Supplemental Digital Content is available in the text
Abstract
Prolactin has different functions, including cytokine secretion and inhibition of the suppressor effect of regulatory T (Treg) cells in healthy individuals. Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by defects in the functions of B, T, and Treg cells. Prolactin plays an important role in the physiopathology of SLE. Our objective was to establish the participation of prolactin in the regulation of the immune response mediated by Treg cells from patients with SLE. CD4+CD25hiCD127−/low cells were purified using magnetic beads and the relative expression of prolactin receptor was measured. The functional activity was evaluated by proliferation assay and cytokine secretion in activated cells, in the presence and absence of prolactin. We found that both percentage and function of Treg cells decrease in SLE patients compared to healthy individuals with statistical significance. The prolactin receptor is constitutively expressed on Treg and effector T (Teff) cells in SLE patients, and this expression is higher than in healthy individuals. The expression of this receptor differs in inactive and active patients: in the former, the expression is higher in Treg cells than in Teff cells, similar to healthy individuals, whereas there is no difference in the expression between Treg and Teff cells from active patients. In Treg:Teff cell cocultures, addition of prolactin decreases the suppressor effect exerted by Treg cells and increases IFNγ secretion. Our results suggest that prolactin plays an important role in the activation of the disease in inactive patients by decreasing the suppressor function exerted by Treg cells over Teff cells, thereby favoring an inflammatory microenvironment.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease characterized by widespread inflammation, alteration in T cell activation, and overproduction of autoantibodies. This disease is most commonly observed in women. The course of the disease is characterized by remissions and exacerbation. The exacerbation of the disease has been linked to the activity of the immune system.1 Autoreactive T cells assist autoreactive B cells and infiltrate into the target organs to promote inflammation via cytokine secretion, which causes damage. Thus, autoreactive T cells are key players in the pathogenesis of SLE.2
Hyperprolactinemia has been reported in several autoimmune diseases, including SLE.3–6 Prolactin (PRL) can be synthesized in an extra-pituitary fashion by cells from the immune system, such as B and T cells, which also express the PRL receptor.7,8 During an immune response, PRL promotes the proliferation, growth, activation, and differentiation of T cells9,10 and intervenes in the expression of CD69 and CD154 by CD4+ T cells.11 In human CD4+ T cell cultures activated with phorbol myristate acetate and subjected to PRL blockade by using an anti-PRL antibody, IL2 and IFNγ secretion is decreased, indicating a role for PRL in the regulation of cytokine secretion.12 Furthermore, PRL can decrease the function of regulatory T (Treg) cells13 in healthy individuals. These studies show the importance of PRL in the regulation of the immune system.
The pathogenesis of SLE involves complex interactions between genetic and environmental factors and the adaptive and innate immune systems. The breakdown of immunologic self-tolerance results in the development of autoimmune diseases.14,15 Other alterations could also be involved in regulating the immune response mediated by Treg cells. There are 2 types of Treg cells: natural Treg cells, which are generated in the thymus, and inducible Treg cells, which are generated in peripheral sites. Both cells exhibit the same CD4+CD25hiCD127low/−FoxP3+ phenotype.16,17 Treg cells exert an inhibitory effect on CD4+CD25−CD127+ conventional or effector T (Teff) cells.18 A numerical defect in Treg cells has been observed in autoimmune pathologies such as thyroiditis19 and diabetes,20 whereas in SLE, decreased21–26 as well as normal27–30 Treg cell numbers have been reported. Moreover, in SLE patients, conventional T cells exhibit reduced sensitivity to Treg cell inhibition.22,31,32
The objective of our work was to determine whether PRL participates in the regulation of the immune response mediated by Treg cells in patients with SLE. We found that both percentage and function of Treg (CD4+CD25hiCD127−/lowFoxP3+) cells were decreased in SLE patients compared to healthy individuals. The expression of PRL receptor was found to be constitutive in both Treg and Teff cells in patients with SLE and this expression was increased compared to that in healthy individuals. PRL receptor expression varied among SLE patients; in inactive patients, the expression of the receptor was higher in Treg cells compared to Teff cells, similar to what was observed in healthy individuals. However, there was no difference in the expression of the receptor between Treg and Teff cells among active SLE patients. We also found that PRL affects the function of Treg cells. The addition of prolactin to Treg:Teff cocultures decreased the suppressor effect in Treg cells and increased IFNγ secretion. These results suggest that PRL increases IFNγ secretion, favoring an inflammatory environment, and decreases the suppressor function of Treg cells; this, in addition to the decrease in the number of Treg cells, contributes to the expansion of autoreactive lymphocytes, favoring disease activation.
METHODS
Study Group
The Ethics Committee of Human Research of the Instituto Mexicano del Seguro Social (IMSS) and the Ethics and Research Committees of the Hospital General de México approved this study (2009-785-028). It was conducted according to the Declaration of Helsinki. Informed consent was obtained from all participants. The samples were obtained from 17 healthy women in the reproductive age (18–50 years) without menstrual disorders and with normal levels of serum prolactin (<20 ng/ml). Since Treg is a rare cell population, the cells from 1 patient are inadequate for all experiments; therefore, from a total of 68 patients with SLE (25–50 years of age), we used samples from an average of 13 patients with inactive lupus and 13 patients with active lupus for each experiment. All patients with SLE fulfilled the American College of Rheumatology (ACR) criteria for SLE.33
Disease activity was measured by SLEDAI (systemic lupus erythematosus disease activity index). Inactive lupus was considered when the SLEDAI value was equal to 0; lupus was considered to be active when the SLEDAI value was ≥4. The samples were obtained between 08:00 and 11:00 am from the cubital vein.
Prolactin
The human PRL used in this study was kindly provided by Dr. A.F. Parlow, from the National Hormone & Pituitary Program (NHPP; Harbor UCLA Medical Center, Los Angeles, CA).
Antibodies
The following antibodies were used: mouse anti-human CD4-APC (OKT4), CD25-PE-Cy5 (BC96), CD127-FITC (eBioRDR5), FoxP3-PE (PCH101), and CD25-APC (BC96), all from eBioscience (San Diego, CA); mouse anti-PRL receptor (ECD, 1A2B1) from Invitrogen (Carlsbad, CA); and Biotin Rat Anti-Mouse IgG2b (R12-3) from BD Pharmingen (San Jose, CA). The biotinylated secondary antibody was detected using streptavidin–phycoerythrin–Cy5.5 from BD Biosciences (Mountain View, CA).
Treg and Teff Cell Purification
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood samples by density centrifugation using Lymphoprep (Axis Shield, Oslo, Norway). Treg cells were isolated from PBMCs by using a CD4+CD25+CD127dim/− Regulatory T cell Isolation Kit II (Miltenyi Biotec, Bergish Gladbach, Germany), according to the manufacturer's instructions. The purity of the cells ranged from 93% to 97% (Supplemental Content 1).
Cell Culture and Proliferation Assays
Cells were cultured in AIM-V liquid medium (Gibco BRL, NY, New York) supplemented with 50 units/ml penicillin and 50 μg/ml streptomycin (Gibco BRL). Treg cells (CD4+CD25hiCD127low/−) were plated at a density of 4.0 × 104 cells/well in 96-well U-bottomed plates (Nunc, Roskilde, Denmark) with or without 8.0 × 104 Teff cells (CD4+CD25−CD127+) and cultured in synthetic serum-free medium (AIM-V, Gibco BRL). We standardized the optimum ratio of Treg:Teff cells required to generate a response by using a standard curve illustrating the following ratios: 0.5:1, 1:1, 2:1, and 4:1. The suppressor effect was observed under all conditions; thus, we decided to use a 0.5:1 Treg:Teff cell ratio, on the basis of the percentage of circulating Treg cells and the feasibility of obtaining sufficient quantities for all tests.
Treg Suppression Inspector human (anti-CD2/CD3/CD28 beads; Miltenyi Biotec, Germany) was used for the functional characterization of human Treg cells by in vitro suppression assays in the presence and absence of 50 ng/ml human PRL (NHPP, Los Angeles, CA). The concentrations of Inspector and PRL were obtained using a dose–response curve. Cells were cultured for 5 days, and 1 μCi [3H]-thymidine (Hartmann Analytical, Braunschweig, Germany) was added 18 hours before harvesting. Thymidine incorporation was determined using a liquid scintillation analyzer (Packard 1900 TR, Meriden, Connecticut), and the percentage of proliferation suppression was determined. All conditions were previously standardized and optimized.
Cytokine Detection
Cell culture supernatants were collected on day 5, and cytokine levels were measured using a commercial BD Cytometric Bead Array (CBA) Human Th1/Th2/Th17 Cytokine Kit (IL2, IL4, IL10, IL6, TNF, IFNγ, and IL17A) by BD Biosciences.
Real-Time PCR Assay
Total RNA was extracted from purified Treg and Teff cells by using TRIzol Reagent (Invitrogen), according to the manufacturer's instructions. RNA concentration was determined using UV spectrophotometry, and 1 μg of total RNA was used to generate cDNA with SuperScript II reverse transcriptase (Invitrogen). The PRL receptor and β actin were then amplified by real-time PCR using a LightCycler TaqMan Master kit (Roche Diagnostic, Mannheim, Germany), hydrolysis probes, and primers designed by Roche Diagnostic; all reactions were performed according to the manufacturer's specifications. The primers and probes used are as follows: number 8 probe from the Universal Probe Library for PRL receptor determination, forward primer CTT TCC ACA TGA ACC CTG AAG and reverse primer GCA GAT GCC ACA TTT TCC TT, and number 64 probe from Universal Probe Library for β-actin determination, forward primer CCA ACC GCG AGA AGA TGA and reverse primer CCA GAG GCG TAC AGG GAT AG. Reactions were carried out in a final volume of 10 μl, and a LightCycler 1.5 instrument was used (Roche Diagnostic). The PCR conditions were as follows: 10 minutes at 95°C, followed by 45 cycles of 10 seconds at 95°C, 30 seconds at 59°C, and 1 seconds at 72°C, with a final cycle for 30 seconds at 40°C. The samples were normalized to β-actin gene expression. The relative expression of PRL and its receptor was calculated using the 2ΔCT formula.
Cell Surface Staining and Flow Cytometry
To determine the percentage of peripheral blood Treg cells, PBMCs were incubated with fluorescently labeled antibodies (anti-CD4, CD25, CD127, and PRL receptor or unrelated antibody) for 20 minutes at 4°C in staining buffer (phosphate-buffered saline [PBS] with 0.5% bovine serum albumin [BSA] and 0.01% sodium azide). The cells were then washed and fixed in 2% PBS–paraformaldehyde (Sigma Aldrich, St. Louis, MO). Data were obtained using a MACSQuant Analyzer 10 flow cytometer (Miltenyi Biotec, Auburn, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR).
Intracellular Staining for FoxP3
After the superficial staining, the cells were fixed and permeabilized with the Foxp3 Staining Buffer Set (eBioscience) for 18 hours, and stained with fluorescent antibodies. After washing, the stained cells were assayed in a MACSQuant Analyzer 10 flow cytometer and the data were analyzed with FlowJo software.
Statistical Analysis
Statistical analysis was performed using the SPSS package, version 20.0 (SPSS, Inc., Chicago, IL). Normality of the data was checked using the Kolmogorov–Smirnoff test, followed by an analysis using the relevant parametric or nonparametric test. The suppressor function among the groups was assessed using the Kruskal–Wallis test. Comparisons between individual groups were tested using the unpaired Mann–Whitney U or paired Wilcoxon matched-pairs test, at a significance level of P < 0.05.
RESULTS
Percentage of Treg Cells
The percentage of Treg (CD4+CD25hiCD127low/−FoxP3+) cells was determined based on PBMCs from healthy individuals and SLE patients (active and inactive). We found that the percentage of Treg cells decreased in a statistically significant way (P < 0.001) in patients with active and inactive SLE, compared to healthy individuals (χ¯=2.95%), but no difference was observed between the inactive (χ¯=1.67%) and active (χ¯=1.19%) patients, suggesting that the number of Treg cells is less in SLE patients (active and inactive) (Figure 1).
FIGURE 1.
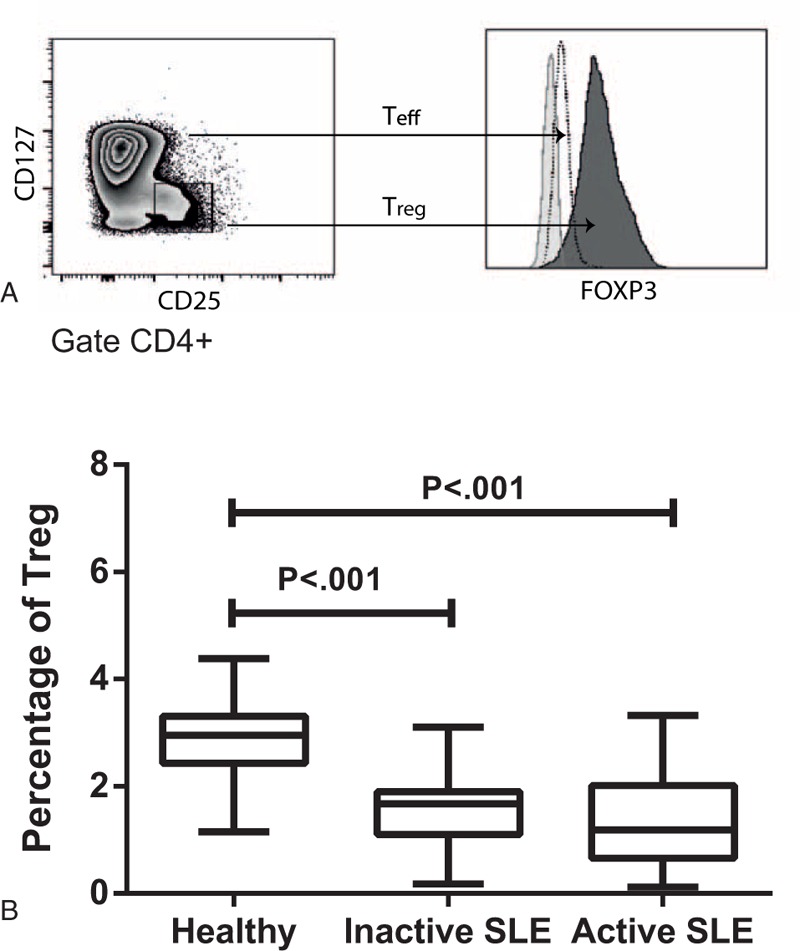
Percentage of Treg CD4+CD25hiCD127−/lowFOXP3+ cells, PBMCs were stained with CD4, CD25, CD127, and FOXP3 antibodies, and the percentage of Treg cells was determined by flow cytometry. (A) Analysis strategy for determining the percentage of Treg cells from the CD4+ gate. (B) Percentage of Treg cells in healthy individuals as well as patients with active and inactive SLE. The graph shows the median value; P < 0.001.
PRL Receptor Expression in Treg and Teff Cells
Our results showed that Treg cells from SLE patients express the PRL receptor even in absence of stimuli and that both mRNA (relative expression) and protein (FMI = mean fluorescence intensity) expression by Treg cells from active and inactive SLE patients was higher than that in Treg cells from healthy individuals (Table 1). This result showed a statistically significant difference (Figure 2 A and B), although no statistically significant difference were observed between the active and inactive patients. We found that the expression of PRL receptor mRNA and protein in Teff cells from active and inactive SLE patients was higher than that in cells from healthy individuals, with a statistically significant difference (Figure 2C and D). There was no difference in the expression of PRL receptor between active and inactive patients. Moreover, the expression of PRL receptor was higher in Treg cells compared to Teff cells from patients with inactive SLE, similar to that observed in healthy individuals. However, in patients with active SLE, there was no difference in the expression of the receptor between Treg and Teff cells and the expression of the PRL receptor in Teff cells from patients with SLE was higher than in healthy controls (Figure 2).
TABLE 1.
Expression of Prolactin Receptor
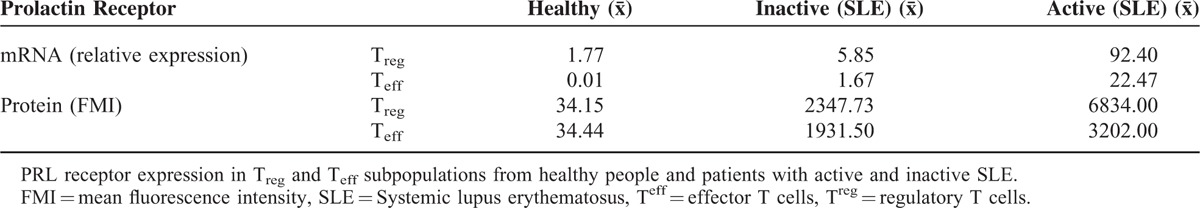
FIGURE 2.
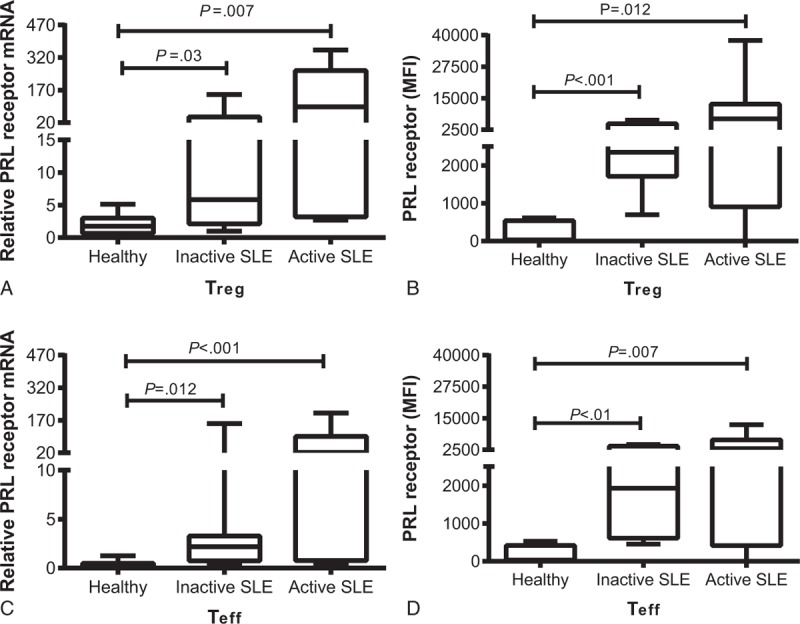
Expression of PRL receptor, Teff (CD4+CD25−CD127+) and Treg (CD4+CD25hiCD127−/low) cell subpopulations from healthy individuals and SLE patients were purified from the PBMCs by using magnetic beads. The relative mRNA expression of PRL receptor was determined in (A) Treg and (B) Teff cells by PCR-RT. Flow cytometry was used to determine the protein expression in (C) Treg and (D) Teff cells. The graph shows the median value.
PRL Function Regarding Treg Cell-Meditated Regulation
The suppressor capacity of Treg cells stimulated with “Treg Suppression Inspector human” (anti-CD2/CD3/CD28 beads) in the presence and absence of PRL was evaluated through in vitro cellular proliferation studies. The proliferation of Teff cells from healthy individuals is shown in Figure 3A. We observed that the addition of PRL did not exert any effect on the proliferation of these cells when Treg cells were added (coculture Treg:Teff), but the cells exerted suppressor activity over Teff cells by decreasing their proliferation in a significant manner (P = 0.001). The addition of PRL to this coculture interfered with the activity of Treg cells, reestablishing the proliferative capacity of Teff cells to levels similar to that of Teff cells in the absence of Treg cells. PRL did not affect the proliferation of Teff cells from patients with inactive SLE. The suppressor effect exerted by Treg cells over Teff cells was observed in most patients (Supplemental Content 2). However, when considering the entire group, we did not find any statistically significant difference (P = 0.08) in the suppressor effect of Treg cells over Teff cells. Similar to healthy subjects, PRL does not increase the proliferation of Teff cells from inactive SLE patients. In Treg:Teff coculture, the addition of PRL decreased the regulatory effect of Treg cells, thus causing an increase in the proliferation of Teff cells, with a statistically significant difference (P = 0.001; Figure 3B). In contrast, in cells from patients with active SLE, PRL activity increased the proliferation of Teff cells in a statistically significant manner (P = 0.006). The Treg cells from these patients did not have the capacity to exert their suppressor activity over the Teff cells, although the addition of PRL to the Treg:Teff coculture tended to increase the proliferation of Teff cells, with no statistically significant difference (P = 0.06; Figure 3C). This result suggests that the function of Treg cells is no longer adequate under this condition (Table 2).
FIGURE 3.
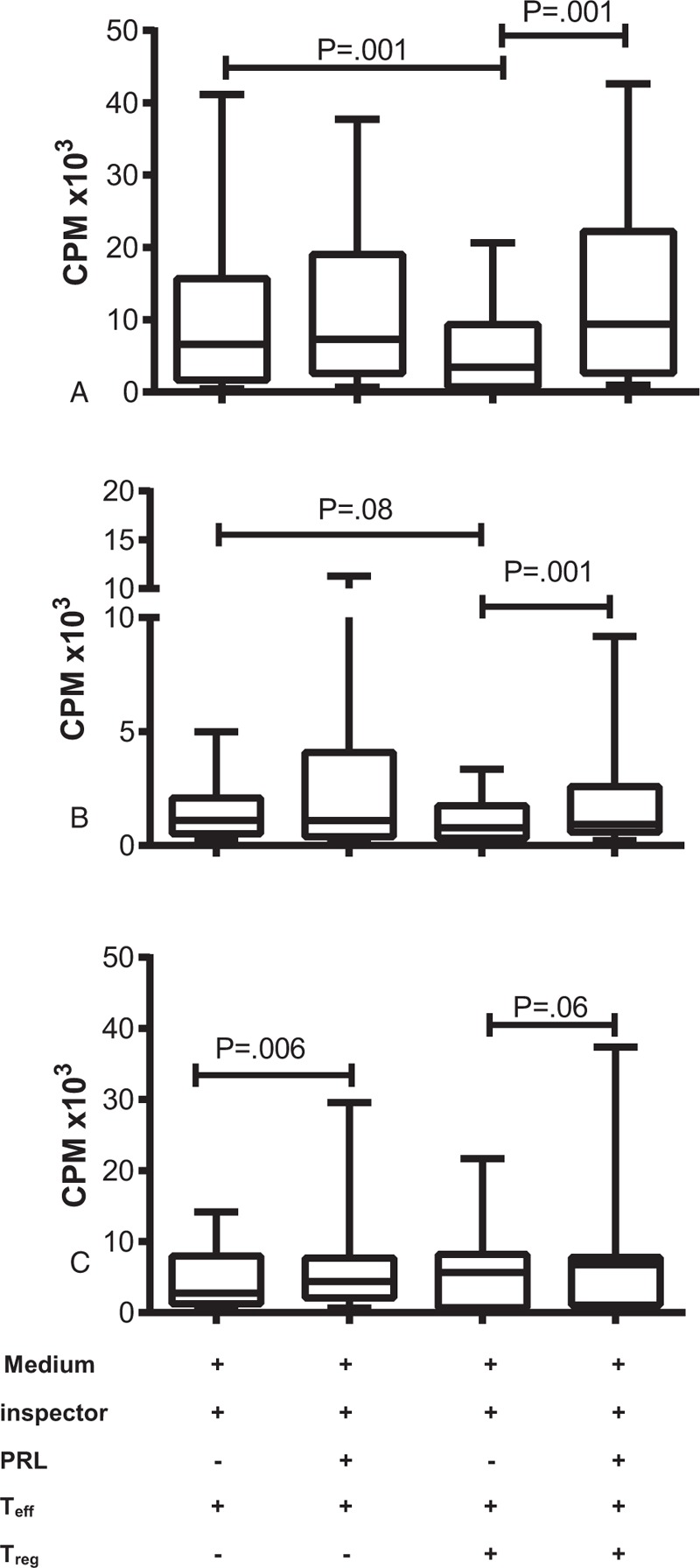
Effects of PRL on the functions of Teff and Treg cells, Teff (CD4+CD25−CD127+) and Treg (CD4+CD25hiCD127−/low) cells from healthy individuals and SLE patients were stimulated with “Treg Suppression Inspector human” (anti-CD2/CD3/CD28 beads) in the presence and absence of PRL (50 ng/ml). Cell proliferation was measured by incorporating [3H]-thymidine in the cells from (A) healthy individuals, (B) inactive SLE patients, and (C) active SLE patients. The median value of 12 independent trials for each group is presented. The assays were performed in triplicate (statistical significance, P ≤ 0.05).
TABLE 2.
Cell Proliferation
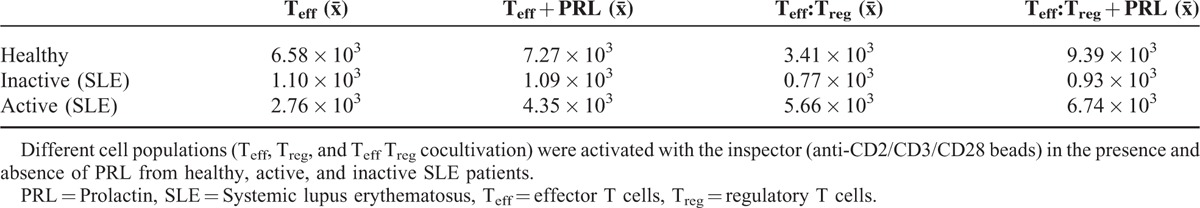
Cytokine Secretion by Teff Cells Cultured in the Presence and Absence of PRL
Cytokine concentrations were determined in Teff culture supernatants stimulated with “Treg Suppression Inspector human” (anti-CD2/CD3/CD28 beads) in the presence and absence of PRL. As shown in Figure 4A, we observed that IL10 secretion from Teff cells from patients with active or inactive SLE was decreased with respect to that from the cells from healthy individuals, with a statistically significant difference (P = 0.05), but there were no differences between inactive and active patients. The addition of PRL to the Teff cell culture did not modify the secretion pattern of IL10 when using cells from any of the 3 studied groups. No difference in the secretion of IL17A, TNF, or IFNγ was observed for the Teff cells from the 3 groups, and the addition of PRL did not affect IL17A and TNF secretion. However, PRL treatment increased IFNγ secretion from Teff from inactive patients, with a statistically significant difference (P = 0.01) (Figure 4B); meanwhile, in active patients, only an increase was observed, without any statistically significant difference (P = 0.08; Table 3).
FIGURE 4.
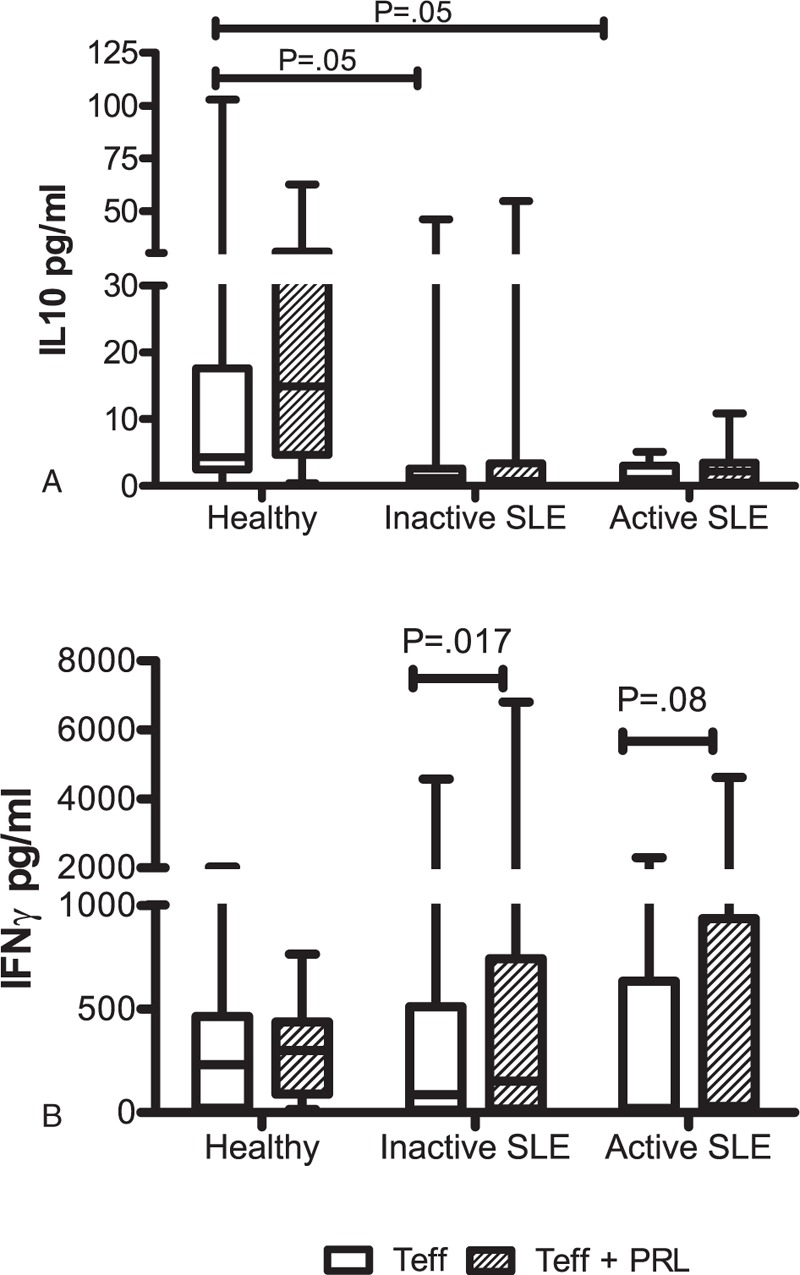
Cytokine secretion profile of Teff in the presence and absence of PRL, Teff cells from healthy persons and SLE patients were stimulated with “Treg Suppression Inspector human” (anti-CD2/CD3/CD28 beads) in the presence and absence of PRL. The secretion of (A) IL10, and (B) IFNγ was determined by CBA. The median value is presented for each group (statistical significance, P ≤ 0.05).
TABLE 3.
Cytokine Secretion by Teff Cells
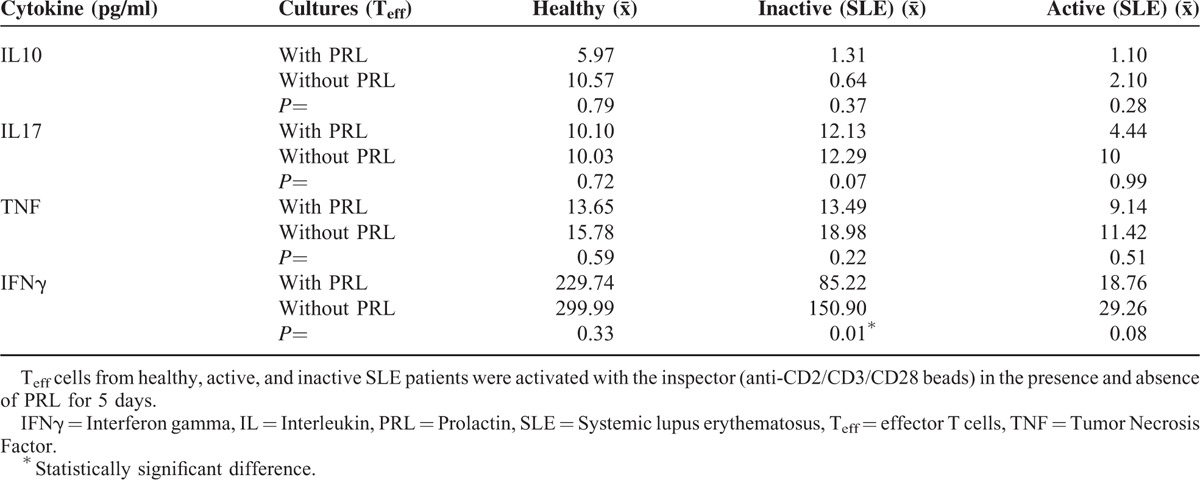
Cytokine Secretion by Treg and Teff Cell Coculture in the Presence and Absence of PRL
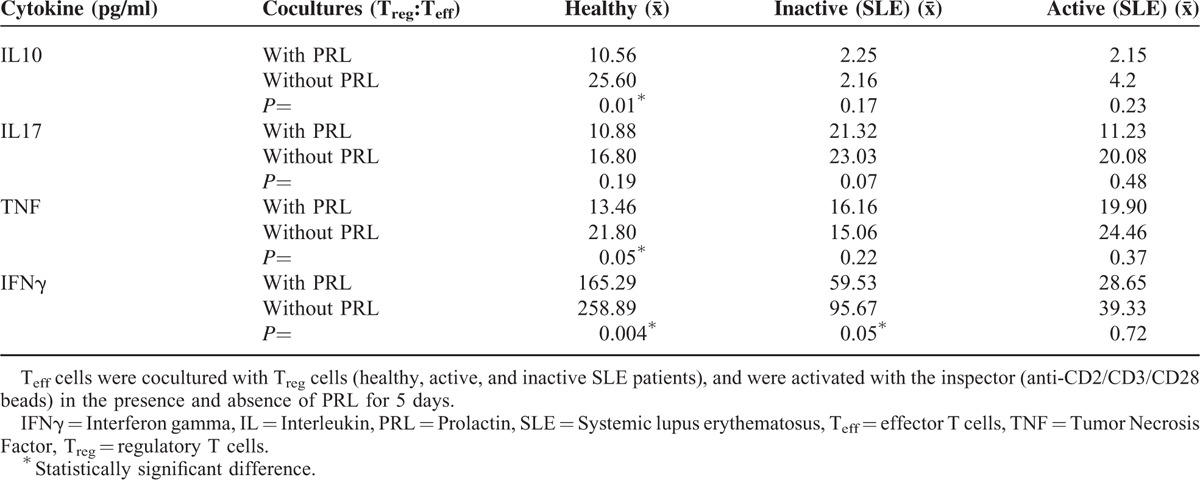
Cytokine secretion was determined in the presence and absence of PRL by using Treg:Teff cocultures from the 3 groups being studied. The addition of PRL to the Treg:Teff coculture from healthy individuals significantly increased the secretion of IL10, TNF, and IFNγ, whereas IL17A secretion was unaffected. Meanwhile, PRL significantly increased IFNγ secretion in Treg:Teff cocultures using cells from patients with inactive SLE (P = 0.05) and IL17A secretion increased in most patients. However, we did not find any statistically significant difference (P = .07) in case of the entire group; there was no difference in TNF and IL10 secretion. Cytokine secretion was not affected by the addition of PRL to the cocultures using cells from patients with active SLE (Figure 5, Table 4).
FIGURE 5.
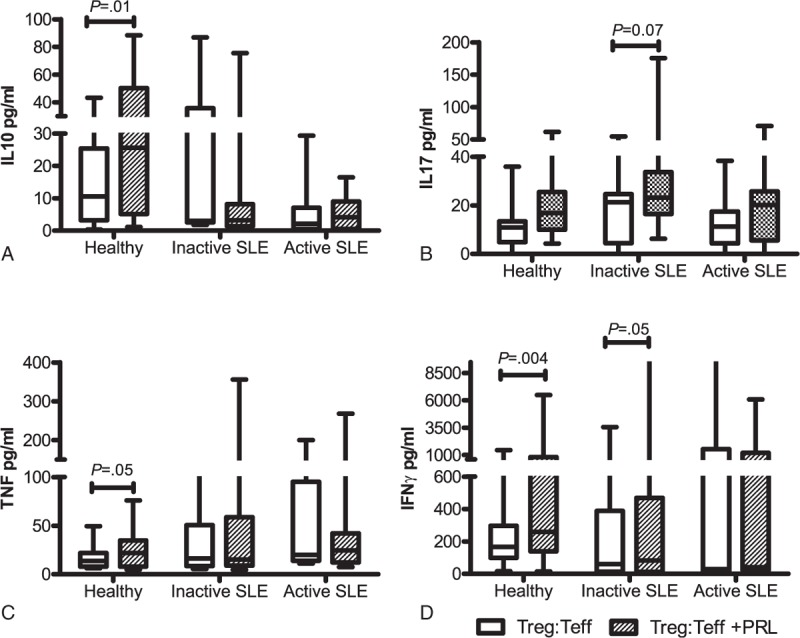
Cytokine secretion profile in Treg:Teff coculture in the presence and absence of PRL, Treg:Teff cocultures using cells from healthy persons and SLE patients were stimulated with “Treg Suppression Inspector human” (anti-CD2/CD3/CD28 beads) in the presence and absence of PRL. The secretion of (A) IL10, (B) IL17, (C) TNF, and (D) IFNγ was determined by CBA. The median value is presented for each group (statistical significance, P ≤ 0.05).
TABLE 4.
Cytokine Secretion by Teff:Treg Cocultures
DISCUSSION
Sex hormones such as PRL play an important role in the modulation of immune response, which depends on the type of cell expressing the PRL receptor.7,34 Moreover, PRL has an immune-stimulating effect and promotes autoimmunity,5 interfering with the tolerance of B cells35 and increasing the production of antibodies.5,36 We previously reported that the PRL receptor is constitutively expressed in the Treg cells of healthy individuals (females), whereas the expression increases in Teff cells in response to a stimulus.13 The results of this study showed that compared to healthy individuals, the expression of PRL receptor was higher in the Treg and Teff cells from patients with SLE (females), with the receptor being expressed even in the absence of a stimulus. This expression tended to increase in cells from active patients compared to that from inactive patients, suggesting higher activity in the disease, along with higher expression of the receptor. Which occurs in B cells from mice that developed lupus (MRL, MRL/lpr), whereby the expression of the receptor increased with the manifestation of the disease.37,38 In addition, the expression patterns of Teff and Treg cells differed between active and inactive patients. In inactive patients, the expression of the receptor was higher in Treg cells compared to Teff cells, a behavior similar to that observed in healthy individuals. However, there was no difference in the expression of the receptor between Teff and Treg cells from active patients, most likely because the Teff cells were already active, increasing the expression of PRL receptor. This would be similar to the phenomenon in Teff cells from healthy individuals: when activated, the cells increase the expression of the receptor to a level higher than that in Treg cells.13
Treg cells are a component of one of the peripheral tolerance mechanisms, which fail in autoimmune diseases such as SLE; therefore, these cells are important in the pathogenesis of the disease.2 However, available data on the number and function of Treg cells in SLE are contradictory, and the definitive role of Treg cells in SLE remains unclear.29 Therefore, we decided to explore, the percentage of Treg cells in patients with active and inactive SLE, and the role played by PRL in the regulatory function of these cells ex vivo. A statistically significant decrease was found in the percentage of Treg (CD4+CD25hiCD127−/low FOXP3+) cells from patients with SLE, both active and inactive, compared to that in healthy individuals, supporting the findings of previous studies.22,24,39,40 Additionally, the suppressor function exerted by Treg cells over Teff cells depends on the stage of the disease. In patients with inactive SLE, we observed 2 behaviors; first, Treg cells did not present any defects in their suppressor activity (majority of the patients), and second, Treg cells did not present a suppressor function in another group of patients (minority of the patients). Although the patients are clinically inactive, their immune system is probably active, and therefore, Treg cells no longer exert their suppressor effect, as observed in active patients where we did not observe Treg suppressor function, as has been reported. The decrease in the number and function of Treg cells in SLE patients favors the activation of autoreactive clones, and thus, disease manifestation.26,40,41
Because Treg cells from SLE patients express high levels of PRL receptor, we studied whether an interaction with its PRL receptor could affect the suppressor effect of Treg cells, especially those from inactive patients, possessing suppressor function. In these patients, PRL blocked the suppressor effect of Treg cells on Teff cells, a behavior similar to healthy individuals.13 The loss of suppressor effect cannot be attributed to the notion that PRL increases the proliferation of Teff cells, because the addition of PRL to the Teff cell culture did not increase the proliferation of these cells. It might be due to the presence of proinflammatory cytokines (IFNα, IFNγ, and TNF),42–45 as their presence in the culture reduces the suppressor effect of Treg cells. It is also known that PRL promotes the secretion of cytokines such as IFNγ, IL2, IL12, and TNF.12,46,47 Our results showed an increase in IFNγ levels in the cocultures incubated with PRL (Treg:Teff of inactive patients), and although an increase in IL17 levels was observed in these cultures, it was not statistically significant. The increase in IFNγ levels by the addition of PRL was also observed in Teff cell cultures (expressing PRL receptor), which makes us hypothesize that interaction of PRL with its receptor on Teff cells increases IFNγ secretion, and that the presence of this cytokine in the culture decreases the suppressor function of Treg cells in patients with inactive SLE, because this cytokine is known to inhibit the generation and/or function of Treg cells.44,48,49 It is also possible that IFNγ is secreted by Treg cells, as reported in patients with type I diabetes and rheumatoid arthritis, diseases in which Treg cells that secrete proinflammatory cytokines as IFNγ and IL17.50–52 Unfortunately because of the low number of Treg cells purified from patients, we could not verify whether PRL favors IFNγ secretion in these cells. It will be interesting to show whether PRL favors the presence of Treg IFNγ-secreting cells, especially because this has been reported for other autoimmune diseases.50–52
Our results show that both Treg and Teff cells in women with inactive SLE constitutively express the PRL receptor, and therefore, an increase in serum PRL levels will favor the interaction of PRL and its receptor and, in turn, the malfunctioning of the Treg cells, probably because of presence of IFNγ. This malfunction, added to the decrease in the cell number, will contribute to the expansion of autoreactive T-lymphocytes, favoring disease activation. In patients with active SLE, different from those with inactive SLE, PRL increased the cellular proliferation of Teff cells. Thus, PRL in active patients could help in maintaining the disease active by favoring the proliferation of Teff cells among those that are autoreactive.
It is worth mentioning that in our study, we did not use antigen-presenting cells (APCs); only Treg cells were coincubated with Teff cells to observe the suppressor effect of Treg cells. Other models using APCs as a suppressor of the function of Treg cells have been reported. In this sense, it has been proposed that the APCs can block Treg cell activity via overproduction of pro-inflammatory cytokines such as IFNα.42 It would be interesting to determine whether APCs express PRL receptor, and whether PRL favors the secretion of IFNα and other inflammatory cytokines, thereby aiding the malfunction of Treg cells in SLE patients.
CONCLUSIONS
Our results showed that Treg cells from patients with SLE differed from those from healthy individuals with regard to number and function. In inactive patients, PRL acts on Teff cells, which constitutively express the receptor, increasing IFNγ secretion and encouraging an inflammatory microenvironment and Treg cell malfunction. The decrease in the number of T reg cells and their malfunction can contribute to the expansion of autoreactive T-lymphocytes to favor disease activation. Additionally, in active patients, PRL increases the proliferation of inspector-stimulated Teff cells, which can further aid the Teff cells to be more resistant to regulation by Treg cells. It will be interesting to study whether PRL decreases the function of different subpopulations of Treg cells and whether this decrease occurs because PRL favors the plasticity of Treg cells toward a Th1 profile.
Supplementary Material
Acknowledgment
We thank Dr. A.F. Parlow from the National Hormone & Pituitary Program, Harbor-UCLA Medical Center, for donating human PRL (hPRL).
Footnotes
Abbreviations: IFNγ = interferon gamma, PBMCs = peripheral blood mononuclear cells, PRL = prolactin, SLE = systemic lupus erythematosus, SLEDAI = systemic lupus erythematosus disease activity index, Teff = T effector cells, Treg = T regulatory cells.
The authors have no conflicts of interest to disclose.
This research was supported in part by Consejo Nacional de Ciencias y Tecnología (CONACYT 113815) and Fondo de Investigación en Salud, IMSS (FIS/IMSS/PROT/G10/834).
Authors’ contributions: All authors were involved in drafting the article or critically revising it for important intellectual content, and all authors approved the final version to be published. LHMV had full access to the data of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, as well as manuscript drafting. BFF and ChRK: Study conception and design, statistical analysis, interpretation of the results, and revisions of the manuscript. ChSL: Acquisition and interpretation of data. ZGE and BVR: Analysis of the data, and revision of the manuscript. CCH, BFL, and AHE: Classification, review, and monitoring of patients. All authors have read and approved the final manuscript.
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011; 365:2110–2121. [DOI] [PubMed] [Google Scholar]
- 2.Crispin JC, Kyttaris VC, Terhorst C, et al. T cells as therapeutic targets in SLE. Nat Rev Rheumatol 2010; 6:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanco-Favela F, Quintal-Alvarez G, Leaños-Miranda A. Association between prolactin and disease activity in systemic lupus erythematosus. Influence of statistical power. J Rheumatol 1999; 26:55–59. [PubMed] [Google Scholar]
- 4.Orbach H, Shoenfeld Y. Hyperprolactinemia and autoimmune diseases. Autoimmun Rev 2007; 6:537–542. [DOI] [PubMed] [Google Scholar]
- 5.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun Rev 2012; 11:A465–A470. [DOI] [PubMed] [Google Scholar]
- 6.Ugarte-Gil MF, Gamboa-Cardenas RV, Zevallos F, et al. High prolactin levels are independently associated with damage accrual in systemic lupus erythematosus patients. Lupus 2014; 23:969–974. [DOI] [PubMed] [Google Scholar]
- 7.Bole-Feysot C, Goffin V, Edery M, et al. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev 1998; 19:225–268. [DOI] [PubMed] [Google Scholar]
- 8.Freeman ME, Kanyicska B, Lerant A, et al. Prolactin: structure, function, and regulation of secretion. Physiol Rev 2000; 80:1523–1631. [DOI] [PubMed] [Google Scholar]
- 9.Carreño PC, Sacodon R, Jimenez E, et al. Prolactin affects both survival and differentiation of T-cell progenitors. J Neuroimmunol 2005; 160:135–145. [DOI] [PubMed] [Google Scholar]
- 10.Dongming X, Ling L, Xiahong L, et al. Immunoregulation of autocrine prolactin: suppressing the expression of costimulatory molecules and cytokines in T lymphocytes by prolactin receptor knockdown. Cell Immunol 2010; 263:71–78. [DOI] [PubMed] [Google Scholar]
- 11.Chávez-Rueda K, Legorreta-Haquet MV, Cervera-Castillo H, et al. Effect of prolactin on lymphocyte activation from systemic lupus erythematosus patients. Ann N Y Acad Sci 2007; 1108:157–165. [DOI] [PubMed] [Google Scholar]
- 12.Chávez-Rueda K, Hernández J, Zenteno E, et al. Identification of prolactin as a novel immunomodulator on the expression of co-stimulatory molecules and cytokine secretions on T and B human lymphocytes. Clin Immunol 2005; 116:182–191. [DOI] [PubMed] [Google Scholar]
- 13.Legorreta-Haquet MV, Chávez-Rueda K, Montoya-Díaz E, et al. Prolactin down-regulates CD4+CD25hiCD127low/- regulatory T cell function in humans. J Mol Endocrinol 2012; 48:77–85. [DOI] [PubMed] [Google Scholar]
- 14.Akiko O, Keishi F, Tomohisa O, et al. Regulatory T-cell-associated cytokines in systemic lupus erythematous. J Biomed Biotechnol 2011; 2011:463412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lleo A, Invernizzi P, Gao B, et al. Definition of human autoimmunity autoantibodies versus autoimmune disease. Autoimmun Rev 2010; 9:A259–A266. [DOI] [PubMed] [Google Scholar]
- 16.Annunziato F, Cosmi L, Lazzeri E, et al. Phenotype, localization, and mechanism of suppression of CD4+ CD25+ human thymocytes. J Exp Med 2002; 196:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le NT, Chao N. Regulating regulatory T cells. Bone Marrow Transplant 2007; 39:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Josefowicz SZ, Li-Fan Lu, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30:531–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bossowski A, Moniuszko M, Dąbrowska M, et al. Lower proportions of CD4+CD25(high) and CD4+FoxP3, but not CD4+CD25+CD127(low) FoxP3+ T cell levels in children with autoimmune thyroid diseases. Autoimmunity 2013; 46:222–230. [DOI] [PubMed] [Google Scholar]
- 20.Glisic-Milosavljevic S, Waukau J, Jailwala P, et al. At-risk and recent-onset type 1 diabetic subjects have increased apoptosis in the D4+CD25+high T-cell fraction. PLoS ONE 2007; 2:e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fathy A, Mohamed RW, Tawfik GA, et al. Diminished CD4+CD25+ T-lymphocytes in peripheral blood of patients with systemic lupus erythematosus. Egypt J Immunol 2005; 12:25–31. [PubMed] [Google Scholar]
- 22.Lee HY, Hong YK, Yun HJ, et al. Altered frequency and migration capacity of CD4+CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology (Oxford) 2008; 47:789–794. [DOI] [PubMed] [Google Scholar]
- 23.Crispin JC, Martinez A, Alcocer-Varela J. Quantification of regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2003; 21:273–276. [DOI] [PubMed] [Google Scholar]
- 24.Miyara M, Amoura Z, Parizot C, et al. Global natural regulatory T cell depletion in active systemic lupus erythematosus. J Immunol 2005; 175:8392–8400. [DOI] [PubMed] [Google Scholar]
- 25.Konstantia MC, Michael RE. Regulatory T-cells in systemic lupus erythematosus and rheumatoid arthritis. FEBS Lett 2011; 585:3603–3610. [DOI] [PubMed] [Google Scholar]
- 26.Bonelli M, Gösch L, Blüm S, et al. Quantitative and qualitative deficiencies of regulatory T cells in patients with systemic lupus erythematosus (SLE). Int Immunol 2008; 20:861–868. [DOI] [PubMed] [Google Scholar]
- 27.Yates J, Whittington A, Mitchell P, et al. Natural regulatory T cells: number and function are normal in the majority of patients with lupus nephritis. Clin Exp Immunol 2008; 153:44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azab NA, Bassyouni IH, Emad Y, et al. CD4+CD25+ regulatory T cells (TREG) in systemic lupus erythematosus (SLE) patients: the possible influence of treatment with corticosteroids. Clin Immunol 2008; 127:151–157. [DOI] [PubMed] [Google Scholar]
- 29.Alvarado-Sanchez B, Hernandez-Castro B, Portales-Perez D, et al. Regulatory T cells in patients with systemic lupus erythematosus. J Autoimmun 2006; 27:10–18. [DOI] [PubMed] [Google Scholar]
- 30.Ohl K, Tenbrock K. Regulatory T cells in systemic lupus erythematosus. Eur J Immunol 2014; 45:344–355. [DOI] [PubMed] [Google Scholar]
- 31.Venigalla RK, Tretter T, Krienke S, et al. Reduced CD4+, CD25- T cell sensitivity to the suppressive function of CD4+, CD25high, CD127-/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum 2008; 58:2120–2130. [DOI] [PubMed] [Google Scholar]
- 32.Monk CR, Spachidou M, Rovis F, et al. MRL/Mp CD4+CD25- T cells show reduced sensitivity to suppression by CD4+CD25+ regulatory T cells in vitro: a novel defect of T cell regulation in systemic lupus erythematosus. Arthritis Rheum 2005; 52:1180–1184. [DOI] [PubMed] [Google Scholar]
- 33.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–1277. [DOI] [PubMed] [Google Scholar]
- 34.Kelley KW, Weigentc DA, Kooijmand R. Protein hormones and immunity. Brain Behav Immun 2007; 21:384–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha S, Gonzalez J, Rosenfeld G, et al. Prolactin alters the mechanisms of B cell tolerance induction. Arthritis Rheum 2009; 60:1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeganathana V, Peevab E, Diamond B. Hormonal milieu at time of B cell activation controls duration of autoantibody response. J Autoimmun 2014; 53:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ledesma-Soto Y, Blanco-Favela F, Fuentes-Pananá EM, et al. Increased levels of prolactin receptor expression correlate with the early onset of lupus symptoms and increased numbers of transitional-1 B cells after prolactin treatment. BMC Immunol 2012; 13:11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legorreta-Haquet MV, Flores-Fernández R, Blanco-Favela F, et al. Prolactin levels correlate with abnormal B cell maturation in MRL and MRL/lpr mouse models of systemic lupus erythematosus-like disease. Clin Dev Immunol 2013; 2013:287469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mellor-Pita S, Citores MJ, Castejon R, et al. Decrease of regulatory T cells in patients with systemic lupus erythematosus. Ann Rheum Dis 2006; 65:553–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horwitz AD. Regulatory T cells in systemic lupus erythematosus: past, present and future. Arthritis Res Ther 2008; 10:227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valencia X, Yarboro C, Illei G, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol 2007; 178:2579–2588. [DOI] [PubMed] [Google Scholar]
- 42.Yan B, Ye S, Chen G, et al. Dysfunctional CD4+, CD25+ regulatory T cells in untreated active systemic lupus erythematosus secondary to interferon-α-producing antigen-presenting cells. Arthritis Rheum 2008; 58:801–812. [DOI] [PubMed] [Google Scholar]
- 43.Paasela M, Kolho KL, Vaarala O, et al. Lactose inhibits regulatory T-cell-mediated suppression of effector T-cell interferon-g and IL-17 production. Br J Nutr 2014; 112:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caretto D, Katzman Shoshana SD, Villarino AV, et al. The Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol 2010; 184:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagar M, Jacob-Hirsch J, Vernitsky H, et al. TNF activates a NF-kappa B-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol 2010; 184:3570–3581. [DOI] [PubMed] [Google Scholar]
- 46.Tang C, Li Y, Lin X, et al. Prolactin increases tumor necrosis factor alpha expression in peripheral CD14 monocytes of patients with rheumatoid Arthritis. Cell Immunol 2014; 290:164–168. [DOI] [PubMed] [Google Scholar]
- 47.Sodhi A, Tripathi A. Prolactin and growth hormone induce differential cytokine and chemokine profile in murine peritoneal macrophages in vitro: involvement of p-38 MAP kinase, STAT3 and NF-kappaB. Cytokine 2008; 41:162–173. [DOI] [PubMed] [Google Scholar]
- 48.Chang JH, Kim YJ, Han SH, et al. IFN-gamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur J Immunol 2009; 39:1241–1251. [DOI] [PubMed] [Google Scholar]
- 49.Petrelli A, Wehrens EJ, Scholman RC, et al. Self-sustained resistance to suppression of CD8+ teff cells at the site of atoimmune inflammation can be reversed by tumor necrosis factor and interferon-γ blockade. Arthritis Rheumatol 2016; 68:229–236. [DOI] [PubMed] [Google Scholar]
- 50.Afzali B, Mitchell PJ, Edozie FC, et al. CD161 expression characterizes a subpopulation of human regulatory T cells that produces IL-17 in a STAT3-dependent manner. Eur J Immunol 2013; 43:2043–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pesenacker AM, Bending D, Ursu S, et al. CD161 defines the subset of FoxP3+ T cells capable of producing proinflammatory cytokines. Blood 2013; 121:2647–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McClymont SA, Putnam AL, Lee MR, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol 2011; 186:3918–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


