Abstract
BACKGROUND:
Despite >30 years of clinical use, the literature is still sparse when it comes to comparisons between percutaneous balloon compression (PBC) and percutaneous retrogasserian glycerol rhizolysis (PRGR) as treatments for trigeminal neuralgia.
OBJECTIVE:
To perform a retrospective cohort comparison between PBC and PRGR with regard to therapeutic effect, side effects, and complications.
METHODS:
Medical records and follow-up data from 124 primary PRGRs performed from 1986 to 2000 and 82 primary PBCs performed from 2000 to 2013 were reviewed. All patients had undergone clinical sensory testing and assessment of sensory thresholds. Analyses were performed to compare duration of pain relief, frequency of sensory disturbances, and side effects.
RESULTS:
Median duration of pain relief was 21 months after PRGR and 20 months after PBC. Both methods carried a high risk of hypesthesia/hypalgesia (P < .001) that was partly reversed with time. Decreased corneal sensibility was common after PRGR (P < .001) but not after PBC. Dysesthesia was more common after PRGR (23%) compared after PBC (4%; P < .001). Other side effects were noted but uncommon.
CONCLUSION:
PBC and PRGR are both effective as primary surgical treatment of trigeminal neuralgia. Both carry a risk of postoperative hypesthesia, but in this series, the side effect profile favored PBC. Furthermore, PBC is technically less challenging, whereas PRGR requires fewer resources. Between these 2 techniques, we propose PBC as the primary surgical technique for percutaneous treatment of trigeminal neuralgia on the basis of its lower incidence of dysesthesia, corneal hypesthesia, and technical failures.
ABBREVIATIONS:
MS, multiple sclerosis
PBC, percutaneous balloon compression
PRGR, percutaneous retrogasserian glycerol rhizotomy
TN, trigeminal neuralgia
KEY WORDS: Balloon compression, Glycerol rhizotomy, Trigeminal neuralgia
Since Harris1 successfully attempted to treat trigeminal neuralgia (TN) by percutaneously injecting alcohol into the trigeminal ganglion in 1911, various percutaneous treatments for TN have been developed.2 However, as with all surgery, the percutaneous techniques entail a certain risk of side effects, and the therapeutic effect often is not permanent. In the early 1980s, 2 percutaneous techniques were introduced with the hope of reducing side effects. In 1981, Håkanson3 introduced the percutaneous retrogasserian glycerol rhizotomy (PRGR), and in 1983, Mullan and Lichtor4 introduced the percutaneous balloon compression (PBC). Both techniques have since reached widespread use as routine procedures.
Despite >3 decades of clinical practice, only 2 clinical studies directly comparing PBC and PRGR for general TN have been published,5,6 and the literature still lacks a clinical comparison among substantial groups of comparable patients, leaving the choice between the 2 techniques without satisfactory evidence.
In Umeå, the senior author (A.T.B.) and colleagues have treated patients with TN, using PRGR as the standard percutaneous procedure between 1986 and 2000. Thereafter, the clinic switched to PBC to offer a procedure under general anesthesia, which is easier for patients to undergo. All patients were prospectively evaluated following the same protocol. In the present study, the objective is to compare PBC and PRGR in terms of pain relief and side effects in TN patients without previous surgical treatment.
METHOD
Study Design
Prospectively and continuously collected data from 2 consecutive cohorts of patients with de novo treatments for TN, 1 earlier cohort of PRGR patients and 1 later cohort of PBC patients, were retrospectively reviewed.
Patient Population
All patients included in this study suffered from a painful sensation in the distribution area of 1 or several of the trigeminal nerve branches, with the typical triggerable shooting pain as the main component. Most patients had primary TN (TN type 1), whereas a few had secondary TN after multiple sclerosis (MS).7
Indication for surgery was insufficient pain control or unacceptable side effects from medication. Microvascular decompression was the procedure of choice in patients who were reasonably young and healthy. Elderly or less healthy patients and patients with secondary TN were selected for a percutaneous procedure. These criteria have been consistent at our department since 1986. All patients in this analysis were surgically treated on the affected side for the first time to avoid bias from the fact that at our clinic PBC patients more commonly undergo retreatment than patients treated with PRGR. The operations were performed at the Umeå University Hospital in Umeå, Sweden, between 1986 and 2013 for a comparable number of years for each technique, which is the basis for the study size. The patients were subjected to continuous follow-up after surgery. Recruitment for this study was performed from 2013 to 2014, whereby all currently living patients who underwent a percutaneous de novo treatment during the study period were asked for an informed consent, and a presumed informed consent was used for the deceased patients.
Operative Techniques
PRGR Technique
In all but 12 patients, a freehand technique as described by Håkanson3 and later modified by Bergenheim et al8 was used for the procedure. The patients were awake and in a sitting position. A 22-gauge needle was inserted through the oval foramen using the Härtel trajectory aided by fluoroscopy. After obtaining spontaneous cerebrospinal fluid flow, cisternography was performed by injecting iohexol to verify a correct needle position within the Meckel cave. After the cistern was emptied, 0.20 to 0.35 mL anhydrous glycerol was injected, and the needle was withdrawn. The patient was then kept sitting for an hour with the head positioned according to which trigeminal branch was affected. In 12 cases, the needle positioning was performed stereotactically with the Laitinen9 Trigeminus Stereoguide with or without cisternography.
PBC Procedure
The procedure was performed in the radiology suit on the basis of the original description by Mullan and Lichtor4 and later by Bergenheim et al.10 The patient was positioned in a supine position, sedated, and intubated. Local anesthetics were administered, and a 13-gauge needle with a semisharp stylet was inserted through a stab incision 2 to 3 cm lateral to the angle of the mouth. Using biplanar fluoroscopy, the needle was directed into the oval foramen. The stylet was removed, and a 4F Fogarty balloon catheter (Edwards Lifesciences, Irvine, California) was inserted 17 to 19 mm beyond the tip of the needle. When in place, the balloon was inflated with 0.3 to 0.8 mL iohexol at 300 mg/mL. The pressure was then held for 1.5 to 3 minutes before the contrast was aspirated, and the catheter and needle were withdrawn.
Clinical Evaluation and Follow-up
The patients were clinically evaluated in person before surgery, 1 to 3 days after surgery, and at an outpatient visit 3 to 6 months postoperatively. Trigeminal sensory function was evaluated on the pain side and contralaterally by testing for light touch, pinprick, and corneal reflex. The responses were ranked according to a 4-grade scale (normal, slightly decreased, severely decreased, and totally impaired). Sensory thresholds were quantitatively assessed for 6 sites on each side of the face with the use of a cutaneous electric stimulation technique, sensimetric testing.11 Side effects and pain relief were evaluated on the same occasions. In the pain-free patients, further follow-up was conducted by telephone, often for several years after surgery. A pain-free condition was defined as the patient being completely free, without medication, from trigeminal pain.
Statistical Analysis
Kaplan-Meier analyses with log-rank tests were performed to evaluate the long-term effects of PBC and PRGR. Patients were censored when free of pain at the last follow-up (including lost to follow-up). The sensimetric data were analyzed with paired t tests. Data from the sensibility tests were analyzed with the Wilcoxon signed-ranks test. Other side effects were with the Fisher exact test. Values of P < .05 were considered significant. Only available data were analyzed. Analyses were performed with IBM SPSS Statistics version 22.
Ethics
The study was approved by the Regional Ethical Review Board, Umeå (2013/76-31).
RESULTS
Participants
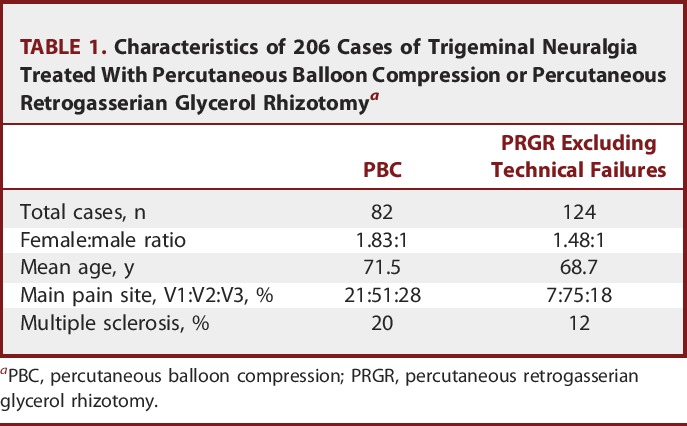
Eighty-three de novo patients receiving PCB and 129 receiving PRGRs were identified as possible participants. Eight of the latter procedures were aborted because of technical failures, ie, either a vasovagal reflex or, more commonly, a failure to verify correct needle positioning owing to an unsuccessful cisternography. One PBC and 5 PRGRs were excluded because of a lack of informed consent. Patient selection is presented in Figure 1. Patient characteristics are presented in Table 1.
FIGURE 1.
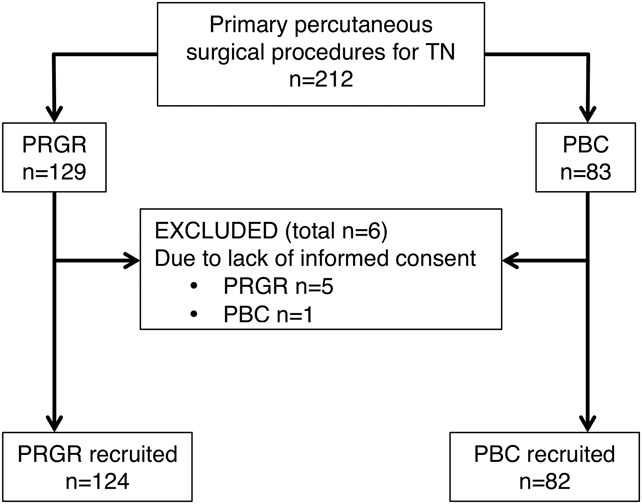
Diagram outlining the patient selection from all patients receiving a percutaneous surgical treatment for trigeminal neuralgia (TN) without a history of previous surgery on the affected side until May 2013 at the Umeå University Hospital. PBC, percutaneous balloon compression; PRGR, percutaneous retrogasserian glycerol rhizotomy.
TABLE 1.
Characteristics of 206 Cases of Trigeminal Neuralgia Treated With Percutaneous Balloon Compression or Percutaneous Retrogasserian Glycerol Rhizotomya
Main Results
There was no significant difference between the pain-free time after PBC and after PRGR (Figure 2). For PBC, the initial success rate was 82%, and the median pain-free time was 20 months. Thirty patients (37%) were free of pain at the last follow-up in this group. For PRGR, with technical failures excluded, the initial success rate was 85%, and the median pain-free time was 21 months, with 47 patients (38%) free of pain at the last follow-up. Eight procedures (6%) in the PRGR group were preceded by technically failed attempts at PRGR, whereas there were no technical failures in the PBC group. When technical failures were included, the median time to pain recurrence was 19 months in the PRGR group, but the difference compared with PBC was not significant (P = .07).
FIGURE 2.
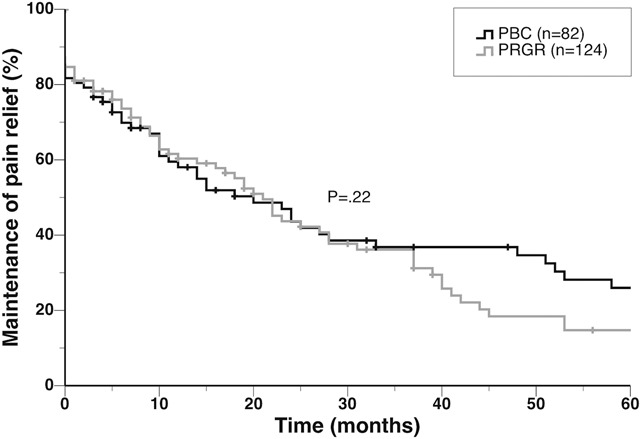
Kaplan-Meier plot illustrating the therapeutic effects after percutaneous balloon compression (PBC) and percutaneous retrogasserian glycerol rhizotomy (PRGR) in months of pain relief without medication. The patients had not previously undergone a completed ipsilateral surgical procedure for trigeminal neuralgia. A log-rank test showed no significant difference between the curves.
Further Analyses
Because the sex distribution between the compared groups was not equal, Kaplan-Meier analyses were performed to reveal whether this variable affected outcome. With PBC, the median time to recurrence was 18 months for men and 23 months for women (P = .54); with PRGR, the median time to recurrence was 12 and 23 months, respectively (P = .09). In a comparison of PBC and PRGR divided into male and female categories, the differences between the treatments were also not significant (P = .23 for men, P = .54 for women).
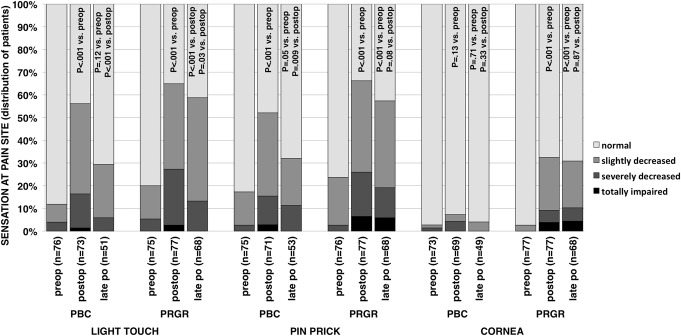
Results from sensimetric testing are presented in Figure 3, and results from clinical examinations at the pain site and cornea are shown in Figure 4. Sensibility data were not collected after technical failures; therefore, all patients analyzed in this respect have been subjected to either a glycerol injection or an inflated balloon. Comparing preoperative results with postoperative and late postoperative results revealed a statistically significant and lasting decrease in perception for all 3 modalities after PRGR. After PBC, the perceptions for touch and pinprick were initially significantly decreased but close to normal at late follow-up. Corneal sensibility was not significantly affected after PBC.
FIGURE 3.
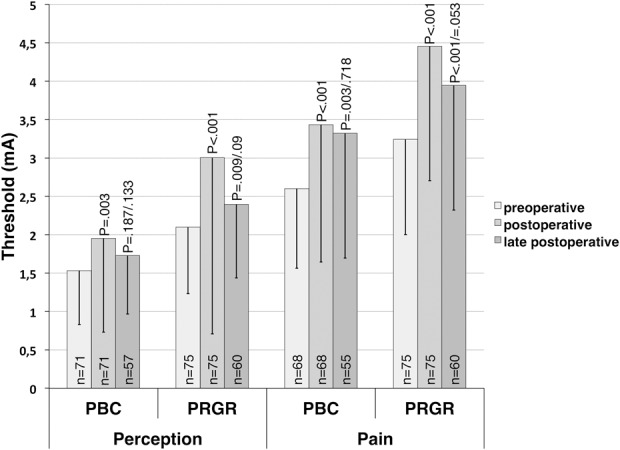
Thresholds in amperage for perception and pain as measured by sensimetric testing in patients treated with percutaneous balloon compression (PBC) and percutaneous retrogasserian glycerol rhizotomy (PRGR) for trigeminal neuralgia. Means for preoperative thresholds are presented from the paired t test with early postoperative thresholds. Means for preoperative thresholds from the paired t test with late postoperative thresholds, thus including fewer cases, are not presented, but they approximate the presented values very well. P values for postoperative thresholds relate to a comparison with preoperative thresholds. P values for late postoperative thresholds are presented for comparisons with both preoperative and postoperative thresholds.
FIGURE 4.
Distribution within groups of patients undergoing percutaneous balloon compression (PBC) and percutaneous retrogasserian glycerol rhizotomy (PRGR) of sensation at the pain site according to a 4-grade scale ranging from normal to totally impaired. Distribution difference between 3 time points for each modality and treatment are examined with Wilcoxon signed-ranks tests. P values in the postoperative bars relates to a comparison with the related preoperative distribution. P values to the left in the late postoperative bar relate to a comparison with the related preoperative distribution, whereas P values to the right in the late postoperative bar relate to a comparison with the related postoperative distribution.
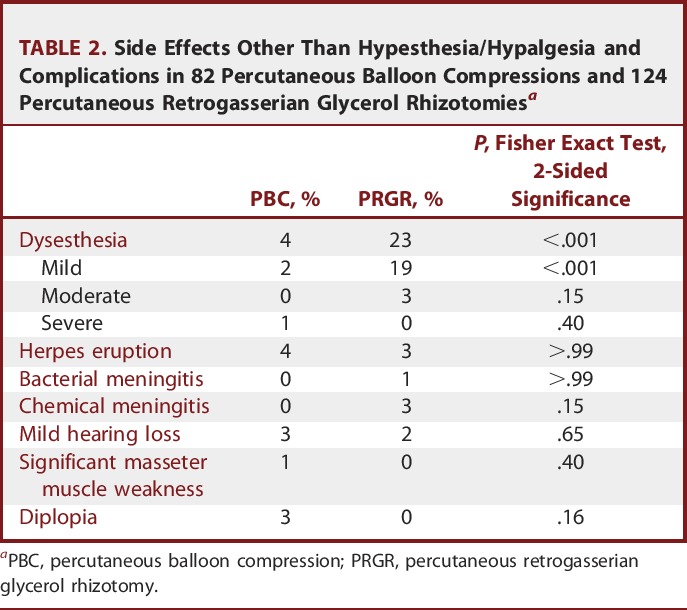
The occurrences of other side effects are presented in Table 2. Postoperative dysesthesia occurred more frequently after PRGR than after PBC. There were no cases of anesthesia dolorosa. The only severe case of dysesthesia occurred after PBC, secondarily to extensive postoperative herpetic eruptions demanding hospitalization.
TABLE 2.
Side Effects Other Than Hypesthesia/Hypalgesia and Complications in 82 Percutaneous Balloon Compressions and 124 Percutaneous Retrogasserian Glycerol Rhizotomiesa
DISCUSSION
Key Results
We have compared 124 PRGRs with 82 PBCs performed on patients not previously operated on and selected for surgery using the same criteria to see how the results differ in terms of pain relief, side effects, and complications. No significant difference in pain-relief was seen between the 2 techniques. There was, however, a 6% rate of aborted procedures in the PRGR group, mainly as a result of failure to verify correct cannula positioning with cisternography. It is the experience at our clinic that PRGR is technically more demanding compared with PBC, not least because patients are awake.
Limitations
Although the data for this study have been collected since 1986, the study design is retrospective. Whereas the indication for percutaneous surgery has not changed during this time, the senior author (A.T.B.) has been involved from the beginning, and the follow-up process has been highly standardized, the procedures reported were performed over a significant amount of time, which could influence the results. PBC also involves general anesthesia and PRGR requires patient cooperation, which might have influenced contraindications for the 2 groups. Furthermore, not all patients in this series were able or willing to participate in the clinical and sensimetric evaluations. The negative result on a possible difference in duration of pain relief between PRGR and PBC could indicate that the study size was too small. However, increasing the study size would also further increase the study period. The series for each technique included a learning curve.
Interpretation
To the best of our knowledge, this is the first study to compare PBC and PRGR as primary treatment when the indications were identical. Fraioli et al6 compared all 3 percutaneous methods, i.e., PBC, PRGR and radiofrequency thermocoagulation, on 681 patients who underwent surgery between 1976 and 1986. They suggested PBC as the treatment of choice for most patients on the basis of good efficiency and low complication rates, but they also concluded that their results for PRGR were less successful than previously reported by others. Furthermore, they reported no actuarial analyses, and some of their patients had a history of previous surgical treatment. Kouzounias et al5 compared 61 PBCs and 92 PRGRs performed between 2004 and 2008. They reported no significant difference with median times to pain recurrence of 13 months for PRGR and 15 months for PBC. They proposed PRGR as a first-line percutaneous treatment in most cases mainly on the basis of a lower complication rate and the avoidance of general anesthesia. The indications for performing PRGR and PBC differed, and PBC was in most cases (92%) chosen for patients in whom previous surgical treatment had been unsuccessful or in whom TN had recurred. MS was also notably more common in the PBC group. It is therefore possible that this method was chosen for their patients who were more difficult to treat.
In 2012, Mallory et al12 reported results from 69 PBCs and 67 PRGRs performed at the Mayo Clinic between 1997 and 2010 on patients with TN associated with MS. The criteria for selecting the patients for either of the 2 techniques were not reported. Freedom from pain without medication was achieved in 33%. Median time to pain recurrence, with or without medication, was 6 months. No significant difference in pain relief was seen between the techniques, but contrary to our results, there was a higher complication rate for PBC and there were no cases of dysesthesia after PRGR. Mohammad-Mohammadi et al13 also recently reported results from a large series of patients with TN secondary to MS, comparing several surgical techniques. PBC and PRGR stood out as the upfront treatments that led to the longest pain relief, with a median of 29 months for PBC and 9 months for PRGR.
PBC and PRGR have further been subjected to comparison in systematic reviews. In 2004, Lopez et al14 reviewed all major surgical options for TN but concluded, with regard to PBC, that there were insufficient good-quality data to compare that technique with the others. Tatli et al,15 on the other hand, found 2 publications on PBC and 2 on PRGR that met the criteria for their review. They concluded that PRGR has a higher recurrence rate than PBC. They did, however, opt to only include studies with a follow-up time of at least 5 years. This by far exceeds the median time to pain recurrence in many published series, and one could therefore expect a bias in favor of series with extraordinary successful results.
Although not statistically significant, women had a much longer duration of pain relief than men in our series of PRGR. Similar results have been reported by North et al.16 After PBC, no notable difference in outcome correlated to sex was found. The female-to-male ratio in larger TN populations has been described as close to 2:1.17,18 This is a higher ratio of women than in our PRGR group; therefore, adjusting these results to the female-to-male ratio of the general TN population may have some impact on the presented overall results for PRGR.
The most common significant side effect of both PBC and PRGR is hypesthesia/hypalgesia. In fact, decreased sensory function has been shown to correlate with a proper surgical technique in PBC and with long-lasting pain relief in PRGR.19-21 Overall, 82% of the preoperative clinical tests for light touch and pinprick were normal, but only 40% were normal postoperatively. At late follow-up, these tests were partially normalized, seemingly more so after PBC than after PRGR. From a diagnostic point of view, it is also interesting to note that not all patients diagnosed with TN had normal sensibility as required by the International Classification of Headache Disorders, Second Edition.22 This classification, however, is currently under revision and will perhaps be adapted to the evidence in the literature that supports that a subset of patients with TN also have an associated sensory deficit.23-28
Almost a third of the patients who underwent PRGR had a seemingly persistent corneal hypesthesia compared with very few in the PBC group. Because of the topographical relation between the different trigeminal branches and the porus trigeminus in a sitting position, one could expect PRGR to be most effective on V3 and less effective on V1. However, this can be overcome by flexing the neck of the patient, as previously described by Bergenheim et al,8 although perhaps at the cost of corneal sensory deficit. A very low frequency of corneal hypesthesia after PBC has also been reported by others.14,15
Dysesthesia is a known complication for both of these procedures, and compiled data have indicated rates of 8.3% to 17.8% for PRGR and 19.1% for PBC, but with declining rates for PBC as the technique has been refined with shorter compression times over the years.5,10,14,20,29 We report a rate of 23% for PRGR and 4% for PBC. The rate of dysesthesia after PRGR was high, even though the vast majority were mild cases and generally a lot less troublesome for the patient than the TN. The only case of severe dysesthesia in this series was a result of a severe herpetic eruption after a PBC. The reported incidence of postoperative herpetic eruptions was comparable between the techniques at 3% to 4%. In our experience, however, most cases appear only after a few days, when the patients have left the hospital, and are not troublesome enough for patients to seek medical counsel. The incidence of mild herpetic eruptions might therefore be higher.
Chemical meningitis is rarely reported after PRGR, with an incidence of 0.12% to 1.7%.20,30-34 In our series, however, the rate was 3%. The mechanism behind this complication is not yet known.20 Chemical meningitis has also been reported after PBC and radiofrequency rhizotomy but appears less frequently in the literature.35,36 Our experience indicates that chemical meningitis usually resolves quickly and without subsequent deficits. Even rarer but more troublesome are cases of bacterial meningitis, which have been reported after all 3 types of commonly performed percutaneous procedures. It has been suggested to result from a penetration of the buccal mucosa by the cannula, with an overall incidence of 0.15%.20,32,34,37-43
Diplopia after PBC has been reported,12,40,44-51 and a report on 6 patients (2 of whom are included in this study) by Bergenheim and Linderoth44 focused on this complication. Although most often transient, it is, compared with PRGR, quite specific to PBC. It has been suggested to arise from a compression of preferentially the fourth cranial nerve.
Rare cases of mild subjective hearing loss after PBC have been reported by others.37,52 Two patients in our series of PBC noted this postoperatively. We also noted 2 previously reported cases of hearing loss after PRGR.20 One possible reason could be surgical trauma to the motor fibers innervating the tensor tympani. In the case of PRGR, it is also possible that small amounts of glycerol overflowed into the posterior fossa, but the technique of flexing the patient's neck during surgery should prevent this event.
Masseter muscle weakness has been reported to be a common side effect after PBC,52-54 but there was only 1 case of clinically significant masseter weakness in our series. Furthermore, we had no serious side effects, which is in line with current opinion that these procedures are generally safe if performed judiciously.
Generalizability
The limitations of this study, as described above, should be considered, not least of which is the time aspect. However, we believe that because the operative techniques and postoperative care have been kept standardized throughout the study period, both at our clinic and globally, the results should be applicable to future use of these techniques at other centers, especially when it comes to the more pronounced differences in side effects, complications, and technical failures. However, one has to be aware that the results from both techniques rely strongly on the skill of the surgeon.
CONCLUSION
We have compared 2 percutaneous methods, PRGR and PBC, for the treatment of TN. Throughout the years, we have had a consistent indication for those surgical options, and by limiting our study to review only primary surgical treatments, we believe that we have achieved a satisfactory comparison. We propose PBC over PRGR as the percutaneous technique of choice for the primary treatment of TN because of its pain-relieving effect similar to that of PRGR but with fewer side effects and complications, especially dysesthesia and corneal hypesthesia.
Disclosures
This study was supported by the Research Foundation of Clinical Neuroscience, Umeå University. Apart from this financial support, the authors have no personal, financial, or institutional interest in drugs, materials, or devices described in this article.
Acknowledgments
We thank Kristin Nyman and Lars Sandman at the Institution of Clinical Neuroscience, Umeå University, Sweden, for assistance in collecting and administrating data for this study. We are grateful to Lauri Laitinen, who introduced glycerol rhizotomy in Umeå, and Bengt Linderoth, who taught us the balloon compression technique.
COMMENTS
The authors report a series of 124 patients who underwent trigeminal glycerol rhizolysis and 82 patients who underwent percutaneous balloon microcompression. This is an interesting study, providing further information to an already rich literature comparing the outcomes of procedures for trigeminal neuralgia. The authors show that the median duration of relief, approximately 20 months, was equivalent between the 2 techniques. However, the risk for adverse effects was significantly different between groups. Glycerol rhizolysis presented with a higher rate of reduced corneal sensitivity and facial dysesthesia. The authors indicate that, over time, their practice changed on the basis of their experience, and glycerol rhizolysis was replaced as the procedure of choice with balloon microcompression under general anesthesia.
As for any retrospective study, a few limitations should be considered. Glycerol rhizolysis was the preferred procedure before 2000, when the preference switched to balloon microcompression. This difference in time may have added a bias to the study because patient selection criteria may have changed over time, including the criteria for offering microvascular decompression instead of percutaneous procedures. Learning curve effects and experience may also have influenced results. Furthermore, the authors have not controlled for some variables that may also influence outcome such as duration of disease. Regardless, this is a large cohort and provides valuable information about 2 procedures commonly performed to manage medically refractory trigeminal neuralgia.
Andre Machado
Cleveland, Ohio
The authors present a retrospective analysis of 2 standard percutaneous treatments for first-time trigeminal neuralgia, percutaneous balloon compression and glycerol rhizotomy. Glycerol rhizotomy was their technique of choice from 1986 to 2000, after which time they switched to percutaneous balloon compression given their preference for performing the procedure under general anesthesia. Clinical measures of success (initial success rate and pain-free percentage at last follow-up) were nearly identical, whereas complication rates, including decreased corneal sensation and dysesthesias, were significantly more common in the glycerol rhizotomy cohort.
Because the 2 procedures were performed at their institution during different time periods, one may question the general applicability of these results to other experienced centers that routinely perform glycerol rhizotomy. It should also be noted that glycerol rhizotomy can be performed under general anesthesia as well, albeit with the caveat that the patient be placed in a seated position and extubated while still under anesthesia.
Because it is unlikely that a prospective randomized study of these procedures will be performed, this information provides a worthwhile contribution to the literature regarding the safety and efficacy of percutaneous interventions for trigeminal neuralgia.
Alon Y. Mogilner
New York, New York
REFERENCES
- 1.Harris W. Three cases of alcohol injection of the gasserian ganglion for trigeminal neuralgia. Proc R Soc Med. 1912;5(clin sect):115-119. [PMC free article] [PubMed] [Google Scholar]
- 2.Wilkins R. Trigeminal neuralgia: historical overview, with emphasis on surgical treatment. In: Burchiel K, ed. Surgical Management of Pain. New York, NY: Thieme; 2002:288-301. [Google Scholar]
- 3.Håkanson S. Trigeminal neuralgia treated by the injection of glycerol into the trigeminal cistern. Neurosurgery. 1981;9(6):638-646. [DOI] [PubMed] [Google Scholar]
- 4.Mullan S, Lichtor T. Percutaneous microcompression of the trigeminal ganglion for trigeminal neuralgia. J Neurosurg. 1983;59(6):1007-1012. [DOI] [PubMed] [Google Scholar]
- 5.Kouzounias K, Lind G, Schechtmann G, Winter J, Linderoth B. Comparison of percutaneous balloon compression and glycerol rhizotomy for the treatment of trigeminal neuralgia. J Neurosurg. 2010;113(3):486-492. [DOI] [PubMed] [Google Scholar]
- 6.Fraioli B, Esposito V, Guidetti B, Cruccu G, Manfredi M. Treatment of trigeminal neuralgia by thermocoagulation, glycerolization, and percutaneous compression of the gasserian ganglion and/or retrogasserian rootlets: long-term results and therapeutic protocol. Neurosurgery. 1989;24(2):239-245. [DOI] [PubMed] [Google Scholar]
- 7.Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53(5):1164-1166; discussion 1166-1167. [DOI] [PubMed] [Google Scholar]
- 8.Bergenheim AT, Hariz MI, Laitinen LV. Selectivity of retrogasserian glycerol rhizotomy in the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1991;56(3):159-165. [DOI] [PubMed] [Google Scholar]
- 9.Laitinen LV. Trigeminus Stereoguide: an instrument for stereotactic approach through the foramen ovale and foramen jugulare. Surg Neurol. 1984;22(5):519-523. [DOI] [PubMed] [Google Scholar]
- 10.Bergenheim AT, Asplund P, Linderoth B. Percutaneous retrogasserian balloon compression for trigeminal neuralgia: review of critical technical details and outcomes. World Neurosurg. 2013;79(2):359-368. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen LV, Eriksson AT. Electrical stimulation in the measurement of cutaneous sensibility. Pain. 1985;22(2):139-150. [DOI] [PubMed] [Google Scholar]
- 12.Mallory GW, Atkinson JL, Stien KJ, Keegan BM, Pollock BE. Outcomes after percutaneous surgery for patients with multiple sclerosis-related trigeminal neuralgia. Neurosurgery. 2012;71(3):581-586; discussion 586. [DOI] [PubMed] [Google Scholar]
- 13.Mohammad-Mohammadi A, Recinos PF, Lee JH, Elson P, Barnett GH. Surgical outcomes of trigeminal neuralgia in patients with multiple sclerosis. Neurosurgery. 2013;73(6):941-950; discussion 950. [DOI] [PubMed] [Google Scholar]
- 14.Lopez BC, Hamlyn PJ, Zakrzewska JM. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54(4):973-982; discussion 982-983. [DOI] [PubMed] [Google Scholar]
- 15.Tatli M, Satici O, Kanpolat Y, Sindou M. Various surgical modalities for trigeminal neuralgia: literature study of respective long-term outcomes. Acta Neurochir (Wien). 2008;150(3):243-255. [DOI] [PubMed] [Google Scholar]
- 16.North RB, Kidd DH, Piantadosi S, Carson BS. Percutaneous retrogasserian glycerol rhizotomy: predictors of success and failure in treatment of trigeminal neuralgia. J Neurosurg. 1990;72(6):851-856. [DOI] [PubMed] [Google Scholar]
- 17.Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45(7):1294-1296. [DOI] [PubMed] [Google Scholar]
- 18.Katusic S, Beard CM, Bergstralth E, Kurland LT. Incidence and clinical features of trigeminal neuralgia, Rochester, Minnesota, 1945-1984. Ann Neurol. 1990;27(1):89-95. [DOI] [PubMed] [Google Scholar]
- 19.Asplund P, Linderoth B, Bergenheim AT. The predictive power of balloon shape and change of sensory functions on outcome of percutaneous balloon compression for trigeminal neuralgia. J Neurosurg. 2010;113(3):498-507. [DOI] [PubMed] [Google Scholar]
- 20.Blomstedt PC, Bergenheim AT. Technical difficulties, perioperative complications of retrogasserian glycerol rhizotomy for trigeminal neuralgia. Stereotact Funct Neurosurg. 2002;79(3-4):168-181. [DOI] [PubMed] [Google Scholar]
- 21.Laitinen LV, Brophy BP, Bergenheim AT. Sensory disturbance following percutaneous retrogasserian glycerol rhizotomy. Br J Neurosurg. 1989;3(4):471-477; discussion 477-478. [DOI] [PubMed] [Google Scholar]
- 22.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(suppl 1):9-160. [DOI] [PubMed] [Google Scholar]
- 23.Bennett MH, Jannetta PJ. Evoked potentials in trigeminal neuralgia. Neurosurgery. 1983;13(3):242-247. [DOI] [PubMed] [Google Scholar]
- 24.Bowsher D, Miles JB, Haggett CE, Eldridge PR. Trigeminal neuralgia: a quantitative sensory perception threshold study in patients who had not undergone previous invasive procedures. J Neurosurg. 1997;86(2):190-192. [DOI] [PubMed] [Google Scholar]
- 25.Lewy F, Grant F. Physiopathologic and pathoanatomic aspects of major trigeminal neuralgia. Arch Neurpsychol. 1938;40(6):1126-1134. [Google Scholar]
- 26.Mursch K, Schäfer M, Steinhoff BJ, Behnke-Mursch J. Trigeminal evoked potentials and sensory deficits in atypical facial pain: a comparison with results in trigeminal neuralgia. Funct Neurol. 2002;17(3):133-136. [PubMed] [Google Scholar]
- 27.Nurmikko TJ. Altered cutaneous sensation in trigeminal neuralgia. Arch Neurol. 1991;48(5):523-527. [DOI] [PubMed] [Google Scholar]
- 28.Sinay VJ, Bonamico LH, Dubrovsky A. Subclinical sensory abnormalities in trigeminal neuralgia. Cephalalgia. 2003;23(7):541-544. [DOI] [PubMed] [Google Scholar]
- 29.Nurmikko TJ, Eldridge PR. Trigeminal neuralgia: pathophysiology, diagnosis and current treatment. Br J Anaesth. 2001;87(1):117-132. [DOI] [PubMed] [Google Scholar]
- 30.Cappabianca P, Spaziante R, Graziussi G, Taglialatela G, Peca C, De Divitiis E. Percutaneous retrogasserian glycerol rhizolysis for treatment of trigeminal neuralgia: technique and results in 191 patients. J Neurosurg Sci. 1995;39(1):37-45. [PubMed] [Google Scholar]
- 31.Jagia M, Bithal PK, Dash HH, Prabhakar H, Chaturvedi A, Chouhan RS. Effect of cerebrospinal fluid return on success rate of percutaneous retrogasserian glycerol rhizotomy. Reg Anesth Pain Med. 2004;29(6):592-595. [DOI] [PubMed] [Google Scholar]
- 32.Pickett GE, Bisnaire D, Ferguson GG. Percutaneous retrogasserian glycerol rhizotomy in the treatment of tic douloureux associated with multiple sclerosis. Neurosurgery. 2005;56(3):537-545; discussion 537-545. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Xu M, Zou Y. Treatment of trigeminal neuralgia with percutaneous glycerol injection into Meckel's cavity: experience in 4012 patients. Cell Biochem Biophys. 2010;58(2):85-89. [DOI] [PubMed] [Google Scholar]
- 34.Burchiel KJ. Percutaneous retrogasserian glycerol rhizolysis in the management of trigeminal neuralgia. J Neurosurg. 1988;69(3):361-366. [DOI] [PubMed] [Google Scholar]
- 35.Brown JA, McDaniel MD, Weaver MT. Percutaneous trigeminal nerve compression for treatment of trigeminal neuralgia: results in 50 patients. Neurosurgery. 1993;32(4):570-573. [DOI] [PubMed] [Google Scholar]
- 36.Kanpolat Y, Savas A, Bekar A, Berk C. Percutaneous controlled radiofrequency trigeminal rhizotomy for the treatment of idiopathic trigeminal neuralgia: 25-year experience with 1600 patients. Neurosurgery. 2001;48(3):524-532; discussion 532-534. [DOI] [PubMed] [Google Scholar]
- 37.Abdennebi B, Bouatta F, Chitti M, Bougatene B. Percutaneous balloon compression of the gasserian ganglion in trigeminal neuralgia: long-term results in 150 cases. Acta Neurochir (Wien). 1995;136(1-2):72-74. [DOI] [PubMed] [Google Scholar]
- 38.Aspevall O, Hillebrant E, Linderoth B, Rylander M. Meningitis due to Gemella haemolysans after neurosurgical treatment of trigeminal neuralgia. Scand J Infect Dis. 1991;23(4):503-505. [DOI] [PubMed] [Google Scholar]
- 39.James EA, Kibbler CC, Gillespie SH. Meningitis due to oral streptococci following percutaneous glycerol rhizotomy of the trigeminal ganglion. J Infect. 1995;31(1):55-57. [DOI] [PubMed] [Google Scholar]
- 40.Lobato RD, Rivas JJ, Sarabia R, Lamas E. Percutaneous microcompression of the gasserian ganglion for trigeminal neuralgia. J Neurosurg. 1990;72(4):546-553. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell RG, Teddy PJ. Meningitis due to Gemella haemolysans after radiofrequency trigeminal rhizotomy. J Clin Pathol. 1985;38(5):558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torroba L, Moreno S, Lorenzana L, Buzon L. Purulent meningitis after percutaneous radiofrequency trigeminal rhizotomy. J Neurol Neurosurg Psychiatry. 1987;50(8):1081-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward L, Khan M, Grieg M, Dolin SJ. Meningitis after percutaneous radiofrequency trigeminal ganglion lesion: case report and review of literature. Pain Med. 2007;8(6):535-538. [DOI] [PubMed] [Google Scholar]
- 44.Bergenheim AT, Linderoth B. Diplopia after balloon compression of retrogasserian ganglion rootlets for trigeminal neuralgia: technical case report. Neurosurgery. 2008;62(2):E533-E534; discussion E534. [DOI] [PubMed] [Google Scholar]
- 45.Chen JF, Tu PH, Lee ST. Repeated percutaneous balloon compression for recurrent trigeminal neuralgia: a long-term study. World Neurosurg. 2012;77(2):352-356. [DOI] [PubMed] [Google Scholar]
- 46.Corrêa CF, Teixeira MJ. Balloon compression of the gasserian ganglion for the treatment of trigeminal neuralgia. Stereotact Funct Neurosurg. 1998;71(2):83-89. [DOI] [PubMed] [Google Scholar]
- 47.Lichtor T, Mullan JF. A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg. 1990;72(1):49-54. [DOI] [PubMed] [Google Scholar]
- 48.Park SS, Lee MK, Kim JW, Jung JY, Kim IS, Ghang CG. Percutaneous balloon compression of trigeminal ganglion for the treatment of idiopathic trigeminal neuralgia: experience in 50 patients. J Korean Neurosurg Soc. 2008;43(4):186-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skirving DJ, Dan NG. A 20-year review of percutaneous balloon compression of the trigeminal ganglion. J Neurosurg. 2001;94(6):913-917. [DOI] [PubMed] [Google Scholar]
- 50.Urculo E, Alfaro R, Arrazola M, Astudillo E, Rejas G. Trochlear nerve palsy after repeated percutaneous balloon compression for recurrent trigeminal neuralgia: case report and pathogenic considerations. Neurosurgery. 2004;54(2):505-508; discussion 508-509. [DOI] [PubMed] [Google Scholar]
- 51.Kefalopoulou Z, Markaki E, Constantoyannis C. Avoiding abducens nerve palsy during the percutaneous balloon compression procedure. Stereotact Funct Neurosurg. 2009;87(2):101-104. [DOI] [PubMed] [Google Scholar]
- 52.de Siqueira SR, da Nóbrega JC, de Siqueira JT, Teixeira MJ. Frequency of postoperative complications after balloon compression for idiopathic trigeminal neuralgia: prospective study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(5):e39-e45. [DOI] [PubMed] [Google Scholar]
- 53.Brown JA, Pilitsis JG. Percutaneous balloon compression for the treatment of trigeminal neuralgia: results in 56 patients based on balloon compression pressure monitoring. Neurosurg Focus. 2005;18(5):E10. [DOI] [PubMed] [Google Scholar]
- 54.Chroni E, Constantoyannis C, Prasoulis I, et al. Masseter muscle function after percutaneous balloon compression of trigeminal ganglion for the treatment of trigeminal neuralgia: a neurophysiological follow-up study. Clin Neurophysiol. 2011;122(2):410-413. [DOI] [PubMed] [Google Scholar]