Abstract
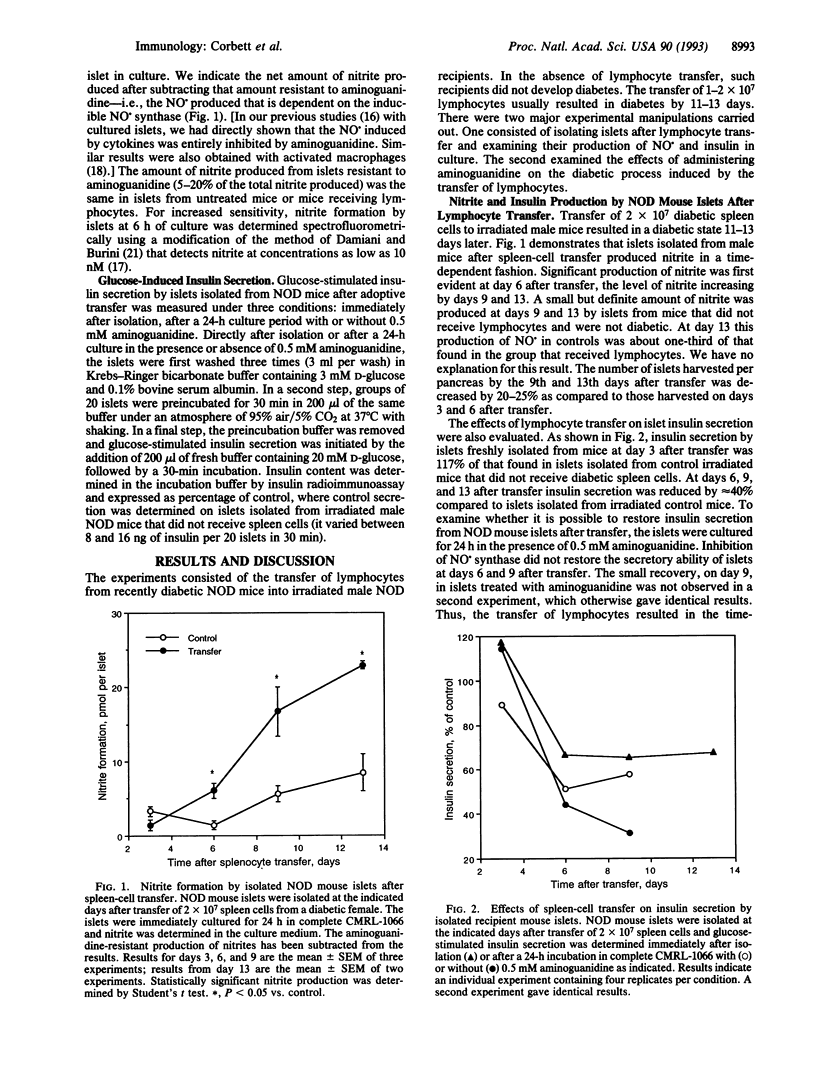
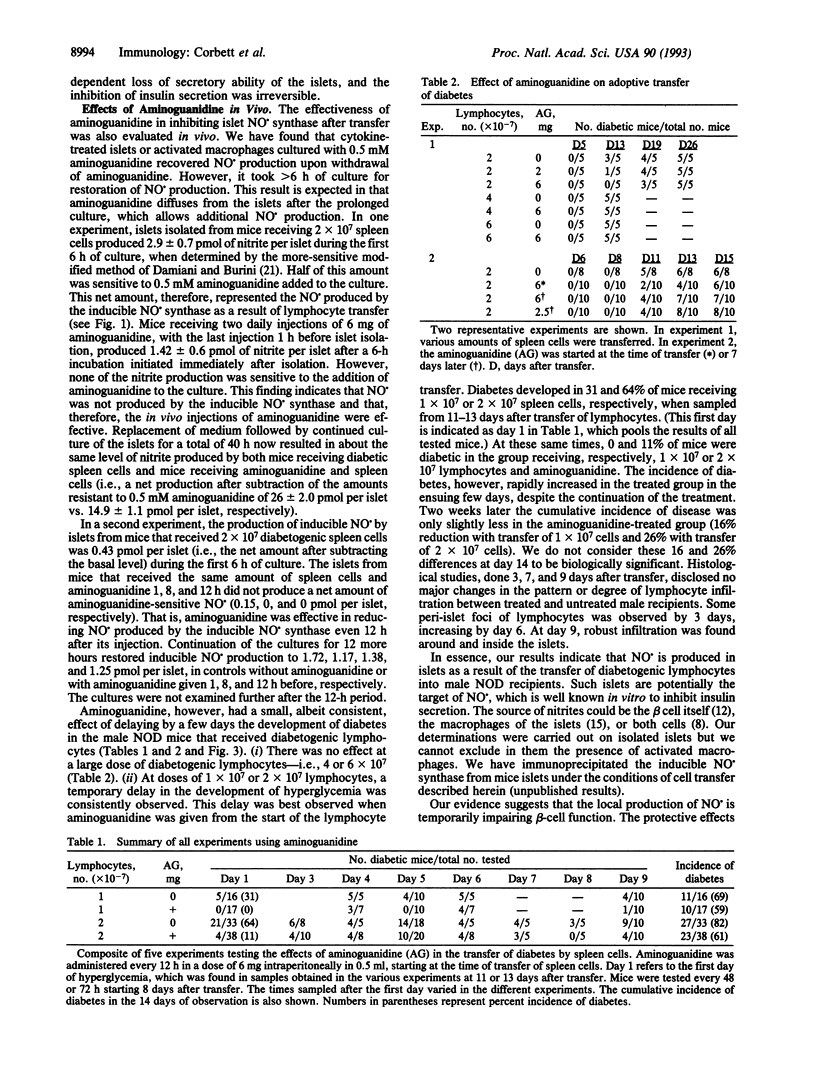
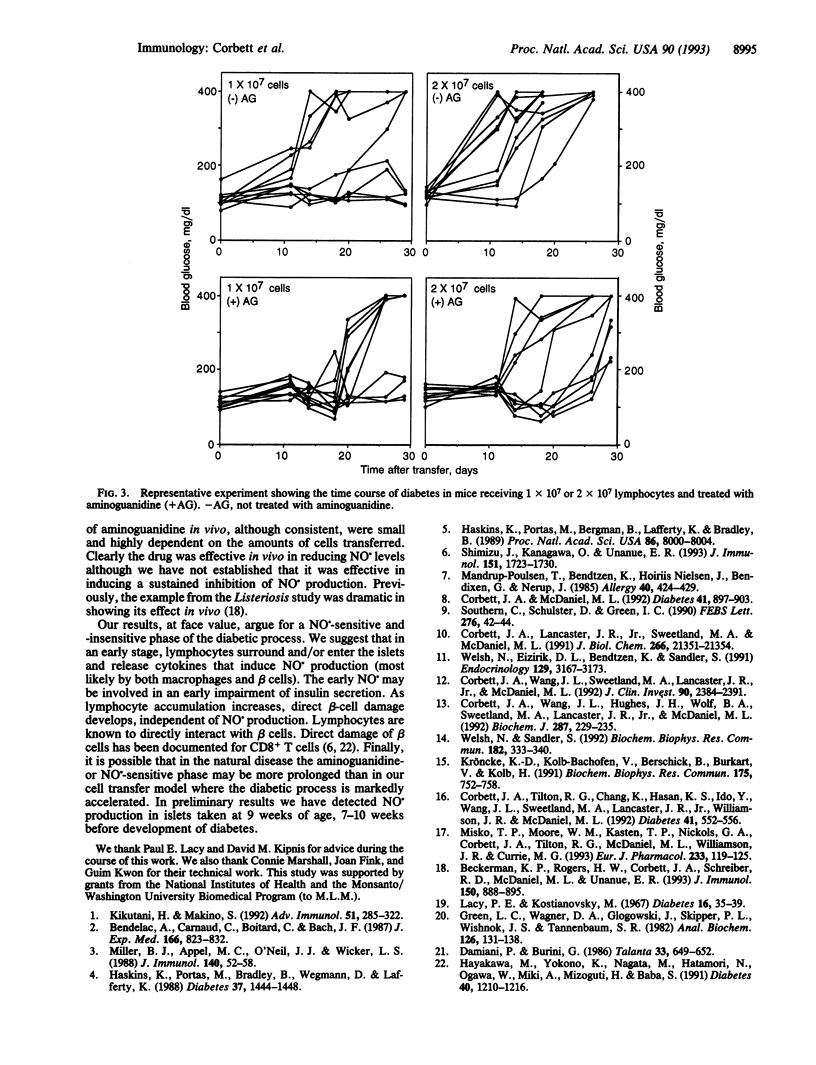
The role of nitric oxide (NO.) in the development of immunologically induced diabetes was examined. Transfer of spleen cells obtained from diabetic female nonobese diabetic (NOD) mice to nondiabetic irradiated males induced diabetes 11-13 days after transfer. Islets isolated from recipient male mice produced NO. in a time-dependent fashion. The production of nitrite was initially detected at day 6 after transfer, with increasing levels by days 9 and 13. Under similar conditions glucose-induced insulin secretion by isolated NOD mouse islets was irreversibly reduced by approximately 40% at days 6, 9, and 13 after transfer of spleen cells. The number of islets harvested per pancreas by the 9th and 13th day after transfer was decreased by 20-25% as compared to controls. Treatment of male NOD mice with aminoguanidine, an inhibitor of the inducible form of NO. synthase, reduced the production of NO. in islets and delayed the development of diabetes by 3-8 days. The temporary inhibition by aminoguanidine was dependent on both inhibitor concentration and number of spleen cells transferred. These results indicate that NO. is produced in NOD islets as a result of an immunological diabetogenic process and suggests a role of this compound in the immunological diabetic process.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckerman K. P., Rogers H. W., Corbett J. A., Schreiber R. D., McDaniel M. L., Unanue E. R. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol. 1993 Feb 1;150(3):888–895. [PubMed] [Google Scholar]
- Bendelac A., Carnaud C., Boitard C., Bach J. F. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987 Oct 1;166(4):823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Corbett J. A., McDaniel M. L. Does nitric oxide mediate autoimmune destruction of beta-cells? Possible therapeutic interventions in IDDM. Diabetes. 1992 Aug;41(8):897–903. doi: 10.2337/diab.41.8.897. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Wang J. L., Hughes J. H., Wolf B. A., Sweetland M. A., Lancaster J. R., Jr, McDaniel M. L. Nitric oxide and cyclic GMP formation induced by interleukin 1 beta in islets of Langerhans. Evidence for an effector role of nitric oxide in islet dysfunction. Biochem J. 1992 Oct 1;287(Pt 1):229–235. doi: 10.1042/bj2870229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett J. A., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, McDaniel M. L. Interleukin 1 beta induces the formation of nitric oxide by beta-cells purified from rodent islets of Langerhans. Evidence for the beta-cell as a source and site of action of nitric oxide. J Clin Invest. 1992 Dec;90(6):2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bergman B., Lafferty K., Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8000–8004. doi: 10.1073/pnas.86.20.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins K., Portas M., Bradley B., Wegmann D., Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988 Oct;37(10):1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- Hayakawa M., Yokono K., Nagata M., Hatamori N., Ogawa W., Miki A., Mizoguti H., Baba S. Morphological analysis of selective destruction of pancreatic beta-cells by cytotoxic T lymphocytes in NOD mice. Diabetes. 1991 Sep;40(9):1210–1217. doi: 10.2337/diab.40.9.1210. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Makino S. The murine autoimmune diabetes model: NOD and related strains. Adv Immunol. 1992;51:285–322. doi: 10.1016/s0065-2776(08)60490-3. [DOI] [PubMed] [Google Scholar]
- Kröncke K. D., Kolb-Bachofen V., Berschick B., Burkart V., Kolb H. Activated macrophages kill pancreatic syngeneic islet cells via arginine-dependent nitric oxide generation. Biochem Biophys Res Commun. 1991 Mar 29;175(3):752–758. doi: 10.1016/0006-291x(91)91630-u. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Mandrup-Poulsen T., Bendtzen K., Nielsen J. H., Bendixen G., Nerup J. Cytokines cause functional and structural damage to isolated islets of Langerhans. Allergy. 1985 Aug;40(6):424–429. doi: 10.1111/j.1398-9995.1985.tb02681.x. [DOI] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Shimizu J., Kanagawa O., Unanue E. R. Presentation of beta-cell antigens to CD4+ and CD8+ T cells of non-obese diabetic mice. J Immunol. 1993 Aug 1;151(3):1723–1730. [PubMed] [Google Scholar]
- Southern C., Schulster D., Green I. C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990 Dec 10;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- Welsh N., Eizirik D. L., Bendtzen K., Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991 Dec;129(6):3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]
- Welsh N., Sandler S. Interleukin-1 beta induces nitric oxide production and inhibits the activity of aconitase without decreasing glucose oxidation rates in isolated mouse pancreatic islets. Biochem Biophys Res Commun. 1992 Jan 15;182(1):333–340. doi: 10.1016/s0006-291x(05)80149-4. [DOI] [PubMed] [Google Scholar]


