Abstract
In about 30–50% of patients with posttraumatic stress disorder (PTSD), symptoms persist after treatment. Although neurobiological research has advanced our understanding of PTSD, little is known about the neurobiology underlying persistence of PTSD. Two functional MRI scans were collected from 72 war veterans with and without PTSD over a 6- to 8-month interval, during which PTSD patients received trauma-focused therapy. All participants performed a trauma-unrelated emotional processing task in the scanner. Based on post-treatment symptom severity, a distinction was made between remitted and persistent patients. Behavioral and imaging measures of trauma-unrelated emotional processing were compared between the three groups (remitted patients, N=21; persistent patients, N=22; and combat controls, N=25) with repeated-measures (pre- and post-treatment) analyses. Second, logistic regression was used to predict treatment outcome. Before and after treatment, persistent patients showed a higher dorsal anterior cingulate cortex (dACC) and insula response to negative pictures compared with remitted patients and combat controls. Before treatment, persistent patients showed increased amygdala activation in response to negative pictures compared with remitted patients. The remitted patients and combat controls did not differ on the behavioral or imaging measures. Finally, higher dACC, insula, and amygdala activation before treatment were significant predictors of symptom persistence. Our results highlight a pattern of brain activation that may predict poor response to PTSD treatment. These findings can contribute to the development of alternative or additional therapies. Further research is needed to elucidate the heterogeneity within PTSD and describe how differences in neural function are related to treatment outcome. Such approaches are critical for defining parameters to customize PTSD treatment and improve treatment response rates.
INTRODUCTION
In about 30–50% of patients with posttraumatic stress disorder (PTSD), symptoms persist after treatment (Bradley et al, 2005) and severely impact the life of the patient, that of their families, and society in general (Calhoun et al, 2002; Kessler, 2000). To improve response rates, it is important to increase our understanding of (the neurobiology underlying) persistence of PTSD.
According to international guidelines (Foa et al, 2009), the treatment of choice for PTSD is trauma-focused therapy. This therapy relies on extinction of the learned fear by means of exposure to the traumatic memory (Izquierdo et al, 2004; Shipherd and Salters-Pedneault, 2008). Extinction learning is justified by emotional processing theory (Foa and Kozak, 1986), which assumes that three aspects are essential for succesful extinction: activation of the traumatic memory, attention to contextual (safety) information, and integration of this new information such that new associations can be established (Foa and Kozak, 1986; Rauch and Foa, 2006). When investigating persistence of PTSD after trauma-focused therapy, it seems relevant to not only study the brain circuit involved in fear learning and extinction, including the amygdala (leDoux, 2000), the hippocampus (Phillips and leDoux, 1992), and the ventromedial prefrontal cortex (vmPFC; Milad et al, 2007), but also consider the brain regions of the salience network, important for directing attention and detecting salient stimuli, that is, the dorsal anterior cingulate cortex (dACC) and insula (Phan et al, 2002; Seeley et al, 2007).
Over the past decades, neurobiological research has advanced our understanding of PTSD by demonstrating increased amygdala, dACC, and insula activation and decreased prefrontal cortex activation in PTSD patients (eg, Bryant et al, 2005; Geuze et al, 2007; Liberzon et al, 1999; Milad et al, 2007; Rauch et al, 2000; Shin et al, 2005). In contrast, little is known about the neurobiology underlying persistence of PTSD after treatment. Probing regions of interest (ROIs) or the function of circuits provides important information about mechanisms underlying the observed psychopathology. Although some fMRI studies have investigated treatment response in PTSD (eg, Bryant et al, 2008; Felmingham et al, 2007; Thomaes et al, 2012), none of these studies has scanned a trauma control group both pre- and post-treatment. Including a control group in longitudinal studies is highly important, because the fMRI signal is vulnerable to substantial within-subject variability between scan sessions (Zandbelt et al, 2008). Furthermore, a control group is needed to disentangle the effect of treatment from time, habituation, and learning effects.
In the present pre- and post-treatment study, two functional MRI scans were collected, when war veterans with and without PTSD viewed trauma-unrelated emotional pictures. Trauma-related pictures would induce an actual fear response to the traumatic event; however, it would, therefore, bias the post-treatment measurements as remitted patients would not show the fear response to the traumatic event at the post-treatment scan. The International Affective Picture System (IAPS) pictures are known to activate our ROIs (van Rooij et al, 2014), that is, the amygdala (LeDoux, 2000), dACC (Beckmann et al, 2009), insula, and hippocampus (Phillips et al, 2003) without provoking a trauma-related fear response in the PTSD patients. We hypothesized that patients in whom symptoms persist after 6–8 months of treatment (persistent patients) show increased activation of the amygdala, dACC, insula, vmPFC, and hippocampus in response to negative pictures when compared with patients who are in remission (remitted patients). Second, we postulated that pretreatment brain activation levels in response to negative pictures could predict treatment outcome.
MATERIALS AND METHODS
Participants
A total of 72 war veterans were included in the study. Forty-seven of them were diagnosed with PTSD by a psychologist or psychiatrist at one of the four Military Mental Healthcare outpatient clinics in the Netherlands. All patients were about to start trauma-focused therapy at the time of inclusion. Trauma-focused therapy represents a broad class of psychotherapeutic interventions that include trauma-focused cognitive behavioral therapy (tfCBT), cognitive processing therapy, eye-movement desensitization and reprocessing (EMDR), among others. Patients received treatment as usual, consisting of tfCBT and/or EMDR, previously demonstrated to be equally effective in treating PTSD (Bisson et al, 2007). Twenty-five male war veterans without a current psychiatric disorder were included as combat controls. All veterans had been deployed at least once. Two functional MRI scans within a 6- to 8-month interval were collected from all participants. The clinician-administered PTSD scale (CAPS; Blake et al, 1990) was applied by a trained researcher to quantify the severity (or confirm absence) of PTSD symptoms at both time points. Severity of PTSD was assessed with total CAPS score. A differentiation was made between PTSD patients in remission (remitted patients) and patients in whom PTSD persisted after 6–8 months of treatment (persistent patients). PTSD in remission was defined as a post-treatment CAPS score below 45, as this has previously been found to indicate the absence of clinically significant PTSD symptoms (Weathers et al, 1999). To examine (comorbid) psychiatric disorders at both timepoints, the structured clinical interview for DSM-IV axis I disorders (SCID-I; First et al, 1997) was administered. Subjects with a history of neurological illness were excluded. Participants received monetary compensation for participation. Written informed consent was obtained from all participants after they had received a complete written and verbal explanation of the study, in accordance with procedures approved by the University Medical Center Utrecht ethics committee and the Declaration of Helsinki. Results described here are part of a larger study (for more information see Supplementary Materials S1).
Emotional Processing Task
Ninety-six pictures from the IAPS (Lang et al, 1997) were presented to participants. Based on the validated valence ratings, pictures were categorized as neutral, negative, or positive. To investigate trauma-unrelated emotional processing, pictures with a war-related content were excluded, as all participants served in combat. Participants were instructed to view each picture for 2 s. Participants were asked to rate the picture as neutral, negative, or positive by pressing a button when the evaluation screen was shown (maximum 2 s). After each response, a fixation-cross appeared for the remaining trial duration. The task consisted of four blocks of 96 s in which 24 pictures, 8 from each condition, were presented in pseudorandomized order. Each block was followed by a rest block in which a fixation-cross was presented (32 s). All subjects performed the task with their right hand. The task and experimental procedures were identical to those described before (van Buuren et al, 2011; van Rooij et al, 2014; Vink et al, 2014), and are briefly explained in Figure 1.
Figure 1.
Trauma-unrelated emotional processing task. Neutral (example left), positive (middle), and negative pictures from the International Affective Picture System (IAPS; Lang et al, 1997) were presented to participants. They were instructed to rate the picture when the evaluation screen was shown, after which a fixation-cross appeared for the remaining trial duration.
Behavioral Analyses
For each category (neutral, negative, positive), the number of subject's ratings that matched the IAPS ratings was calculated, and was taken as a measure of behavioral performance. Repeated-measures analyses of variance (ANOVA) using category and group as factors were used to investigate group differences on behavioral performance. To confirm that participants had actually seen the picture (eg, did not have their eyes closed), and had perceived the picture the way it was analyzed (based on IAPS rating), we included only congruent trials for the functional MRI analyses.
Functional MRI
Functional images were acquired using a 3.0 T whole-body magnetic resonance imaging scanner (Philips Medical System, Best, The Netherlands). A total of 322 whole brain, T2*-weighted echo planar images with blood oxygen-level-dependent contrast (voxel size 4 mm isotropic; repetition time (TR)=1600 ms; echo time (TE)=23 ms; flip angle=72.5°) were collected in a single run. A T1-weighted image (200 slices; TR=10 ms; TE=3.8 ms; flip angle=8° field of view=240 × 240 × 160 mm3; matrix of 304 × 299) was used for within-subject registration purposes. Functional MRI data were preprocessed and analyzed with SPM 5 (http://www.fil.ion.ucl.ac.uk/spm). Preprocessing included slice time correction, realignment, coregistration of the anatomical image to the mean functional image, spatial normalization to Montreal Neurological Institute template brain, and smoothing (using a 8 mm full-width at half-maximum (FWHM) Gaussian kernel).
A general linear model (GLM) regression analysis was used to estimate task effects on brain activation. Three conditions were created for the neutral, negative, and positive pictures (only congruent trials). The onset and duration (2 s) of these three conditions were modeled as factors in the design matrix of the regression model. To correct for head motion, the six realignment parameters were included in the design matrix as regressors of no interest. A high-pass filter with a cutoff frequency of 0.0058 Hz was applied to the data to correct for low-frequency scanner drift.
Three first-level contrasts were created: (1) the neutral pictures compared with rest (neutral>rest; baseline contrast), (2) the negative pictures compared with the neutral pictures (negative>neutral; negative contrast), and (3) the positive pictures compared with the neutral pictures (positive>neutral; positive contrast). The negative and positive contrasts were created in this way to measure the effect of valence correcting for attentional and visual processes.
For each contrast, mean activation levels were extracted from predefined ROIs, separately for left and right. The dACC (Brodmann's area 32), insula, and amygdala ROIs were based on the WFU Pick Atlas. The hippocampus was defined using the LONI Probabilistic Brain Atlas with a probability threshold of 80%. A 6 mm sphere (MNI coordinates: 4, 44, -4) in the vmPFC was used as a fifth ROI. This was the peak voxel in a recent study showing reduced vmPFC activation in PTSD compared with controls during inhibition (Jovanovic et al, 2013).
To investigate group differences outside the ROIs, whole brain group analyses were performed for the three contrasts. The resulting maps were tested for significance at a cluster-defining threshold of p<0.001, and a p<0.05 family-wise error (FWE)-corrected critical cluster size was calculated separately for each contrast. The cluster sizes were determined using SPM and a script (CorrClusTh.m, http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/scripts/spm), which uses estimated smoothness (estimated FWHM: 8 mm) and Random Field Theory to find these corrected thresholds.
Statistical Analyses
To test the first hypothesis that activation levels in our ROIs differ between remitted and persistent patients, group by time (pre- and post-treatment) by hemisphere repeated-measures GLM analyses were performed for the baseline, negative, and positive contrast for each ROI. Post hoc tests were performed when the main effect of group or the group by time (by hemisphere) interaction was significant.
For the second hypothesis, a logistic regression was used to investigate if pretreatment brain activation levels can predict treatment outcome (remission vs persistence of PTSD). The effect was analyzed for activation levels of each ROI separately to circumvent multicollinearity. The confounding influence of pretreatment PTSD severity, comorbidity and pharmacotherapy status, number of treatment sessions, age, education level, and early traumatic experiences on the predictive value of the ROIs was tested in each regression model. In accordance with ‘change in estimate strategy,' only significant confounders (change in B>10%) were used in the final regression models (Greenland, 1989; Maldonado and Greenland, 1993). Post hoc linear regression analyses were performed to investigate if actual post-treatment CAPS score could be predicted with the same models.
RESULTS
Participants
Participant characteristics are presented in Table 1. Four patients were excluded from the analyses: three had not performed the task correctly by responding at the wrong moments or by using inappropriate buttons, and one patient did not receive treatment in between the two scans. None of the participants displayed excessive scan-to-scan head movement (>4 mm). In total, 43 PTSD patients and 25 combat controls were included in the analyses. Based on post-treatment PTSD severity, 21 patients were classified as remitted and 22 as persistent. These two groups and the combat control group were comparable in age, education level, early traumatic experiences, months since deployment, number of missions, and blast exposure (Table 1). Pretreatment total CAPS score was higher in persistent patients, which was because of the presence of more hyperarousal symptoms. Re-experiencing and avoidance/numbing symptoms did not significantly differ between remitted and persistent patients pretreatment. The number of total treatment sessions did not differ between remitted and persistent patients (Table 2). Most of the patients received EMDR (N=35), some received tfCBT (N=15), and seven (persistent) patients received both. Neither the number of patients who received EMDR vs tfCBT nor the total number of sessions for each treatment differed significantly between the two patient groups. At pretreatment, remitted and persistent patients were comparable on medication use; however, post-treatment, more of the persistent patients used SSRIs. At pretreatment, a higher number of persistent patients compared with remitted patients fulfilled the criteria for a comorbid anxiety disorder, whereas at post-treatment the number of patients with a comorbid anxiety disorder did not differ between the groups. At pretreatment, the number of remitted and persistent patients with a comorbid mood disorder did not differ. However, post-treatment, a higher number of persistent patients had a comorbid mood disorder compared with remitted patients (Table 2).
Table 1. Participant Characteristics of Combat Controls, and Remitted and Persistent PTSD Patients.
| Combat controls (N=23) | Remitted patients (N=21) | Persistent patients (N=22) | Test statistic | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 37.3±10.4 | 35.2±9.3 | 38.3±8.9 | F=0.58 | 0.56 |
| Education level (ISCED) | |||||
| Own | 3.3±2.0 | 3.7±1.4 | 3.1±0.9 | F=0.67 | 0.52 |
| Father | 3.8±1.8 | 3.7±1.7 | 3.5±2.2 | F=0.13 | 0.88 |
| Mother | 2.7±1.5 | 2.7±1.5 | 2.2±1.5 | F=0.73 | 0.47 |
| Months since deployment | 78.4±83.3 | 89.0±109.9 | 114.7±96.9 | F=0.84 | 0.44 |
| Number of missions | 2.5±1.4 | 3.1±4.3 | 2.2±1.6 | F=0.66 | 0.52 |
| (1/2/3 />3) | (8/7/4/6) | (8/5/4/4) | (11/3/4/4) | ||
| Blast exposure (number) | 3 | 2 | 3 | χ2=0.18 | 0.92 |
| Early traumatic experiences | 3.1±3.0 | 4.2±3.7 | 5.2±4.7 | F=1.55 | 0.22 |
| PTSD symptoms pretreatment | |||||
| Re-experiencing (CAPS B) | 0.7±1.2 | 21.9±4.8 | 23.7±5.8 | F=211.4 | <0.001 |
| Avoiding (CAPS C) | 1.0±2.3 | 21.9±9.8 | 25.1±8.9 | F=71.1 | <0.001 |
| Hyperarousal (CAPS D) | 3.2±3.1 | 22.5±5.3* | 25.7±4.1* | F=203.3 | <0.001* |
| Total (CAPS Total) | 4.8±4.5 | 66.3±12.6* | 74.4±13.1* | F=308.4 | <0.001* |
| PTSD symptoms post-treatment | |||||
| Re-experiencing (CAPS B) | 1.4±2.2 | 6.7±6.4* | 21.9±6.3* | F=95.6 | <0.001* |
| Avoiding (CAPS C) | 0.6±1.6 | 6.4±5.5* | 20.7±8.3* | F=75.6 | <0.001* |
| Hyperarousal (CAPS D) | 3.2±2.7 | 11.2±5.9* | 23.4±5.8* | F=98.3 | <0.001* |
| Total (CAPS Total) | 5.3±4.2 | 24.3±14.1* | 66.0±15.3* | F=154.6 | <0.001* |
Abbreviations: CAPS, Clinician Administered PTSD scale (Blake et al, 1990); ISCED, International Standard Classification of Education (Schneider, 2013); PTSD, posttraumatic stress disorder.
Data are presented as means±SD.
Values with an asterisk (*) indicate that the remitted and persistent patients differ significantly.
Early traumatic experiences were investigated with the Early Trauma Inventory (Bremner et al, 2007).
The p-value used to indicate significance is p<0.05.
Table 2. Treatment, Medication Use, and Comorbid Disorders of Remitted and Persistent Patients.
| Remitted patients (N=21) | Persistent patients (N=22) | Test statistic | p-Value | |||
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Total sessions | 9.3±6.7 | 9.5±4.5 | t=−0.15 | 0.88 | ||
| EMDR | N=16 | N=19 | 0.39 | |||
| Total sessions | 5.3±2.5 | 6.6±4.0 | t=−1.12 | 0.24 | ||
| tfCBT | N=5 | N=10 | 0.14 | |||
| Total sessions | 10.6±6.6 | 7.3±5.1 | t=1.08 | 0.30 | ||
| Pre | Post | Pre | Post | p-Value pre | p-Value post | |
| Medication (no.) | 10 | 8 | 8 | 12 | 0.46 | 0.28 |
| SSRI | 4 | 3 | 6 | 11 | 0.52 | 0.01 |
| Benzodiazepine | 6 | 6 | 3 | 2 | 0.23 | 0.10 |
| SARI | 0 | 1 | 2 | 1 | 0.16 | 0.97 |
| Antipsychotics | 0 | 1 | 1 | 3 | 0.32 | 0.32 |
| Nicotine antagonist | 1 | 0 | 0 | 0 | 0.30 | 1.00 |
| β-Blocker | 1 | 0 | 1 | 0 | 0.97 | 1.00 |
| Comorbid disorders (no.) | 12 | 3 | 19 | 10 | 0.03 | 0.03 |
| Mood | 10 | 1 | 14 | 6 | 0.29 | 0.05 |
| Anxiety | 3 | 2 | 11 | 5 | 0.01 | 0.24 |
| Somatic | 1 | 0 | 1 | 1 | 0.97 | 0.32 |
Abbreviations: EMDR, eye-movement desensitization and reprocessing; pre, pretreatment; post, post-treatment; tfCBT, trauma-focused cognitive behavioral therapy.
Numbers for total treatment sessions represent group means±SD.
The p-values for the number of patients (EMDR, tfCBT, medication, and comorbid disorder) are based on χ2 analyses.
The p-value used to indicate significance is p<0.05.
Behavioral Results
A main effect of group was found for the number of subject's ratings that matched the IAPS ratings (F(2,65)=4.81, p=0.011). Overall, persistent patients rated fewer pictures according to standard rating compared with combat controls (p=0.003). Remitted patients did not significantly differ from the other two groups. Post hoc analyses showed that persistent patients rated more of the neutral pictures as negative (F(2,65)=5.76, p=0.005) compared with both combat controls (p=0.002) and remitted patients (p=0.014). Furthermore, they rated more of the positive pictures as neutral (F(2,65)=3.81, p=0.027) compared with combat controls (p=0.008). This pattern was consistent over time, as there was neither a main effect of time nor a significant time by group interaction.
Functional MRI
Group results
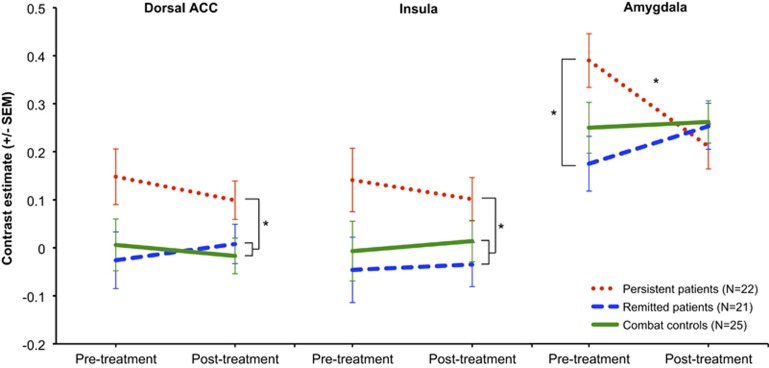
Results of the ROI analyses are presented in Figure 2. A significant main effect of group was observed in the dACC (F(2,65)=4.60, p=0.015) and in the insula (F(2,65)=3.92, p=0.025) for the negative contrast. Persistent patients showed a higher bilateral dACC and insula response across both time points compared with combat controls (dACC, p=0.011; insula, p=0.045) and remitted patients (dACC, p=0.012; insula, p=0.009). These differences were not affected by time or treatment, as no main effects of time or interaction effects were observed. Also, bilateral effects are reported, because the group by time by hemisphere interaction was not significant.
Figure 2.
Pre- and post-treatment functional magnetic resonance imaging (MRI) results. Pre- and post-treatment activation in response to negative pictures is displayed for the dorsal anterior cingulate cortex (ACC) (left panel), insula (middle panel), and amygdala (right panel). Mean contrast estimates are shown separately for persistent patients (red dotted lines), remitted patients (blue striped lines), and combat controls (green solid lines). Error bars show the SEs for each group mean. An asterisk (*) indicates a significant group difference of p<0.05.
In the amygdala, a significant group by time interaction was observed (F(2,65)=4.57, p=0.014) for the negative contrast. Post hoc analyses revealed a significant group difference pretreatment (F(2,65)=3.70, p=0.030), with persistent patients demonstrating a larger bilateral amygdala response compared with remitted patients (p=0.010). Neither of the PTSD groups differed from the combat control group pretreatment. Furthermore, the three groups did not differ at the post-treatment measurement. The persistent group showed a significant decrease in amygdala response from pre- to post-treatment (t(21)=2.15, p=0.043), whereas amygdala response of combat controls and remitted patients did not change from pre- to post-treatment. Again, no group by time by hemisphere interaction was observed and effects for bilateral amygdala are therefore described.
No group differences were observed in the hippocampus or the vmPFC. Also, no group differences were observed for the neutral pictures vs rest (baseline contrast) in any of the ROIs. Furthermore, neither a main effect of group nor a significant group by time (by hemisphere) interaction for the positive contrast was found in any of the ROIs. Finally, no group differences were observed in the corrected whole brain analyses, but uncorrected results were consistent with the ROI results.
Predicting treatment outcome
Pre-treatment activation of the bilateral dACC, insula, and amygdala in response to negative stimuli were significant predictors for persistence of symptoms, even after controlling for potential confounding factors, such as pretreatment PTSD severity, comorbidity, pharmacotherapy status, number of treatment sessions, age, education level, and early traumatic experiences. The final regression models for the dACC, insula, and amygdala are presented in Table 3. Post hoc linear regression analyses revealed that the same models with the dACC, insula, and amygdala also significantly predict actual post-treatment CAPS score (dACC, F(3,42)=3.66, p=0.020, R=0.47, R2=0.22; insula, F(3,42)=2.91, p=0.046, R=0.43, R2=0.18; amygdala, F(1,42)=7.42; p=0.009, R=0.39, R2=0.15).
Table 3. Logistic Regression.
| Independent variable | B | SE | Wald | p-Value | Exp(B) |
|---|---|---|---|---|---|
| dACC | 3.32 | 1.43 | 5.36 | 0.02 | 27.69 |
| CAPS total | 0.05 | 0.03 | 2.69 | 0.10 | 1.06 |
| Comorbidity | 1.52 | 0.92 | 2.75 | 0.10 | 4.59 |
| Model χ2 | 13.25 | ||||
| Nagelkerke R2 | 0.35 | ||||
| p-Value | 0.004 | ||||
| Insula | 2.12 | 1.19 | 3.15 | 0.08 | 8.29 |
| Comorbidity | 1.97 | 0.95 | 4.33 | 0.04 | 7.16 |
| Age | −0.03 | 0.05 | 0.32 | 0.57 | 0.98 |
| Model χ2 | 8.64 | ||||
| Nagelkerke R2 | 0.24 | ||||
| p-Value | 0.04 | ||||
| Amygdala | 3.20 | 1.48 | 4.69 | 0.03 | 24.42 |
| Model χ2 | 6.43 | ||||
| Nagelkerke R2 | 0.19 | ||||
| p-Value | 0.01 |
Abbreviations: CAPS, clinician administered PTSD scale (Blake et al, 1990); dACC, dorsal anterior cingulate cortex.
In accordance with ‘change in estimate strategy', only significant confounders (change in B >10%) were used in the final regression models (Greenland, 1989; Maldonado and Greenland, 1993), and are presented here.
Results from post-hoc analyses
Additional analyses were performed to control for the effect of medication. Including medication status as a covariate in the repeated-measures analyses did not change the results. Again, a main effect of group for the dACC (F2,64=4.422, p=0.016) and insula (F2,64=3.878, p=0.026), and a group by time interaction effect in the amygdala (F2,64=4.479, p=0.015) was observed. Furthermore, no significant group by time or group effects were observed in the hippocampus and vmPFC when medication was included as a covariate.
Correlation analyses were performed to investigate if the neural differences between the remitted and persistent patients could be explained by baseline differences between the groups. Results are presented in Supplementary Material S2A. No significant correlation was observed between any of the ROIs and pretreatment total CAPS score or one of the CAPS clusters. It is therefore unlikely that our results are explained by the baseline group differences.
To investigate from whence the main effect of group over time is arising, and to investigate if the main effect of group is reflecting baseline differences, group analyses for dACC and insula were performed separately for the two time points. The F-values for the two ROIs were comparable for the two time points (Supplementary Material S2B), supporting the conclusion that the main effect of group is not driven by the baseline differences in CAPS score between the two groups.
Finally, correlation analyses between percent change in CAPS score and changes in activation for the five regions of interest were performed. A significant negative correlation between percent change in CAPS score and amygdala activation was observed (r=−0.340, p=0.026). This correlation can be explained by the change in amygdala activation observed in the persistent group only. This finding is also reflected in the pre-treatment differences in amygdala activation between the remitted and persistent patients. No significant correlations with other regions of interest were observed.
DISCUSSION
Here, we demonstrate that increased activation of the dACC, insula, and amygdala in response to trauma-unrelated negative stimuli predicts persistence of PTSD after trauma-focused therapy. Second, we showed differences in brain activation between remitted and persistent patients before treatment. This demonstrates that PTSD is a very heterogeneous disorder, even though the field often acts as if it is a unified phenomenon (Galatzer-Levy and Bryant, 2013). Moreover, it shows that this difference is of great consequence as these individuals who are neurobiologically distinct are less likely to recover. Here, we provide potential predictive biomarkers for individual prognosis of PTSD, which is relevant for an early differentiation between patients who are likely to recover and those patients for whom alternative or additional treatment might be beneficial (Galatzer-Levy et al, 2013).
Trauma-focused therapy is based on extinction learning, for which three aspects are essential: activation of the traumatic memory, attention to contextual safety information, and integration of this new information such that new associations can be established (Foa and Kozak, 1986; Rauch and Foa, 2006). Amygdala activation is associated with activation of the traumatic memory (Liberzon et al, 1999). However, overengagement of the amygdala is disadvantageous, because it can prevent processing of other relevant information (Rauch and Foa, 2006). In our sample of persistent patients, hyperactivation of the amygdala to trauma-unrelated negative stimuli may reflect such an overengagement. This finding is consistent with data from Bryant et al (2008), who showed increased amygdala activation in response to fearful faces in a small group of non-responders (N=7) compared with responders (N=7), measured only pre-treatment (Bryant et al, 2008).
The dACC is implicated in the regulation of both cognitive and emotional processing (Etkin et al, 2011; Phan et al, 2002). The insula is involved in awareness of internal bodily states and the functional integration with emotional experience (Critchley et al, 2004; Simmons et al, 2013; Zaki et al, 2012). The dACC and insula are the core nodes of the salience network (Menon and Uddin, 2010; Seeley et al, 2007). The salience network is important for detecting biologically relevant stimuli from a range of external and internal stimuli, and is involved in involuntarily orienting attention to these stimuli to guide behavior (Menon and Uddin, 2010; Seeley et al, 2007). Aberrant functioning of the salience network has been associated with PTSD as patients showed heightened activation in the dACC, insula, and amygdala, as well as other regions of the salience network (Patel et al, 2012). We support and extend these findings by showing that this hyperactivation is particularly important for persistence of PTSD. Furthermore, structural (Long et al, 2013) and functional resting state connectivity studies (Sripada et al, 2012) have revealed increased connectivity within the salience network in PTSD patients. A recent graph analysis study showed a relationship between insula decoupling and re-experiencing symptoms in PTSD patients, indicating disrupted salience determination (Spielberg et al, 2015). Moreover, PTSD patients show increased coupling between the salience network and the default mode network (Sripada et al, 2012), a network that engages in internally focused tasks (Buckner et al, 2008). Based on these findings, it has been hypothesized that in PTSD patients there is an imbalance between salience detection and internally focused thought (Sripada et al, 2012), resulting in an attentional bias to external stimuli. Indeed, an attentional bias to threat, associated with increased dACC activation, has previously been observed in PTSD patients (Fani et al, 2012a; Pannu Hayes et al, 2009). Additionally, there is a relationship between attentional bias and decreased extinction learning in PTSD (Fani et al, 2012b). As has previously been suggested, although a threat-orienting attention style is useful when presented with actual threat, in a safe environment it can prevent adequate processing of other relevant environmental information, thereby reducing extinction learning (Fani et al, 2012b). Building upon this work, we postulate that hyperactivation of the core nodes of the salience network, the dACC and insula, could imply an attentional bias to negative stimuli, which prevents processing of safety information during therapy.
Our behavioral findings support the hypothesis that persistent PTSD patients have an attentional bias toward negative stimuli, because these patients rated more of the neutral pictures as negative, and more of the positive as neutral. However, because we did not use a task to directly measure attentional bias, this hypothesis must be confirmed in future studies. Our findings correspond with those of a previous study that showed that distorted perception of threat, such as negative interpretation of intrusions and anger cognitions, explained persistence of PTSD symptoms after treatment (Mayou et al, 2002).
Hippocampus and vmPFC have often been implicated in PTSD; however, in the current study, no differences between patients and controls, or remitted and persistent patients in these regions were observed. The hippocampus and the vmPFC are particularly involved in fear extinction recall (Milad et al, 2007), because of which these brain regions are thought to be implicated in trauma-focused therapy. However, the emotional processing task used here does not present fear-evoking cues and does not measure extinction learning and recall. Therefore, it does not rely so much on the fear and extinction learning circuit (including the vmPFC and hippocampus), but recruits areas for directing attention and detecting salient stimuli, that is, the salience network. This very likely explains the absence of group differences in the hippocampus and vmPFC. Therefore, treatment studies that investigate fear inhibition are needed to improve our understanding of the role of the vmPFC and hippocampus in trauma-focused therapy.
Even though amygdala activation predicted persistence of symptoms and differed between remitted and persistent PTSD patients, neither of the two PTSD groups differed from the control group at either time point (pre- or post-treatment). This is in accordance with the absence of group differences in amygdala response between patients and controls as observed in our pretreatment study (van Rooij et al, 2014) and a previous PET study (Phan et al, 2006). It is supported by our earlier conclusion that heightened amygdala reactivity, often observed in PTSD in response to trauma-related cues (Etkin and Wager, 2007; Patel et al, 2012), does not extend to trauma-unrelated negative stimuli. In contrast to our study and Phan et al (2006), in which only males were included, two studies with predominantly female PTSD patients did observe an increased amygdala response to negative IAPS pictures. This could indicate an effect of gender on the amygdala response, as was suggested previously (van Rooij et al, 2014). The difference in amygdala activation between patient groups was no longer observed post-treatment, because only persistent patients showed a habituation effect at the level of the amygdala. This finding suggests that in the persistent patients trauma-focused therapy has biological effects on the (aberrant) amygdala response, but this is not sufficient for recovery, potentially due to the alterations in the salience network (dACC and insula).
Our results indicate that PTSD patients cannot be considered a homogenous patient group. Persistent PTSD patients are a specific subgroup of patients with distinct neurobiological characteristics (see also van Rooij et al, 2015). Although all patients within the study qualified as a ‘PTSD case,' those who do not recover are distinct in their symptom presentation and concordantly have different brain functioning. Individuals who have elevated hyperarousal do not respond to trauma-focused therapy, because key regions associated with this circuit are functioning differently. For such individuals, one would hypothesize that new treatments that alter the functioning of these regions are warranted. The information of the current study can be used to consider alternative treatments, such as targeting the dACC with cognitive training, neurofeedback, or brain stimulation, as previously proposed by Etkin (2012).
Limitations, Strengths, and Future Directions
This study is limited in several respects. First, all patients received trauma-focused therapy, but we did not intervene in the treatment procedure and were thus studying treatment as usual. Although this allows for better generalization to actual treatment, it limits us in making specific predictions about the number of sessions or type of treatment. It is important to note that groups did not differ with respect to type of treatment and number of treatment sessions. Furthermore, we did not distinguish tfCBT and EMDR, because we were interested in the shared mechanism of exposure. However, investigating the two treatments separately could potentially increase our understanding of mechanisms specific to one of these treatments, and is suggested when treatment is controlled.
Second, differentiating remitted and persistent patients resulted in some pretreatment group differences, that is, persistent patients had more hyperarousal symptoms resulting in a larger pretreatment CAPS score and more persistent patients fulfilled the criteria for a comorbid anxiety disorder. As this is a naturalistic study, we were not able to control these factors beforehand; however, it is unlikely that these differences influenced our findings. The baseline activation levels did not correlate with baseline CAPS (cluster) scores. Second, the main effect of group for the dACC and insula is a reflection of a group difference at both time points as the group differences are comparable for both time points separately. Finally, we analyzed the effect of potential confounding factors in the regression model, and pretreatment activation of the dACC, insula, and amygdala significantly predicted treatment outcome even after controlling for pretreatment CAPS score, comorbidity, and other potential confounding factors.
Childhood trauma is often associated with PTSD, but in this study there are no indications that childhood trauma had a significant role in PTSD or in the ability to recover. One possible explanation is the relatively low levels of childhood trauma in our sample. Therefore, research conducted in a sample with higher levels of childhood trauma exposure should investigate its relationship with treatment outcome. Additionally, future studies should replicate this study in a civilian and female sample, because in the current study only male war veterans were included.
Even though a control group is interviewed and scanned at both time points to control for the effects of time and repeated testing in general, future studies should include a waitlist control group to account for the effects of time, regression to the mean, and natural symptom fluctuations. Moreover, replication and substantiation are necessary before these results are used as actual predictive biomarkers for clinical purposes. As Galatzer-Levy et al (2013) observed that remitted patients could be distinguished from trauma controls and persistent patients as early as 10 days post-trauma, it would be relevant to study trauma survivors soon after trauma exposure to investigate if neural correlates can contribute to an early identification of patients who will and who will not recover.
CONCLUSION
The present study has potential implications for the prognosis and prediction of treatment success for individuals with PTSD. We highlight a pattern of brain activation that could be considered a marker for persistence of PTSD after trauma-focused therapy. Increased activation of the dACC, insula, and amygdala, the core nodes of the salience network, indicate an attentional bias to negative stimuli. This hyperactivation can prevent the processing of safety information, which is essential for successful trauma-focused therapy. Results from this study can therefore be used to explore alternative or additional treatment options for these patients. Furthermore, this study shows that patients cannot be considered a homogenous group, and further research is needed to elucidate this heterogeneity and investigate its relation to treatment outcome. Differences in neural function represent a promising avenue for such research. These approaches constitute a critical step towards the identification of parameters to individualize PTSD treatment in the future.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest.
Acknowledgments
We thank Mariët van Buuren and Anca Rapcencu for their help with the setup of the task and data collection. Furthermore, we also thank Alieke Reijnen, Iris Eekhout, and Thomas Crow for their valuable suggestions. The study was financially supported by the Dutch Ministry of Defence (Dr Geuze, Principal Investigator).
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Beckmann M, Johansen-Berg H, Rushworth MFS (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. J Neurosci 29: 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson JI, Ehlers A, Matthews R, Pilling S, Richards D, Turner S (2007). Psychological treatments for chronic post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry 190: 97–104. [DOI] [PubMed] [Google Scholar]
- Blake D, Weathers F, Nagy L, Kaloupek D, Klauminzer G, Charney DA (1990). Clinican rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther 13: 187–188. [Google Scholar]
- Bradley R, Greene J, Russ E, Dutra L, Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 162: 214–227. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer EA (2007). Psychometric properties of the Early Trauma Inventory—Self Report. Jf Nerv Mental Dis 195: 211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A et al (2008). Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med 38: 555–561. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Kemp AH, Barton M, Peduto AS, Rennie C et al (2005). Neural networks of information processing in posttraumatic stress disorder: a functional magnetic resonance imaging study. Biol Psychiatry 58: 111–118. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL (2008). The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun PS, Beckham JC, Bosworth HB (2002). Caregiver burden and psychological distress in partners of veterans with chronic posttraumatic stress disorder. J Trauma Stress 15: 205–212. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004). Neural systems supporting interoceptive awareness. Nat Neurosci 7: 189–195. [DOI] [PubMed] [Google Scholar]
- Etkin A (2012). Neurobiology of anxiety: from neural circuits to novel solutions? Depr Anxiety 29: 355–258. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB et al (2012. a). Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 90: 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Tone EB, Phifer J, Norrholm SD, Bradley B, Ressler KJ et al (2012. b). Attention bias toward threat is associated with exaggerated fear expression and impaired extinction in PTSD. Psychol Med 42: 533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci 18: 127–129. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1997). Structured Clinical Interview for DSM-IV Axis I disorders. SCID-I/P. Clinician Version (Administration Booklet).
- Foa EB, Keane TM, Friedman MJ, Cohen JA. (2009) Effective Treatments for PTSD: Practice Guidelines from the International Society of Traumatic Stress Studies. Guilford Press: New York, NY. [Google Scholar]
- Foa EB, Kozak MJ (1986). Emotional processing of fear: exposure to corrective information. Psychol Bull 99: 20–35. [PubMed] [Google Scholar]
- Galatzer-Levy IR, Ankri Y, Freedman S, Israeli-Shalev Y, Roitman P, Gilad M et al (2013). Early PTSD symptom trajectories: persistence, recovery, and response to treatment: results from the Jerusalem Trauma Outreach and Prevention Study (J-TOPS). PLoS One 8: e70084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galatzer-Levy IR, Bryant B (2013). 636 120 Ways to have posttraumatic stress disorder. Perspect Psychol Sci 8: 651–662. [DOI] [PubMed] [Google Scholar]
- Geuze E, Westenberg HM, Jochims A, de Kloet CS, Bohus M, Vermetten E et al (2007). Altered pain processing in veterans with posttraumatic stress disorder. Arch Gen Psychiatry 64: 76–85. [DOI] [PubMed] [Google Scholar]
- Greenland S (1989). Modeling and variable selection in epidemiologic analysis. Am J Public Health 79: 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo I, Cammarota M, Vianna MRM, Bevilaqua LRM (2004). The inhibition of acquired fear. Neurotoxicity Res 6: 175–188. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ely T, Fani N, Glover EM, Gutman D, Tone EB et al (2013). Reduced neural activation during an inhibition task is associated with impaired fear inhibition in a traumatized civilian sample. Cortex 49: 1884–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC (2000). Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry 61: 4–14. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. (1997) International Affective Picture System (IAPS): Technical Manual and Affective Ratings. The Center for Research in Psychophysiology, University of Florida: Gainesville, FL. [Google Scholar]
- LeDoux JE (2000). Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S et al (1999). Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 45: 817–826. [DOI] [PubMed] [Google Scholar]
- Long Z, Duan X, Xie B, Du H, Li R, Xu Q et al (2013). Altered brain structural connectivity in post-traumatic stress disorder: a diffusion tensor imaging tractography study. J Affect Disord 150: 798–806. [DOI] [PubMed] [Google Scholar]
- Maldonado G, Greenland S (1993). Simulation study of confounder-selection strategies. Am J Epidemiol 138: 923–936. [DOI] [PubMed] [Google Scholar]
- Mayou RA, Ehlers A, Bryant B (2002). Posttraumatic stress disorder after motor vehicle accidents: 3-year follow-up of a prospective longitudinal study. Behav Res Ther 40: 665–675. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, LaBar KS, Petty CM, McCarthy G, Morey RA (2009). Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry ResNeuroimag 172: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Archiv Gen Psychiatry 63: 184–192. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I (2002). Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R (2003). Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285. [DOI] [PubMed] [Google Scholar]
- Rauch S, Foa E (2006). Emotional processing theory (EPT) and exposure therapy for PTSD. J Contemp Psychother 36: 61–65. [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB et al (2000). Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47: 769–776. [DOI] [PubMed] [Google Scholar]
- Schneider SL (2013). The International Standard Classification of Education 2011. Compar Soc Res 30: 365–379. [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B et al (2005). A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archiv Gen Psychiatry 62: 273–281. [DOI] [PubMed] [Google Scholar]
- Shipherd JC, Salters-Pedneault K (2008). Attention, memory, intrusive thoughts, and acceptance in PTSD: an update on the empirical literature for clinicians. Cogn Behav Pract 15: 349–363. [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P (2013). Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp 34: 2944–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, Salat DH (2015). Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry 78: 210–216. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS et al (2012). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 74: 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, De Ruiter MB, Elzinga BM, Van Balkom AJ et al (2012). Treatment effects on insular and anterior cingulate cortex activation during classic and emotional Stroop interference in child abuse-related complex post-traumatic stress disorder. Psychol Med 42: 2337–2349. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Vink M, Rapcencu AE, Kahn RS (2011). Exaggerated brain activation during emotion processing in unaffected siblings of patients with schizophrenia. Biol Psychiatry 70: 81–87. [DOI] [PubMed] [Google Scholar]
- van Rooij SJH, Kennis M, Sjouwerman R, van den Heuvel MP, Kahn RS, Geuze E (2015). Smaller hippocampal volume as a vulnerability factor for the persistence of post-traumatic stress disorder. Psychol Med; FirstView: 1–10, doi:10.1017/S0033291715000707. [DOI] [PubMed]
- van Rooij SJH, Rademaker AR, Kennis M, Vink M, Kahn RS, Geuze E (2014). Neural correlates of trauma-unrelated emotional processing in war veterans with PTSD. Psychol Med 45: 575–587. [DOI] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS (2014). Functional differences in emotion processing during adolescence and early adulthood. NeuroImage 91: 70–76. [DOI] [PubMed] [Google Scholar]
- Weathers F, Ruscio AM, Keane TM (1999). Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess 11: 124–133. [Google Scholar]
- Zaki J, Davis JI, Ochsner KN (2012). Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62: 493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandbelt BB, Gladwin TE, Raemaekers M, van Buuren Mt, Neggers SF, Kahn RS et al (2008). Within-subject variation in BOLD-fMRI signal changes across repeated measurements: quantification and implications for sample size. NeuroImage 42: 196–206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.