Significance
Lifespan of animals can be extended by genetic and environmental interventions, which often also induce resistance to toxins. This association has given rise to the Green Theory of Aging, which suggests that the ability to remove toxins is limiting for lifespan. To test this idea, we genetically increased resistance to toxins in Drosophila, but found no consequent increase in lifespan. Furthermore, we could block the xenobiotic resistance of genetically long-lived flies without reducing their lifespan. It will be important to understand whether the xenobiotic resistance of long-lived mice is also a correlated, rather than a causal, trait, and to understand the functional significance of the common increase in xenobiotic resistance in long-lived animals.
Keywords: lifespan, xenobiotic resistance, IIS, nuclear hormone receptor, DHR96
Abstract
Lifespan of laboratory animals can be increased by genetic, pharmacological, and dietary interventions. Increased expression of genes involved in xenobiotic metabolism, together with resistance to xenobiotics, are frequent correlates of lifespan extension in the nematode worm Caenorhabditis elegans, the fruit fly Drosophila, and mice. The Green Theory of Aging suggests that this association is causal, with the ability of cells to rid themselves of lipophilic toxins limiting normal lifespan. To test this idea, we experimentally increased resistance of Drosophila to the xenobiotic dichlordiphenyltrichlorethan (DDT), by artificial selection or by transgenic expression of a gene encoding a cytochrome P450. Although both interventions increased DDT resistance, neither increased lifespan. Furthermore, dietary restriction increased lifespan without increasing xenobiotic resistance, confirming that the two traits can be uncoupled. Reduced activity of the insulin/Igf signaling (IIS) pathway increases resistance to xenobiotics and extends lifespan in Drosophila, and can also increase longevity in C. elegans, mice, and possibly humans. We identified a nuclear hormone receptor, DHR96, as an essential mediator of the increased xenobiotic resistance of IIS mutant flies. However, the IIS mutants remained long-lived in the absence of DHR96 and the xenobiotic resistance that it conferred. Thus, in Drosophila IIS mutants, increased xenobiotic resistance and enhanced longevity are not causally connected. The frequent co-occurrence of the two traits may instead have evolved because, in nature, lowered IIS can signal the presence of pathogens. It will be important to determine whether enhanced xenobiotic metabolism is also a correlated, rather than a causal, trait in long-lived mice.
The aging process can be ameliorated by genetic and environmental interventions, which can also delay or prevent age-related loss of function and pathology (1–4). Notably, the lifespans of the nematode worm (Caenorhabditis elegans), the fruit fly (Drosophila melanogaster), and the mouse (Mus musculus) can be extended by reduced activity of the insulin/insulin-like growth factor signaling (IIS) network (1–4), which may also be important in human aging (5). This evolutionary conservation indicates that at least some aspects of mammalian aging can be understood by work with invertebrates, with their short lifespans and ease of genetic manipulation.
In C. elegans and Drosophila, the single forkhead box O (FOXO) transcription factor is essential for the increased lifespan upon reduced IIS (6–8), suggesting that altered transcription of the direct or indirect targets of FOXO mediates the changes in physiology required for longer life. In Drosophila, most of the pleiotropic traits induced by lowered IIS are merely correlated with, rather than causal for, extension of lifespan, because they are still present in the absence of Drosophila forkhead box O (dFOXO) (7). Only extended lifespan and increased resistance to xenobiotics of IIS mutants have been demonstrated to require the presence of dFOXO (6–8), suggesting that lowered IIS may extend lifespan through increased detoxification of endobiotic and xenobiotic compounds.
The metabolism of xenobiotics is divided into three phases: (i) modification, (ii) conjugation, and (iii) excretion. Genome-wide transcript profiles from long-lived animals, including IIS mutant worms and flies (9, 10), long-lived mutant Ames and Little dwarf mice (11), and mice from crowded litters, subjected to dietary restriction or treated with rapamycin (12) all show increased expression of genes involved in phase 1 and 2 drug and xenobiotic metabolism (13). Little mice are also resistant to toxicity from xenobiotic compounds (14), indicating that the gene expression profiles are physiologically relevant. The link between increased lifespan and xenobiotic metabolism has led to the “Green Theory,” which suggests that aging results from an accumulation of xenobiotic and endobiotic toxicity as a consequence of a declining detoxification response with age (15).
We have found that, in Drosophila, aging and xenobiotic metabolism are independently controlled. We identified a nuclear hormone receptor, DHR96, as required for the increased xenobiotic resistance of long-lived IIS mutants. However, IIS mutants that lack DHR96 are equally long-lived without enhanced resistance to xenobiotics, demonstrating that the association between increased lifespan and xenobiotic metabolism is not causal.
Results
Increased Resistance to the Insecticide Dichlordiphenyltrichlorethan Does Not Increase Lifespan.
In Drosophila, increased lifespan from reduced IIS is consistently associated with resistance to the insecticide dichlordiphenyltrichlorethan (DDT), and both traits require the presence of dFOXO (7). We first investigated whether enhanced resistance to DDT would extend lifespan, by using artificial selection or overexpression of a cytochrome P450-encoding gene that enhances resistance to DDT (16).
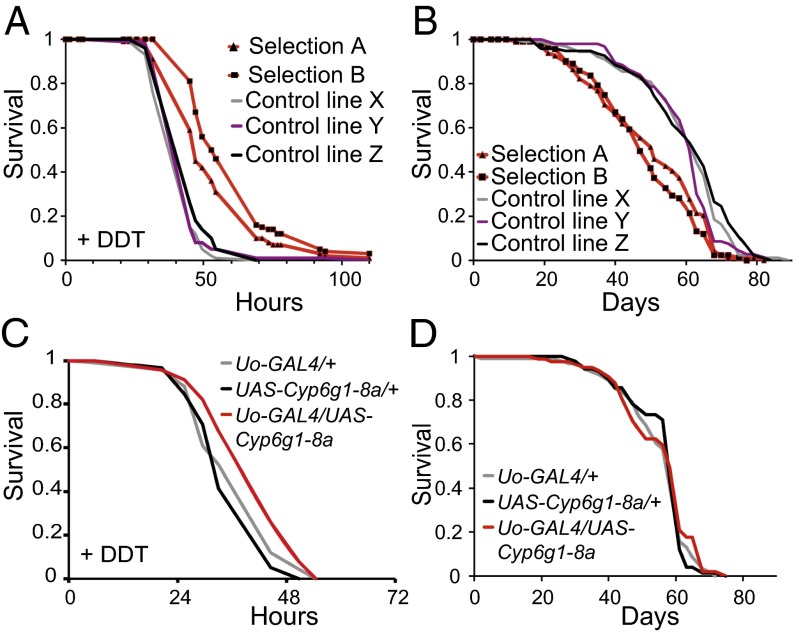
Two large populations of Drosophila (sel-A and sel-B) were artificially selected for resistance to DDT, and both showed a response to selection (Fig. 1A). However, in the absence of DDT, the DDT-resistant lines were short-lived compared with controls (Fig. 1B). Detoxification enzymes expressed in the insect excretory Malpighian tubules play an important role in xenobiotic metabolism (17). DDT resistance was induced by overexpression of the cytochrome P450-encoding Cyp6g1 in the Malpighian tubules (Fig. 1C and see repeated experiment shown in Fig. S3A). However, the lifespan of the flies in the absence of DDT was unaffected (Fig. 1D). Hence, resistance to DDT per se is not sufficient to extend lifespan.
Fig. 1.
Enhancing DDT resistance by artificial selection or overexpression of Cyp6g1 in Malpighian tubules did not extend fly lifespan. (A) Both selection lines (selection A and selection B) showed significant DDT resistance compared with three control populations (control lines X, Y, and Z) that had been maintained in parallel under nonselection conditions. (B) Lifespans of the same lines as in A, in the absence of DDT. The DDT-selected lines were shorter-lived than controls (P < 0.005 in all comparisons of selection vs. control populations, log-rank test). (C and D) Uo-GAL4 drove expression of Cyp6g1 in Malpighian tubules. This intervention increased resistance to DDT (P = 0.040 for comparison with Uo-GAL4/+ and P = 0.001 for comparison with UAS-Cyp6g1-8a/+, log-rank test) (C) but did not affect longevity (D) (P > 0.3 for all experimental lines vs. controls, log-rank test).
Fig. S3.
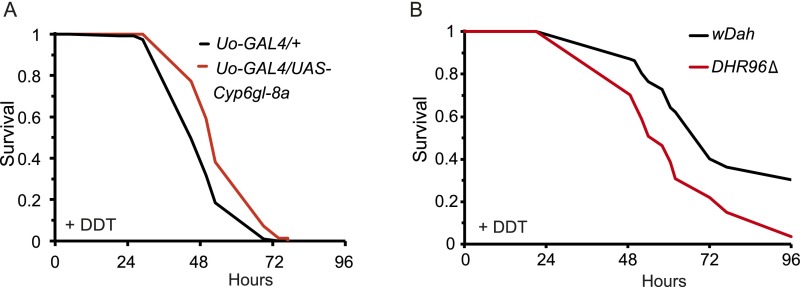
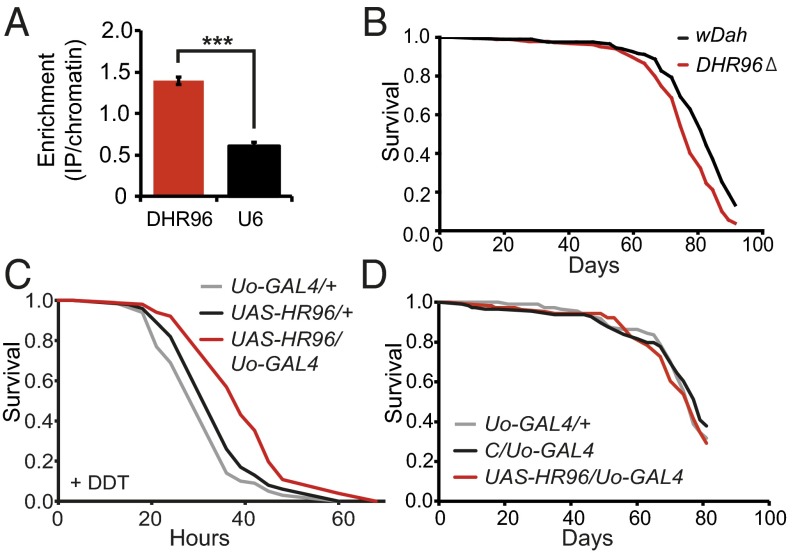
Cyp6g1-8a and DHR96 are important mediators of the response to DDT. (A) Flies overexpressing Cyp6g1-8a in the Malpighian tubules were resistant to DDT compared with driver control (P < 0.05, log-rank test; repeat of the experiment shown in Fig. 1C). (B) Flies with genetic deletion of the DHR96 gene were sensitive to DDT compared with control wild-type flies (wDah) (P < 0.05, log-rank test).
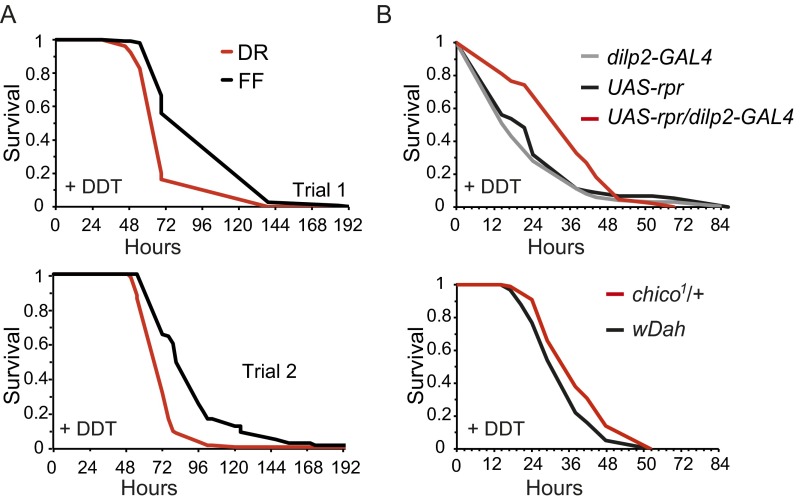
Dietary restriction (DR) increases lifespan in diverse organisms, including Drosophila (4) where the increased longevity from DR is dFOXO-independent (18). Interestingly, we found that flies subjected to DR were not resistant to DDT (Fig. S1A). This result cannot be explained by increased consumption of the DDT-dosed food by the DR flies, because DR flies do not differ from fully fed flies in food intake (19, 20). This finding demonstrates that DDT resistance is not necessary for increased longevity and is associated only with particular interventions that extend lifespan.
Fig. S1.
DR flies were not DDT-resistant, whereas IIS mutant flies showed increased DDT resistance. (A) Long-lived, dietarily restricted flies were not resistant to DDT. Age-synchronized female flies were maintained under dietary restriction (DR) or fully fed (FF) conditions as described in Grandison et al. (45). On day 7 of adult life, flies were transferred to the same food containing DDT. FF flies were significantly longer lived than DR flies under DDT stress (P < 0.001 in both trials, log-rank test). (B) Long-lived chico1-heterozygote and insulin-producing mNSC-ablated flies were resistant to DDT (for any comparison of mutant versus control in either trial of resistance to either compound, P < 0.03, log-rank test).
Transcriptional Signatures of Long-Lived IIS Mutants Identify DHR96 as Mediating Xenobiotic Resistance.
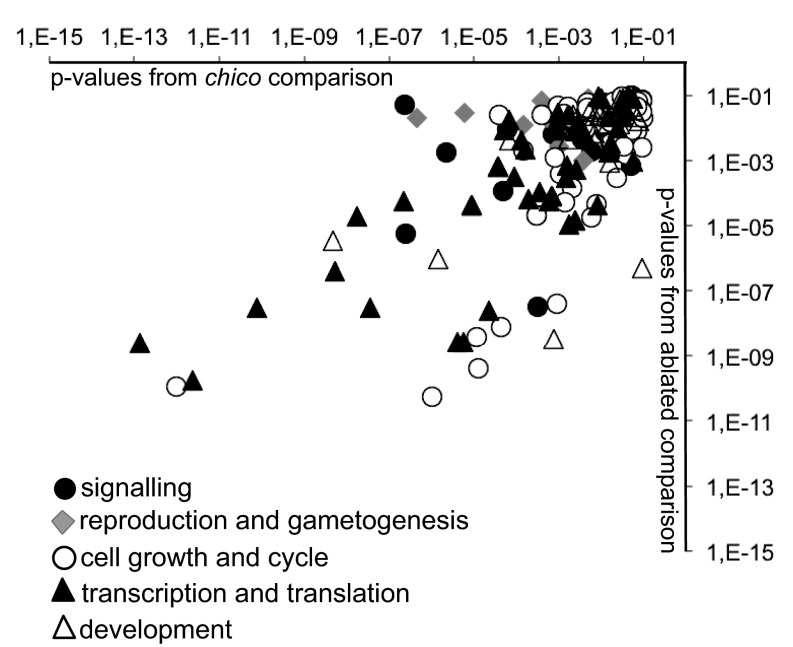
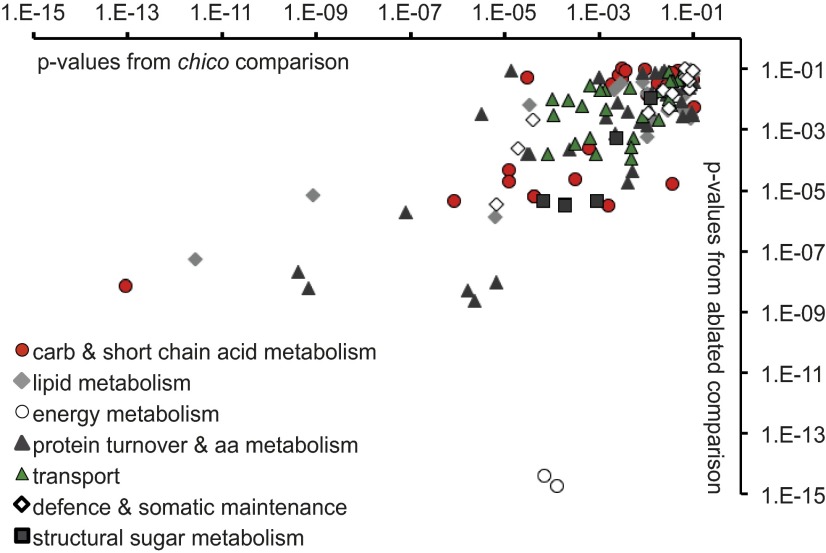
If IIS mutants are long-lived because of enhanced xenobiotic metabolism, a broader spectrum of detoxification activities than those induced by either artificial selection to one xenobiotic compound or Cyp6g1 overexpression may be necessary. To address this hypothesis, we identified candidate transcription factors that could mediate the increased resistance to xenobiotics of long-lived IIS mutant flies. We profiled transcripts from flies of two different IIS mutants: (i) ablation of median neurosecretory cells (mNSC) in the brain that produce insulin-like ligands (21) and (ii) heterozygous loss of the insulin receptor substrate chico (22). Both of these mutants exhibited increased resistance to DDT (Fig. S1B). Genes that were down-regulated in the long-lived mutants were enriched for functions in growth (including nucleic acid biosynthesis and translation), development, and reproduction including gametogenesis (Fig. S2). Genes with increased expression were enriched for functions in energy metabolism (including amino acid, carbohydrate, and lipid catabolism), protein turnover (numerous peptidases), transmembrane transport, and defense, including metabolism of toxic compounds (Fig. 2). These changes in gene expression correlate well with the phenotypes of IIS mutants (7). Within the enriched defense category, 72 up-regulated genes met our significance cutoff and were associated to metabolism of toxic compounds (Dataset S1). The majority of these genes were regulated in response to heterozygous loss of chico (55 in total) with the remainder regulated in mNSC-ablated flies. In concordance with previous comparative studies (13) we detected clear differences between the transcriptional profiles, although the overlap between them was significant.
Fig. S2.
CATMAP categories from microarray data for chico and mNSC-ablated flies. Similar functional groups of genes identified by CAPMAP (44) were down-regulated in both long-lived IIS mutants. The P values for functional group changes that were found in common between the two mutants are plotted (P < 0.1), for chico1 on the x axis and the mNSC ablation on the y axis.
Fig. 2.
Functionally related changes in gene expression in IIS mutants. Microarray data from chico1 and mNSC-ablated females were analyzed by using CATMAP, which retrieves significant changes in functionally related groups of genes (44). The P values for genes with increased expression in common between the two mutants are plotted (P < 0.1, chico1 compared with wild-type Dahomey control, mNSC-ablated flies compared with UAS-rpr control), where one data point represents a single functionally related gene, and the genes are labeled with the higher-level categories shown in the legend. P values from the chico1 comparison are plotted on the x axis, those from the mNSC-ablation comparison are on the y axis. The equivalent data for genes with lower expression in common in the two mutants are shown in Fig. S2.
Using the program Clover (23), we identified overrepresented transcription factor binding sites (Table S1) in the promoters of genes with altered expression. Most of the putative, cognate transcription factors have documented roles in development, but only a few have known roles in adult flies. Despite this pattern, transcripts of all but two of the genes encoding these transcription factors (CG10348 and Grn) were expressed at reliably detectable levels during adulthood. Of these transcription factors, two groups are involved in immunity (the GATA-binding and AP-1 transcription factors), in accordance with the enriched GO category in the IIS mutants and the resistance to bacterial infections of chico1 mutant flies (24). We also identified a binding site corresponding to the sequence bound by mammalian pregnane X receptor (PXR) (25, 26), a nuclear receptor that regulates multiple genes involved in the metabolism of endobiotic and xenobiotic toxins (27). This PXR binding site was enriched near genes with higher expression in both long-lived IIS mutants, including those genes with a proposed role in toxin metabolism (Dataset S2).
Table S1.
Transcription factor binding sites found overrepresented in the promoters of genes with higher expression in the long-lived IIS mutant flies
| TRANSFAC | Drosophila TF binding to site | Function summary |
| AP-1 | Jra Kayak | Cytoskeletal rearrangement in development, immune response, wound healing |
| DR1 | PPAR ortholog unknown in flies | Control aspects of fat tissue formation and metabolism in mammals |
| Evi-1 | Putatively CG10348 and Hamlet | Neuronal development |
| GATA | Pnr, Srp, Grn, GATAd GATAe | Hematopoeisis, cardiac development, endoderm development and adult immunity |
| HNF4 | Hnf4 | CNS development |
| Pbx | Extradenticle | Developmental leg patterning |
| PXR | DHR96 | Req for normal regulation of detox enzymes |
| TFAM | mtTF A | mitDNA replication and maintenance |
| TTF1 | Putatively Vnd | Ventral nerve system development |
| Zeste | Zeste | Regulation of homeotic genes |
Known proteins or predicted orthologs of proteins binding to DNA element. See also Dataset S2.
PXR is phylogenetically related to Drosophila DHR96, one of 18 nuclear receptors in flies (28). Interestingly, null mutation in DHR96 causes flies to become lean and sensitive to treatment with xenobiotic toxins (29, 30). DHR96 is also a direct target of dFOXO, which is required for basal transcript levels of DHR96 (10). We validated the previously published dFOXO chromatin immunoprecipitation (ChIP) binding data by quantitative PCR (qPCR) and found, compared with U6 control (a nonpolII-transcribed gene), a significant enrichment of DNA neighboring DHR96 in samples immunoprecipitated with a dFOXO antibody (Fig. 3A). Thus, the loss of resistance to xenobiotics in IIS mutant flies lacking dFOXO could be attributable to loss of normal expression of DHR96. DHR96 was thus selected as a candidate for mediating the enhanced xenobiotic resistance of IIS mutants.
Fig. 3.
DHR96 is a direct target of dFOXO and required for normal xenobiotic response and lifespan. (A) Relative enrichment of chromatin immunoprecipitated with a dFOXO-specific antibody. Higher levels in the precipitate of DNA neighboring DHR96 versus U6, a nonpolII-transcribed gene, indicate direct binding of dFOXO to DNA adjacent to the gene (P < 0.001, Welch t test). Relative enrichment was calculated as proportion of chromatin recovered in the IP for each region divided by the average of the two regions (HR96 and U6) for each chromatin (arbitrary scale). (B) Genetic deletion of DHR96 modestly decreased lifespan of female flies (P < 0.0001, log-rank test). (C and D) Tissue-specific overexpression of DHR96 in the Malpighian tubules (Uo-GAL4 driver) increased DDT resistance (C; P < 0.005, log-rank test), but did not affect lifespan (D).
DHR96 Mediates Xenobiotic Resistance of IIS Mutants.
We first investigated the role of DHR96 in xenobiotic resistance of adult flies. We subjected mutant DHR96 null flies (29) to treatment with DDT and found them to be sensitive (Fig. S3). In contrast, removal of DHR96 caused only a mild reduction in lifespan under nonstressed conditions (Fig. 3B). Ubiquitous overexpression of DHR96 resulted in developmental lethality (Fig. S4), but overexpression in the Malpighian tubules increased resistance to DDT (Fig. 3C), without affecting lifespan (Fig. 3D), again showing that an increase in DDT resistance does not necessarily increase longevity. DHR96 thus has an important role in xenobiotic metabolism of adult flies.
Fig. S4.

Constitutive overexpression of DHR96 in the whole body caused developmental lethality. Overexpression of DHR96 using the daughterless-GAL4 driver resulted in lethality in different stages of Drosophila development and few survivors. Flies reared at 18 °C showed increased survival.
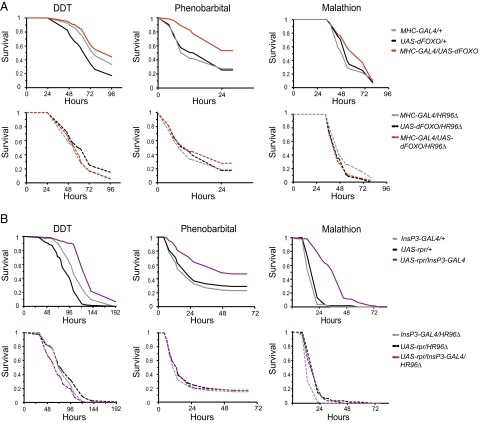
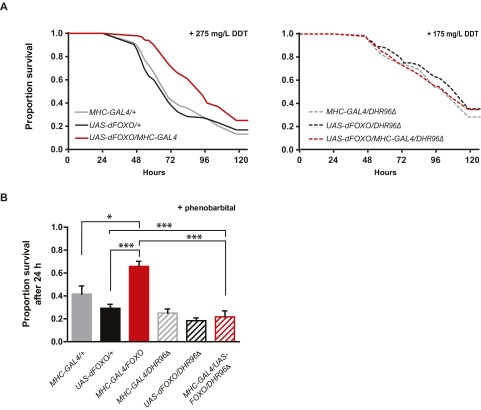
To test whether DHR96 mediates the xenobiotic resistance of IIS mutant flies, we introduced a DHR96 null mutant into two IIS mutants: overexpression of dFOXO in muscle (31) or targeted deletion of the mNSC cells (32). Overexpression of dFOXO (Fig. 4A, repeated experiment in Fig. S5) and targeted ablation of the insulin-like peptide-producing mNSC cells (Fig. 4B, repeated experiment in Fig. S6) both significantly increased resistance to the xenobiotics DDT, phenobarbital (PB), and malathion. Strikingly, this resistance to all three xenobiotics was lost in a DHR96 null background (see Table S2 for Cox Proportional Hazards statistics). DHR96 is thus a key mediator of the enhanced xenobiotic resistance of long-lived IIS mutants.
Fig. 4.
Analysis of the effects of DHR96 on the xenobiotic resistance of two IIS mutants. (A) Muscle-specific overexpression of dFOXO significantly enhanced resistance to DDT, phenobarbital, and malathion compared with control lines (Upper, log-rank test, P values for all comparisons with the matching driver and UAS lines <0.001, except for comparison of DDT resistance of dFOXO overexpressors with the MHC-GAL4 line, P = 0.61). Enhanced resistance was lost, when dFOXO was overexpressed in a DHR96 null background (Lower; P values for all comparisons with the matching driver and UAS lines >0.05). Cox proportional hazards (CPH) was used to test for a statistical interaction between the effects of dFOXO overexpression and genomic deletion of DHR96, and revealed that each significantly affected stress resistance, with a significant interaction between them (P < 0.01; Table S2). (B) Deletion of the mNSC cells significantly enhanced resistance to the three xenobiotics (Upper, log-rank test, P values for all comparisons with the matching driver and UAS lines <0.001), and this resistance was lost in a DHR96 null background (P values for all comparisons with the matching driver and UAS lines >0.05). CPH analysis revealed a significant interaction between the effect of mNSC ablation and genomic DHR96 deletion, indicating that xenobiotic resistance was significantly blocked by the genomic deletion of DHR96 (CPH, P < 0.001; Table S2).
Fig. S5.
Repeat xenobiotic stress assays with dFOXO overexpressing flies in wild-type and DHR96 null background. (A) dFOXO overexpressing flies were resistant to DDT (Left; 275 mg/L DDT, P values for all comparisons with the matching driver and UAS lines <0.001, log-rank test), whereas dFOXO over-expression in a DHR96 null background did not increase DDT resistance (Right; 175 mg/L DDT, P values for all comparisons with the matching driver and UAS lines >0.05). (B) dFOXO overexpressing flies in a wild-type or DHR96 null background were exposed to PB. dFOXO overexpression increased PB resistance, which entirely depended on the presence of DHR96. Two-way ANOVA revealed a significant interaction term (P = 0.016 for two-way ANOVA against the driver control and P = 0.0005 against the UAS control). Individual pairwise comparisons used Tukey’s multiple comparisons test (*P < 0.05; **P < 0.01; ***P < 0.001).
Fig. S6.
Repeat xenobiotic stress assay with mNSC-ablated flies in wild-type and DHR96 null background. Ablation of mNSCs enhanced DDT resistance but this resistance was lost when mNSCs were ablated in a DHR96 null background because two-way ANOVA revealed a significant interaction term (P = 0.0004 for two-way ANOVA against the driver control and P < 0.0001 against the UAS control). Individual pairwise comparisons used Tukey’s multiple comparisons test (*P < 0.05; **P < 0.01; ***P < 0.001).
Table S2.
Cox Proportional Hazard statistics
| Relevant figure | Experiment | Coefficient | Estimate | SE | P value |
| Fig. 4A | Stress assay | dFOXO oe status | 0.0967 | 0.0433 | 0.0237 |
| DDT | DHR96 status | 0.4358 | 0.0441 | <0.0001 | |
| DHR96 status > dFOXO oe status | −0.1329 | 0.0433 | 0.0021 | ||
| Stress assay | dFOXO oe status | 0.2411 | 0.0490 | <0.0001 | |
| Phenobarbital | DHR96 status | 0.2622 | 0.0490 | <0.0001 | |
| DHR96 status > dFOXO oe status | −0.1377 | 0.0489 | 0.0046 | ||
| Stress assay | dFOXO oe status | 0.0846 | 0.0506 | 0.0902 | |
| Malathion | DHR96 status | 0.4224 | 0.0511 | <0.0001 | |
| DHR96 status > dFOXO oe status | −0.1816 | 0.0507 | 0.0003 | ||
| Fig. 4B | Stress assay | mNSC ablation status | −0.425 | 0.0727 | <0.0001 |
| DDT | DHR96 status | 0.9092 | 0.0730 | <0.0001 | |
| DHR96 status > mNSC status | 0.5854 | 0.0726 | <0.0001 | ||
| Stress assay | mNSC ablation status | −0.2000 | 0.0521 | <0.0001 | |
| Phenobarbital | DHR96 status | 0.4428 | 0.053 | <0.0001 | |
| DHR96 status > mNSC status | 0.2014 | 0.0508 | <0.0001 | ||
| Stress assay | mNSC ablation status | −0.3484 | 0.0607 | <0.0001 | |
| Malathion | DHR96 status | 0.3035 | 0.0586 | <0.0001 | |
| DHR96 status > mNSC status | 0.2694 | 0.0599 | <0.0001 | ||
| Fig. 6A | Lifespan assay | dfoxo oe status | 0.2583 | 0.0362 | <0.001 |
| DHR96 status | 0.2009 | 0.0362 | <0.001 | ||
| DHR96 status > dfoxo oe status | 0.0662 | 0.0356 | 0.0909 | ||
| Fig. 6B | Lifespan assay | mNSC ablation status | −0.309 | 0.0383 | <0.001 |
| DHR96 status | 0.1518 | 0.0369 | <0.001 | ||
| DHR96 status > mNSC status | −0.0377 | 0.0368 | 0.3055 |
Interaction was tested between the effect of reduced IIS [dFOXO overexpression (oe) status or mNSC ablation status] and the effect of DHR96 gene deletion (DHR96 status). The estimate of the coefficient states the natural log of the hazard ratio. A beneficial effect on survival is displayed by a negative value. “>” in column 3 indicates that interaction between two status was tested.
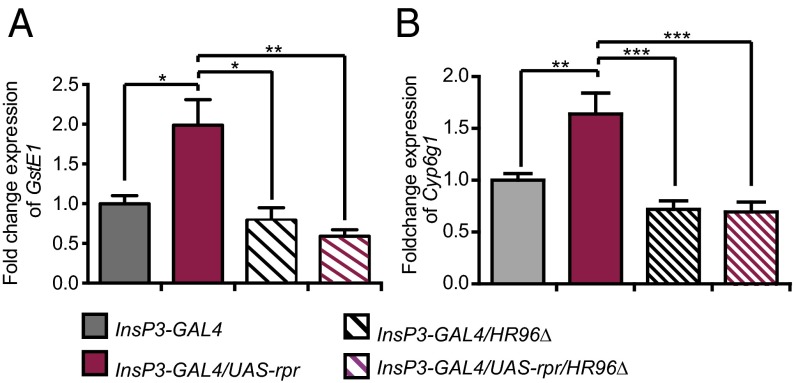
If DHR96 mediates xenobiotic resistance of IIS mutant flies, then it should regulate expression of genes directly involved in xenobiotic metabolism in the tissues responsible for detoxification. With the help of the software tool FIMO (33), we identified the putative binding motif of DHR96 six times in the flanking region of the gluthatione S transferase gene GstE1 (region 2 kb upstream and 2 kb downstream of the gene, P ≤ 0.00096) and 10 times in the flanking region of the cytochrome P450 gene Cyp6g1 (P ≤ 0.00096). Furthermore, GstE1 and Cyp6g1 expression is induced by PB (29). We therefore investigated the role of IIS and DHR96 in regulating their expression in gut and Malpighian tubules. GstE1 and Cyp6g1 were both up-regulated in mNSC-ablated flies (Fig. 5 A and B) but not in dFOXO overexpressors (Fig. S7A). The up-regulation of GstE1 and Cyp6g1 in mNSC-ablated flies was lost in a DHR96 null background, suggesting the response was DHR96-dependent (P = 0.027 for GstE1 and P = 0.011 for Cyp6g1, two-way ANOVA; Fig. 5 A and B). DHR96 thus mediated the increased expression of both detoxification genes.
Fig. 5.
DHR96 mediates the increased expression of detoxification genes in IIS mutants. mRNA expression of GstE1 (A) and Cyp6g1 (B) in the gut of mNSC-ablated flies was assessed by qRT-PCR to determine whether it was regulated by IIS or DHR96. Results represent fold changes in mRNA levels relative to the InsP3-GAL4 control (mean ± SEM). GstE1 and Cyp6g1 were significantly up-regulated in mNSC-ablated flies in a wild-type but not a DHR96 null background. Two-way ANOVA revealed a significant interaction term (P = 0.027 for GstE1 and P = 0.011 for Cyp6g1) with the response of both genes in the mNSC-ablated flies being entirely dependent on DHR96 (n ≥ 4). Individual pair-wise comparisons used Tukey’s multiple comparisons test (*P < 0.05, **P < 0.01, ***P < 0.001).
Fig. S7.
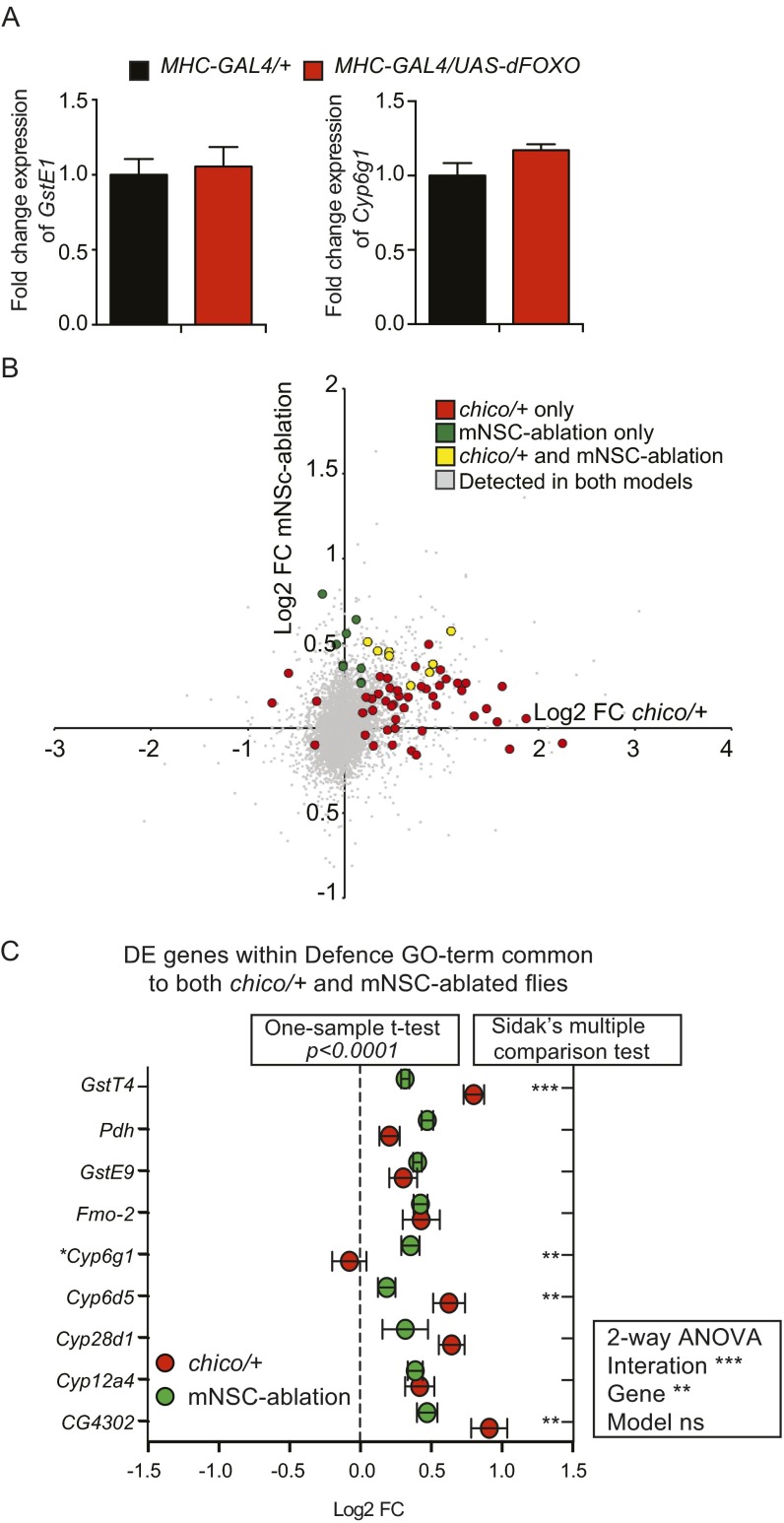
Regulation of detoxification genes by IIS is both common and model-specific. (A) Fold changes in mRNA expression of GstE1 and Cyp6g1 in guts and Malpighian tubules was assessed by qRT-PCR in dFOXO overexpessing flies and driver controls. dFOXO overexpression did not affect mRNA expression of either gene (P > 0.05 for both GstE1 and Cyp6g1, Student’s t test). (B) Correlation of fold changes in expression of genes within the GO term Defense in chico/+ and mNSC-ablated flies. Fifty-five genes were differentially regulated in chico/+ (green) and 17 in mNSC-ablated flies (red) with 8 being regulated in both datasets (yellow) with a significant overlap between them (P = 0.0085, Fisher’s exact test). (C) Differentially expressed (DE) genes within the GO term Defense common to both chico/+ and mNSC-ablated flies were generally up-regulated in both mutants (P < 0.0001 for both mutants, one-sample t test). Expression changes were significantly different for specific genes in the two mutants as revealed by Sidak’s multiple comparison test (P < 0.01). Two-way ANOVA revealed a significant interaction term between differentially regulated genes and the mutant genotype (P < 0.0001), showing that the two mutants produced different changes in expression of genes within the GO term Defense. *Although only differently regulated in mNSC-ablated flies, data for Cyp6g1 are included in this figure because this gene enhanced xenobiotic resistance when overexpressed in the Malpighian tubules (Fig. 1C and Fig. S3).
To further investigate the differences in expression of genes involved in xenobiotic metabolism in different IIS mutants, we reinterrogated our chico/+ and mNSC-ablated array data. In total, 72 genes associated to xenobiotic response were regulated in at least one array dataset, with the majority of those genes being up-regulated (Fig. S7B), indicating a common functional response across different models. However, the two models show overlapping, but distinct, transcriptional profiles, 55 genes were regulated in the heterozygous chico flies, and 17 in the mNSC-ablated flies, with only 8 being regulated in both (Fig. S7C). Two-way ANOVA of these common genes confirmed a significant (P < 0.0001) interaction, showing a mutant-specific response to reduced IIS. Our qPCR data, together with the statistical analysis of the microarray data, thus demonstrate that reduced IIS can induce cellular detoxification by regulation of both common and distinct sets of genes, as is also the case for IIS mutants in different model organisms (13).
DHR96 Does Not Mediate the Increased Lifespan of IIS Mutant Flies.
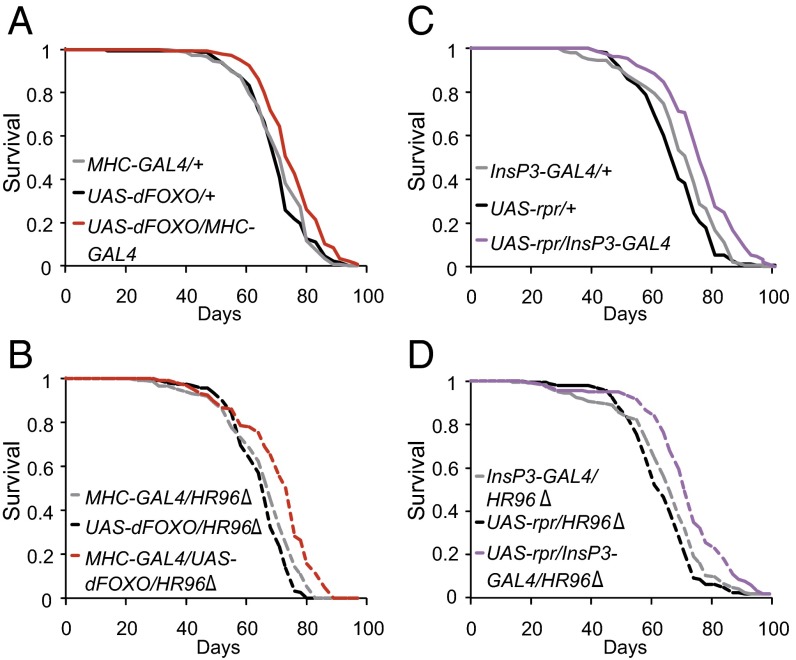
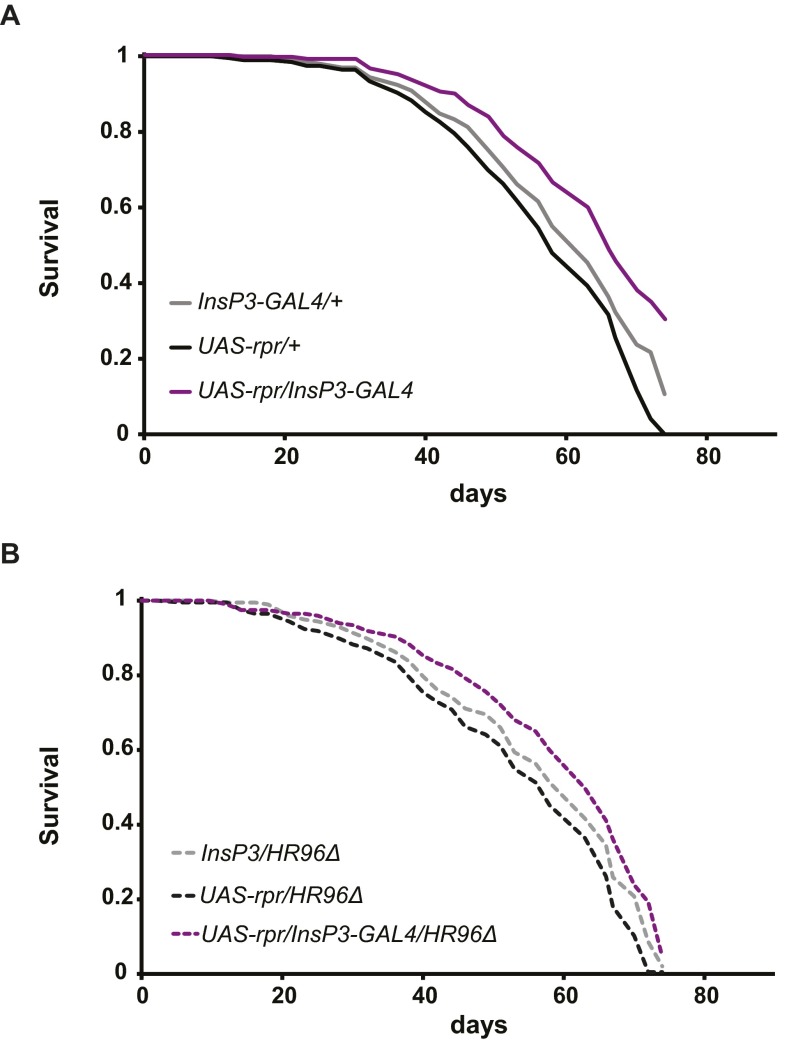
To determine whether the increased lifespan of IIS mutant flies was mediated by DHR96, we measured adult survival of flies with dFOXO overexpression in muscle or ablation of the mNSC, in the presence or the absence of DHR96. Consistent with published data (31), muscle-specific overexpression of dFOXO significantly extended lifespan compared with controls (Fig. 6A; see Table S2 for Cox Proportional Hazards statistics). However, this lifespan extension was unaffected by null mutation of DHR96 (Fig. 6B). Lifespan was also significantly increased by the ablation of mNSC cells (Fig. 6C, repeated experiment in Fig. S8A) and, again, this extension was unaffected by the absence of DHR96 (Fig. 6D, repeated experiment in Fig. S8B). DHR96 thus played no role in the extension of lifespan by reduced IIS.
Fig. 6.
Lifespan extension by lowered IIS is independent of DHR96. Lifespan of females was significantly increased by muscle-specific overexpression of dFOXO or by targeted ablation of mNSC cells in both a wild-type (A and C, respectively) and a DHR96 null background (B and D, respectively) (P values for all comparisons with the matching driver and UAS lines <0.001, log-rank test). CPH analysis revealed that genomic DHR96 and overexpression of dFOXO or ablation of mNSC each significantly affected lifespan, but these effects did not show a significant interaction (Table S2).
Fig. S8.
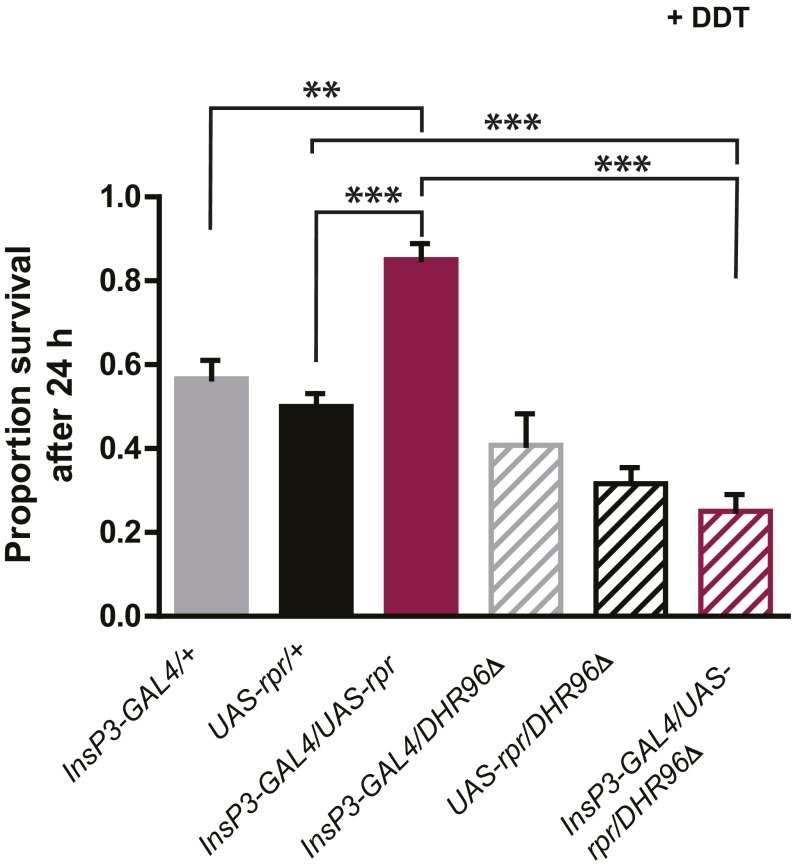
Repeat of lifespan experiment with mNSC-ablated flies in wild-type and DHR96 null background. Ablation of mNSCs significantly increased lifespan in a wild-type background (A; P values for all comparisons with the matching driver and UAS lines <0.0001, log-rank test) and this lifespan extension was not affected by DHR96 null mutation (B; P = 0.017 compared with driver control InsP3-GAL3/DHR96Δ and P < 0.001 compared with UAS control UAS-rpr/DHR96Δ, log-rank test).
Discussion
The IIS mutants used in this study showed both enhanced expression of genes involved in xenobiotic metabolism and resistance to xenobiotics. Cognate observations have led to the proposal that enhanced detoxification processes could act as an evolutionarily conserved mechanism for lifespan extension (12, 13, 15, 34). Indeed, there is evidence from both worms and flies that enhanced expression of GST-encoding genes can increase longevity (35, 36). These findings led us to investigate whether experimentally enhancing xenobiotic detoxification could also promote longevity. However, although artificial selection for DDT resistance and overexpression of the cytochrome P450 Cyp6g1 in a key detoxification tissue, the Malpighian tubule, both increased DDT resistance, neither intervention increased lifespan and, indeed, artificial selection even decreased lifespan. Such costs of selection-induced insecticide resistance have been reported (37). However, dietary restriction increased fly lifespan but not DDT resistance. Thus, xenobiotic resistance and lifespan could clearly be uncoupled from each other.
A search for binding motifs of transcription factors differentially regulated in IIS mutants revealed a significantly enriched sequence corresponding to the binding site of mammalian PXR (Pregnane X receptor), the homolog of Drosophila DHR96. We also confirmed DHR96 as a direct target of dFOXO, which is required for basal transcript representation of DHR96. We confirmed the sensitivity to xenobiotics of DHR96 null mutant flies and showed that they are also also short-lived, both characteristics shared by dFOXO null mutants. Overexpression of DHR96 in the Malpighian tubules increased DDT resistance, demonstrating the role of DHR96 in mediating xenobiotic resistance in adult flies. Interestingly, however, DHR96 overexpression did not increase lifespan, again showing that the two traits can be uncoupled. We showed that DHR96 mediates the resistance of IIS mutants to the xenobiotics that we tested, because this resistance was completely lost when DHR96 was absent. Furthermore, we demonstrated, using microarray data, that detoxification genes are up-regulated in two different models of reduced IIS and that up-regulation of two of these genes in mNSC-ablated flies depends on DHR96. Interestingly, the up-regulated genes were model-specific, but coalesced into a protective response evident in the resistance to the three xenobiotics that we tested. These model-specific differences agree with previously published studies that have led to the proposal that enhanced detoxification processes could act as an evolutionarily conserved mechanism for lifespan extension (12, 13, 15, 34). Interestingly, the mammalian DHR96 homologs CAR (constitutive androstane receptor) and PXR are also key regulators of phenobarbital-induced xenobiotic response (38, 39), but it is not yet known whether they function downstream of IIS. It will be important to investigate whether the increased expression of genes involved in xenobiotic metabolism and xenobiotic resistance of long-lived mammals is causal in their increased lifespan.
Importantly, we showed that, at least for the three xenobiotics that we tested, the increased xenobiotic resistance and lifespan of IIS mutants are independently mediated traits with no direct, causal connection between them. Increased expression of genes involved in xenobiotic metabolism together with xenobiotic resistance are, nonetheless, common correlates of lifespan extension (7, 10, 11, 13–15), raising the question of why this association is so frequent. Interestingly, genes involved in xenobiotic metabolism are indirectly activated by toxic by-products of microbes and pathogens, through the cellular surveillance-activated detoxification and defense (cSADD) system (40), which senses xenobiotics through the dysfunction in cellular processes that they cause, including decreased host translation and altered metabolism (41). Importantly, microbes and pathogens can alter metabolism in the gut, resulting in lower IIS (42). Organisms may hence have evolved systems to sense lowered IIS as an indirect signal of the presence of pathogens and mount cSADD as a defense response, thus inducing a form of hormesis. Many of the interventions that can increase lifespan involve altered signal transduction of pathways linked to metabolism, and activation of cSADD could provide a common mechanism.
Materials and Methods
Fly Strains and Maintenance.
The control white Dahomey (wDah) was derived by backcrossing w1118 into the outbred, wild-type Dahomey background. All transgenic lines were maintained with periodic backcrossing into wDah and are summarized in Table S3. The DHR96 null mutant was a generous donation by Carl Thummel, Department of Human Genetics, University of Utah, Salt Lake City. Generation of mNSC-ablated flies and construction of transgenic lines and of DDT selection lines is described in SI Materials and Methods, sections 1–3.
Table S3.
Drosophila strains and transgenic lines
| Fly strain | Source | Details |
| Wild-type, balancer and mutant flies | ||
| White Dahomey wolbachia plus (wDah w+) | 12 | Wild-type Drosophila stock |
| wDah w+; CyO | Bloomington Drosophila Stock Center | Balancer fly on the second chromosome, homozygous lethal, Curly wings |
| wDah w+;; TM3Sb | Bloomington Drosophila Stock Center | Balancer fly on the third Chromosome, homozygous lethal |
| wDah w+; chico1 | 13 | A Drosophila insulin receptor substrate protein |
| wDah w+;; DHR96Δ | 1 | DHR96 null mutation on the third chromosome |
| GAL4 driver lines | ||
| wDah w+;; mhc-GAL4 | Bloomington Drosophila Stock Center | Muscle-specific driver, chromosome 3 |
| wDah w+;; mhc-Gal4/ DHR96 Δ | This study | Muscle-specific driver in a DHR96 null background |
| wDah w+; dilp2-GAL4 | 14 | mNSC-specific driver (median neurosecretory cell) |
| wDah w+;; InsP3-GAL4 | 15 | mNSC-specific driver (median neurosecretory cell) |
| wDah w+;; InsP3-GAL4/DHR96 Δ | This study | mNSC-specific driver (median neurosecretory cell) in a DHR96 null background |
| wDah w+; Uo-GAL4 | 16 | Malpighian Tubule-specific driver |
| UAS-responder lines | ||
| wDah w+; UAS-Cyp6g1-8a | 17 | Cytochrome P450 6g1 |
| wDah w+; UAS- DHR96-WT | This study | Wild type UAS-DHR96 line on chromosome 2 |
| wDah w+; UAS-dFOXO | 18 | dFOXO inserted through attp40 sites into the second Chromosome |
| wDah w+; UAS-dFOXO; DHR96Δ | This study | UAS-dFOXO in a DHR96 null background |
| wDah w+, UAS-rpr | 14 | UAS-reaper |
| wDah w+, UAS-rpr;; DHR96Δ | This study | UAS-reaper in a DHR96 null background |
Lifespan Measurement.
Lifespans were performed as described in Bass et al. (43). Lifespan experiments included 100–200 female flies per genotype that were allowed to mate for 48 h before the start of the experiment and transferred to fresh food three times weekly. Experiments were performed at least twice with the exception of the dFOXO overexpression epistasis experiment (Fig. 6), which was performed only once. Lifespan measurements and statistical analyses are described in SI Materials and Methods, section 4.
Stress Assays.
Flies for stress assays were prepared in the same way as for lifespan experiments. At least 100 females from each cross were sorted into wide plastic vials, 20 flies per vial containing 1× sugar/yeast/agar (SYA) food, and transferred to fresh food three times a week. Stress resistance was assayed at age 10 d. Stock solutions of DDT (Greyhound) and phenobarbital (Sigma Aldrich) were dissolved in ethanol, and stock solution of malathion (FLUKA) was dissolved in isopropanol. Final concentration was 175 mg/L or 275 mg/L for DDT (see SI Materials and Methods, section 5 for details), 5% (wt/vol) for phenobarbital, and 7.5 µM for malathion. Nearly all stress assays were performed twice; independent repeats of the experiments are in SI Materials and Methods.
Microarrays.
In total, cRNA derived from five biological replicates of each IIS mutant genotype and control (Dahomey; chico1/+, UAS-rpr/+, and UAS-rpr/dilp2-Gal4) were hybridized to Quintuplicate Affymetrix Dros2 microarrays. We chose a q value <0.15 as significance cutoff to consider a gene to be differentially regulated. A detailed description of the microarray experimental procedures and data analysis is summarized in SI Materials and Methods, section 6.
Chromatin Immunoprecipitation.
ChIP was performed on three biological repeats of chromatin as described in refs. 10, 13, and 22, and DNA was quantified by qPCR using the primers Hr96 56 (CAAAGAGAGCATATTTAGGATACCAAG) with Hr96 36 (CACAGAACCCAC GCTTCCAAG).
Quantitative Real-Time PCR.
For the gene expression analysis of GSTE1 and Cyp6d5, guts including Malpighian tubules of 10–15 female flies per sample were dissected and expression was quantified by qPCR using Taqman probes (Applied Biosystems) for GstE1 (Dm01826984), Cyp6g1 (Dm01819889), Actin5C (Dm02361909), and Rpl32 (Dm02151827) using the ΔΔCt method, n ≥ 3 for all experiments.
SI Materials and Methods
Fly Strains and Maintenance.
Flies were kept in glass bottles (13.5 cm × 6 cm diameter) on a standard 1× sugar/yeast/agar (SYA) medium in a controlled temperature room with a 12:12 light:dark cycle, 65% humidity, and a temperature of 18 °C for stock maintenance and 25 °C for experiments. mNSC-ablated flies were generated by crossing UAS-reaper to dilp2-GAL4 (21) for the microarray experiments (Fig. 2) and stress assays (Fig. S1) or by crossing UAS-reaper to InsP3-GAL4 (32) for the qPCR (Fig. 5), stress assays (Fig. 4 and Figs. S5 and S6) and lifespan measurements (Fig. 6). See Table S3 for a list of fly stocks used in this study.
Construction of Transgenic Lines.
Construction of UAS-DHR96.
Cloned DHR96 coding sequence (kind gift from Tony Southall, Department of Life Sciences, Imperial College London, South Kensington Campus, London) was used as a template to PCR amplify the wild-type DHR96 coding sequence (HR96), using the following primers:
Hr96-51- NotI (ACGCGGCCGCATGTCGCCGCCGAAGAAC)
Hr96-31Stop-XbaI (GTCTAGACTAGTGATTTTTCAAATCGAATATTTC)
PCR product was inserted into the pUAST vector via the restriction sites NotI and XbaI. pUAST-DHR96 was injected into Drosophila embryos and resultant UAS-DHR96 transgenics were backcrossed for at least eight generations into the wDah wolbachia+ background.
Generation of UAS-dFOXO and of MHC-GAL4 in a DHR96 null background.
UAS-dFOXO is inserted at the attp40 locus on the second chromosome, and flies are marked with the mini white gene and balanced over CyO. The deletion in DHR96 null flies is located on the third chromosome and mutants are white-eyed, but marked with GFP-expressing eyes (29) and balanced over TM3Sb. Positive UAS-dFOXO; DHR96 null were identified by orange, GFP expressing eyes and were crossed to homozygosity.
Both MHC-GAL4 and DHR96 null are on the third chromosome and were recombined. Both were balanced over TM3Sb before recombining them. After screening for GFP, positive +; MHC-GAL4/DHR96 null were crossed to homozygosity.
Generation of UAS-reaper and InsP3-GAL4 in a DHR96 null background.
The crossing for InsP3-GAL4 in a DHR96 null background was performed as for the MHC-GAL4; DHR96 null, as the driver is inserted on the third chromosome. UAS-reaper is integrated into the X chromosome and was maintained over FM6.
Construction of DDT Selection Lines.
From our large population cages containing wDah wolbachia+, six groups of several hundred flies were removed and randomly assigned to one of six new population cages. Every week, three bottles containing 30 mL of fresh food (1× SYA Brewer’s) were introduced into the cages and the three oldest bottles removed. This procedure was continued throughout selection so that at all times each cage contained 11 bottles of different ages: three within 1 wk old, three between 1 and 2 wk old, three between 2 and 3 wk old and two between 3 and 4 wk old. Three control cages were always fed normal food, whereas the three selection cages were fed food containing 1× SYA Brewer’s containing DDT at increasing concentrations over time. During the course of 5 mo, the DDT dose was incremented in the following steps (wt/vol food): 0.001%, 0.0025%, 0.005%, 0.006%, 0.008%, 0.01%, 0.012%, 0.015%, 0.018%, and 0.021%. During the transition from 0.018 to 0.021%, one of the treatment populations died out.
Lifespan Experiments.
Experimental flies were raised at a density of 200–300 flies per bottle containing 70 mL 1× SYA medium. Upon emergence, flies were transferred to fresh bottles for 48 h to standardize mating status. Subsequently, females were counted for experiments under light CO2 anesthesia and transferred to glass vials, 10 flies per vial, and transferred to fresh food three times weekly. Statistics were performed by using JMP statistical software (SAS Institute). Differences in death rates at all ages were assessed by log-rank test, and significance for values of maximum lifespan (final surviving 10% for each population) was assessed by the nonparametric median test. Cox Proportional Hazards was performed in JMP to test for an interaction between IIS and DHR96. The model included two covariates in all analyses: the status of reduced IIS (dFOXO overexpression or mNSC ablation status versus controls) and DHR96 status (wild-type DHR96 versus DHR96 null).
Stress Assays.
All xenobiotics were added to 1× SYA food after cooling it down to 55 °C. Flies exposed to drugs were not tipped into new vials because they died within a few days and no progeny developed. Dead flies were counted every 4–8 h. Measurement was stopped when flies were dead or response to xenobiotic ceased.
Note.
In the first stress assay where flies were treated with DDT (Fig. S5), we used two different concentrations for dFOXO overexpressors in a wild-type background (275 mg/L) and dFOXO overexpressors in a DHR96 null background (175 mg/L). For DHR96 mutant flies, a lower DDT concentration was used because they were known to be sensitive (29) and we were afraid that we would not see differences between dFOXO overexpressors in a DHR96 null background and the uninduced controls in a DHR96 null background. But even with this low concentration, we were unable to detect a protective effect of dFOXO overexpression and, therefore, decided to use the standard DDT concentration (275 mg/L).
Microarray Data Analyses.
Experimental procedure.
For sampling, flies were snap-frozen in liquid nitrogen at 3:00 PM on day 7 after eclosion. For each array, RNA from 20 to 30 whole flies was extracted by using TRIzol (Gibco) and purified with RNeasy columns (Qiagen) following the manufacturer’s instructions. The quality and concentration of RNA was confirmed by using an Agilent Bioanalyzer 2100 (Agilent Technologies), and further procedures followed the standard Affymetrix protocol. All samples were hybridized to the Drosophila Genome 2.0 Genechip in quintuplicates.
Data analysis.
All individual probes were mapped against all known and predicted transcripts of the D. melanogaster genome release version 5.4. This mapping allowed for up to one alignment error for either perfect match or mismatch of each individual probe, and a composite score was calculated for each probe set. This strategy allowed each probe set to be assigned a qualitative category: perfect (all probes match a single target gene with no mismatches), promiscuous (some or all probes within a probe set map to more than one gene in the genome), weak (the probe set maps to a single gene, but some probes may have mismatches or may not map to the gene), or orphan (no probes in the probe set map to any known or predicted gene in the genome). Both promiscuous and orphan probe sets were excluded from further analysis. FlyBase gene IDs were mapped to Gene Ontology (GO) IDs (version 1.107).
Raw data (cel files) were processed to correct for probe-sequence biases, and R's implementation of the Affymetrix’s MicroArray Suite 5.0 software was used to determine present target transcripts (46). A transcript was considered present if the P value was <0.111, and absent otherwise. The data were normalized by eight different methods (47), and the statistical analysis of each normalization was combined to identify a robust set of differentially expressed genes. The R code from ref. 46 was altered to exclude absent probe sets before the final Loess normalization to reduce the number of false positives associated with the absent probe sets.
Because lowered IIS can extend lifespan without reduction of fertility (48), we removed ovary-specific transcripts after the first round of normalization. Ovary-specific transcripts were identified as follows. Tissue-specific Affymetrix array data from 11 tissues dissected from the adult fly were downloaded from the FlyAtlas webpage (49). As above, the raw data were preprocessed to correct for probe-sequence biases, and R's implementation of the Affymetrix’s MicroArray Suite 5.0 software was used to determine present target transcripts (46). A probeset was considered to be ovary specific if it was called present in ovary but not in any of the other tissues. The microarray data for chico1 heterozygotes have been reported in ref. 13, but were reanalyzed here to account for software updates. The data for the mNSC-ablated flies was generated for this study. We also analyzed chico1 homozygous flies, which show a great lifespan extension than do heterozygotes, but the effect of the mutant on the transcriptome was so large that the array data could not be normalized adequately for comparison with any of the other groups.
For functional analysis using all expressed genes, we used the Wilcoxon rank sum test in CATMAP (44). Ranks of genes were based on the Bayes t statistic for differential expression and, for a given functional category, the significance of the rank sum for all genes in the category was calculated analytically based on a random gene-rank distribution.
The Clover program (23) was used to identify overrepresentation of TRANSFAC (26) motifs in the 1,000 bp upstream of the transcriptional start site, as defined by Ensembl (50).
Supplementary Material
Acknowledgments
We thank Dr. Paul Essers for helping with the statistical analysis of microarray data, Dr. Carl Thummel and Dr. Kirst King-Jones for the kind donation of the DHR96 null flies, Dr. Susan Broughton for helping with fly work, and Dr. Adam Antebi and Dr. Dan Magner for advice and scientific discussion. We acknowledge funding from the Max Planck Society; The Royal Society, UK Grants UF100158 and RG110303; the Biotechnology and Biological Sciences Research Council, UK Grant BB/I011544/1; MaxNetAging (J.M.H.); Bundesministerium für Bildung und Forschung Grant SyBACol 0315893A-B (to L.S.T. and L.P.); European Research Council under the European Union’s Seventh Framework Programme Grant FP7/2007-2013/ERC Grant Agreement 268739; and Wellcome Trust Strategic Award (WT081394).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1515137113/-/DCSupplemental.
References
- 1.Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 2.Partridge L, Thornton J, Bates G. The new science of ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366(1561):6–8. doi: 10.1098/rstb.2010.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broer L, et al. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J Gerontol A Biol Sci Med Sci. 2015;70(1):110–118. doi: 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 7.Slack C, Giannakou ME, Foley A, Goss M, Partridge L. dFOXO-independent effects of reduced insulin-like signaling in Drosophila. Aging Cell. 2011;10(5):735–748. doi: 10.1111/j.1474-9726.2011.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto R, Tatar M. Insulin receptor substrate chico acts with the transcription factor FOXO to extend Drosophila lifespan. Aging Cell. 2011;10(4):729–732. doi: 10.1111/j.1474-9726.2011.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem. 2004;279(43):44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- 10.Alic N, et al. Genome-wide dFOXO targets and topology of the transcriptomic response to stress and insulin signalling. Mol Syst Biol. 2011;7:502. doi: 10.1038/msb.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amador-Noguez D, Yagi K, Venable S, Darlington G. Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell. 2004;3(6):423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 12.Steinbaugh MJ, Sun LY, Bartke A, Miller RA. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am J Physiol Endocrinol Metab. 2012;303(4):E488–E495. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McElwee JJ, et al. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8(7):R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amador-Noguez D, et al. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell. 2007;6(4):453–470. doi: 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gems D, McElwee JJ. Broad spectrum detoxification: The major longevity assurance process regulated by insulin/IGF-1 signaling? Mech Ageing Dev. 2005;126(3):381–387. doi: 10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Daborn PJ, et al. A single p450 allele associated with insecticide resistance in Drosophila. Science. 2002;297(5590):2253–2256. doi: 10.1126/science.1074170. [DOI] [PubMed] [Google Scholar]
- 17.Dow JA. Insights into the Malpighian tubule from functional genomics. J Exp Biol. 2009;212(Pt 3):435–445. doi: 10.1242/jeb.024224. [DOI] [PubMed] [Google Scholar]
- 18.Min KJ, Yamamoto R, Buch S, Pankratz M, Tatar M. Drosophila lifespan control by dietary restriction independent of insulin-like signaling. Aging Cell. 2008;7(2):199–206. doi: 10.1111/j.1474-9726.2008.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong R, Piper MD, Wertheim B, Partridge L. Quantification of food intake in Drosophila. PLoS One. 2009;4(6):e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong R, Piper MD, Blanc E, Partridge L. Pitfalls of measuring feeding rate in the fruit fly Drosophila melanogaster. Nat Methods. 2008;5(3):214–215, author reply 215. doi: 10.1038/nmeth0308-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broughton SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102(8):3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clancy DJ, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 23.Frith MC, et al. Detection of functional DNA motifs via statistical over-representation. Nucleic Acids Res. 2004;32(4):1372–1381. doi: 10.1093/nar/gkh299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libert S, Chao Y, Chu X, Pletcher SD. Trade-offs between longevity and pathogen resistance in Drosophila melanogaster are mediated by NFkappaB signaling. Aging Cell. 2006;5(6):533–543. doi: 10.1111/j.1474-9726.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 25.Vyhlidal CA, Rogan PK, Leeder JS. Development and refinement of pregnane X receptor (PXR) DNA binding site model using information theory: Insights into PXR-mediated gene regulation. J Biol Chem. 2004;279(45):46779–46786. doi: 10.1074/jbc.M408395200. [DOI] [PubMed] [Google Scholar]
- 26.Matys V, et al. TRANSFAC: Transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31(1):374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr. 2003;133(7) Suppl:2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- 28.King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6(4):311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- 29.King-Jones K, Horner MA, Lam G, Thummel CS. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4(1):37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 30.Sieber MH, Thummel CS. The DHR96 nuclear receptor controls triacylglycerol homeostasis in Drosophila. Cell Metab. 2009;10(6):481–490. doi: 10.1016/j.cmet.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buch S, Melcher C, Bauer M, Katzenberger J, Pankratz MJ. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling. Cell Metab. 2008;7(4):321–332. doi: 10.1016/j.cmet.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Grant CE, Bailey TL, Noble WS. FIMO: Scanning for occurrences of a given motif. Bioinformatics. 2011;27(7):1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shore DE, Ruvkun G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013;23(9):409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seong KH, Ogashiwa T, Matsuo T, Fuyama Y, Aigaki T. Application of the gene search system to screen for longevity genes in Drosophila. Biogerontology. 2001;2(3):209–217. doi: 10.1023/a:1011517325711. [DOI] [PubMed] [Google Scholar]
- 36.Ayyadevara S, et al. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005;4(5):257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- 37.Coustau C, Chevillon C, ffrench-Constant R. Resistance to xenobiotics and parasites: Can we count the cost? Trends Ecol Evol. 2000;15(9):378–383. doi: 10.1016/s0169-5347(00)01929-7. [DOI] [PubMed] [Google Scholar]
- 38.Honkakoski P, Zelko I, Sueyoshi T, Negishi M. The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol. 1998;18(10):5652–5658. doi: 10.1128/mcb.18.10.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sueyoshi T, Negishi M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- 40.Melo JA, Ruvkun G. Inactivation of conserved C. elegans genes engages pathogen- and xenobiotic-associated defenses. Cell. 2012;149(2):452–466. doi: 10.1016/j.cell.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McEwan DL, Kirienko NV, Ausubel FM. Host translational inhibition by Pseudomonas aeruginosa Exotoxin A Triggers an immune response in Caenorhabditis elegans. Cell Host Microbe. 2012;11(4):364–374. doi: 10.1016/j.chom.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hang S, et al. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe. 2014;16(5):592–604. doi: 10.1016/j.chom.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bass TM, et al. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62(10):1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Breslin T, Edén P, Krogh M. Comparing functional annotation analyses with Catmap. BMC Bioinformatics. 2004;5:193. doi: 10.1186/1471-2105-5-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462(7276):1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuster EF, Blanc E, Partridge L, Thornton JM. Correcting for sequence biases in present/absent calls. Genome Biol. 2007;8(6):R125. doi: 10.1186/gb-2007-8-6-r125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choe SE, Boutros M, Michelson AM, Church GM, Halfon MS. Preferred analysis methods for Affymetrix GeneChips revealed by a wholly defined control dataset. Genome Biol. 2005;6(2):R16. doi: 10.1186/gb-2005-6-2-r16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giannakou ME, et al. Dynamics of the action of dFOXO on adult mortality in Drosophila. Aging Cell. 2007;6(4):429–438. doi: 10.1111/j.1474-9726.2007.00290.x. [DOI] [PubMed] [Google Scholar]
- 49.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39(6):715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 50.Hubbard T, et al. Ensembl 2005. Nucleic Acids Res. 2005;33(Database issue):D447–D453. doi: 10.1093/nar/gki138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.