Abstract
Adiponectin is a pleiotropic adipokine implicated in obesity, metabolic syndrome and cardiovascular disease. Recent studies have identified adiponectin as a negative regulator of tissue fibrosis. Wnt/β-catenin signaling has also been implicated in metabolic syndrome and can promote tissue fibrosis, but the extent to which adiponectin cross-regulates Wnt/β-catenin signaling is unknown. Using primary human dermal fibroblasts and recombinant purified proteins, we show that adiponectin can limit β-catenin accumulation and downstream gene activation by inhibiting Lrp6 phosphorylation, a key activation step in canonical Wnt signaling. Inhibition of Wnt3a-mediated Lrp6 phospho-activation is relatively rapid (e.g., by 30 minutes), and is not dependent on established adiponectin G-protein coupled receptors, AdipoR1 and R2, suggesting a more direct relationship to Lrp6 signaling. In contrast, the ability of adiponectin to limit Wnt-induced and baseline collagen production in fibroblasts requires AdipoR1/R2. These results suggest the possibility that the pleiotropic effects of adiponectin may be mediated through distinct cell surface receptor complexes. Accordingly, we propose that the anti-fibrotic activity of adiponectin may be mediated through AdipoR1/R2 receptors, while the ability of adiponectin to inhibit Lrp6 phospho-activation may be relevant to other recently established roles for Lrp6 signaling in glucose metabolism and metabolic syndrome.
Introduction
Adiponectin is a pleiotropic cytokine produced primarily by adipocytes [1]. Adiponectin is implicated in a constellation of diseases from obesity [2] and metabolic syndrome [3] to cardiovascular disease [4,5,6] and fibrosis [7,8]. At the cellular level, adiponectin regulates a number of metabolic processes, such as glucose flux, lipid catabolism, insulin sensitivity, energy balance and mitochondrial uncoupling reviewed in [9]. Currently, there are three established cell surface receptors mediating the diverse effects of adiponectin: the G-protein-couple receptors, Adipo R1 and R2, and the GPI-linked cell-cell adhesion protein, T-cadherin [10,11,12]. Whether the diverse cellular outcomes of adiponectin signaling are independently or coordinately mediated by these, or presently unidentified, surface receptors, remains incompletely understood.
Our interest in adiponectin stems from its recently characterized anti-fibrotic activities [7,8]. Organ fibrosis is a growing global health concern that is considered to contribute up to 45% of all deaths worldwide [13]. Gene expression profiling of fibrotic tissue reveals striking similarities to the same tissue during developmental morphogenesis, suggesting that aberrant regulation of core developmental signaling pathways (e.g., TGF-β, Wnt, Hh) may contribute to the persistent nature of fibrosis [14]. Indeed, we have shown that signaling via the Wnt co-receptor, Lrp5, is a driver of lung fibrosis [15], in part through promoting β-catenin-dependent nuclear signaling activities that favor fibroblast differentiation, proliferation, migration and matrix secretion [16,17,18].
In our efforts to understand the mechanistic basis for the anti-fibrotic effects of adiponectin, we focused on the structurally similarity of adiponectin to complement C1q, a serum protein known for its role in the complement cascade [10]. C1q was recently implicated in age-related organ fibrosis through its ability to cleave Wnt co-receptors, Lrp5/6, resulting in activation of β-catenin signaling [19]. This raised the possibility that some of the anti-fibrotic activities of adiponectin might involve limiting Wnt/β-catenin signaling at the level of Lrp5/6. Therefore, we sought to evaluate adiponectin as an inhibitor of Wnt/β-catenin signaling, and the degree to which the anti-fibrotic effects of adiponectin might be mediated through Wnt co-receptor-versus AdipoR1/R2-signaling.
Results
Adiponectin inhibits Wnt/β-catenin signaling
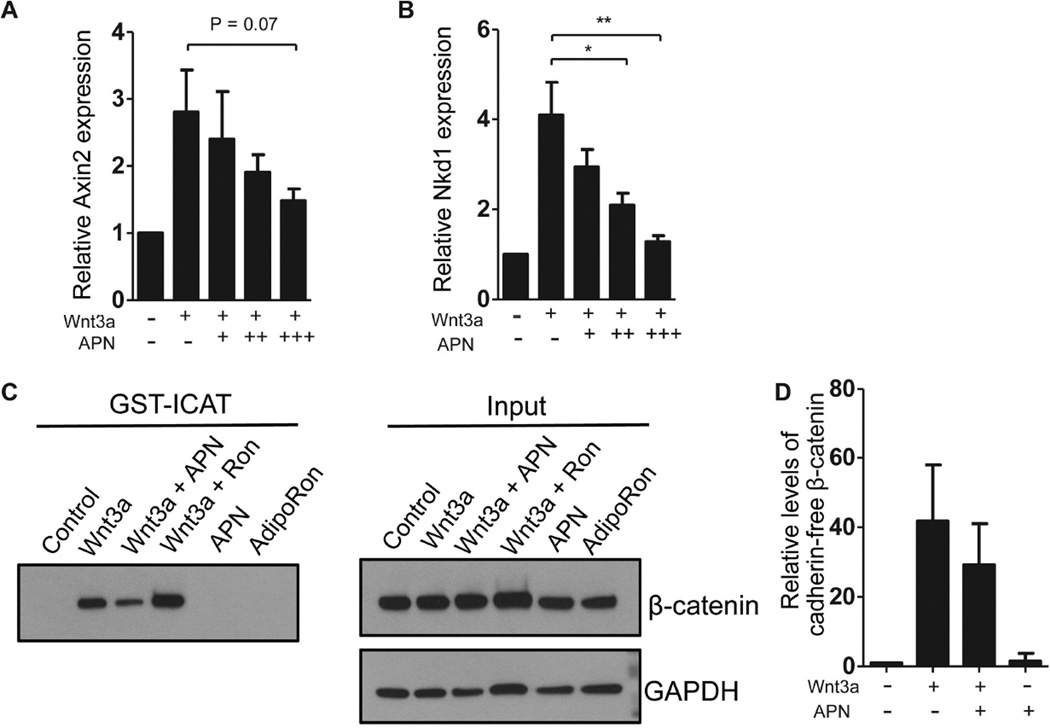
To understand how adiponectin inhibits fibrosis, we sought to determine its effects on Wnt/β-catenin signaling. Using primary skin fibroblasts treated with recombinant Wnt3a as a model system [20], we found that adiponectin (high molecular weight) caused a dose-dependent inhibition of two canonical Wnt/β-catenin target genes, AXIN2 and NAKED1, which are known to function as negative feedback regulators of Wnt signaling (Fig. 1A&B). Adiponectin treatment appears to be sufficient to limit β-catenin signaling broadly, as levels of the cadherin-free, cytoplasmic “signaling form” of β-catenin were significantly reduced by adiponectin, assessed using an established affinity-precipitation assay that targets the Wnt-stabilized pool of β-catenin [21] (Fig. 1C&D). Thus, adiponectin can limit Wnt-dependent accumulation of β-catenin and transcription of nuclear target genes in primary fibroblast cultures.
Figure 1. Adiponectin inhibits Wnt/β-catenin signaling.
Primary human skin fibroblasts were grown to confluency and serum-starved overnight. Cells were then treated with recombinant adiponectin (APN; 1 or 5 µg/ml). Twenty-four hours later, cells were treated with Wnt3a (100ng/ml). After 24 hours, RNA was isolated and qPCR was conducted on β-catenin target genes, Axin2 (A) and Naked1 (B). Graphs represent means from independent experiments and error bars represent standard deviation (SD) (n = 4 or 3 in A or B, respectively). C&D. Twenty-four hours after Wnt3a treatment, lysates were collected and GST-ICAT pull-down assays were used to measure levels of cadherin-free β-catenin. The graph in D shows relative levels of cadherin-free β-catenin normalized to total β-catenin from 4 independent experiments. Panel C shows representative blot from those graphed in D. Asterisks * and ** represent statistical significance by Student’s t-test, p<0.05 and <0.01, respectively).
Adiponectin inhibits Lrp6 phospho-activation
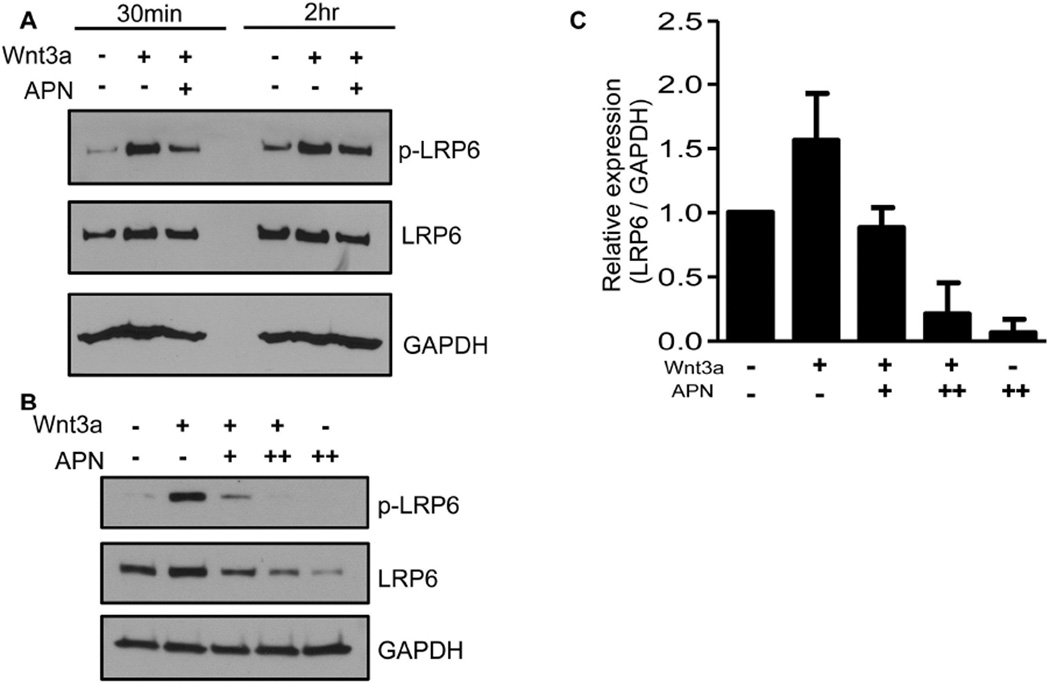
To understand how adiponectin negatively regulates Wnt signaling, we sought to interrogate one of the most upstream steps in Wnt-receptor activation by focusing on Lrp6 phosphorylation. We were particularly interested in Lrp6 in light of evidence that a structurally related factor, complement C1q, could activate Wnt signaling by promoting Lrp5/6 cleavage [19]. A series of experiments in primary dermal fibroblasts showed that adiponectin strongly inhibited Lrp6 phosphorylation induced by Wnt3a (Fig. 2A&B), as early as 30 minutes after treatment (Fig. 2A). This inhibition is specific to adiponectin and not simply due to co-incubation of Wnt3a-treated cells with a similarly abundant protein, since treatment of cells with an equivalent molar amount of BSA did not inhibit Lrp6 phosphorylation (Fig. S1). Although apparently similar reductions in total Lrp6 were observed after stripping and reprobing these immunoblots with an antibody that recognizes all forms of Lrp6 (Fig. 2A&B), this reduction likely reflects incomplete stripping of high affinity anti-Lrp6 phospho-serine epitopes, leading to reduced detection by pan-Lrp6 antibodies that recognize overlapping epitopes. Efforts to capture pLrp6 and pan-Lrp6 simulaneously using LiCOR Odyssey imaging were unsuccessful due to the reduction in sensitivity of the latter method and apparent competition for similar epitopes (not shown). We found no evidence that adiponectin treatment could promote Lrp6 endocytosis and trafficking to a lysosome, which would be expected if the reduction in pan-Lrp6 levels were real (not shown). Thus, adiponectin can limit β-catenin accumulation and signaling by functioning as a rapid and robust inhibitor of Lrp6 phospho-activation by Wnt3a.
Figure 2. Adiponectin inhibits phosphorylation of the Wnt co-receptor LRP6.
Primary human skin fibroblasts serum-starved overnight, then treated with adiponectin (APN; 1 or 5 µg/ml) and 24 hours later treated with Wnt3a (100ng/ml). Lysates were collected after 30 minutes and 2 hours (A), or after five days (B), and immunoblotted to assess levels of phospho-and total LRP6. GAPDH served as a loading control. The bar graph in C depicts the average relative total LRP6 levels 30 minutes after Wnt3a treatment from two independent experiments, including that shown in B. Error bars represent SD.
Adiponectin inhibits β-catenin signaling independently of AdipoR1/R2 and AMPK signaling
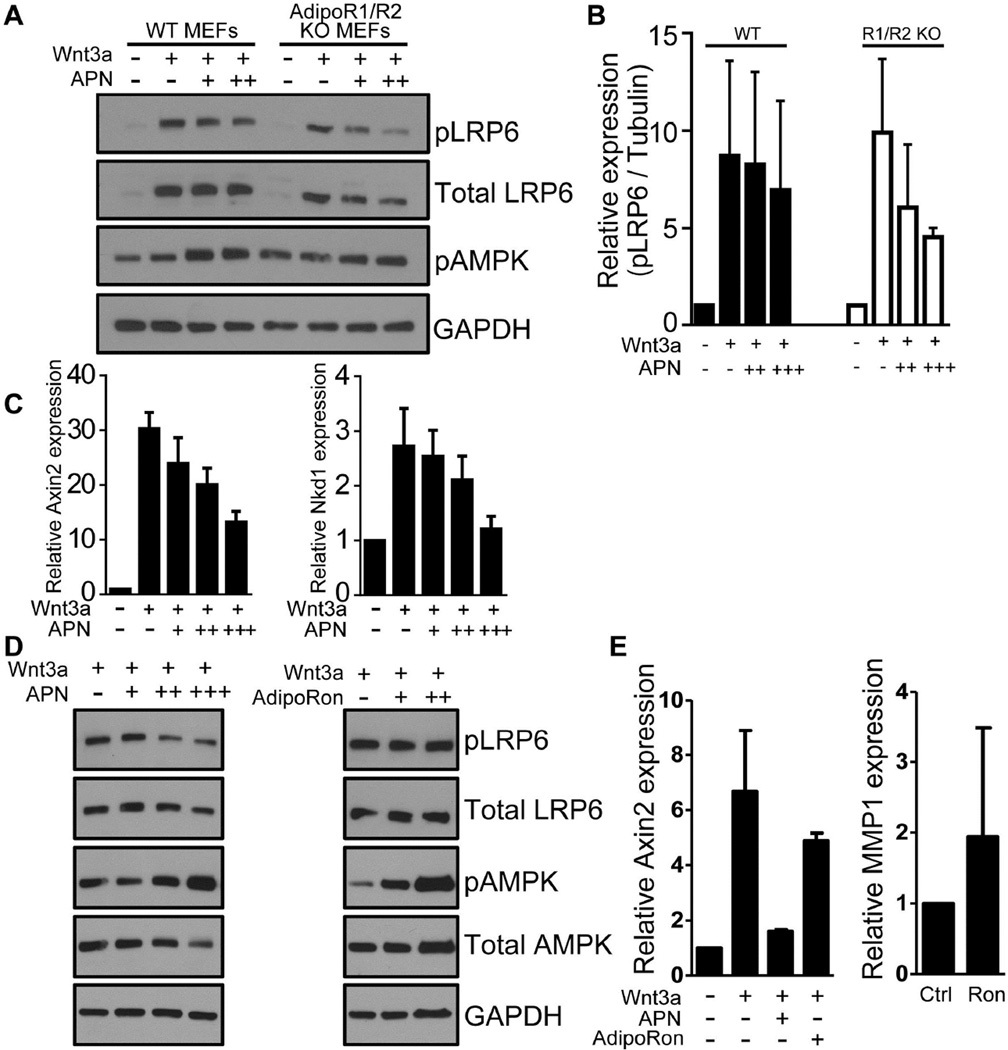
To determine whether the ability of adiponectin to limit Lrp6 phosphorylation and β-catenin signaling requires its G-protein coupled receptors, AdipoR1 and AdipoR2, we took advantage of readily available double-knockout MEFs [22]. We validated AdipoR1 and AdipoR2 gene disruption in these cells by genomic PCR (Fig. S2). Of interest, adiponectin efficiently inhibited Wnt3a-mediated Lrp6 phospho-activation in both wild-type and AdipoR1 and R2 knock-out MEFs (Fig. 3A&B). Moreover, adiponectin inhibited canonical targets of β-catenin signaling, AXIN2 and NAKED1 in double-knockout cells (Fig. 3C). These findings suggest that adiponectin is not working through its known receptors, AdipoR1 and R2, to repress Wnt/β-catenin signaling. Consistent with these data, pharmacologic activation of these receptors with the small molecule, AdipoRon, failed to inhibit either Wnt-induced Lrp6 phospho-activation or β-catenin target gene expression, even though AdipoRon and adiponectin were both competent to promote AMPK phospho-activation, and AdipoRon could induce expression of MMP1, a known adiponectin target gene [23,24] (Fig. 3D&E). Furthermore, adiponectin inhibited Lrp6 phosphorylation and β-catenin target gene expression even in the absence of AMPK, as shown using MEFs derived from AMPK knock-out animals (Fig. S3A&B), or a chemical inhibitor of AMPK, Compound C (Fig. S3C&D). Thus altogether, these data show that adiponectin’s capacity to inhibit Lrp6 phosphorylation and β-catenin signaling does not require signaling through the established AdipoR1/R2-AMPK signaling pathway.
Figure 3. AdipoR1 and AdipoR2 receptors are not required for adiponectin-mediated inhibition of LRP6 phosphorylation and Wnt/β-catenin target gene expression.
A-C) Mouse embryonic fibroblasts (WT or AdipoR1/R2 KO) were serum-starved overnight. In A and B, cells were treated with adiponectin (APN; 1, 5, or 10 µg/ml). Two hours later, cells were treated with Wnt3a (100ng/ml), and 30 minutes after Wnt3a treatment, lysates were subjected to immunoblot analysis with antibodies to phosho- and total LRP6. GAPDH served as a loading control. Phosphorylation of AMPK (pAMPK) served as a positive control for the addition of adiponectin (n = 3 independent experiments, including that shown in A). Error bars represent SD. In C, AdipoR1/R2 KO MEFs were treated with APN (1, 5, or 10 µg/ml) 24 hours before treatment with Wnt3a (100ng/ml). Twenty-four hours after Wnt3a treatment, RNA was isolated and qRT-PCR conducted to measure Wnt target genes, including Axin2 (left) and Nkd1 (right). In D and E, fibroblasts were serum-starved overnight and then treated with adiponectin (APN; 1, 5, or 10 µg/ml) or the small-molecule AdipoR1/R2 agonist AdipoRon (20µM or 50µM) two hours before treatment with Wnt3a. (D) Thirty minutes after Wnt3a treatment, lysates were subject to immunoblot analysis. (E) Twenty-four hours after Wnt3a treatment, RNA was isolated and qRTPCR was conducted to measure expression of Wnt target genes including Axin2 (left). Expression of the AMPK target gene MMP1 (right) was assessed to demonstrate effectiveness of AdipoRon at activating signaling via AdipoR1/R2. Bar graphs depict averages of two independent experiments. Error bars represent SD.
AdipoR1/R2 mediate the anti-fibrotic effects of adiponectin
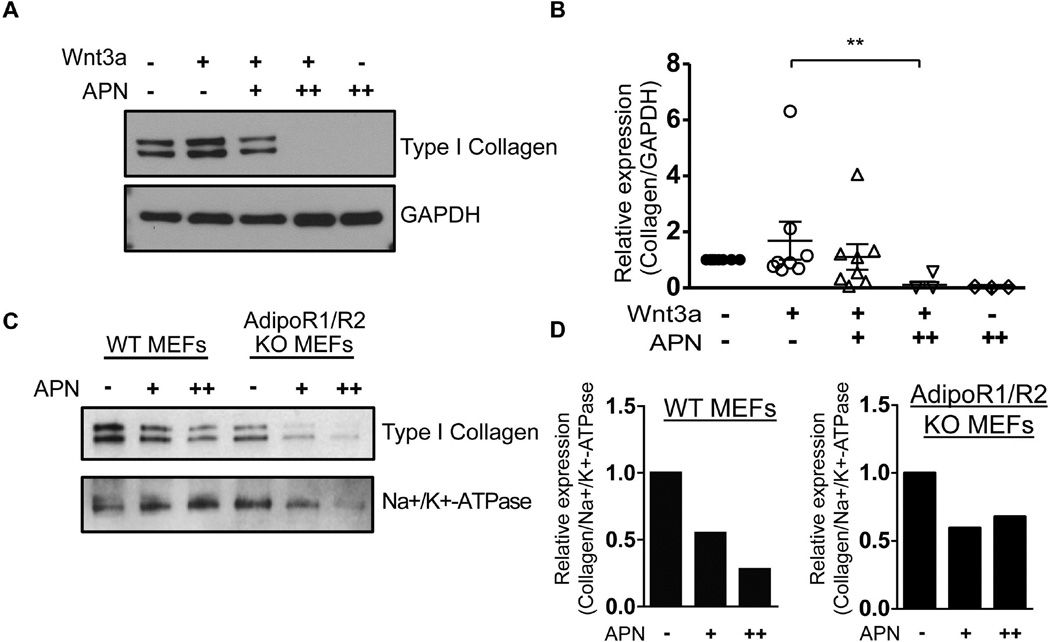
Previous studies suggested that adiponectin has anti-fibrotic activities [7,8], while unrestrained Wnt signaling can promote fibrosis [15,25,26,27,28,29]. However, whether the anti-fibrotic effects of adiponectin are mediated through adiponectin receptors R1/R2, or through antagonizing Wnt/Lrp5/6 signaling is unknown. In primary human skin fibroblasts, adiponectin was a potent inhibitor of type 1 collagen synthesis (Fig. 4A&B). Remarkably, adiponectin not only attenuated the Wnt3a-induced stimulation of collagen production, but also reduced baseline collagen expression to undetectable levels, suggesting that adiponectin may be limiting collagen production through a more general, Wnt-independent mechanism. Consistent with this idea, adiponectin failed to inhibit collagen production in AdipoR1/R2 null fibroblasts relative to WT fibroblasts (Fig. 4C&D), suggesting that its anti-fibrotic activities largely depend on signaling from these receptors.
Figure 4. Adiponectin represses Type I Collagen expression independently of AdipoR1/R2 receptors.
A and B) Primary human skin fibroblasts were serum-starved overnight and then treated with adiponectin (APN; 1 or 5 µg/ml). Twenty-four hours later, cells were treated with Wnt3a (100ng/ml). Five days after Wnt3a treatment, lysates were subjected to immunoblot analysis to measure type I collagen. The graph in B shows relative collagen expression from at least four independent experiments. The Mann-Whitney U test shows that Wnt3a causes a statistically significant decrease in collagen (p = .0016). Panel A depicts a representative blot from those graphed in panel B. GAPDH served as a loading control. (C and D) Wild-type (WT) or AdipoR1/R2 KO MEFs were serum-starved overnight and then treated with adiponectin (APN; 5 or 10 µg/ml). Five days later lysates were subjected to immunoblot analysis for type I collagen. Bands shown in panel C are quantified in panel D. Na+/k+-ATPase immunoblot served as a loading control.
Discussion
Adiponectin is a pleiotropic fat-derived cytokine broadly implicated in the regulation of metabolism, and its dysregulation has been linked to metabolic syndrome [1] and tissue fibrosis [7,8]. Since a familial mutation in the Wnt co-receptor, Lrp6, has been recently implicated in metabolic syndrome and reduced sensitivity to insulin signaling [30,31] and Wnt/β-catenin signaling can promote tissue fibrosis in a number of systems [15,25,26,27,28,29], we reasoned that adiponectin and Wnt/β-catenin signaling might be coordinated, and that some of the anti-fibrotic effects of adiponectin might involve inhibition of Wnt/β-catenin signaling at the level of Lrp5/6. Indeed, we found that adiponectin inhibits Wnt3a-mediated β-catenin accumulation and activation of established target genes in a dose-dependent manner. We showed that inhibition by adiponectin is reasonably rapid, and occurs at the level of Lrp6 phosphorylation, a key upstream step in Wnt-co-receptor activation [32,33]. The precise mechanism by which adiponectin limits Wnt3a-mediated Lrp6-phosphorylation remains unclear. Interestingly, adiponectin is structurally related to complement C1q, a factor recent found to activate β-catenin signaling by promoting cleavage of the Lrp5/6 extracellular domain [19], where the remaining transmembrane and cytoplasmic domain is known to manifest robust, constitutive β-catenin signaling [34]. Although Lrp5/6 cleavage is not a canonical mechanism of receptor activation, it is possible that adiponectin may limit Lrp6 phosphorylation through hindering access of Wnts to the Lrp5/6-Frizzled receptor complex, or by promoting a conformation of Lrp6 that limits is accessibility to kinases that activate it [32,33]. While direct evidence that Lrp6 is a bona fide receptor for adiponectin remains to be established, the kinetics of inhibition are relatively rapid, and are independent of adiponectin receptors AdipoR1 and R2, suggesting a more proximal relationship to signaling inhibition.
Our previous work indicated that Wnt/β-catenin signaling promotes lung and skin fibrosis [15,17] and adiponectin antagonizes fibrosis [8]. Together with the present results showing that adiponectin inhibits Wnt/β-catenin signaling at the level of Lrp6 phospho-activation, this raised the possibility that some of the anti-fibrotic effects of adiponectin might be through limiting Wnt/β-catenin signaling. Indeed, while we found that Wnt3a can increase collagen production in fibroblasts, consistent with previous studies [18], adiponectin treatment not only abrogated this increase, but also potently reduced the baseline expression of collagen. Since this inhibition required adiponectin receptors R1 and R2, it appears that at least one anti-fibrotic effect of adiponectin is largely mediated through R1/R2. Interestingly, we found that the ability of adiponectin to inhibit Lrp6 phosphoryation appeared independent of adiponectin receptors R1/R2, and its downstream mediator AMP kinase, suggesting that adiponectin’s action towards Lrp6 signaling serves an alternate function. Given that a familial mutation in Lrp6 has been associated with metabolic syndrome and cardiovascular disease [30,35], together with recent evidence that this mutation drives reduced sensitivity to insulin signaling [31], we reason that future studies into how adiponectin improves glucose metabolism should be considered in the context of its ability to antagonize Lrp6 phosphorylation and Wnt/β-catenin signaling.
Methods
Cell culture and reagents
Primary cultures of neonatal fibroblasts were established by explantation from human foreskin samples, as previously described [20]. Embryonic fibroblasts from AMPK−/− and wild-type mice were a gift from Yu-Ying He (University of Chicago). Embryonic fibroblasts from AdipoR1/R2 double-knockout mice were a gift from Philipp Scherer (University of Texas Southwestern). Unless indicated otherwise, fibroblasts lines and human embryonic kidney 293 cells were maintained at 37°C at an atmosphere of 5% CO2 in Dulbecco’s Modified Eagle’s Medium (DMEM) with 4.5gm/L glucose and L-glutamine with sodium pyruvate (Mediatech, Manassass, VA) plus 10% Fetal Bovine Serum (Hyclone, Waltham, MA) and penicillin/streptomycin (Mediatech). In select experiments, recombinant murine Wnt3a (PeproTech, Rocky Hill, NJ), recombinant full-length human Adiponectin (BioVendor, Candler, NC), the AMP-activated protein kinase (AMPK) inhibitor Compound C (Sigma, St Louis, MO), or the small molecule AdipoR1/R2 agonist AdipoRon (Xcess Biosciences, San Diego, CA) was added to the cultures at the indicated concentrations. Imaging was performed using Zeiss Axioskop with Cri Nuance multispectral camera (Thornton, NY).
Plasmids
The previously described HA-AdipoR1 and Flag-AdipoR2 constructs [36] were gifts from Louis Luttrell (Medical University of South Carolina).
Quantification of mRNA
Total RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. cDNA was generated by reverse transcription using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Real-time reverse-transcription PCR (qRT-PCR) was performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA) in a Bio-Rad CFX96 Real-Time Detection System. mRNA levels were normalized to levels of GAPDH, and results are expressed as the fold change of Ct values (mean of 3 replicates) relative to controls, using the 2−ΔΔCt formula. Primer sequences are as follows: Human Axin2 forward primer 5’-CCAACACCAGGCGGAACGAAG and reverse primer 5’- CGCCCAATAAGGAGTGTAAGGAC; human Nkd1 forward primer 5’- GGG AAA CTTCACTCCAAGCCG and reverse primer 5’- GTCTCCCGATCCACTCCTCG; human GAPDH forward primer 5’- CCTGGGCTACACTGAGCACC and reverse primer 5’- GTGGTCGTTGAGGGCAATGC; human MMP1 forward primer 5’- GCACAAATCCCTTCTACCCG and reverse primer 5’- TGAACAGCCCAGTACTTATTCC; mouse Axin2 forward primer 5’ - ACC TCA AGT GCA AAC TCT CAC CCA and reverse primer 5’ - AGC TGT TTC CGT GGA TCT CAC ACT; mouse Nkd1 forward primer 5’ - GAC AAC AAT GGC AAA GTG ACC CGT and reverse primer 5’ - TGC TCT GCA GAT CGG TAT GGT TGA; mouse RPL19 forward primer 5’-AGCCTGTGACTGTCCATTC and reverse primer 5’- ATCCTCATCCTTCTCATCCAG.
Genotyping of AdipoR1/R2 double-knockout MEFs
Genomic DNA was isolated from MEFs using the Blood & Cell Culture DNA Kit (Qiagen) according to the manufacturer’s instructions. PCR reactions were conducted using GoTaq DNA Polymerase (Promega), and PCR products were run on 2% agarose gels.
Antibodies
Antibodies used in this study are as follows: AdipoR1 (Sigma-Aldrich, Saint Louis, MO); AdipoR2 (Novus Biologicals, Littleton, CO); pAMPK, Total AMPK, pLRP6, and Total LRP6 (Cell Signaling Technology, Danvers, MA); β-catenin (BD Transduction Laboratories, Franklin Lakes, NJ); GAPDH (Santa Cruz Biotechnology Inc., Santa Cruz, CA); α-Tubulin (Sigma-Aldrich); Type I Collagen (Southern Biotech, Birmingham, AL).
GST-ICAT pulldown assays
Intracellular levels of cadherin-free β-catenin were determined by pull-down assays, using glutathione S-transferase [37]/β-catenin–interacting protein (ICAT), as previously described [21] with slight modifications. In brief, cells were harvested in 1% Triton X-100 lysis buffer. Total protein from cell lysates was incubated with GST-ICAT and a 50% suspension of glutathione-coupled Sepharose beads (GE Healthcare) for 2 h at 4°C. Precipitated proteins were washed and subjected to SDS-PAGE and immunoblot analysis using standard procedures. Densitometric analysis was performed using ImageJ (National Institutes of Health).
Statistics
Unless indicated otherwise, data are presented as means ± SD. Statistical analysis was performed by Student’s t test or one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test. A p value of less than 0.05 was considered significant. Statistical analyses were performed using GraphPad Prism software (Graph Pad Software Inc, La Jolla, CA).
Supplementary Material
Highlights.
Adiponectin inhibits Wnt-dependent β-catenin accumulation and target-gene expression
Adiponectin antagonizes Lrp6 phosphorylation
Inhibition of Lrp6 phosphorylation is independent of adiponectin receptors, AdipoR1 and R2
Ability adiponectin to inhibit Wnt/β-catenin signaling has implications for adiponectin’s roles in tissue fibrosis and metabolic syndrome
Acknowledgments
We thank Philipp Scherer and William Holland (University of Texas, Southwestern, Dallas) for Adiponectin R1 and R2 double KO MEFs and PCR validation scheme. We also thank Yu-Ying He (University of Chicago) for AMPK KO MEFs. LR was supported by a NIH/NIAMS Ruth L. Kirschstein National Research Service Award (2 T32 AR007611-11A1) a Department of Defense Award (PR110276), APL by K08HL093216 and P30HL101292, JV and CJG by DOD W81XWH-12-1-0471 and C.J.G. by GM076561 and HL094643.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C, Bianco A, Daniele A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007;18:263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 4.Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Essick EE, Ouchi N, Wilson RM, Ohashi K, Ghobrial J, Shibata R, Pimentel DR, Sam F. Adiponectin mediates cardioprotection in oxidative stress-induced cardiac myocyte remodeling. Am J Physiol Heart Circ Physiol. 2011;301:H984–H993. doi: 10.1152/ajpheart.00428.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maia-Fernandes T, Roncon-Albuquerque R, Jr, Leite-Moreira AF. Cardiovascular actions of adiponectin: pathophysiologic implications. Rev Port Cardiol. 2008;27:1431–1449. [PubMed] [Google Scholar]
- 7.Chiarugi P, Fiaschi T. Adiponectin in health and diseases: from metabolic syndrome to tissue regeneration. Expert Opin Ther Targets. 2010;14:193–206. doi: 10.1517/14728220903530712. [DOI] [PubMed] [Google Scholar]
- 8.Fang F, Liu L, Yang Y, Tamaki Z, Wei J, Marangoni RG, Bhattacharyya S, Summer RS, Ye B, Varga J. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Res Ther. 2012;14:R229. doi: 10.1186/ar4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shehzad A, Iqbal W, Shehzad O, Lee YS. Adiponectin: regulation of its production and its role in human diseases. Hormones (Athens) 2012;11:8–20. doi: 10.1007/BF03401534. [DOI] [PubMed] [Google Scholar]
- 10.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 11.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 12.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam AP, Herazo-Maya JD, Sennello JA, Flozak AS, Russell S, Mutlu GM, Budinger GR, DasGupta R, Varga J, Kaminski N, Gottardi CJ. Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:185–195. doi: 10.1164/rccm.201401-0079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, Budinger GR, Feghali-Bostwick CA, Varga J, Gottardi CJ. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, Gottardi CJ, MacDougald OA, Varga J. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis Rheum. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, Gottardi CJ, Atit R, Whitfield ML, Varga J. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012;64:2734–2745. doi: 10.1002/art.34424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei J, Ghosh AK, Sargent JL, Komura K, Wu M, Huang QQ, Jain M, Whitfield ML, Feghali-Bostwick C, Varga J. PPARgamma downregulation by TGFss in fibroblast and impaired expression and function in systemic sclerosis: a novel mechanism for progressive fibrogenesis. PLoS One. 2010;5:e13778. doi: 10.1371/journal.pone.0013778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koskinen A, Juslin S, Nieminen R, Moilanen T, Vuolteenaho K, Moilanen E. Adiponectin associates with markers of cartilage degradation in osteoarthritis and induces production of proinflammatory and catabolic factors through mitogen-activated protein kinase pathways. Arthritis Res Ther. 2011;13:R184. doi: 10.1186/ar3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YA, Ji HI, Lee SH, Hong SJ, Yang HI, Chul Yoo M, Kim KS. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: implications for arthritic joints. Exp Mol Med. 2014;46:e72. doi: 10.1038/emm.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009 doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henderson WR, Jr, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, Borok Z, Knight DA, Kahn M. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA. Axin pathway activity regulates in vivo pY654-beta-catenin accumulation and pulmonary fibrosis. J Biol Chem. 2012;287:5164–5172. doi: 10.1074/jbc.M111.322123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh R, De Aguiar RB, Naik S, Mani S, Ostadsharif K, Wencker D, Sotoudeh M, Malekzadeh R, Sherwin RS, Mani A. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab. 2013;17:197–209. doi: 10.1016/j.cmet.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature. 2005;438:873–877. doi: 10.1038/nature04185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature. 2005;438:867–872. doi: 10.1038/nature04170. [DOI] [PubMed] [Google Scholar]
- 34.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keramati AR, Singh R, Lin A, Faramarzi S, Ye ZJ, Mane S, Tellides G, Lifton RP, Mani A. Wild-type LRP6 inhibits, whereas atherosclerosis-linked LRP6R611C increases PDGF-dependent vascular smooth muscle cell proliferation. Proc Natl Acad Sci U S A. 2011;108:1914–1918. doi: 10.1073/pnas.1019443108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee MH, Klein RL, El-Shewy HM, Luttrell DK, Luttrell LM. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry. 2008;47:11682–11692. doi: 10.1021/bi801451f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt SL, Nambiar AM, Tayob N, Sundaram B, Han MK, Gross BH, Kazerooni EA, Chughtai AR, Lagstein A, Myers JL, Murray S, Toews GB, Martinez FJ, Flaherty KR. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J. 2011;38:176–183. doi: 10.1183/09031936.00114010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.