Abstract
Background
Dynapenia (age-associated loss of muscle strength not caused by neurologic or muscular diseases) and functional limitations (e.g. climbing stairs, chair rising) are important problems in elderly persons. Whole body vibration, used as an adjunct to classical resistance training or even as a stand-alone alternative, might help to reduce these problems. Its value might be highest in elderly persons with very low function, where whole body vibration can be used as a skilling up training until more conventional exercise types are possible. This systematic review and meta-analysis summarized the current evidence for whole-body vibration interventions on isometric maximum voluntary contraction, dynamic strength, power, rate of force development and functional strength in elderly categorised in different subgroups based on function levels.
Methods
An extensive literature search was carried out in February 2014 and repeated in February 2015 at PubMed, Cochrane Central Register of Controlled Trials, Physiotherapy Evidence Database and CINAHL electronic databases. The International Clinical Trials Registry Platform from the World Health Organization was also searched. Randomized controlled trials measuring isometric maximum voluntary contraction, dynamic strength, power, rate of force development and functional strength in studies using WBV intervention in 65 years or older elderly individuals were included. The methodological quality of included studies was assessed using the Cochrane Collaboration’s tool for assessing Risk of Bias. Studies were classified based on the level of physical capacitiy of the participants as “Go-Go”, “Slow-Go” or “No-Go”. Data were pooled using a random effects model.
Results
Thirty-eigth articles of moderate methodological quality were included. The vibration modes for sinusoidal vertical whole-body vibration was between 25 and 40 Hz, the amplitude varied from 2 to 4 mm. Sinusoidal side-alternating -whole-body vibration revealed frequencies from 2.5 to 35 Hz with amplitudes ranging from 0.05 to 12 mm. Stochastic resonance whole-body vibration used frequencies between 3 and 6 Hz. Effect sizes in Go-Go were moderate after vertical sinusoidal Whole-body vibration compared to non-training control groups for isometric maximum voluntary contraction with effect size 0.48 (95 % CI 0.33 to 0.63) and for Dynamic Strength with effect size 0.47 (95 % CI 0.06 to 0.88). Side-alternating sinusoidal whole body vibration showed moderate effect sizes with 0.69 (95 % CI 0.32 to 1.06) for isometric maximum voluntary contraction, 0.50 (95 % CI 0.07 to 0.92) for power, 0.40 (95 % CI 0.16 to 0.64) for Rate of Force Development and 0.42 (95 % CI 0.13 to 0.71) for Functional Strength compared to non-exercise control. The analysis for Slow-Go showed for stochastic resonance whole-body vibration and Functional Strength an effect size of 0.97 (95 % CI −0.07 to 2.00) compared to non-exercise control in one study. No-Go showed for stochastic resonance whole-body vibration a moderate effect size with 0.50 (95 % CI −0.32 to 1.33) for Functional Strength compared to non-exercise control.
Conclusions
Whole-body vibration shows beneficial effects, mainly in the No-Go group elderly compared to non-training control and conventional strength training groups. The results suggest that WBV can be used as a skilling-up exercise in participants not able to perform standard exercises. Further studies with the various types of WBV in various sub-populations of elderly persons are needed to determine the most effective vibration modes.
Trial registration
Registration number: CRD42013006489.
Electronic supplementary material
The online version of this article (doi:10.1186/s11556-015-0158-3) contains supplementary material, which is available to authorized users.
Keywords: WBV, Isometric maximum voluntary contraction, Dynamic maximum voluntary contraction, Power, Rate of force development, Functional strength
Background
Aging is associated with a decrease of muscle strength and power [1–3]. The term dynapenia, coined by Manini and Clark [3–5], best describes the condition of decreased muscle strength and power instead of the term sarcopenia. The latter only refers to an age-related loss in skeletal muscle mass. Muscle weakness is related to falls, lower walking speed, functional limitation, a decrease in mobility, and disability [6–8]. In this context, the elderly are viewed as a group of people in need [9]. When physical functioning is concerned there often is a mismatch between chronological age and biological age. Chronological age is not necesarilly related to physical capabilities. For this reason, a classification of elderly based on physical abilities; e.g. physical and mental functions is more appropriate.
Zeyfang and Braun [10] classified older adults as “being an independent person” (Go-Go); “being a needy person with a slight handicap” (Slow-Go); and “being a person in need of care with severe functional limitation” (No-Go). The need for care may be defined as depending permanently on assistance (No-Go) or depending on support in everyday activities such as dressing, body care, eating, using the toilet, mobility, and planning the day (Slow-Go) [11].
The ability of elderly individuals to perform basic activities of daily life is crucial for their ability to exist independently [12]. To improve and/or enable performance of basic activities of daily life, exercise programs are indicated. Sensorimotor training and resistance exercises are effective methods to increase muscle mass and strength in the elderly [13]. Whole-body vibration (WBV) can be used as a sensorimotor training regimen. The impact of WBV on the body is low according to indicators such as blood pressure, heart rate, lactate, and O2 uptake [14–16]. Systematic reviews concluded that, compared to more demanding interventions, WBV might be a safer and less fatiguing type of exercise [17] with a beneficial effect on movement skills [18].
Three types of WBV are used based on the amount of vibrating plates [18, 19]. Sinusoidal vertical whole-body vibration (SV-WBV) and sinusoidal side-alternating whole-body vibration (SS-WBV) use a single vibrating platform, whereas stochastic resonance whole-body vibration (SR-WBV) expects the trainees to stand on two platforms. During sinusoidal WBV the participants stand on a platform that vibrates vertically (SV-WBV) or to the side alternating (SS-WBV) with a high frequency between 20 and 50 Hz and an amplitude between 2 and 14 mm [20]. SR-WBV vibrates with frequencies between 1 and 12 Hz and an amplitude between 3 and 6 mm while the feet of the participants are placed on two independent powered and stochastic vibrating platforms [20].
In recent years, WBV has been introduced as a training method to improve muscle power and strength [21–23]. Several systematic reviews [24, 25] report on strength-related outcomes. However, no review has considered muscle strength related outcomes in a comparison of WBV against non-exercising control or conventional exercise groups and no review evaluated the effects separately for groups differing in initial levels of physical functioning, e.g. the three groups “Go-Go, Slow-Go and No-Go”. This is of relevance, however, since training principles would let us expect that those with the lowest level of fitness have greatest room for improvement. In other words, improvement in the outcome of interest will be greatest in those with lower initial values [26]. Furthermore, no review includes stochastic resonance WBV.
For clinicians, a systematic overview about the relevance and indication for application of SV-WBV, SS-WBV or SR-WBV and how it might be applied for Go-Go, Slow-Go and No-Go elderly individuals is lacking. Therefore, the aims of this systematic review are to provide 1) an overview of the current studies on WBV, 2) to determine the effects of WBV on strength or power in Go-Go, Slow-Go and No-Go elderly individuals and 3) give recommendations on available evidence for practical use. We hypothesized that WBV differently effects on measures of strength and power in Go-Go, Slow-Go and No-Go.
Methods
Data sources and searches
Inclusion criteria and analysis methods were developed and documented in a protocol prior to the current review. Included were elderly over 65 years of age; excluded were Geriatric diseases (Parkinson disease, Stroke, Multiple sclerosis), studies applying electrical current vibration or vibration with shoe insoles. More detailed information on the protocol including a link to the search strategy can be found on http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013006489 (PROSPERO registration number 2013:CRD42013006489). This systematic review and meta-analysis followed the PRISMA guidelines [27].
A first literature search of electronic databases was repeatedly carried out from January 2013 to February 2014 in the PubMed, CENTRAL (Cochrane Central Register of Controlled Trials), Physiotherapy Evidence Database (PEDro) and CINAHL electronic databases. The International Clinical Trials Registry Platform from the World Health Organization (WHO) was also searched. In February 2015 the search was repeated shortly before submission of the manuscript to ensure inclusion of most recent relevant material in the review. Additionally, a manual search of the reference lists of retrieved publications was conducted. English and German language restrictions were imposed upon the search.
Systematic search
The following keywords and combinations according to the PICO-model [28] were used in the search strategy:
Population: elderly, aged, dwelling home, nursing home, human research.
Intervention: Whole Body Vibration, WBV, noise, random vibration, RCT.
Comparator: WBV against control intervention (non-exercise or exercise on a level too low to effect on muscle [29]), WBV against conventional strength training intervention.
Outcome: strength, maximal voluntary contraction, power, rate of force development, performance, falls. Our search terms are detailed in Additional file 1.
Based on the four PICO components, a final question was stated as: For an older adult with diminishing physical capacity, will whole body vibration (WBV) exercise as compared to non- or conventionally exercising older adults improve muscle strength and/or power?
The following aspects were operationalized: 1) assessment of the quality and internal validity of the studies reviewed; 2) description of the assessments used to document the effect of WBV on isometric maximal voluntary contraction (IMVC), dynamic strength (DS), power, rate of force development (RFD) and functional strength (FS); 3) composition of the WBV training parameters; and 4) conclusion about clinical relevance in general.
Study selection
Five independent reviewers (CJ, CW, NJS, SR, RH) screened the titles and abstracts for eligibility. They screened for randomized controlled trials (RCTs) measuring maximal voluntary contraction, power and rate of force development in studies using WBV intervention in elderly individuals (mean age at least 65 years). Full text articles in English or German were eligible for inclusion. Healthy elderly participants and all clinical outcome measures of IMVC, DS, power, RFD, and FS were included in this review. Studies describing vibrations applied by electrical current or vibrating insoles, and patient series were excluded.
In the event of missing data, additional information was requested from the corresponding authors in order to include these data in our meta-analysis.
Data extraction
In addition, general characteristics of the studies were extracted. Five authors (CJ, CW, NJS, SR, RH) independently abstracted the following information from each of the studies included in this review: 1) design and sample; 2) inclusion criteria; 3) training parameters (i. e. duration, frequency, intensity of WBV); 4) type of vibration plate; 5) change in strength, power, RFD; 6) conclusions of the studies and statistical significance.
Methodological quality assessment of studies
The methodological quality of the included articles was rated with the “Cochrane Collaboration tool for assessing risk of bias” (RoB) [30] to assess the risk of over- or under-estimating the effects of an intervention [31].
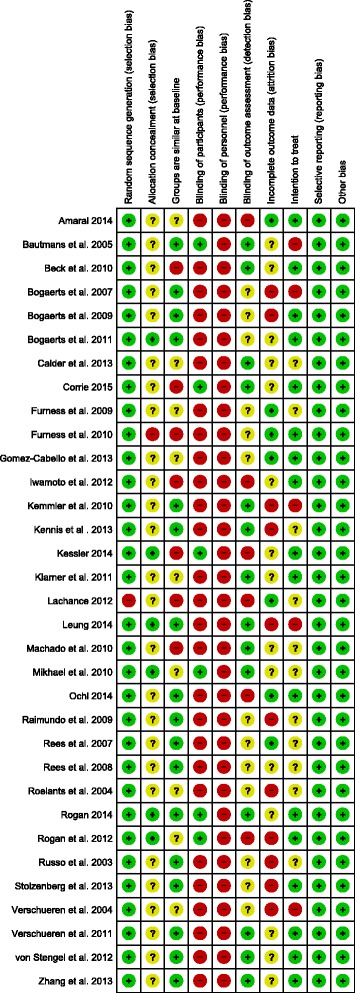
Nine items, with each having three rating categories, were scored and divided into six domains of bias (Fig. 2): (1) low ROB, (2) unclear ROB and (3) high ROB. Rating (1) is unlikely to alter the results significantly, (2) raises some doubt about the results and (3) seriously weakens confidence in the results. With insufficient information on an item, the score given was “high risk”. The arbitration of a third reviewer was used in the event of any disagreement between the reviewers (YB, RH) for both ratings.
Fig. 2.
Risk of bias
Data synthesis and analysis
Most outcomes of interest were presented as continuous data (mean values and SD or mean changes). For the meta-analysis of the present study the standardized mean difference (SMD) and 95 % confidence interval (CIs) of the post-intervention values or changes in scores were used for all comparisons. SMDs were pooled with a random effects model. The magnitude of the effect sizes for the between groups comparisons, calculated by SMDs are interpreted as follows: an effect size (d) around 0.2 indicates a small effect size, around 0.5 a medium effect size, and around 0.8 a large effect size [32]. If only one study was identified or data were not presented in a format that allowed inclusion in the dataset, results of individual studies are presented. If studies reported more than one IMVC, DS, power or RFD, and FS; then we only extracted the first outcome data on a hierarchy of outcomes:
IMVC: 1. isometric knee extension, 2. isometric hip extension, 3. isometric leg press; DS: 1. dynamic leg press, 2. dynamic knee extension (lowest speed), 3. dynamic hip extension; Power: 1. leg press, 2. knee extension; RFD: 1. counter movement jump, 2. squat jump, 3. leg press,; FS: 1. chair rise time, 2. chair rise repetition, 3. chair rise power, 4. stair climb, 5. wall squat. Subgroup analyses were undertaken to assess the effects of WBV on IMVC, DS, power, RFD and FS in Go-Go, Slow-Go and No-Go.
Heterogeneity was assessed by forest plots and the I2 statistics. Values >25 % indicate small, >50 % middle and >75 % considerable heterogeneity [33]. All other information was summarized and analysed qualitatively. Stata (version 13) was used for all meta-analyses.
Results
Study selection
The literature search yielded 1383 studies (PubMed n = 394, Central n = 163, Cinahl = 446, Embase = 174, Pedro n = 144, ScienceDirect n = 29, WHO International Clinical Trials Registry Platform n = 33). After identification of 367 duplicates, 1016 titles and abstracts were screened. Seventy-nine studies remained for further full-text analysis. Subsequently, 41 studies were excluded because they did not address strength, power or rate of force development or included participants with diseases. Finally, 38 full text papers [20, 23, 34–64] were included for this review and 37 were used for the meta-analysis (Fig. 1).
Fig. 1.
Flow diagram
Study characteristics
The major characteristics of the included studies are summarized in Table 1. Three papers investigated the effects on force by vertical and side-alternating sinusoidal WBV [35, 46, 66]. Five studies included more than 100 participants. Leung et al. [66] (n = 596), Boegarts et al. [38] (n = 180), Kemmler et al. [44] (n = 151) von Stengel [59] (n = 151) and Sitjà-Rabert et al. [64] (n = 117). The other included studies had a small sample size of less than 100 participants. The used strength outcomes varied across all included studies.
Table 1.
Study characteristics of the included studies
| Study | Participants (N, sex distribution); mean age (± SD) | Protocol exercise | Outcome measures | Within GE | Between GE |
|---|---|---|---|---|---|
| (yes/no) | (yes/no) | ||||
| Vertical sinusoidal vibration | |||||
| Amaral et al. [78] | WBV: 9 ♀; 76.6 (±11.8) | WBV: isometric squat | FS: CR over 30 s | No | No |
| Con: 9 ♀; 78.6 (±10.4) | Con: daily activities routines | ||||
| Álvarez- Barbosa et al. [62] | WBV: 15, 12 ♀; 84.0 (±3.0) | WBV: dynamic exercise lunge, squat, calf raises, left and right pivot in a front and lateral position, step up and down. | FS: CR over 30 s | Yes | Yes |
| Con: 15, 11 ♀; 86.0 (±7.5) | Con: no change lifestyle | ||||
| Bautmans et al. [34] | WBV: 10, 8 ♀; 76.6 (±11.8) | WBV: static position exercises (lunge squats, squats, deep squats, wide stance squats, calves, calves deep) druing WBV | DS: (N) | Yes | NO |
| Power: (W), work (J) | |||||
| Sham: 11, 6 ♀; 78.6 (±10.4) | RFD: (N/s) at 40 and 60 cm/s | ||||
| Beck et al. [35] | WBV: 15 ♀; 68.5 (±8.6) | Pos: full extension | FS: wall squat with dominant leg and non dominant leg & CR over five repetitions (s) | Yes | No |
| Con: 15 ♀; 74.2 (±8.1) | Con: no vibration | ||||
| Bogaerts et al. [37] | WBV: 25 ♂; 66.9 (±0.7) | WBV: squat, deep squat, wide stance squat, toesstand, toes-stand deep, one-legged squat, and lunge. | IMVC: knee extension (Nm) | Yes | Yes |
| Ex: 25 ♂; 67.4 (±0.9) | |||||
| Con: 32 ♂; 68.6 (±1.0) | Ex: cardio exercise, strength and balance training, flexibility exercise | RFD: CMJ (cm) on a contact mat. | |||
| Con: no change lifestyle | |||||
| Bogaerts et al. [38] | WBV: 70; 66.8 | WBV: exercises for upper and lower body | IMVC: knee extension (Nm) | Yes | No |
| Ex: 49: 66.8 | Ex: cardio exercise, strength and balance training, flexibility exercise | ||||
| Con: 61; 67.8 (ratio ♂:♀ = 1.5:1 for the total sample) | Con: no change life style | ||||
| Boegarts [36] | WBV 1: 26 ♀; 80.3 (±5.3) | WBV: squat, deep squat, wide stance squat, toes stand and one legged squat | Physiological Profile Assessment [76]: | Yes | No |
| WBV 2: 28 ♀; 79.8 (±5.3) | Con 1 & 2: no change life style | IMVC: knee extension (kg) | |||
| Con 1: 29 ♀; 78.7 (±5.6) | |||||
| Con 2: 28 ♀; 79.6 (±5.2) | |||||
| Corrie et al. [65] | WBV: 21, 13 ♀; 81.9 (±5.7) | WBV: standing position, with bent knees and Otago Exercise programm | Power: (W/kg body weight) leg press | Yes (power & CR) | Yes (power) |
| Sham: 20, 16 ♀; 79.1 (±7.8) | Sham: Otago Exercise program which consisted of 6 weekly visits | RFD: CMJ (N/kg body weight | |||
| FS: CR over five repetitions | |||||
| Gomez-Cabello [42] | WBV: 24 | WBV: squat position | Senior Fitness Test battery and Eurofit Testing Battery [77]: | Yes | No |
| Con: 25 (20 ♂, 29 ♀) | Con: no change life style | FS: CR over 30 s (repetition) | |||
| Kemmler et al. [44] | WBV: 50 ♀; 68.8 (±3.6) | WBV: static and dynamic exercise (toe stand, squat) | IMVC: leg press (N) | Yes | Yes |
| Ex: 50 ♀; 68.6 (±3.0) | Ex: static and dynamic exercise (toe stand, squat) without vibration | Power: leg press (W/kg) | |||
| Con: 51 ♀; 68.1 (±2.7) | Con: exercise and relaxation program once a week (30× 60 min) | RFD: leg press (N/ms) & Squat jump (jump height, cm) | |||
| Kennis et al. [45] | WBV: 23 ♂ | WBV: static and dynamic squat, deep squat, wide stance squat, 1-legged squat, lunge, toes-stand, toes-stand deep, moving heels. | IMVC: at 120° knee extension (Nm) | Yes | No |
| Ex: 20 ♂ | |||||
| Con: 29 ♂ | Ex: 60–90 min aerobic, resistance, balance, and flexibility exercises | DS: concentric dynamic knee extension (Nm) at a movement velocity at 120°/s. | |||
| Con: no change life style | RFD: counter movement jump (high, cm) | ||||
| Klarner et al. [46] | WBV: 36 ♀; 68.1 (±4.0) | WBV: dynamic exercises | IMVC: with leg press (N) | Yes | Yes |
| Con: 36 ♀; 67.6 (±4.13) | Con: 1/w low gymnastic exercise & relaxation exercise | RFD: with Counter movement jump (CMJ, jump height, cm) | |||
| Lachance [47] | WBV: 26; 70.4 (±7.7) | WBV: static squats (60°), lunges (60°) and heel raises. | FS: CR over 30 s | Yes | No |
| Ex: 29; 75.9 (±7.2) | Ex: static squats (60°), lunges (60°) heel raises, bicep curls, tricep extensions, | ||||
| (33 ♂, 22 ♀) | Exercises were progressive in nature by safely increasing the number of repetitions completed and/or weight of the dumbbells. | ||||
| Leung et al. [66] | WBV: 280 ♀; 74.2 (±7.0) | WBV: standing upright without knee banding | IMVC: knee extensor (kg) | Yes | Yes |
| Con: 316 ♀; 71.0 (±7.0) | Non: no change life style | ||||
| Machado et al. [48] | WBV: 13 ♀; 79.3 (±7.3) | WBV: static and dynamic exercise (half-squat (120–130°), deep squat (knee angle 90°), a wide-stance squat and calves. | IMVC: leg extensor (N) | Yes | Yes |
| Con: 13 ♀; 76.2 (±8.4) | Con: no change life style | Power: output at three relatives loads: 20, 40, 60 % of the IMVC. | |||
| Mikhael et al. [49] | WBV1: 6, 4 ♀; 63.3 (±7.6) | WBV: WBV1 with flexed knees at 20° and WBV 2 with extended knees. | DS: one repetition maximum (1RM) leg press (N), relative strength (kg/kg), leg press strength (kg) | Yes | Yes |
| WBV2: 5, 3 ♀; 69.0 (±7.6) | Sham: flexed knees at 20° without vibration | Power: (W) and velocity (cm/s) were measured at 20, 30, 40, 50, 60, 70, 80, 90, and 100 % of current 1RM. | |||
| Sham: 8, 4 ♀; 62.3 (±8.8) | FS : CR over 30 s | ||||
| Roelants et al. [53] | WBV: 30 ♀; 64.6 (±0.7) | WBV: high squat (120° and 130°, deep squat (90°), wide-stance squat and lunge. | IMVC: (0°/s) torque (Nm) of knee extensor | Yes | No |
| Ex: 30 ♀; 63.9 (±0.8) | Ex: resistance exercise | DS: dynamic extension-flexion movements (torque: N/m) between 90 and 160° at a velocity of 50, 100 and 150°/s. | |||
| Con: 29 ♀; 64.2 (±0.6) | Con: no change life style | RFD: jump height (mm) on a contact mat | |||
| Sitjà-Rabert et al. [64] | WBV: 59; 64.6 (±0.7) | WBV: static/dynamic exercises. | FS: CR over five repetitions | Yes | No |
| Ex: 58; 63.9 (±0.8) | Ex: static and dynamic exercise | ||||
| (Total sample 67 % ♀) | |||||
| Verschueren et al. [58] | WBV: 25 ♀; 64.6 (±3.3) | WBV: static and dynamic knee-extensor exercises like squat, deep squat, wide-stance squat, one-legged squat and lunge. | IMVC: knee-extension | Yes | Yes |
| Ex: 22 ♀; 63.9 (±3.8) | Ex: warm-up, resistance training knee-extensor on a leg extension and a leg press machine. Designed to the guideline of the American College of Sports Medicine | DS: isokinetic extension-flexion movements for maximal DS (peak torque N/m) at a velocity of 100°/s between of 90 and 160° joint angle. | |||
| Con: 24 ♀; 64.2 (±3.1) | Con: no change life style | ||||
| Verschueren et al. [57] | WBV: 28 ♀; 79.8 (±5.3) | WBV: static and dynamic knee-extensor exercises like squat, deep squat, wide-stance squat, one-legged squat and toe-stance. | IMVC: Knee-extension (Nm) | Yes | No |
| Con: 28 ♀; 79.6 (±5.2) | Con: no change in life style | DS: Knee-extension (Nm). | |||
| Sidealternating sinusoidal vibration | |||||
| Beck et al. [35] | WBV: 17 ♀; 68.9 (±70) | Pos: static with slightly bended knees | FS: wall squat dominant leg (DL) and non dominant leg (NDL) & CR over five repetitions (s) | Yes = | No |
| Con: 15 ♀; 74.2 (±8.1) | Con: no vibration | ||||
| Calder et al. [39] | N: 41, 30 ♀; 80.1 | WBV: stand with slightly bended knees (35° flexion) & Physiotherapy | FS: CR | Yes | No |
| Con: Physiotherapy | |||||
| Corrie et al. [65] | WBV: 21, 16 ♀; 81.9 (±5.7) | WBV: standing position, with bent knees and Otago Exercise | Power: (W/kg body weight) leg press | No | No |
| Sham: 20, 8 ♀; 79.1 (±7.8) | Sham: Otago Exercise program which consisted of 6 weekly visits | RFD: CMJ (N/kg body weight | |||
| FS: CR over five repetitions | |||||
| Furness and Maschette [40] | WBV1: 18 (1/week) | WBV: static with 70 knee flexion | FS: CR | Yes (for WBV2 and WBV3) | No |
| WBV2: 18 (2/week) | Con: no vibration | ||||
| WBV3: 19 (3/week) | |||||
| Age: 72 (±8) | |||||
| Con: 18 (0/wk) | |||||
| (Total sample 38 ♂, 35 ♀) | |||||
| Furness et al. [41] | WBV: 19 | WBV: static with 70° Kneeflexion | FS: CR | Yes | No |
| Con: 18 | Con: no exercise | ||||
| (Total sample 16 ♂, 21 ♀) | |||||
| Iwamoto et al. [43] | WBV: 26 ♀; 72.4 (±8.1) | WBV: stands with bended knee and hips | FS: CR over 5 times | Yes | No |
| Con: 26 ♀; 76.0 (±7.4) | Con. No exercise | ||||
| Klarner et al. [40] | WBV: 36 ♀; 67.9 (±3.78) | WBV: dynamic exercises | IMVC: Hip & Knee extension (N) | Yes | Yes |
| Con: 36 ♀; 67.6 (±4.13) | Con: 1/w low gymnastic exercise & relaxation exercise | RFD: CMJ (jump height, cm) | |||
| Ochi et al. [67] | WBV: 10 ♀; 80.9 (±2.8) | WBV: dynamic exercises | IMVC: Quadiceps muscle dominant leg | Yes | No |
| Ex: 10 ♀; 80.2 (±3.3) | Con: dynamic exercise: half squat, heel rise, toe up. | ||||
| Raimundo et al. [50] | WBV: 14 ♀; 66 (±6) | WBV: static with knee angle 120° | DS: dynamic maximal unilateral strength at 60 and 300 °/s for concentric and eccentric at 60 °/s (Peak torque (Nm/kg). | Yes | Yes |
| Ex: 13 ♀; 66 (±4) | Ex: walk-based-programme | Power (W). | |||
| Walking over 60 m with two sets with 70–75 % of their maximal heart rate. | RFD: mixed counter movement jump on | ||||
| Ergo Jump Platform (Bosco System, Italy) | |||||
| FS: CR over three repetitions. | |||||
| Rees et al. [51] | WBV: 15; 74.3 (±5.0) | WBV: static squats over 4 weeks, than dynamic squats and calf raises over 4 weeks. | DS: angular velocity 60°/s for knee and hip and the angle joint was tested at 30 °/s. | Yes | Yes |
| Ex: 13; 73.1 (±4.1) | Ex: static squats over 4 weeks, than dynamic squats and calf raises over 4 weeks, without vibration. | FS: CR over five repetition | |||
| Con: 15; 73.1 (±4.6) | |||||
| (Total sample 23 ♂, 20 ♀) | |||||
| Rees et al. [52] | WBV: 15; 74.3 (±5.0) | Con: only walking WBV: static and dynamic exercise (squats, calf raises) | DS: as torque (Nm/kg) | Yes | No |
| Ex: 13; 73.1 (±4.1) | Ex: same exercise without vibration | maximum isokinetic power (W/kg) angular velocity for the hip and knee was 60°/s, with the ankle joint tested at 30°/s. | |||
| (No sex distribution information) | |||||
| Russo et al. [55] | WBV: 17 ♀ | WBV: static, knees slightly flexed | DS: strength (N), acceleration of the centre of gravity (COG) was calculated as the ratio of force (N) and body mass (kg). | Yes | No |
| Age: 60.7 (±6.1) | Con: no change in life style | RFD: starting from a standstill, jumped as high as possible and landed (W). | |||
| Con: 16 ♀ | |||||
| Age: 61.4 (±7.3) | |||||
| Sievänen et al. [61] | WBV: 8, 7 ♀ | WBV: dynamic exercise such as slight squatting, toe raises, lateral weigth transfer. | FS: SPPB | Yes | NO |
| Age: 84.4 (±6.3) | Ex: light squatting, toe raises or weight transfer forward and lateral weight transfer on WBV. | ||||
| Sham: 7, 5 ♀ | |||||
| Age: 83.6 (±8.9) | |||||
| Stolzenberg et al. [56] | WBV: 30 ♀; 67.5 (±3.8) | WBV: static standing with slightly bent knees and hips, continuous squatting from erect standing to 90° knee flexion or static stance in 90° knee flexion | Power: CMJ (W/kg) | Yes | No |
| Con: 30 ♀; 65.5 (±4.3) | Con: balance exercise like Romberg, tandem and single-leg stance. | RFD: CMJ jump height (cm) | |||
| FS: 1-leg hopping and CR over five repetitions | |||||
| von Stengel et al. [59] | WBV: 50♀; 68.8 (±3.6) | WBV: heel rise, one-legged deep squat, and leg abduction | MVC: leg press (N) | Yes | Yes |
| Ex: 50 ♀; 68.6 (±3.0) | Ex: heel rise, one-legged deep squat, and leg abduction without vibration | RFD: CMJ (W/Kg) | |||
| Con: 51 ♀; 68.1 (±2.7) | Con: exercise and relaxation program once a week in blocks of 10 weeks with breaks | ||||
| Zhang et al. [60] | WBV: 19, 2 ♀; 85.8 (±3.6) | WBV: different to their function. Who could stand: partial squat position with slight hip, knee and ankle joint flexion. Who could not stand independently, same position, but were allowed to hold the support bar with their hands. | IMVC: M. quadriceps | Yes | Yes |
| Con: 18, 3 ♀; 84.7 (±3.7) | Ex: usual care, physical therapy (ultrasound therapy, electrical stimulation, etc.) and routine exercises, such as pedalling training with regular dosage and time of treatments. | FS: CR over 30 s | |||
| Stochastic resonance vibration | |||||
| Kessler et al. [23] | WBV: 10, 8 ♀; 77 (±7.7) | static (e.g. normal stance, semi-tandem, one leg stance) and dynamic standing (e.g. squat) | IMVC: knee-extension (N) | Yes | Yes |
| Sham:10, 8 ♀; 81 (±5.7) | RFD: knee-extension (N/s) | ||||
| FS: SPPB | |||||
| Rogan et al. [54] | WBV: 10; 77 (±7.7) | static standing with slightly bent knees and hips | FS: CR (1 time) | Yes | No |
| Sham:10; 81 (±5.7) | |||||
| (No sex distribution information) | |||||
| Rogan et al. [20] | WBV: 5; 77 (±7.7) | static standing with slightly bent knees and hips | IMVC: knee-extension (N) | Yes | No |
| Sham: 4; 81 (±5.7) | RFD: knee-extensor (N/s) | ||||
| (Total sample 4 ♂, 5 ♀) | FS: SPPB | ||||
Abbreviation: Con control group, EX exercise group, Sham sham group; SD standard deviation, GE group effests, mo month, wk week, WBV whole-body vibration, Pos position, s seconds, IMVC isometric maximal voluntary contraction, DS dynamic maximal strength, RFD rate of force development, FS functional strength, CR chair rising, CMJ counter movement jump, SPPB Short Physical Performance Battery Test, cm centimetre, mm millimetre, N newton; N/s Newton/seconds, Nm Newton-metre, Nm/kg Newton-meter/kilogram, N/ms Newton/milliseconds, kg kilogram, J Joule, W watt, W/kg watt/kilogram
Table 2 shows the training parameters. All authors prescribed two to three WBV sessions per week. Intervention duration of six trials were lasting more than 1 year [37, 38, 44, 46, 59, 67]. The duration of ten trials [35, 36, 42, 43, 50, 55–58, 53] was between 6 months and 1 year. The other trials lasted less than 6 months while one study examined strength effects immediately after a single WBV intervention [20]. The training parameters such as amplitude, frequency and sets of WBV varied across all studies investigating sinusoidal WBV. In contrast, studies with SR-WBV were more homogenous.
Table 2.
Overview of trainings parameter within each study
| Study | Duration/(Session/per week) | Amplitude | Frequency | Sets, Duration, Rest between set |
|---|---|---|---|---|
| Vertically sinusoidal vibration | ||||
| Amaral et al. [78] | 12 weeks/(3/week) | 2–4 mm | 30–40Hz | 3 sets × 30–45 s. |
| Álvarez- Barbosa et al. [62] | 8 weeks/(3/week) | 4 mm | F: 30–35Hz | 6–12 sets, 12–17 min total time, 45 s rest between set |
| Bautmans et al. [34] | 6 weeks/(3/week) | 2–5 mm | 30–40 Hz | 4 sets × 30–60 s, 30–60 s rest between set |
| Beck et al. [35] | 32 weeks/(2/week) | 0.3 g | 30 Hz | 15 min (1 session), no rest |
| Bogaerts et al. [37] | 47 weeks/(3/week) | 2.5–5 mm | 30–40 Hz | 4 sets sets × 30 s – 15 × 30 s, 15–30 s rest between set |
| Bogaerts et al. [38] | 48 weeks mo/(3/week) | NA | NA | NA |
| Boegarts [36] | 24 weeks/(3/week) | 1.6–2.2 g | 30–40 Hz | 3 sets × 15–60 s, 60–5 s rest between set |
| Corrie et al. [65] | 12 weeks/(3/week) | 1.3 mm | 30 Hz | 3 to 6 sets × 20 to 60 s, 60 s rest between set |
| Gomez-Cabello [42] | 44 weeks mo/(3/week) | 2 mm | 40 Hz | 10 sets × 45 s, 60 s rest between set |
| Kemmler et al. [44] | 88 weeks/(2/week) | NA | 25–35 Hz | NA |
| Kennis et al. [45] | 2.5–5 mm | 30–40 Hz | 4 sets × 30 s till 15 sets × 30 s, 15–30 s rest between set | |
| Klarner et al. [46] | 48 weeks mo/(3/week) | 35 Hz | 7 sets × 90 s, 40 s rest between set | |
| Lachance [47] | 8 weeks/(2/week) | 2 mm | 35 Hz | NA |
| Leung et al. [66] | 72 weeks mo/(5/week) | 2 mm | 35 Hz | 20 min, rest (NA) |
| Machado et al. [48] | 10 week/(3–5/week) | 2–4 mm | 20–40 Hz | 3–8 sets × 30–60 s, rest (NA) |
| Mikhael et al. [49] | 12 weeks/(3/week) | 1 mm | 12 Hz | 10 sets × 60 s, 60 s rest between set |
| Roelants et al. [53] | 24 weeks/(3/week) | 2.5–5 mm | 35–40 Hz | 1–3 sets × 30–60 s of one exercise, 60 to 5 s rest between set |
| Sitjà-Rabert et al. [64] | 6 weeks/(3/week) | 2–4 mm | 30–35 Hz | 3 sets × 30–60 s of one exercise, 60 to 5 s rest between set |
| Verschueren et al. [58] | 24 weeks/(3/week) | 1.7–2.5 mm | 35–40 Hz | NA |
| Verschueren et al. [57] | 18 weeks/(3/week) | 1.6–2.2 g | 30–40 Hz | 15–60 s × Pos. exercise, 60 s till 5 min rest between exercises |
| Sidealternating sinusoidal vibration | ||||
| Beck et al. [35] | 32 weeks/(2/week) | 2 mm | 12.5 Hz | 2 sets × 3 min, 60 s rest between set |
| Calder et al. [39] | 6 weeks | 2 mm | 20 Hz | 4 sets × 75 s, 90 s rest between set |
| Corrie et al. [65] | 12 weeks/(3/week) | 2.9 mm | 30 Hz | 3 to 6 sets × 20 to 60 s, 60 s rest between set |
| Furness and Maschette [40] | 6 weeks | 0.05 mm | 15–25 Hz | 5 sets × 60 s, 60 s rest between set |
| Furness et al. [41] | 6 weeks/(3/week) | 1 mm | 15–25 Hz | 5 sets × 60 s, 60 s rest between sets |
| Iwamoto et al. [43] | 18 weeks/(2/week) | NA | 20 Hz | 4 min, NA rest |
| Klarner et al. [40] | 48 weeks/(3/week) | 3–7 mm | 12,5 Hz | 7 sets × 90 s, 40 s rest between set |
| Ochi et al. [67] | 12 weeks/(3/week) | 12 mm | 2,5 Hz | 180 s, no rest |
| Raimundo et al. [50] | 32 weeks/(3/week) | 6 mm | 20–30 Hz | 3 sets × 60 s, 60 s rest between set |
| Rees et al. [51] | 8 weeks/(3/week) | 5–8 mm | 26 Hz | 6 sets × 45 up to 60 s, 5 × 45 up to 80 s rest between set |
| Rees et al. [52] | 8 weeks/(3/week) | 5–8 mm | 26 Hz | 6 sets × 45–80 s, 45–80 s rest between set |
| Russo et al. [55] | 24 weeks/(2/week) | NA | 12–28 Hz | 3 sets × 60–120 s, 60 s rest between set |
| Sievänen et al. [61] | 10 week/(2/week) | 2–8 mm | 12 and 18 Hz | 1–5 sets × 60–120 s, 60 s rest between set |
| Stolzenberg et al. [56] | 36 weeks/(2/week) | NA | 22–26 Hz | 60–90 s, rest (NA) |
| von Stengel et al. [59] | 74 weeks/(2weeks) | 1.7–2 mm | 25–35 Hz | 6 sets × 60 s, 60 s rest between set |
| Zhang et al. [60] | 8 weeks/(3–5/week) | 1–3 mm | 25–35 Hz | 4–5 sets × 60 s, 60 s rest between set |
| Stochastic resonance vibration | ||||
| Kessler et al. [23] | 4 weeks/(3/week) | – | 3–6 Hz (Noise 4) | 5 sets × 60 s, 60 s rest between set |
| Rogan et al. [54] | 4 weeks/(3/week) | – | 5 Hz (Noise 4) | 5 sets × 60 s, 60 s rest between set |
| Rogan et al. [20] | Immediately (acute effects) | 6 Hz (Noise 4) | 5 sets × 60 s, 60 s rest between set | |
Abbreviation: mo month, wk week, s seconds, Hz hertz, NA not available
Study methodological quality
All studies included in Fig. 2 were at risk of bias according to the “Cochrane Collaboration’s tool for assessing risk of bias”. Most studies lacked allocation concealment, blinding, and presented incomplete data.
Meta-analysis
For the meta-analysis 37 studies were included and data were available for IMVC, DS, power, RFD or FS outcome measurements. The effect sizes for these outcomes are summarized in Figs. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 13.
Fig. 3.
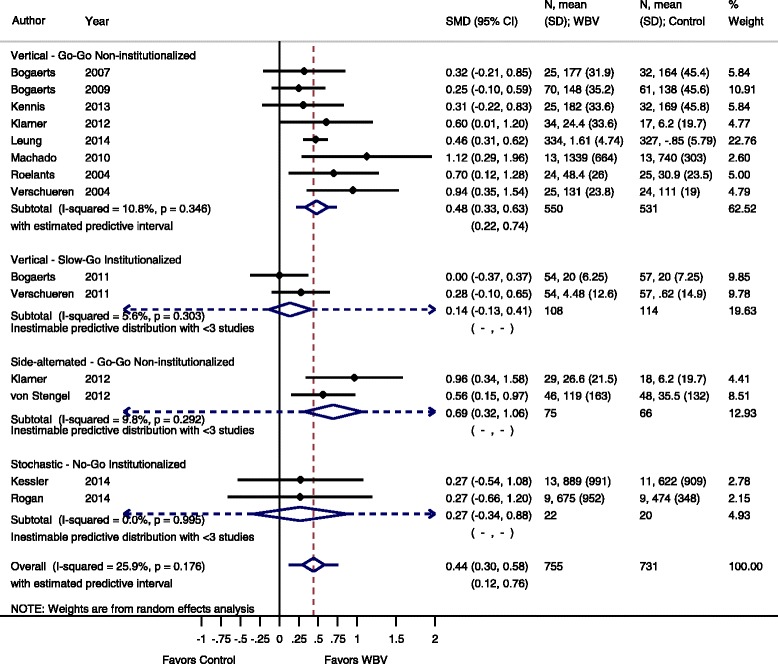
Comparison of WBV versus control group (i.e. no exercise), outcome: maximal voluntary isometric contraction. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 4.
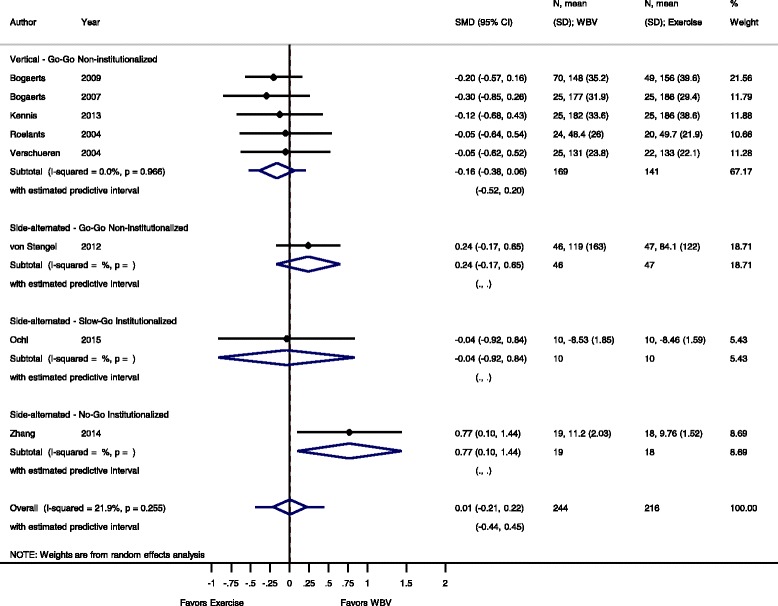
Comparison of WBV versus exercise group (i.e. no exercise), outcome: maximal voluntary isometric contraction. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 5.
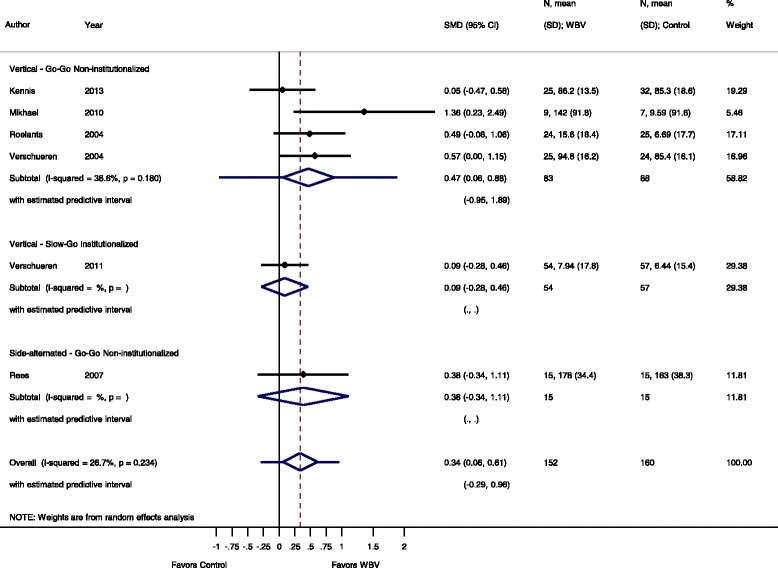
Comparison of WBV versus control group (i.e. no exercise) outcome: dynamic strength. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 6.
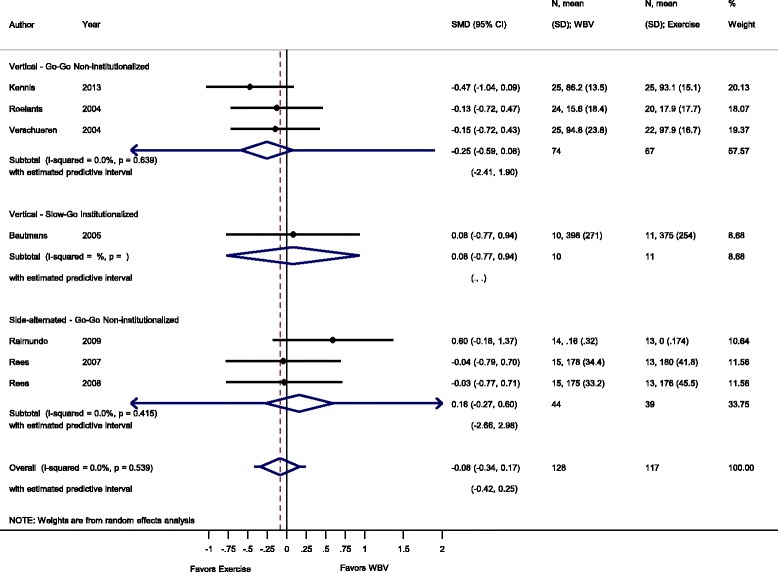
Comparison of WBV versus exercise group; outcome: dynamic strength. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 7.
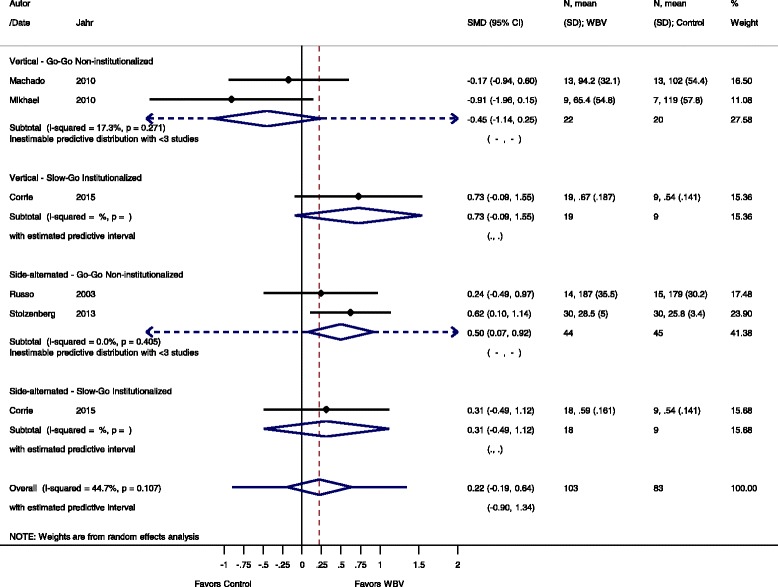
Comparison of WBV versus control group (i.e. no exercise) outcome: power. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2: statistic for heterogeneity, WBV whole-body vibration
Fig. 8.
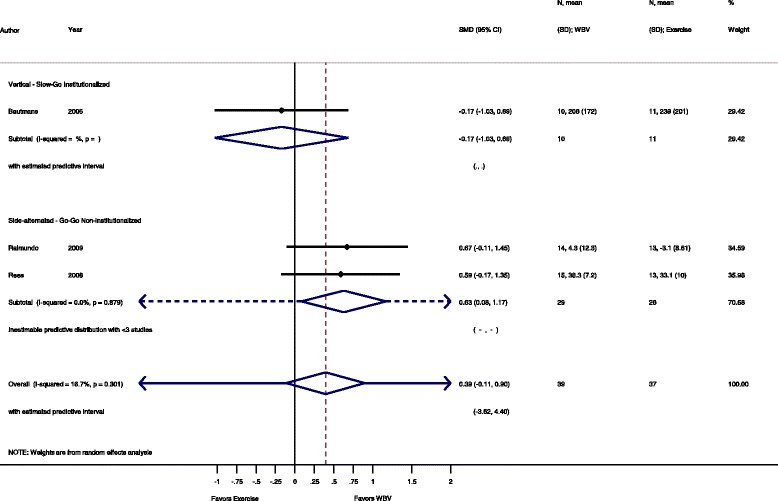
Comparison of WBV versus exercise group; outcome: power. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 9.
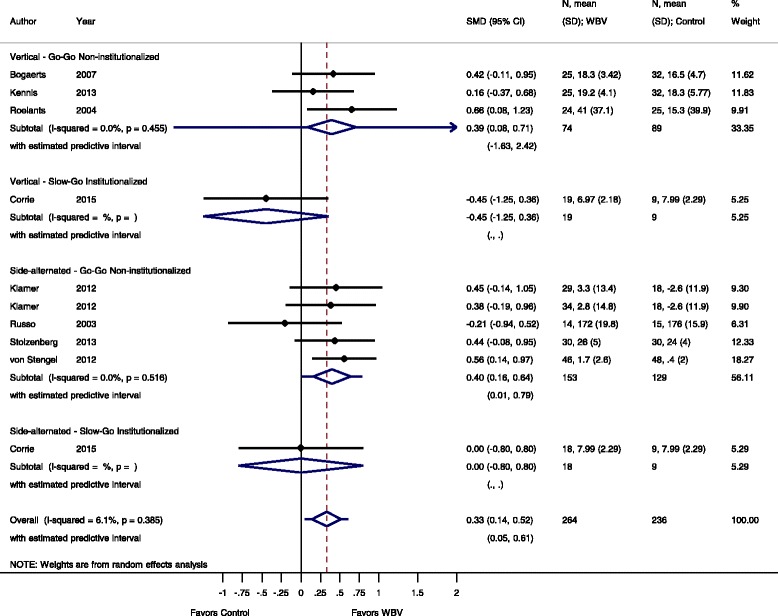
Comparison of WBV versus control group (i.e. no exercise), outcome: rate of force development. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 10.
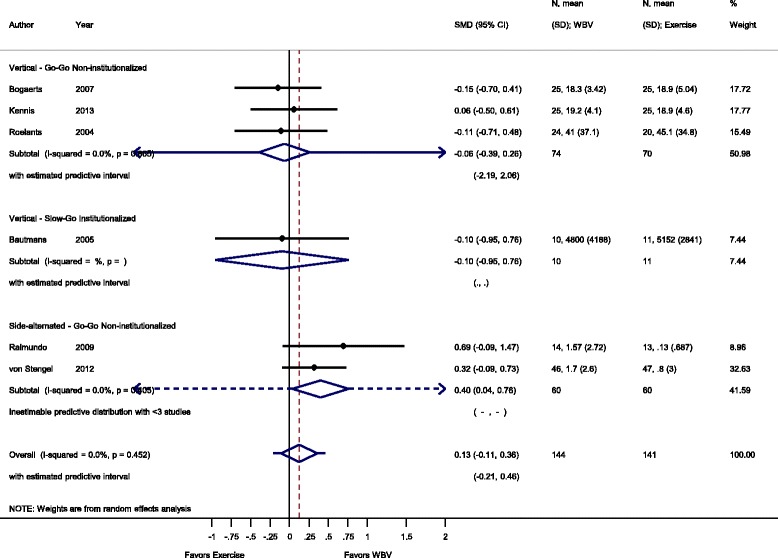
Comparison of WBV versus exercise group, outcome: rate of force development. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 11.
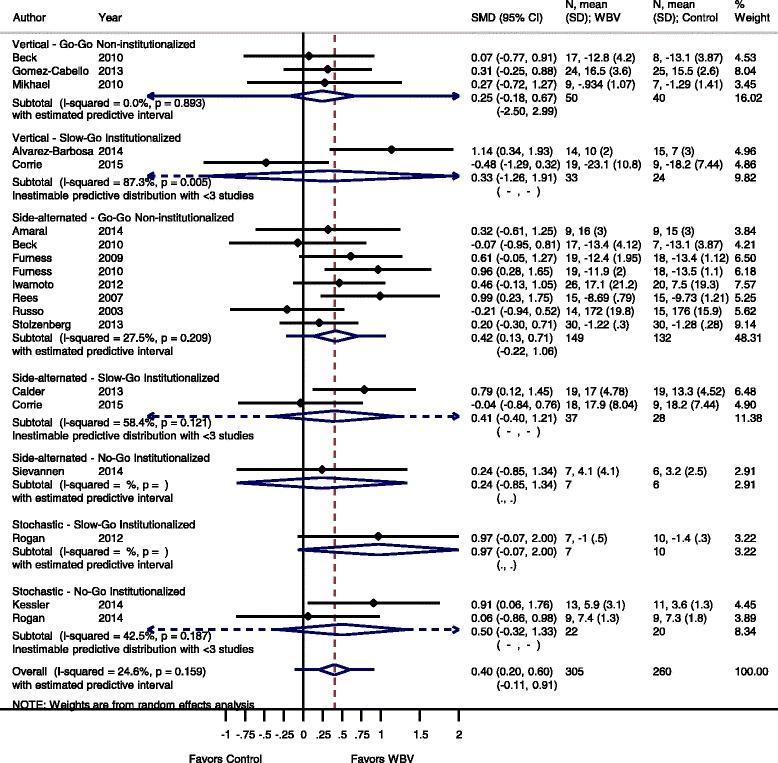
Comparison of WBV versus control group (i.e. no exercise), outcome: functional strength. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 12.
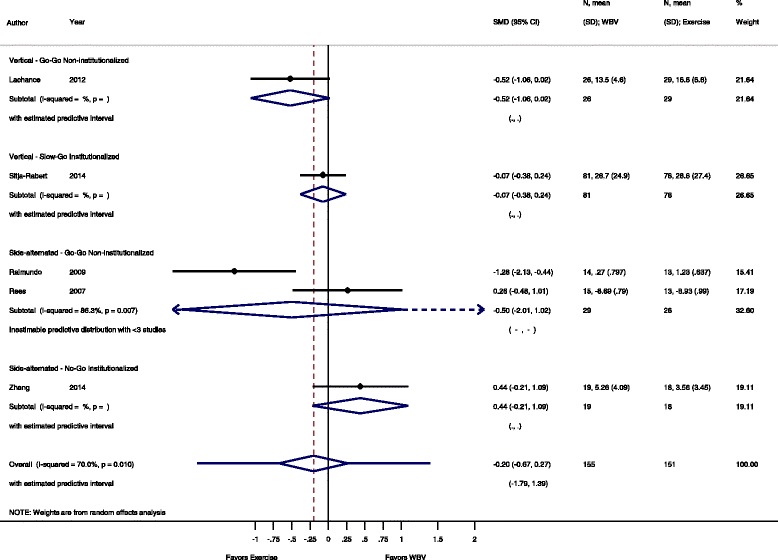
Comparison of WBV versus exercise group, outcome: functional strength. SMD standardized mean difference, SD standard deviation, 95 % CI confidence interval, I 2 statistic for heterogeneity, WBV whole-body vibration
Fig. 13.
Funnel plot over all analysis
Isometric maximal voluntary contraction: WBV vs. non-exercise control group
Thirteen studies [20, 23, 36–38, 45, 46, 48, 53, 57–59, 67], including 1468 participants, reported data contributing to the comparison WBV vs. non-exercise control group, one study had two WBV-arms (vertical and side-alternating) and one control arm [46] (the number of participants in the control groups was cut in half to obtain correct numbers for the pooled analysis). The pooled overall SMD was 0.44 (95 % CI 0.30 to 0.58) in favor of WBV with low heterogeneity, I2 25.9 % (p = 0.176).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of 0.48 (95 % CI 0.33 to 0.63) with a low heterogeneity (I2 10.8 % (p = 0.346)); and for SS-WBV-Go-Go a SMD of 0.69 (95 % CI 0.32 to 1.06) with a low heterogeneity, I2 9.8 % (p = 0.292).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go revealed a SMD of 0.14 (95 % CI −0.13 to 0.41) with a low heterogeneity, I2 5.6 % (p = 0.303).
No-Go
The subgroup analysis for SR-WBV-No-Go showed a SMD of 0.27 (95 % CI −0.34 to 0.88) in favour of SR-WBV with no heterogeneity, I2 0.0 % (p = 0.995) (Fig. 3).
Isometric maximal voluntary contraction: WBV vs. exercise group
For the comparison WBV versus conventional exercise eight studies [37, 38, 45, 53, 58–60, 68] contributed 460 participants. The pooled overall SMD was 0.01 (95 % CI −0.21 to 0.22) with a low heterogeneity, I2 = 21.9 % (p = 0.255).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of −0.16 (95 % CI −0.38 to 0.06) with a low heterogeneity, I2 0.0 % (p = 0.966); SS-WBV-Go-Go showed an SMD of 0.24 (95 % CI −0.17 to 0.65) in favour of SS-WBV.
Slow-Go
The subgroup analysis for SS-WBV-Slow-Go showed a SMD of −0.04 (95 % CI −0.92 to 0.84) in favour of conventional exercise.
No-Go
The subgroup analysis for SS-WBV-No-Go showed a SMD of 0.77 (95 % CI 0.10 to 1.44) in favour of SS-WBV.
Dynamic strength: WBV vs. non-exercise control
Comparing dynamic strength in WBV versus non-exercise controls, six studies [45, 49, 51, 53, 57, 58] contributed with a total of 312 participants. The pooled overall SMD was 0.34 (95 % CI 0.06 to 0.61), which was statistically significant in favour of the WBV group, with low heterogeneity (I2 26.7 %, p = 0.234).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of 0.47 (95 % CI 0.06 to 0.88) in favour of SV-WBV with a low heterogeneity, I2 38.6 %, p = 0.180. The subgroup analysis for SS-WBV-Go-Go showed a SMD of 0.38 (95 % CI −0.34 to 1.11) in favour of SS-WBV.
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of 0.09 (95 % CI −0.28 to 0.46).
Dynamic strength: WBV vs. conventional exercise
For the comparison WBV versus conventional exercise seven studies [45, 50–52, 58, 59, 53] contributed with a total of 245 participants. The pooled overall SMD was 0.08 (95 % CI −0.34 to 0.17), statistically non-significant, thus, not in favour of a particular group, with no heterogeneity (I2 0.0 %, p = 0.539).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of −0.25 (95 % CI −0.59 to 0.08) in favour of exercise, with no heterogeneity, I2 0.0 % (p = 0.639). The subgroup analysis for SS-WBV-Go-Go showed a SMD of 0.16 (95 % CI −0.27 to 0.60) statistically not significant in favour of SS-WBV with no heterogeneity, I2 0.0 % (p = 0.415).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of 0.08 (95 % CI −0.77 to 0.94).
Power: WBV vs. non-exercise control
For the outcome power and the comparison WBV versus non-exercise control five studies [48, 49, 55, 56, 66] contributed with a total of 186 participants. The control group of the Corrie et al. [65] study contributed to two subgroup analyses, therefore, we cut the number of participants in the control group in half to avoid incorrect standard errors.
Go-Go
The pooled overall SMD was 0.22 (95 % CI −0.19 to 0.64), statistically non-significant in favour of the WBV group, with a moderate heterogeneity (I2 44.7 %, p = 0.107).
The subgroup analysis for SV-WBV-Go-Go showed a SMD of −0.45 (95 % CI −1.14 to 0.25), with low heterogeneity, I2 17.3 % (p = 0.271). SS-WBV-Go-Go showed a SMD of 0.50 (95 % CI 0.07 to 0.92) statistically significant in favour of SS-WBV with no heterogeneity, I2 0.0 % (p = 0.405).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of 0.73 (95 % CI −0.09 to 1.55). SS-WBV-Slow-Go showed a SMD of 0.31 (95 % CI −0.49 to 1.12) statistically not significant in favour of SS-WBV.
Power: WBV vs. conventional exercise
Three studies [34, 50, 52] with 76 participants contributed to the comparison SS-WBV versus conventional exercise. The SMD was 0.39 (95 % CI −0.11 to 0.90), statistically not significant in favour of the WBV group.
Go-Go
The subgroup analysis for SS-WBV-Go-Go showed a SMD of 0.63 (95 % CI 0.08 to 1.17), statistically significant in favour of SS-WBV with no heterogeneity, I2 0.0 % (p = 0.879).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of −0.17 (95 % CI −1.03 to 0.69), statistically not significant in favour of conventional exercise.
Rate of force development: WBV vs. non-exercise control
For the outcome rate of force development and the comparison WBV versus non-exercise control eight studies [34, 37, 45, 46, 55, 56, 66, 53] contributed with a total of 500 participants. The control groups of two studies [46, 66] contributed to two subgroup analyses, therefore, we divided the number of participants in the control groups in half. The pooled overall SMD was 0.33 (95 % CI 0.14 to 0.52), statistically significant in favour of the WBV group with low heterogeneity (I2 6.1 %, p = 0.385).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of 0.39 (95 % CI 0.08 to 0.71) in favour of conventional exercise with no heterogeneity, I2 0.0 % (p = 0.455). SS-WBV-Go-Go showed a SMD of 0.40 (95 % CI 0.16 to 0.64) and statistically significant in favour of SS-WBV with no heterogeneity, I2 0.0 % (p = 0.516).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of −0.45 (95 % CI −1.25 to 0.36). The subgroup analysis for SS-WBV-Slow-Go showed a SMD of 0.00 (95 % CI −0.80 to 0.80).
Rate of force development: WBV vs. conventional exercise
For the comparison WBV versus conventional exercise six studies [34, 37, 45, 50, 59, 53] contributed with a total of 285 participants. The pooled overall SMD was 0.13 (95 % CI −0.11 to 0.36), statistically non-significant in favour of the WBV group, with no heterogeneity (I2 0.0 %, p = 0.452).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of −0.06 (95 % CI −0.39 to 0.26) in favour of exercise, with no heterogeneity, I2 0.0 % (p = 0.805). SS-WBV-Go-Go showed a SMD of 0.40 (95 % CI 0.04 to 0.76), statistically significant in favour of SS-WBV with no heterogeneity, I2 0.0 % (p = 0.405).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of −0.10 (95 % CI −0.95 to 0.76).
Functional strength: WBV vs. non-exercise control
For the outcome functional strength and the comparison WBV versus non-exercise control 17 studies [20, 23, 35, 37, 39–43, 45, 49, 51, 54–56, 59, 64, 53] contributed with a total of 565 participants (one study has SS-WBV and SV-WBV versus control, therefore we cut the number of participants of the control group in half) [35]. The pooled overall SMD was 0.40 (95 % CI 0.20 to 0.60), statistically non-significant in favour of the WBV group with low heterogeneity (I2 24.6 %, p = 0.159).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed an SMD of 0.25 (95 % CI −0.189 to 0.67), statistically non-significant in favour of exercise with no heterogeneity, I2 0.0 % (p = 0.893).SS-WBV-Go-Go showed a SMD of 0.42 (95 % CI 0.13 to 0.71) statistically significant in favour of SS-WBV with low heterogeneity, I2 27.5 % (p = 0.209).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed an SMD of −0.33 (95 % CI −1.26 to 1.91) with high heterogeneity, I2 87.3 % (p = 0.005). The subgroup analysis for SS-WBV-Slow-Go showed a SMD of 0.41 (95 % CI −0.40 to 1.21), statistically non-significant in favour of SS-WBV with moderate heterogeneity, I2 58.4 % (p = 0.121).
No-Go
The subgroup analysis for SS-WBV-No-Go showed a SMD of 0.24 (95 % CI −0.85 to 1.34). The subgroup analysis for SR-WBV-Slow-Go showed a SMD of 0.97 (95 % CI −0.07 to 2.00). The subgroup analysis for SR-WBV-No-Go showed a SMD of 0.50 (95 % CI −0.32 to 1.33), statistically non-significant in favour of SR-WBV, with a moderate heterogeneity, I2 42.5 % (p = 0.187).
Functional strength: WBV vs. conventional exercise
For the comparison WBV versus conventional exercise five studies [47, 50, 51, 60, 64] contributed with a total of 306 participants. The pooled overall SMD was −0.20 (95 % CI −0.67 to 0.27), statistically non-significant in favour of the conventional exercise group with substantial heterogeneity (I2 70.0 %, p = 0.010).
Go-Go
The subgroup analysis for SV-WBV-Go-Go showed a SMD of −0.52 (95 % CI −1.06 to 0.02), statistically non-significant in favour of conventional exercise. The subgroup analysis for SS-WBV-Go-Go showed a SMD of −0.50 (95 % CI −2.01 to 1.02) with high heterogeneity, I2 86.3 % (p = 0.007).
Slow-Go
The subgroup analysis for SV-WBV-Slow-Go showed a SMD of −0.07 (95 % CI −0.38 to 0.24).
No-Go
The subgroup analysis for SS-WBV-No-Go showed a SMD of 0.44 (95 % CI −0.21 to 1.09), statistically non-significant in favour of SS-WBV.
Over all comparisons, only the comparisons of WBV versus non-exercise control for the outcomes isometric strength had a significant Egger’s test (p = 0.002) for small study bias. Funnel plots for individual comparison are not shown. Figure 13 shows a panel of the funnel plots for all comparisons.
The effect sizes in the studies with participants in the No-Go group (SMD of 0.47, 95 % CI 0.16 to 0.78, I2 0.00 %, p <0.792) were higher compared to the Go-Go (SMD 0.26, 95 % CI 0.15 to 0.63, I2 48.9 %, p <0.001) and the Slow-Go groups (SMD of 0.14, 95 % CI −0.04 to 0.33, I2 26.6 %, p = 0.121) (see Table 3 and Additional files 2, 3 and 4).
Table 3.
Overview of WBV utilization on physical performance status
| Go-Go | Slow-Go | No-Go | |||
|---|---|---|---|---|---|
| VS-WBV | VS-WBV | ||||
| 1 | Amaral et al. [78] (SA) FS | 1 | Alvarez et al. [62] FS | ||
| 2 | Beck et al. [35] FS | 2 | Bautmans [34] DS, Power, RFD | ||
| 3 | Boegarts et al. [37] IMVC, RFD | 3 | Boegarts et al. [36] IMVC | ||
| 4 | Boegarts et al. [38] IMVC | 4 | Corrie et al. [6] Power, FS, RFD | ||
| 5 | Gomez-Cabello et al. [42] FS | 5 | Sitja-Rabert et al. [68] FS | ||
| 6 | Kemmler et al. [44] IMVC, Power, | 6 | Verscheuern et al. [57] IMVC, DS | ||
| 7 | RFD | ||||
| 8 | Kennis et al. [45] IMVC, DS, RFD | ||||
| 9 | Klarner et al. [46] IMVC, RFD | ||||
| 10 | Lachane [47] FS | ||||
| 11 | Leung et al. [66] IMVC | ||||
| 12 | Machado et al. [48] MVC, Power | ||||
| 13 | Mikhael et al. [49] DS, Power, FS | ||||
| 14 | Roelants et al. [53] IMVC, DS, RFD | ||||
| 15 | Verscheuren et al. [58] IMVC, DS | ||||
| SS-WBV | SS-WBV | SS-WBV | |||
| 1 | Beck et al. [35] FS | 1 | Calder et al. [39] FS | 1 | Sievänen et al. [61] FS |
| 2 | Furness and Maschette [40] FS | 2 | Corrie et al. [65] Power, RFD, FS | 2 | Zhang et al. [60] IMVC, FS |
| 3 | Furness et al. [41] FS | 3 | Ochi et al. [67] IMVC | ||
| 4 | Iwamoto et al. [43] FS | ||||
| 5 | Klarner et al. [46] IMVC, RFD | ||||
| 6 | Raimundo et al. [50] DS, Power, RFD; FS | ||||
| 7 | Rees et al. [51] DS, FS | ||||
| 8 | Rees et al. [52] DS, Power | ||||
| 9 | Russo et al. [55] DS, RFD | ||||
| 10 | Stolzenberg et al. [56] Power, RFD, FS | ||||
| 11 | von Stengel et al. [59] IMVC, RFD | ||||
| SR-WBV | SR-WBV | ||||
| 1 | 1 | Rogan et al. [54] FS | 1 | Kessler et al. [23] IMVC, RFD, FS | |
| 2 | 2 | Rogan et al. [20] IMVC, IRFD, FS | |||
| SMD 0.26 (95 % CI 0.15 to 0.63) | SMD of 0.14 (95 % CI −0.04 to 0.33) | SMD of 0.47 (95 % CI 0.16 to 0.78) | |||
| I2 48.9 %, p <0.001 | I2 26.6 %, p = 0.121 | I2 0.00 %, p <0.792 |
Abbreviation: VS-WBV vertical sinusoidal whole-body vibration, SS-WBV side-alternating whole-body vibration; SR-WBV stochastic resonance whole-body vibration, IMVC isometric maximal voluntary contraction, DS dynamic maximal strength, RFD rate of force development, IRFD isometric rate of force development, FS functional strength, SMD, I 2 I2 –statistic for heterogeneity
Discussion
We hypothesized that WBV differently effects on measures of strength and power in Go-Go, Slow-Go and No-Go. This systematic review on muscle-strength-related outcomes of WBV in healthy elderly participants included 37 studies in a final analysis, most of which were studies with small sample sizes. The main findings were that WBV showed low to moderate effects in Go-Go, Slow-Go and No-Go when compared to non-exercising control groups on proxies of muscle strength in older adults. Furthermore, compared to groups performing more conventional types of exercise, WBV had only small and mostly non-significant advantages. Although only a few studies evaluated the effects of WBV in samples of elderly participants in need of care (classified as No-Go [10] in this review), the highest effect sizes favouring WBV were found in these studies. The few studies that evaluated SR-WBV also resulted in high effect sizes in favour of this type of WBV in No-Go. These findings seem to confirm our hypothesis. However, when we summarized the effect sizes of the meta-analysis and intended to perform statistical analysis on these data that would either refute or confirm our hypothesis, the amount of data was not big enough and the groups too unbalanced in size to allow a credible analysis to be performed. Further studies in No-Go are, therefore, warranted and needed with the various types of WBV. This means regarding our aim to give recommendations on available evidence for practical use the inference must be that at present no recommendations can be given for the most effective vibration mode in elderly persons.
Our review classified the physical capacities of the included participants (i.e. in “Go-Go”, “Slow-Go”, and “No-Go” [10]) and analysed studies with WBV versus non-exercising control separately from studies comparing WBV versus other types of conventional strength training exercise, as recommended by Orr [68]. Furthermore, we separately analyzed studies using vertical, side-alternating and stochastic resonance WBV. The reason for these distinctions relate to the assumption that initial fitness when entering a training program together with the training content may differently effect on training outcomes. Physical fitness includes health-related (cardiorespiratory endurance, muscular endurance, muscular strength, flexibility and body composition) and skill-related components (agility, coordination, balance, speed, reaction time and power) [69]. Although exercise recommendations have been published for older adults; e.g. the American College of Sports Medicine (ACSM) [70] guidelines recommend that older adults should undertake 30 min of moderate intensity, aerobic exercise or activity, five times per week to incur any health benefits, the complex interactions present in various sub-populations of older adults preclude the definition of specific, detailed exercise prescriptions. Furthermore, the number of older people fulfilling the ACSM requirement is rather small and most likely even lower amongst those with low levels of functioning [71]. It seems fair to assume that when principles of exercise training are applied to the development of exercise protocols, clinicians in practical settings can have greater confidence that non-significant research findings reflect deficiencies in exercise efficacy rather than deficiencies in exercise prescription [72]. It is thereby important, however, to consider low baseline fitness and mobility levels in pre-frail or frail or rather untrained elderly when starting an exercise program. Based on the findings of this systematic review it seems that the use of (SR)-WBV is valuable for untrained or frail elderly where the neuromuscular systems might not be able withstanding higher loading and long training sessions, however, with increasing levels of functioning there is a diminishing effect of the WBV interventions. Considering this it becomes clear that this systematic review only reveals first estimates for the possible effect of WBV in (pre-)frail elderly. An important next step would be the design and implementation of a sufficiently powered WBV exercise study specifically targeting (pre-)frail institutionalised elderly with a training duration of at least 2 months since this is the duration where effects of WBV training in the elderly may be expected [73].
Role of physical capacity level
Altough we did not find a systematic review on WBV that used a classification of participants in different subgroups of functioning, our conclusion is in line with similar previously expressed conclusions. Lau et al. [24] mainly focussed on bone mineral density but also included strength measures. They concluded, that WBV is beneficial in elderly persons to increase muscle strength. However, because they did not use a separate analysis for different groups with different levels of physical capacities it is difficult to determine whether the effects observed are dependent on the baseline fitness of the study participants. Sitja-Rabert et al. [25] concluded that WBV was beneficial in elderly participants to improve strength. Osawa et al. [74] included both young and elderly participants and concluded that WBV in addition to exercises or to a normal lifestyle improved knee extensor strength and countermovement jump performance when compared to identical training conditions without WBV.
WBV as skilling-up exercise
Our findings seem to justify the assumption, that WBV might be applicable as a “skilling-up” exercise for elderly with low physical capacity (i.e. the No-Go group), who are considering to begin with an exercise program, but who are not yet able to perform traditional strength exercises. A short bout of vibration would produce sufficient stimuli to effect on muscle strength, power and functional tasks. There are some theoretical explanations that WBV might improve the neuromuscular drive, which improves muscular function [18, 75]. In contrary to the No-Go group, elderly persons in the Go-Go and the Slow-Go group can perform standard exercises and WBV might be an additional option among all exercise modalities. The measured effects sizes for the Go-Go and Slow-Go groups were not very high and not all outcomes showed significant differences in the included studies. For this reason, WBV should be rather used for “skilling-up” in pre-frail or frail elderly individuals.
Limitations of this study
There are some limitations of this review. The included studies presented moderate to high risk of bias, allocation concealment was not described in most studies, groups were often not similar at baseline (which is often the case in small studies), participants were blinded in only seven studies and outcome assessors were blinded in only 11 studies. With the exception of Leung et al. [66], Boegarts et al. [38], Kemmler et al. [44], von Stengel [59] (n = 151) and Sitjà-Rabert et al. [64], the remaining studies were small. This further increases the risk of bias. Therefore, the quality of the included studies overall was rather low.
The ability to replicate or reproduce experimental results, or reproducibility, is one of the major tenets of the scientific method. SR-WBV results considered in this review come from one research group only. It is, therefore, necessary that the published findings of this group are validated through replication by others. Until replication is done the results for SR-WBV should be interpreted with caution and, hence, this is a limitation of this review. Another limitation of our approach is the possible language bias, we only included studies in English or German and it cannot be excluded that relevant research in different languages exists.
This review included mainly studies with “Go-Go” and “Slow-Go” participants and only few studies with participants from the “No-Go” group. As the effect might be largest in this No-Go group with SMD of 0.47 (95 % CI 0.16 to 0.78), further studies should evaluate WBV in this group of elderly. Only a few studies have evaluated SR-WBV and no study has compared SR-WBV to other vibration modalities in elderly participants.
Conclusions
WBV shows beneficial effects on proxies of muscle strength in older adults, mainly in elderly with lower initial levels of functioning, suggesting that WBV can be used as a skilling-up exercise in participants. However, the review suggests that WBV has no overall treatment effect on muscle strength properties in older women and men across the whole spectrum of physical functioning. Only few studies evaluated WBV in (pre-)frail elderly. No randomized trial has examined the effects of WBV on muscle in older (pre-)frail elderly. Based on this review no recommendations can be given for the most effective vibration mode. Further studies with the various types of WBV in various sub-populations of elderly persons are warranted and needed to determine the most effective vibration modes.
Abbreviations
- CENTRAL
Cochrane Central Register of Controlled Trials
- DS
dynamic strength
- FS
functional strength
- Go-Go
independent person
- IMVC
isometric maximum voluntary contraction
- No-Go
person depending permanently on assistance with severe functional limitation
- PEDro
Physiotherapy Evidence Database
- RFD
rate of force development
- Slow-Go
depending on support in everyday activities such as dressing, body care, eating, using the toilet, mobility, and planning the day
- SMD
standardised mean differences
- SR-WBV
stochastic resonance whole-body vibration
- SS-WBV
side-alternating sinusoidal whole-body vibration
- SV-WBV
sinusoidal vertical whole-body vibration
- WBV
whole-body vibration
- WHO
World Health Organization
Additional files
Search terms in PubMed. (DOCX 62 kb)
Forest plot overview of Classification Go-Go, outcome: all strength outcomes (IMVC, DS, Power, RFD, FS). (PPTX 165 kb)
Forest plot overview of Classification Slow-Go, outcome: all strength outcomes (IMVC, DS, Power, RFD, FS). (PPTX 105 kb)
Forest plot overview of Classification No-Go, outcome: all strength outcomes (IMVC, DS, Power, RFD, FS). (PPTX 75 kb)
Footnotes
Competing interests
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Authors’ contributions
SR conceived the idea of this study. SR, LR, and RH participated in the conception and design of the study; SR, CJ, CW, NJS, YB, RH collected the data. RH analysed the data. SR, EDB and RH wrote the manuscript. EDB, RDB: Methodology, Supervision, critically revising the manuscript for important intellectual content. LR, CJ, CW, NJS, YB, EDB and RH were involved in drafting or revising the manuscript. All authors read and approved the final manuscript.
Authors’ information
-SO Valais-Wallis).
Contributor Information
Slavko Rogan, Email: slavko.rogan@bfh.ch.
Eling D. de Bruin, Email: eling.debruin@hest.ethz.ch
Lorenz Radlinger, Email: lorenz.radlinger@bfh.ch.
Christine Joehr, Email: christine.joehr@gmail.com.
Christa Wyss, Email: christa.nyffenegger@gmail.com.
Neil-Jerome Stuck, Email: njstuck@bluewin.ch.
Yvonne Bruelhart, Email: yvonne.bruelhart@bfh.ch.
Rob A. de Bie, Email: ra.debie@maastrichtuniversity.nl
Roger Hilfiker, Email: roger.hilfiker@hevs.ch.
References
- 1.Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20(1):49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- 2.Granacher U, Muehlbauer T, Gruber M. A qualitative review of balance and strength performance in healthy older adults: impact for testing and training. J Aging Res. 2012;2012:708905. doi: 10.1155/2012/708905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci. 2012;67(1):28–40. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 5.Clark BC, Manini TM. What is dynapenia? Nutrition. 2012;28(5):495–503. doi: 10.1016/j.nut.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56(5):B209–217. doi: 10.1093/gerona/56.5.B209. [DOI] [PubMed] [Google Scholar]
- 8.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54(1):56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 9.de Kort YAW. Housing for the independendtly living elderly; theoretical backgrounds. Eindhoven: Eindhoven University of Technology; 1995. [Google Scholar]
- 10.Zeyfang A, Braun A. [Guidelines “Diabetes mellitus in the elderly”] MMW Fortschr Med. 2009;151(20):33–35. [PubMed] [Google Scholar]
- 11.Swiss Academy Of M Treatment and care of elderly persons who are in need of care. Swiss Med Wkly. 2004;134(41–42):618–626. doi: 10.4414/smw.2004.10848. [DOI] [PubMed] [Google Scholar]
- 12.Walter-Ginzburg A, Guralnik JM, Blumstein T, Gindin J, Modan B. Assistance with personal care activities among the old-old in Israel: a national epidemiological study. J Am Geriatr Soc. 2001;49(9):1176–1184. doi: 10.1046/j.1532-5415.2001.49234.x. [DOI] [PubMed] [Google Scholar]
- 13.Landi F, Marzetti E, Martone AM, Bernabei R, Onder G. Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care. 2014;17(1):25–31. doi: 10.1097/MCO.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 14.Herren K, Holz Hängärtner C, Oberli A, Radlinger L. [Cardiovascular and metabolic strain during stochastic resonance therapy in stroke patients] Physioscience. 2009;5(1):13–17. doi: 10.1055/s-0028-1109140. [DOI] [Google Scholar]
- 15.Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol. 2000;20(2):134–142. doi: 10.1046/j.1365-2281.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 16.Rittweger J, Ehrig J, Just K, Mutschelknauss M, Kirsch KA, Felsenberg D. Oxygen uptake in whole-body vibration exercise: influence of vibration frequency, amplitude, and external load. Int J Sports Med. 2002;23(6):428–432. doi: 10.1055/s-2002-33739. [DOI] [PubMed] [Google Scholar]
- 17.Chanou K, Gerodimos V, Karatrantou K, Jamurtas A. Whole-body vibration and rehabilitation of chronic diseases: a review of the literature. J Sports Sci Med. 2012;11(2):187–200. [PMC free article] [PubMed] [Google Scholar]
- 18.Rogan S, Hilfiker R. [Training methods - increase muscle strength due to whole-body vibration - force with Hz] Sportverletz Sportschaden. 2012;26(4):185–187. doi: 10.1055/s-0032-1333364. [DOI] [PubMed] [Google Scholar]
- 19.Blasimann A, Fleuti U, Rufener M, Elfering A, Radlinger L. Electromyographic activity of back muscles during stochastic whole body vibration. J Musculoskelet Neuronal Interact. 2014;14(3):311–317. [PubMed] [Google Scholar]
- 20.Rogan S, Schmidtbleicher D, Radlinger L. Immediate effects after stochastic resonance whole-body vibration on physical performance on frail elderly for skilling-up training: a blind cross-over randomised pilot study. Aging Clin Exp Res. 2014;26(5):519–27. doi: 10.1007/s40520-014-0212-4. [DOI] [PubMed] [Google Scholar]
- 21.Nordlund MM, Thorstensson A. Strength training effects of whole-body vibration? Scand J Med Sci Sports. 2007;17(1):12–7. doi: 10.1111/j.1600-0838.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 22.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur J Appl Physiol. 2010;108(5):877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 23.Kessler J, Radlinger L, Baur H, Rogan S. Effect of stochastic resonance whole body vibration on functional performance in the frail elderly: a pilot study. Arch Gerontol Geriatr. 2014;59(2):305–311. doi: 10.1016/j.archger.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil. 2011;25(11):975–988. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 25.Sitja-Rabert M, Rigau D, Fort Vanmeerghaeghe A, Romero-Rodriguez D, Bonastre Subirana M, Bonfill X. Efficacy of whole body vibration exercise in older people: a systematic review. Disabil Rehabil. 2012;34(11):883–893. doi: 10.3109/09638288.2011.626486. [DOI] [PubMed] [Google Scholar]
- 26.Ammann BC, Knols RH, Baschung P, de Bie RA, de Bruin ED. Application of principles of exercise training in sub-acute and chronic stroke survivors: a systematic review. BMC Neurol. 2014;14:167. doi: 10.1186/s12883-014-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayers A. Tips and tricks in performing a systematic review--chapter 4. Br J Gen Pract. 2008;58(547):136. doi: 10.3399/bjgp08X277168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American College of Sports Medicine Position Stand The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakebeeke TH, Knols RH, de Bruin ED. Should rehabilitation specialists use external focus instructions when motor learning is fostered? A systematic review. Sports. 2013;1(2):37–54. doi: 10.3390/sports1020037. [DOI] [Google Scholar]
- 32.Cohen J. Statistical power analysis for the behavioral sciences. 2. NJ: Erlbaum, Hillsdale; 1988. [Google Scholar]
- 33.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bautmans I, Van Hees E, Lemper JC, Mets T. The feasibility of Whole Body Vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: a randomised controlled trial [ISRCTN62535013] BMC Geriatr. 2005;5:17. doi: 10.1186/1471-2318-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beck BR, Norling TL. The effect of 8 mos of twice-weekly low- or higher intensity whole body vibration on risk factors for postmenopausal hip fracture. Am J Phys Med Rehabil. 2010;89(12):997–1009. doi: 10.1097/PHM.0b013e3181f71063. [DOI] [PubMed] [Google Scholar]
- 36.Bogaerts A, Delecluse C, Boonen S, Claessens AL, Milisen K, Verschueren SM. Changes in balance, functional performance and fall risk following whole body vibration training and vitamin D supplementation in institutionalized elderly women. A 6 month randomized controlled trial. Gait Posture. 2011;33(3):466–472. doi: 10.1016/j.gaitpost.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 37.Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men: a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630–635. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 38.Bogaerts AC, Delecluse C, Claessens AL, Troosters T, Boonen S, Verschueren SM. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial) Age Ageing. 2009;38(4):448–454. doi: 10.1093/ageing/afp067. [DOI] [PubMed] [Google Scholar]
- 39.Calder CG, Mannion J, Metcalf PA. Low-intensity whole-body vibration training to reduce fall risk in active, elderly residents of a retirement village. J Am Geriatr Soc. 2013;61(8):1424–1426. doi: 10.1111/jgs.12391. [DOI] [PubMed] [Google Scholar]
- 40.Furness TP, Maschette WE. Influence of whole body vibration platform frequency on neuromuscular performance of community-dwelling older adults. J Strength Cond Res. 2009;23(5):1508–1513. doi: 10.1519/JSC.0b013e3181a4e8f9. [DOI] [PubMed] [Google Scholar]
- 41.Furness TP, Maschette WE, Lorenzen C, Naughton GA, Williams MD. Efficacy of a whole-body vibration intervention on functional performance of community-dwelling older adults. J Altern Complement Med. 2010;16(7):795–797. doi: 10.1089/acm.2009.0366. [DOI] [PubMed] [Google Scholar]
- 42.Gomez-Cabello A, Gonzalez-Aguero A, Ara I, Casajus JA, Vicente-Rodriguez G. Effects of a short-term whole body vibration intervention on physical fitness in elderly people. Maturitas. 2013;74(3):276–8. doi: 10.1016/j.maturitas.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Iwamoto J, Sato Y, Takeda T, Matsumoto H. Whole body vibration exercise improves body balance and walking velocity in postmenopausal osteoporotic women treated with alendronate: Galileo and Alendronate Intervention Trail (GAIT) J Musculoskelet Neuronal Interact. 2012;12(3):136–143. [PubMed] [Google Scholar]
- 44.Kemmler W, VS S, Mayer S, Niedermayer M, Hentschke C, Kalender WA. [Effect of whole body vibration on the neuromuscular performance of females 65 years and older. One-year results of the controlled randomized ELVIS study] Z Gerontol Geriatr. 2010;43(2):125–132. doi: 10.1007/s00391-009-0074-0. [DOI] [PubMed] [Google Scholar]
- 45.Kennis E, Verschueren SM, Bogaerts A, Coudyzer W, Boonen S, Delecluse C. Effects of fitness and vibration training on muscle quality: a 1-year postintervention follow-up in older men. Arch Phys Med Rehabil. 2013;94(5):910–918. doi: 10.1016/j.apmr.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Klarner A, von Stengel S, Kemmler W, Kladny B, Kalender W. [Effects of two different types of whole body vibration on neuromuscular performance and body composition in postmenopausal women] Dtsch Med Wochenschr. 2011;136(42):2133–2139. doi: 10.1055/s-0031-1292023. [DOI] [PubMed] [Google Scholar]
- 47.Lachane CC. The effects of whole-body vibration exercise on muscular strength in seniors. Ontario: University of Windsor; 2012. [Google Scholar]
- 48.Machado A, Garcia-Lopez D, Gonzalez-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand J Med Sci Sports. 2010;20(2):200–207. doi: 10.1111/j.1600-0838.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 49.Mikhael M, Orr R, Amsen F, Greene D, Singh MA. Effect of standing posture during whole body vibration training on muscle morphology and function in older adults: a randomised controlled trial. BMC Geriatr. 2010;10:74. doi: 10.1186/1471-2318-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raimundo AM, Gusi N, Tomas-Carus P. Fitness efficacy of vibratory exercise compared to walking in postmenopausal women. Eur J Appl Physiol. 2009;106(5):741–748. doi: 10.1007/s00421-009-1067-9. [DOI] [PubMed] [Google Scholar]
- 51.Rees S, Murphy A, Watsford M. Effects of vibration exercise on muscle performance and mobility in an older population. J Aging Phys Act. 2007;15(4):367–381. doi: 10.1123/japa.15.4.367. [DOI] [PubMed] [Google Scholar]
- 52.Rees S, Murphy A, Watsford ML. Effects of whole-body vibration exercise on lower-extremety muscle strength and power in an older population: a randomized clinical trial. Phys Ther. 2008;88:462–470. doi: 10.2522/ptj.20070027. [DOI] [PubMed] [Google Scholar]
- 53.Roelants M, Delecluse C, Verschueren SM. Whole-body-vibration training increases knee-extension strength and speed of movement in older women. J Am Geriatr Soc. 2004;52(6):901–908. doi: 10.1111/j.1532-5415.2004.52256.x. [DOI] [PubMed] [Google Scholar]
- 54.Rogan S, Hilfiker R, Schmid S, Radlinger L. Stochastic resonance whole-body vibration training for chair rising performance on untrained elderly: a pilot study. Arch Gerontol Geriatr. 2012;55(2):468–473. doi: 10.1016/j.archger.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Russo CR, Lauretani F, Bandinelli S, Bartali B, Cavazzini C, Guralnik JM, et al. High-frequency vibration training increases muscle power in postmenopausal women. Arch Phys Med Rehabil. 2003;84(12):1854–1857. doi: 10.1016/S0003-9993(03)00357-5. [DOI] [PubMed] [Google Scholar]
- 56.Stolzenberg N, Belavy DL, Rawer R, Felsenberg D. Vibration or balance training on neuromuscular performance in osteopenic women. Int J Sports Med. 2013;34(11):956–962. doi: 10.1055/s-0033-1334870. [DOI] [PubMed] [Google Scholar]
- 57.Verschueren SM, Bogaerts A, Delecluse C, Claessens AL, Haentjens P, Vanderschueren D, et al. The effects of whole-body vibration training and vitamin D supplementation on muscle strength, muscle mass, and bone density in institutionalized elderly women: a 6-month randomized, controlled trial. J Bone Miner Res. 2011;26(1):42–49. doi: 10.1002/jbmr.181. [DOI] [PubMed] [Google Scholar]
- 58.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–359. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 59.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effect of whole-body vibration on neuromuscular performance and body composition for females 65 years and older: a randomized-controlled trial. Scand J Med Sci Sports. 2012;22(1):119–127. doi: 10.1111/j.1600-0838.2010.01126.x. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Weng C, Liu M, Wang Q, Liu L, He Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: a pilot randomized controlled trial. Clin Rehabil. 2014;28(1):59–68. doi: 10.1177/0269215513492162. [DOI] [PubMed] [Google Scholar]
- 61.Sievanen H, Karinkanta S, Moisio-Vilenius P, Ripsaluoma J. Feasibility of whole-body vibration training in nursing home residents with low physical function: a pilot study. Aging Clin Exp Res. 2014;26(5):511–517. doi: 10.1007/s40520-014-0206-2. [DOI] [PubMed] [Google Scholar]
- 62.Alvarez-Barbosa F, Del Pozo-Cruz J, Del Pozo-Cruz B, Alfonso-Rosa RM, Rogers ME, Zhang Y. Effects of supervised whole body vibration exercise on fall risk factors, functional dependence and health-related quality of life in nursing home residents aged 80+ Maturitas. 2014;79(4):456–463. doi: 10.1016/j.maturitas.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Osugi T, Iwamoto J, Yamazaki M, Takakuwa M. Effect of a combination of whole body vibration exercise and squat training on body balance, muscle power, and walking ability in the elderly. Ther Clin Risk Manag. 2014;10:131–138. doi: 10.2147/TCRM.S57806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sitja-Rabert M, Martinez-Zapata MJ, Fort Vanmeerhaeghe A, Rey Abella F, Romero-Rodriguez D, Bonfill X. Effects of a Whole Body Vibration (WBV) exercise intervention for institutionalized older people: a randomized, multicentre, parallel, clinical trial. J Am Med Dir Assoc. 2015;16(2):125–31. doi: 10.1016/j.jamda.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 65.Corrie H, Brooke-Wavell K, Mansfield NJ, Cowley A, Morris R, Masud T. Effects of vertical and side-alternating vibration training on fall risk factors and bone turnover in older people at risk of falls. Age Ageing. 2015;44(1):115–122. doi: 10.1093/ageing/afu136. [DOI] [PubMed] [Google Scholar]
- 66.Leung KS, Li CY, Tse YK, Choy TK, Leung PC, Hung VW, et al. Effects of 18-month low-magnitude high-frequency vibration on fall rate and fracture risks in 710 community elderly—a cluster-randomized controlled trial. Osteoporos Int. 2014;25(6):1785–1795. doi: 10.1007/s00198-014-2693-6. [DOI] [PubMed] [Google Scholar]
- 67.Ochi A, Abe T, Yamada K, Ibuki S, Tateuchi H, Ichihashi N. Effect of balance exercise in combination with whole-body vibration on muscle activity of the stepping limb during a forward fall in older women: a randomized controlled pilot study. Arch Gerontol Geriatr. 2015;60(2):244–51. doi: 10.1016/j.archger.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 68.Orr R. The effect of whole body vibration exposure on balance and functional mobility in older adults: a systematic review and meta-analysis. Maturitas. 2015;80(4):342–58. doi: 10.1016/j.maturitas.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 69.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 70.Medicine ACoS. ACSM’s guidelines for exercise testing and prescription. Baltimore, Philadelphia (US): ACSM (Lippincott Raven); 2013. See: https://www.lww.com/product/9781609139551
- 71.Forster A, Lambley R, Young JB. Is physical rehabilitation for older people in long-term care effective? Findings from a systematic review. Age Ageing. 2010;39(2):169–175. doi: 10.1093/ageing/afp247. [DOI] [PubMed] [Google Scholar]
- 72.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46(13):909–916. doi: 10.1136/bjsports-2010-082719. [DOI] [PubMed] [Google Scholar]
- 73.Kawanabe K, Kawashima A, Sashimoto I, Takeda T, Sato Y, Iwamoto J. Effect of whole-body vibration exercise and muscle strengthening, balance, and walking exercises on walking ability in the elderly. Keio J Med. 2007;56(1):28–33. doi: 10.2302/kjm.56.28. [DOI] [PubMed] [Google Scholar]
- 74.Osawa Y, Oguma Y, Ishii N. The effects of whole-body vibration on muscle strength and power: a meta-analysis. J Musculoskelet Neuronal Interact. 2013;13(3):380–390. [PubMed] [Google Scholar]
- 75.Cardinale M, Bosco C. The use of vibration as an exercise intervention. Exerc Sport Sci Rev. 2003;31(1):3–7. doi: 10.1097/00003677-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Lord SR, Menz HB, Tiedemann A. A physiological profile approach to falls risk assessment and prevention. Phys Ther. 2003;83(3):237–252. [PubMed] [Google Scholar]
- 77.Pedrero-Chamizo R, Gomez-Cabello A, Delgado S, Rodriguez-Llarena S, Rodriguez-Marroyo JA, Cabanillas E, et al. Physical fitness levels among independent non-institutionalized Spanish elderly: the elderly EXERNET multi-center study. Arch Gerontol Geriatr. 2012;55(2):406–416. doi: 10.1016/j.archger.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Amaral PC, Rica RL, Evangelista AL, Casarin CAS, Junior JAS, Bocalini DS. Whole-body vibration training does not modify anthropometric parameters and lower limb strength in elderly people. Med Sci Tech. 2014;55:6–10. doi: 10.12659/MST.890178. [DOI] [Google Scholar]


