell.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo WH, Wang YL. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol. Biol. Cell. 2007;18:4519–4527. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison RG. The cultivation of tissues in extraneous media as a method of morphogenetic study. Anat. Rec. 1912;6:181–193. [Google Scholar]
- 58.Harvey PA, Leinwand LA. The cell biology of disease: cellular mechanisms of cardiomyopathy. J. Cell Biol. 2011;194:355–365. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayakawa K, Hosokawa A, Yabusaki K, Obinata T. Orientation of smooth muscle-derived A10 cells in culture by cyclic stretching: relationship between stress fiber rearrangement and cell reorientation. Zool. Sci. 2000;17:617–624. doi: 10.2108/zsj.17.617. [DOI] [PubMed] [Google Scholar]
- 60.Hayakawa K, Sato N, Obinata T. Dynamic reorientation of cultured cells and stress fibers under mechanical stress from periodic stretching. Exp. Cell Res. 2001;268:104–114. doi: 10.1006/excr.2001.5270. [DOI] [PubMed] [Google Scholar]
- 61.Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 62.Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ho CY, Lammerding J. Lamins at a glance. J. Cell Sci. 2012;125:2087–2093. doi: 10.1242/jcs.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hotulainen P, Llano O, Smirnov S, Tanhuanpaa K, Faix J, Rivera C, Lappalainen P. Defining mechanisms of actin polymerization and depolymerization during dendritic spine morphogenesis. J. Cell Biol. 2009;185:323–339. doi: 10.1083/jcb.200809046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Houtchens GR, Foster MD, Desai TA, Morgan EF, Wong JY. Combined effects of microtopography and cyclic strain on vascular smooth muscle cell orientation. J. Biomech. 2008;41:762–769. doi: 10.1016/j.jbiomech.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hsu HJ, Lee CF, Kaunas R. A dynamic stochastic model of frequency-dependent stress fiber alignment induced by cyclic stretch. PLoS One. 2009;4:e4853. doi: 10.1371/journal.pone.0004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu HJ, Lee CF, Locke A, Vanderzyl SQ, Kaunas R. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One. 2010;5:e12470. doi: 10.1371/journal.pone.0012470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Humphries MJ. Integrin structure. Biochem. Soc. Trans. 2000;28:311–339. [PubMed] [Google Scholar]
- 69.Ingber DE. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003;35:564–577. doi: 10.1080/07853890310016333. [DOI] [PubMed] [Google Scholar]
- 70.Jungbauer S, Gao H, Spatz JP, Kemkemer R. Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys. J. 2008;95:3470–3478. doi: 10.1529/biophysj.107.128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kakisis JD, Avgerinos ED, Liapis CD. Analysis of the evidence behind the ESVS guidelines for the invasive treatment of carotid stenosis. Acta Chir. Belg. 2009;109:574–580. doi: 10.1080/00015458.2009.11680491. [DOI] [PubMed] [Google Scholar]
- 72.Kaunas R, Hsu HJ. A kinematic model of stretch-induced stress fiber turnover and reorientation. J. Theor. Biol. 2009;257:320–330. doi: 10.1016/j.jtbi.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 73.Kaunas R, Nguyen P, Usami S, Chien S. Cooperative effects of Rho and mechanical stretch on stress fiber organization. Proc. Natl. Acad. Sci. USA. 2005;102:15895–15900. doi: 10.1073/pnas.0506041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal. 2006;18:1924–1931. doi: 10.1016/j.cellsig.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 75.Khatau SB, Hale CM, Stewart-Hutchinson PJ, Patel MS, Stewart CL, Searson PC, Hodzic D, Wirtz D. A perinuclear actin cap regulates nuclear shape. Proc. Natl. Acad. Sci. USA. 2009;106:19017–19022. doi: 10.1073/pnas.0908686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khatau SB, Kim DH, Hale CM, Bloom RJ, Wirtz D. The perinuclear actin cap in health and disease. Nucleus. 2010;1:337–342. doi: 10.4161/nucl.1.4.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim DH, Cho S, Wirtz D. Tight coupling between nucleus and cell migration through the perinuclear actin cap. J. Cell Sci. 2014;127:2528–2541. doi: 10.1242/jcs.144345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim DH, Wirtz D. Cytoskeletal tension induces the polarized architecture of the nucleus. Biomaterials. 2015;48:161–172. doi: 10.1016/j.biomaterials.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurpinski K, Chu J, Hashi C, Li S. Anisotropic mechanosensing by mesenchymal stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:16095–16100. doi: 10.1073/pnas.0604182103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, Lee RT. Lamins A and C but not lamin B1 regulate nuclear mechanics. J. Biol. Chem. 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 81.Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, Stewart CL, Lee RT. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Invest. 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee CF, Haase C, Deguchi S, Kaunas R. Cyclic stretch-induced stress fiber dynamics—dependence on strain rate, Rho-kinase and MLCK. Biochem. Biophys. Res. Commun. 2010;401:344–349. doi: 10.1016/j.bbrc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 83.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys. J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J. Cell Physiol. 2006;207:187–194. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- 85.Li P, Meinke P, Huong LTT, Wehnert M, Noegel AA. Contribution of SUN1 mutations to the pathomechanism in muscular dystrophies. Hum. Mutat. 2014;35:452–461. doi: 10.1002/humu.22504. [DOI] [PubMed] [Google Scholar]
- 86.Li R, Wu Y, Manso AM, Gu Y, Liao P, Israeli S, Yajima T, Nguyen U, Huang MS, Dalton ND, Peterson KL, Ross RS. beta1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses. Am. J. Pathol. 2012;180:952–962. doi: 10.1016/j.ajpath.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liang WC, Mitsuhashi H, Keduka E, Nonaka I, Noguchi S, Nishino I, Hayashi YK. TMEM43 mutations in emery-dreifuss muscular dystrophy-related myopathy. Ann. Neurol. 2011;69:1005–1013. doi: 10.1002/ana.22338. [DOI] [PubMed] [Google Scholar]
- 88.Liu B, Qu MJ, Qin KR, Li H, Li ZK, Shen BR, Jiang ZL. Role of cyclic strain frequency in regulating the alignment of vascular smooth muscle cells in vitro. Biophys. J. 2008;94:1497–1507. doi: 10.1529/biophysj.106.098574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Livne A, Bouchbinder E, Geiger B. Cell reorientation under cyclic stretching. Nat. Commun. 2014;5:3938. doi: 10.1038/ncomms4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lo SH. Focal adhesions: what’s new inside. Dev. Biol. 2006;294:280–291. doi: 10.1016/j.ydbio.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 91.Lombardi ML, Jaalouk DE, Shanahan CM, Burke B, Roux KJ, Lammerding J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011;286:26743–26753. doi: 10.1074/jbc.M111.233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc. Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 93.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc. Natl. Acad. Sci. USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, Worman HJ, Gundersen GG, Lattanzi G, Wehnert M, Shackleton S. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014;10:e1004605. doi: 10.1371/journal.pgen.1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys. J. 1996;71:3030–3045. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muntoni F, Bonne G, Goldfarb LG, Mercuri E, Piercy RJ, Burke M, Ben Yaou R, Richard P, Recan D, Shatunov A, Sewry CA, Brown SC. Disease severity in dominant Emery Dreifuss is increased by mutations in both emerin and desmin proteins. Brain. 2006;129:1260–1268. doi: 10.1093/brain/awl062. [DOI] [PubMed] [Google Scholar]
- 97.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagayama K, Yamazaki S, Yahiro Y, Matsumoto T. Estimation of the mechanical connection between apical stress fibers and the nucleus in vascular smooth muscle cells cultured on a substrate. J. Biomech. 2014;47:1422–1429. doi: 10.1016/j.jbiomech.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 99.Neidlinger-Wilke C, Grood E, Claes L, Brand R. Fibroblast orientation to stretch begins within three hours. J. Orthop. Res. 2002;20:953–956. doi: 10.1016/S0736-0266(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 100.Neidlinger-Wilke C, Grood ES, Wang JHC, Brand RA, Claes L. Cell alignment is induced by cyclic changes in cell length: studies of cells grown in cyclically stretched substrates. J. Orthop. Res. 2001;19:286–293. doi: 10.1016/S0736-0266(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 101.Ngu H, Feng Y, Lu L, Oswald SJ, Longmore GD, Yin FC. Effect of focal adhesion proteins on endothelial cell adhesion, motility and orientation response to cyclic strain. Ann. Biomed. Eng. 2010;38:208–222. doi: 10.1007/s10439-009-9826-7. [DOI] [PubMed] [Google Scholar]
- 102.Niwa K, Perloff JK, Bhuta SM, Laks H, Drinkwater DC, Child JS, Miner PD. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. 2001;103:393–400. doi: 10.1161/01.cir.103.3.393. [DOI] [PubMed] [Google Scholar]
- 103.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oakes PW, Gardel ML. Stressing the limits of focal adhesion mechanosensitivity. Curr. Opin. Cell Biol. 2014;30:68–73. doi: 10.1016/j.ceb.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oakley C, Brunette DM. The sequence of alignment of microtubules, focal contacts and actin filaments in fibroblasts spreading on smooth and grooved titanium substrata. J. Cell Sci. 1993;106(Pt 1):343–354. doi: 10.1242/jcs.106.1.343. [DOI] [PubMed] [Google Scholar]
- 106.Obbink-Huizer C, Oomens CW, Loerakker S, Foolen J, Bouten CV, Baaijens FP. Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech. Model. Mechanobiol. 2014;13:227–236. doi: 10.1007/s10237-013-0501-4. [DOI] [PubMed] [Google Scholar]
- 107.Ohara PT, Buck RC. Contact guidance in vitro. A light, transmission, and scanning electron microscopic study. Exp. Cell Res. 1979;121:235–249. doi: 10.1016/0014-4827(79)90002-8. [DOI] [PubMed] [Google Scholar]
- 108.Padmakumar VC, Libotte T, Lu WS, Zaim H, Abraham’ S, Noegel AA, Gotzmann J, Foisner R, Karakesisoglou L. The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 2005;118:3419–3430. doi: 10.1242/jcs.02471. [DOI] [PubMed] [Google Scholar]
- 109.Pajerowski JD, Dahl KN, Zhong FL, Sammak PJ, Discher DE. Physical plasticity of the nucleus in stem cell differentiation. Proc. Natl. Acad. Sci. USA. 2007;104:15619–15624. doi: 10.1073/pnas.0702576104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am. J. Physiol Heart Circ. Physiol. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 111.Poh YC, Shevtsov SP, Chowdhury F, Wu DC, Na S, Dundr M, Wang N. Dynamic force-induced direct dissociation of protein complexes in a nuclear body in living cells. Nat. Commun. 2012;3:866. doi: 10.1038/ncomms1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Prodanov L, te Riet J, Lamers E, Domanski M, Luttge R, van Loon JJ, Jansen JA, Walboomers XF. The interaction between nanoscale surface features and mechanical loading and its effect on osteoblast-like cells behavior. Biomaterials. 2010;31:7758–7765. doi: 10.1016/j.biomaterials.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 113.Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: new insights into the dynamics of integrin activation. J. Cell Biol. 2006;175:349–360. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puklin-Faucher E, Sheetz MP. The mechanical integrin cycle. J. Cell Sci. 2009;122:179–186. doi: 10.1242/jcs.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Saez A, Ghibaudo M, Buguin A, Silberzan P, Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. USA. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saito AC, Matsui TS, Ohishi T, Sato M, Deguchi S. Contact guidance of smooth muscle cells is associated with tension-mediated adhesion maturation. Exp. Cell Res. 2014;327:1–11. doi: 10.1016/j.yexcr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 117.Samarel AM. Focal adhesion signaling in heart failure. Pflugers Arch. 2014;466:1101–1111. doi: 10.1007/s00424-014-1456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sands G, Goo S, Gerneke D, LeGrice I, Loiselle D. The collagenous microstructure of cardiac ventricular trabeculae carneae. J. Struct. Biol. 2011;173:110–116. doi: 10.1016/j.jsb.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 119.Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomater. 2005;1:93–100. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 120.Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J. Heart Valve Dis. 1997;6:1–6. [PubMed] [Google Scholar]
- 121.Schwarz US, Gardel ML. United we stand: integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J. Cell Sci. 2012;125:3051–3060. doi: 10.1242/jcs.093716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat. Rev. Mol. Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 123.Smith MA, Blankman E, Deakin NO, Hoffman LM, Jensen CC, Turner CE, Beckerle MC. LIM domains target actin regulators paxillin and zyxin to sites of stress fiber strain. PLoS One. 2013;8:e69378. doi: 10.1371/journal.pone.0069378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith MA, Hoffman LM, Beckerle MC. LIM proteins in actin cytoskeleton mechanoresponse. Trends Cell Biol. 2014;24:575–583. doi: 10.1016/j.tcb.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Standley PR, Cammarata A, Nolan BP, Purgason CT, Stanley MA. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am. J. Physiol Heart Circ. Physiol. 2002;283:H1907–H1914. doi: 10.1152/ajpheart.01043.2001. [DOI] [PubMed] [Google Scholar]
- 127.Starr DA. KASH and SUN proteins. Curr. Biol. 2011;21:R414–R415. doi: 10.1016/j.cub.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tamiello C, Bouten CV, Baaijens FP. Competition between cap and basal actin fiber orientation in cells subjected to contact guidance and cyclic strain. Sci. Rep. 2015;5:8752. doi: 10.1038/srep08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taranum S, Vaylann E, Meinke P, Abraham S, Yang L, Neumann S, Karakesisoglou L, Wehnert M, Noegel AA. LINC complex alterations in DMD and EDMD/CMT fibroblasts. Eur. J. Cell Biol. 2012;91:614–628. doi: 10.1016/j.ejcb.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers—assembly, dynamics and biological roles. J. Cell Sci. 2012;125:1855–1864. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 132.Tomasek JJ, Hay ED. Analysis of the role of microfilaments and microtubules in acquisition of bipolarity and elongation of fibroblasts in hydrated collagen gels. J. Cell Biol. 1984;99:536–549. doi: 10.1083/jcb.99.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tondon A, Hsu HJ, Kaunas R. Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J. Biomech. 2012;45:728–735. doi: 10.1016/j.jbiomech.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 134.Tranquillo RT, Girton TS, Bromberek BA, Triebes TG, Mooradian DL. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials. 1996;17:349–357. doi: 10.1016/0142-9612(96)85573-6. [DOI] [PubMed] [Google Scholar]
- 135.Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: orientation of actin filaments and focal adhesions. J. Biomed. Mater. Res. A. 2005;75:668–680. doi: 10.1002/jbm.a.30478. [DOI] [PubMed] [Google Scholar]
- 136.van Kooten TG, von Recum AF. Cell adhesion to textured silicone surfaces: the influence of time of adhesion and texture on focal contact and fibronectin fibril formation. Tissue Eng. 1999;5:223–240. doi: 10.1089/ten.1999.5.223. [DOI] [PubMed] [Google Scholar]
- 137.Ventre M, Natale CF, Rianna C, Netti PA. Topographic cell instructive patterns to control cell adhesion, polarization and migration. J. R. Soc. Interface. 2014;11:20140687. doi: 10.1098/rsif.2014.0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Versaevel M, Grevesse T, Gabriele S. Regulation of nuclear shape and function with cell elongation. Biophys. J. 2013;104:151A. [Google Scholar]
- 139.Vishavkarma R, Raghavan S, Kuyyamudi C, Majumder A, Dhawan J, Pullarkat PA. Role of actin filaments in correlating nuclear shape and cell spreading. PLoS One. 2014;9:e107895. doi: 10.1371/journal.pone.0107895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 141.Walboomers XF, Ginsel LA, Jansen JA. Early spreading events of fibroblasts on microgrooved substrates. J. Biomed. Mater. Res. 2000;51:529–534. doi: 10.1002/1097-4636(20000905)51:3<529::aid-jbm30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 142.Walboomers XF, Monaghan W, Curtis AS, Jansen JA. Attachment of fibroblasts on smooth and microgrooved polystyrene. J. Biomed. Mater. Res. 1999;46:212–220. doi: 10.1002/(sici)1097-4636(199908)46:2<212::aid-jbm10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 143.Wang JH. Substrate deformation determines actin cytoskeleton reorganization: a mathematical modeling and experimental study. J. Theor. Biol. 2000;202:33–41. doi: 10.1006/jtbi.1999.1035. [DOI] [PubMed] [Google Scholar]
- 144.Wang JH, Goldschmidt-Clermont P, Wille J, Yin FC. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001;34:1563–1572. doi: 10.1016/s0021-9290(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 145.Wang JH, Goldschmidt-Clermont P, Yin FC. Contractility affects stress fiber remodeling and reorientation of endothelial cells subjected to cyclic mechanical stretching. Ann. Biomed. Eng. 2000;28:1165–1171. doi: 10.1114/1.1317528. [DOI] [PubMed] [Google Scholar]
- 146.Wang JH, Grood ES. The strain magnitude and contact guidance determine orientation response of fibroblasts to cyclic substrate strains. Connect. Tissue Res. 2000;41:29–36. doi: 10.3109/03008200009005639. [DOI] [PubMed] [Google Scholar]
- 147.Wang JH, Grood ES, Florer J, Wenstrup R. Alignment and proliferation of MC3T3-E1 osteoblasts in microgrooved silicone substrata subjected to cyclic stretching. J. Biomech. 2000;33:729–735. doi: 10.1016/s0021-9290(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 148.Wang H, Ip W, Boissy R, Grood ES. Cell orientation response to cyclically deformed substrates: experimental validation of a cell model. J. Biomech. 1995;28:1543–1552. doi: 10.1016/0021-9290(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 149.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009;10:75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 150.Wang D, Xie Y, Yuan B, Xu J, Gong P, Jiang X. A stretching device for imaging real-time molecular dynamics of live cells adhering to elastic membranes on inverted microscopes during the entire process of the stretch. Integr. Biol. (Camb.) 2010;2:288–293. doi: 10.1039/b920644b. [DOI] [PubMed] [Google Scholar]
- 151.Wang JH, Yang G, Li Z, Shen W. Fibroblast responses to cyclic mechanical stretching depend on cell orientation to the stretching direction. J. Biomech. 2004;37:573–576. doi: 10.1016/j.jbiomech.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 152.Wei Z, Deshpande VS, McMeeking RM, Evans AG. Analysis and interpretation of stress fiber organization in cells subject to cyclic stretch. J. Biomech. Eng. 2008;130:031009. doi: 10.1115/1.2907745. [DOI] [PubMed] [Google Scholar]
- 153.Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015;17:678–688. doi: 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Weichsel J, Herold N, Lehmann MJ, Krausslich HG, Schwarz US. A quantitative measure for alterations in the actin cytoskeleton investigated with automated high-throughput microscopy. Cytom. Part A. 2010;77A:52–63. doi: 10.1002/cyto.a.20818. [DOI] [PubMed] [Google Scholar]
- 155.Weiss P. Experiments on cell and axon orientation in vitro; the role of colloidal exudates in tissue organization. J. Exp. Zool. 1945;100:353–386. doi: 10.1002/jez.1401000305. [DOI] [PubMed] [Google Scholar]
- 156.Whitehead J, Vignjevic D, Futterer C, Beaurepaire E, Robine S, Farge E. Mechanical factors activate beta-catenin-dependent oncogene expression in APC mouse colon. HFSP. J. 2008;2:286–294. doi: 10.2976/1.2955566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wille JJ, Ambrosi CM, Yin FC. Comparison of the effects of cyclic stretching and compression on endothelial cell morphological responses. J. Biomech. Eng. 2004;126:545–551. doi: 10.1115/1.1798053. [DOI] [PubMed] [Google Scholar]
- 158.Williams C, Xie AW, Yamato M, Okano T, Wong JY. Stacking of aligned cell sheets for layer-by-layer control of complex tissue structure. Biomaterials. 2011;32:5625–5632. doi: 10.1016/j.biomaterials.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 159.Worman HJ. Nuclear lamins and laminopathies. J. Pathol. 2012;226:316–325. doi: 10.1002/path.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yamane M, Matsuda T, Ito T, Fujio Y, Takahashi K, Azuma J. Rac1 activity is required for cardiac myocyte alignment in response to mechanical stress. Biochem. Biophys. Res. Commun. 2007;353:1023–1027. doi: 10.1016/j.bbrc.2006.12.144. [DOI] [PubMed] [Google Scholar]
- 161.Yoshigi M, Clark EB, Yost HJ. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytom. A. 2003;55:109–118. doi: 10.1002/cyto.a.10076. [DOI] [PubMed] [Google Scholar]
- 162.Yoshigi M, Hoffman LM, Jensen CC, Yost HJ, Beckerle MC. Mechanical force mobilizes zyxin from focal adhesions to actin filaments and regulates cytoskeletal reinforcement. J. Cell Biol. 2005;171:209–215. doi: 10.1083/jcb.200505018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 164.Zhang QP, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi QJ, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum. Mol. Genet. 2007;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- 165.Zhang L, Kahn CJ, Chen HQ, Tran N, Wang X. Effect of uniaxial stretching on rat bone mesenchymal stem cell: orientation and expressions of collagen types I and III and tenascin-C. Cell Biol. Int. 2008;32:344–352. doi: 10.1016/j.cellbi.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 166.Zhong Z, Wilson KL, Dahl KN. Beyond lamins other structural components of the nucleoskeleton. Methods Cell Biol. 2010;98:97–119. doi: 10.1016/S0091-679X(10)98005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zimerman B, Arnold M, Ulmer J, Blummel J, Besser A, Spatz JP, Geiger B. Formation of focal adhesion-stress fibre complexes coordinated by adhesive and non-adhesive surface domains. IEE. Proc. Nanobiotechnol. 2004;151:62–66. doi: 10.1049/ip-nbt:20040474. [DOI] [PubMed] [Google Scholar]
- 168.Zuela N, Bar DZ, Gruenbaum Y. Lamins in development, tissue maintenance and stress. Embo Rep. 2012;13:1070–1078. doi: 10.1038/embor.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, Herrmann H, Wallrath LL, Lammerding J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]