Abstract
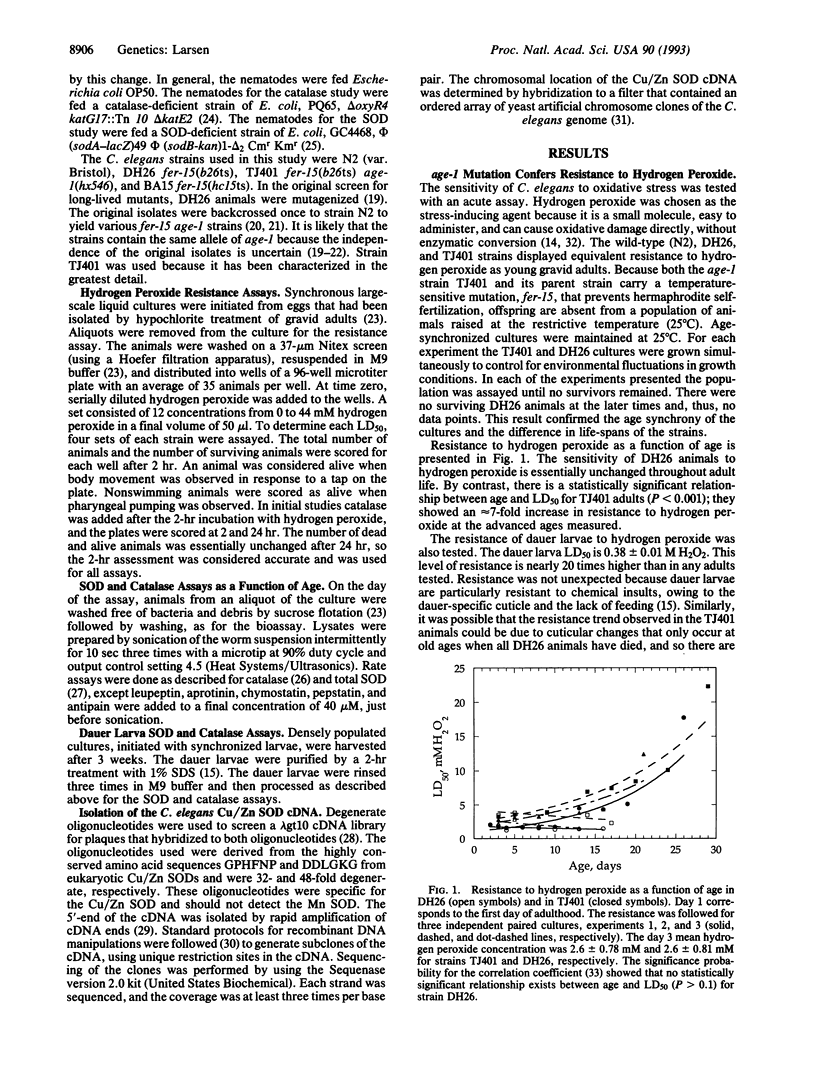
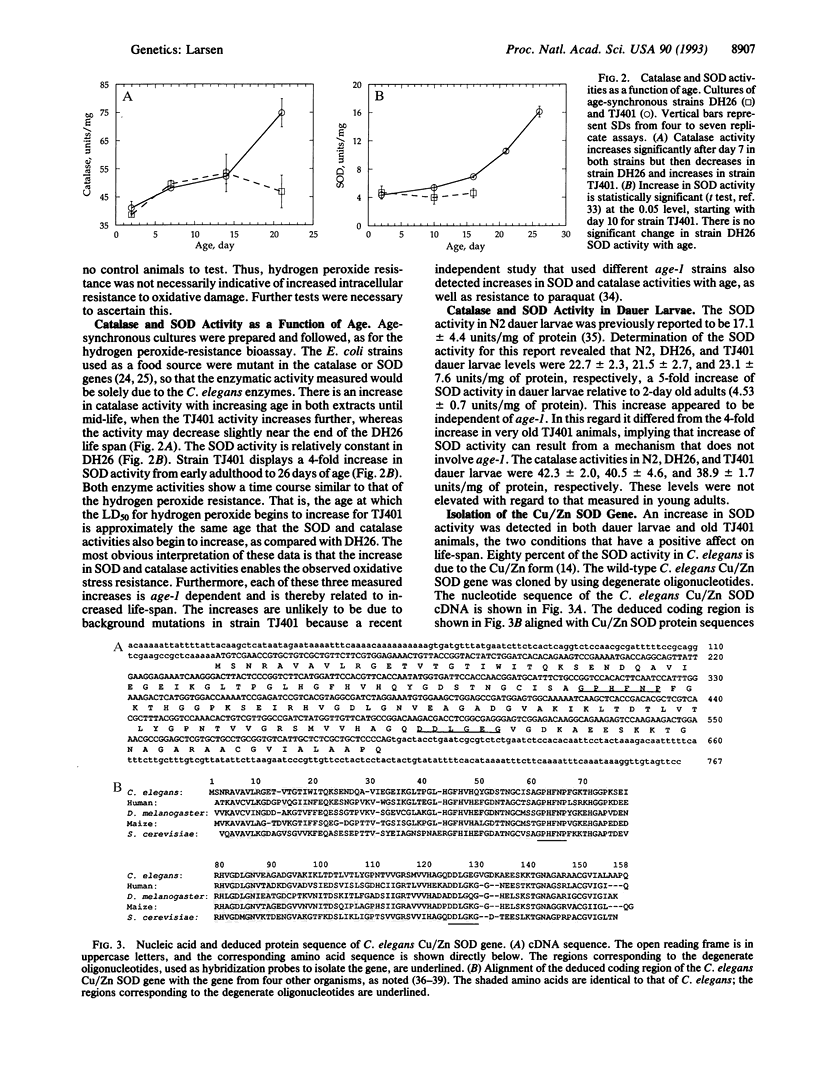
The dauer larva state and the age-1 mutation, both of which extend life-span in Caenorhabditis elegans, were tested for hyperresistance to cellular damage that may be relevant to aging. The age-1 strain TJ401 displayed hyperresistance to oxidative stress relative to its parental strain. The activities of two enzymes that protect cells from oxidative damage, superoxide dismutase (SOD) and catalase, showed an age-dependent increase in mutant animals, which was not seen in the parental strain. These increases in activities paralleled the time course of the hyperresistance. The results are consistent with the age-1 gene product functioning as a negative regulator of SOD and catalase activities. In wild-type and age-1 dauer larvae, elevated levels of SOD activity, but not of catalase activity, were present when compared with young adults. The common increase in SOD activity prompted cloning the C. elegans Cu/Zn SOD gene. Its position on the physical map of the genome was in the region to which the age-1 gene has been genetically mapped, but it is unlikely that a mutation at the SOD locus confers the Age phenotype. Results support the free radical theory of aging by suggesting that the increased resistance to oxidative stress may be among the causes of increased longevity in both strain TJ401 and in the dauer larva.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman R., Saul R. L., Ames B. N. Oxidative damage to DNA: relation to species metabolic rate and life span. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2706–2708. doi: 10.1073/pnas.85.8.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum J., Fridovich I. Superoxide, hydrogen peroxide, and oxygen toxicity in two free-living nematode species. Arch Biochem Biophys. 1983 Apr 1;222(1):35–43. doi: 10.1016/0003-9861(83)90499-x. [DOI] [PubMed] [Google Scholar]
- Bolanowski M. A., Russell R. L., Jacobson L. A. Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech Ageing Dev. 1981 Mar;15(3):279–295. doi: 10.1016/0047-6374(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Cannon R. E., White J. A., Scandalios J. G. Cloning of cDNA for maize superoxide dismutase 2 (SOD2). Proc Natl Acad Sci U S A. 1987 Jan;84(1):179–183. doi: 10.1073/pnas.84.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada R. C., Russell R. L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975 Oct;46(2):326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Coulson A., Kozono Y., Lutterbach B., Shownkeen R., Sulston J., Waterston R. YACs and the C. elegans genome. Bioessays. 1991 Aug;13(8):413–417. doi: 10.1002/bies.950130809. [DOI] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Groner Y. Impaired neurotransmitter uptake in PC12 cells overexpressing human Cu/Zn-superoxide dismutase--implication for gene dosage effects in Down syndrome. Cell. 1988 Jan 29;52(2):259–267. doi: 10.1016/0092-8674(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Friedman D. B., Johnson T. E. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988 Jan;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich O., Quillardet P., Hofnung M. Induction of the SOS response by hydrogen peroxide in various Escherichia coli mutants with altered protection against oxidative DNA damage. J Bacteriol. 1989 Nov;171(11):6141–6147. doi: 10.1128/jb.171.11.6141-6147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay J. A., Linn S. DNA damage and oxygen radical toxicity. Science. 1988 Jun 3;240(4857):1302–1309. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- Ishii N., Takahashi K., Tomita S., Keino T., Honda S., Yoshino K., Suzuki K. A methyl viologen-sensitive mutant of the nematode Caenorhabditis elegans. Mutat Res. 1990 May-Jul;237(3-4):165–171. doi: 10.1016/0921-8734(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Johnson T. E. Increased life-span of age-1 mutants in Caenorhabditis elegans and lower Gompertz rate of aging. Science. 1990 Aug 24;249(4971):908–912. doi: 10.1126/science.2392681. [DOI] [PubMed] [Google Scholar]
- Klass M. R. A method for the isolation of longevity mutants in the nematode Caenorhabditis elegans and initial results. Mech Ageing Dev. 1983 Jul-Aug;22(3-4):279–286. doi: 10.1016/0047-6374(83)90082-9. [DOI] [PubMed] [Google Scholar]
- Klass M. R. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977 Nov-Dec;6(6):413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Klass M., Hirsh D. Non-ageing developmental variant of Caenorhabditis elegans. Nature. 1976 Apr 8;260(5551):523–525. doi: 10.1038/260523a0. [DOI] [PubMed] [Google Scholar]
- Phillips J. P., Campbell S. D., Michaud D., Charbonneau M., Hilliker A. J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G., Xia E., Nadakavukaren M. J., Richardson A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr. 1990 Jun;120(6):602–609. doi: 10.1093/jn/120.6.602. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J. P., Deng H. X. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993 Mar 4;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Salin M. L., McCord J. M. Superoxide dismutases in polymorphonuclear leukocytes. J Clin Invest. 1974 Oct;54(4):1005–1009. doi: 10.1172/JCI107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B. DNA repair enzymes. Annu Rev Biochem. 1988;57:29–67. doi: 10.1146/annurev.bi.57.070188.000333. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins. J Biol Chem. 1990 Jul 25;265(21):12111–12114. [PubMed] [Google Scholar]
- Scott M. D., Meshnick S. R., Eaton J. W. Superoxide dismutase-rich bacteria. Paradoxical increase in oxidant toxicity. J Biol Chem. 1987 Mar 15;262(8):3640–3645. [PubMed] [Google Scholar]
- Seto N. O., Hayashi S., Tener G. M. Overexpression of Cu-Zn superoxide dismutase in Drosophila does not affect life-span. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4270–4274. doi: 10.1073/pnas.87.11.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto N. O., Hayashi S., Tener G. M. The sequence of the Cu-Zn superoxide dismutase gene of Drosophila. Nucleic Acids Res. 1987 Dec 23;15(24):10601–10601. doi: 10.1093/nar/15.24.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L., Dafni N., Lieman-Hurwitz J., Groner Y. Nucleotide sequence and expression of human chromosome 21-encoded superoxide dismutase mRNA. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5465–5469. doi: 10.1073/pnas.80.18.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveley B. E., Phillips J. P., Hilliker A. J. Phenotypic consequences of copper-zinc superoxide dismutase overexpression in Drosophila melanogaster. Genome. 1990 Dec;33(6):867–872. doi: 10.1139/g90-130. [DOI] [PubMed] [Google Scholar]
- Tolmasoff J. M., Ono T., Cutler R. G. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980 May;77(5):2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanfleteren J. R. Oxidative stress and ageing in Caenorhabditis elegans. Biochem J. 1993 Jun 1;292(Pt 2):605–608. doi: 10.1042/bj2920605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth W. G., Riddle D. L. Acidic intracellular pH shift during Caenorhabditis elegans larval development. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8435–8438. doi: 10.1073/pnas.85.22.8435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth W. G., Riddle D. L. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev Biol. 1989 Mar;132(1):167–173. doi: 10.1016/0012-1606(89)90214-5. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]




