Abstract
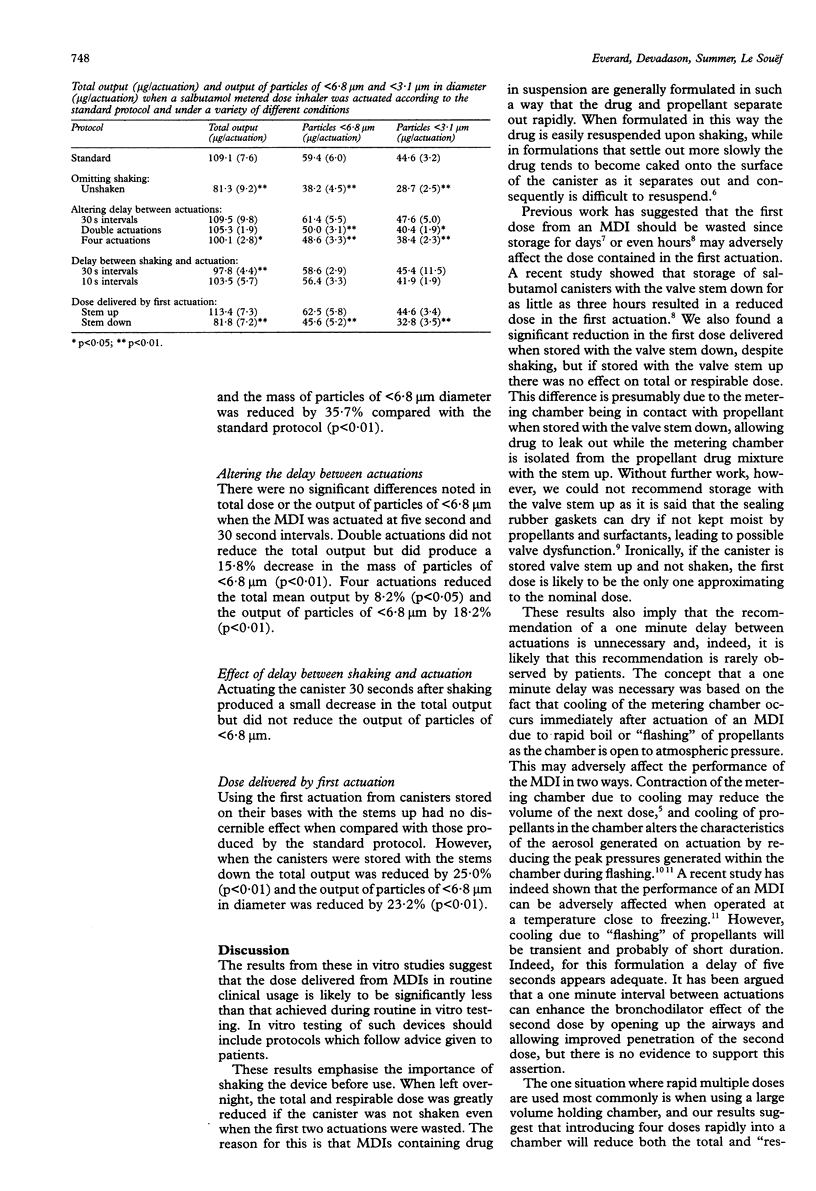
BACKGROUND--Many factors contribute to the high variability of doses delivered to the lungs of patients using metered dose inhalers (MDIs). Relatively little attention has been paid to the contribution to this variability of the way in which the MDI is handled before the inhalation manoeuvre. Instruction leaflets often recommend procedures at odds with those used for in vitro testing of the device. The standard protocol for in vitro assessment of salbutamol MDIs involves shaking the MDI vigorously for 30 seconds and wasting the first two actuations. Subsequent actuations are introduced into the testing device at five second intervals. Patient instructions do not include a recommendation to waste the first two actuations and recommend a delay of one minute between actuations. A series of experiments was performed to determine whether such differences might be important. METHODS--The total and "respirable" doses delivered by a salbutamol MDI (Ventolin, Allen & Hanburys) under various conditions were assessed with a multistage liquid impinger. The quantity of drug deposited on each stage was measured by an ultraviolet spectrophotometric method. The effect on the delivered dose of not shaking the canister, not wasting the first two doses, waiting 30 seconds between actuations, and using multiple rapid actuations was assessed by comparing the results with those obtained using the standard in vitro testing protocol. RESULTS--Compared with a standard protocol, it was found that not shaking the MDI before use reduced the total and "respirable" dose by 25.5% and 35.7%, respectively. The dose delivered when actuating the MDI at 30 second intervals was no different from that when intervals was no different from that when intervals of five seconds were used. Two actuations separated by one second had no effect on the total dose but reduced the "respirable" dose by 15.8%, while four rapid actuations reduced the total and "respirable" doses by 8.2% and 18.2%, respectively. Storing the MDI stem down reduced the total and "respirable" dose delivered in the first actuation by 25.0% and 23.3% despite shaking the MDI before use. CONCLUSIONS--MDIs containing drug in suspension must be shaken before use to resuspend the drug contained in the MDI, but shaking does not alter the composition of the suspension in the metering chamber and hence the dose in the first actuation remains low. Very rapid actuations can reduce the dose delivered per actuation, but salbutamol MDIs can be actuated immediately after a 10 second breath holding pause without affecting the dose delivered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry P. W., O'Callaghan C. Multiple actuations of salbutamol MDI into a spacer device reduce the amount of drug recovered in the respirable range. Eur Respir J. 1994 Sep;7(9):1707–1709. doi: 10.1183/09031936.94.07091707. [DOI] [PubMed] [Google Scholar]
- Clark A. R., Rachelefsky G., Mason P. L., Goldenhersh M. J., Hollingworth A. The use of reservoir devices for the simultaneous delivery of two metered-dose aerosols. J Allergy Clin Immunol. 1990 Jan;85(1 Pt 1):75–79. doi: 10.1016/0091-6749(90)90224-r. [DOI] [PubMed] [Google Scholar]
- Cyr T. D., Graham S. J., Li K. Y., Lovering E. G. Low first-spray drug content in albuterol metered-dose inhalers. Pharm Res. 1991 May;8(5):658–660. doi: 10.1023/a:1015825311750. [DOI] [PubMed] [Google Scholar]
- Edman P. Pharmaceutical formulations--suspensions and solutions. J Aerosol Med. 1994;7(Suppl 1):S3–S6. doi: 10.1089/jam.1994.7.suppl_1.s-3. [DOI] [PubMed] [Google Scholar]
- Everard M. L., Clark A. R., Milner A. D. Drug delivery from holding chambers with attached facemask. Arch Dis Child. 1992 May;67(5):580–585. doi: 10.1136/adc.67.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard M. L., Devadason S. G., Summers Q. A., Le Souëf P. N. Factors affecting total and "respirable" dose delivered by a salbutamol metered dose inhaler. Thorax. 1995 Jul;50(7):746–749. doi: 10.1136/thx.50.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson N. B., Mueller M. P. Cooling of metered-dose inhalers decreases pressure output from canisters. N Engl J Med. 1989 Feb 2;320(5):321–321. doi: 10.1056/NEJM198902023200520. [DOI] [PubMed] [Google Scholar]
- Lourenço R. V., Cotromanes E. Clinical aerosols. I. Characterization of aerosols and their diagnostic uses. Arch Intern Med. 1982 Nov;142(12):2163–2172. [PubMed] [Google Scholar]
- Newman S. P., Clarke S. W. Therapeutic aerosols 1--physical and practical considerations. Thorax. 1983 Dec;38(12):881–886. doi: 10.1136/thx.38.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. F., Mukai D. S., Ahdout J. J. Effect of canister temperature on performance of metered-dose inhalers. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1034–1037. doi: 10.1164/ajrccm/143.5_Pt_1.1034. [DOI] [PubMed] [Google Scholar]


