Abstract
Objective
FMRI activation of the mesial temporal lobe (MTL) may be important for epilepsy surgical planning. We examined MTL activation and lateralization during language fMRI in children and adults with focal epilepsy.
Methods
142 controls and patients with left hemisphere focal epilepsy (Pediatric: epilepsy, n = 17, mean age = 9.9 ± 2.0; controls, n = 48; mean age = 9.1 ± 2.6; Adult: epilepsy, n = 20, mean age = 26.7 ± 5.8; controls, n = 57, mean age = 26.2 ± 7.5) underwent 3T fMRI using a language task (auditory description decision task). Image processing and analyses were conducted in SPM8; ROIs included MTL, Broca’s area, and Wernicke’s area. We assessed group and individual MTL activation, and examined degree of lateralization.
Results
Patients and controls (pediatric and adult) demonstrated group and individual MTL activation during language fMRI. MTL activation was left lateralized for adults but less so in children (p’s < 0.005). Patients did not differ from controls in either age group. Stronger left-lateralized MTL activation was related to older age (p = 0.02). Language lateralization (Broca’s and Wernicke’s) predicted 19% of the variance in MTL lateralization for adults (p = 0.001), but not children.
Significance
Language fMRI may be used to elicit group and individual MTL activation. The developmental difference in MTL lateralization and its association with language lateralization suggests a developmental shift in lateralization of MTL function, with increased left lateralization across the age span. This shift may help explain why children have better memory outcomes following resection compared to adults.
Keywords: Functional neuroimaging, Seizures, Neuropsychological Assessment
Presurgical language and memory assessment help to minimize adverse effects of epilepsy surgery. Neuropsychological assessment and the intracarotid amobarbital procedure are used for this purpose. In children, however, the former is not highly predictive of post-operative outcome1; the latter is invasive and difficult to perform. Functional magnetic resonance imaging (fMRI) provides reliable, noninvasive language mapping for adults and children2; however, using fMRI to directly probe memory function in epilepsy is a nascent technique, with published studies only for adults3–6.
Memory fMRI can be methodologically challenging; therefore, we explored an indirect method of eliciting hippocampal/parahippocampal or mesial temporal lobe (MTL) activation in epilepsy. We used an established pre-operative fMRI task conventionally used for language mapping to glean information regarding MTL functioning7,8. Our rationale for this approach is based on previous data showing that language fMRI predicts post-operative memory outcome in adults with temporal lobe epilepsy (TLE)8,9 and that the MTL supports language functioning10–17. Numerous studies have demonstrated the MTL’s role in language processing, including evidence that confrontation naming depends on hippocampal integrity, and left hippocampal volume and degree of perirhinal cortex asymmetry in TLE predict language and memory performance10. Hippocampal pathology also affects language lateralization13,14,16,17, intrahemispheric language reorganization15, and pre- and post-operative language functioning12.
We address gaps in understanding the MTL’s role in language by determining if 1) language fMRI reliably elicits MTL activation on group and individual bases, 2) MTL lateralization differs between children and adults with and without epilepsy, and 3) MTL lateralization parallels anterior (Broca’s) and posterior (Wernicke’s) language lateralization. We hypothesized that language fMRI would elicit MTL activation on group and individual bases due to evidence that the MTL supports language10–17. Second, as memory encoding in typically developing (TD) adults produces material-specific lateralization (verbal-left MTL, visual-right MTL)4,18,19, we predicted left-lateralized MTL activation during language fMRI in TD adults. Adults with TLE demonstrate greater MTL activation contralateral to seizure foci4,6; in left TLE verbal encoding yields right activation and in right TLE nonverbal encoding yields left activation4. Therefore, we expected reduced left lateralization in left focal epilepsy patients compared to controls. Although there is ongoing strengthening of the lateralization of fronto-temporal language regions across development20,21, the language network itself is fundamentally established by age four20. Children also have demonstrated left parahippocampal activation during language fMRI22, thus we expected similar results for adults and children. Finally, TD adults show material-specific memory lateralization related to language lateralization during memory fMRI23; thus, we predicted MTL lateralization would correlate with Broca’s and Wernicke’s lateralization during language fMRI for adults and children. Overall, children would show a similar pattern to adults with less pronounced lateralization.
METHODS
Participants
This cross-sectional, retrospective review of data from a prospectively acquired study included adult and pediatric participants:
Adult
Seventy-seven native English speaking, right-handed adults participated; all evaluated between 2003–2011 at a tertiary referral epilepsy center. Twenty patients had left-hemisphere focal epilepsy and normal MRI (mean age = 26.7 years, range = 17.0–40.8, SD = 5.8 years; 12 female); 57 were TD controls (mean age = 26.2 years, range = 20.1=56.3, SD = 7.5 years; 29 female).
Pediatric
Sixty-five native English-speaking, right-handed children aged 6–13 participated. Seventeen had left-hemisphere focal epilepsy (mean age = 9.9 years, range = 6.8–12.8 SD = 2.0 years); 48 were TD controls (mean age = 9.1 years, range = 4.2–13.0, SD = 2.6 years). See Supplemental Materials for more information about pediatric and adult participants.
For all patients, clinical features, neurologic examination, ictal video-EEG, and high-resolution epilepsy protocol MRI were used to localize seizure foci. In both groups (adult and pediatric), we included only patients with left hemisphere foci (based on clinical evaluation and ictal video-EEG) who had normal MRI to limit confounding factors and volume averaging effects in patients with sclerotic MTL (see Table 1 for seizure characteristics). The patients were on various AEDs. Detailed AED information was available for 12 of the adult patients, with 11 out of the 12 adult patients on more than one medication (Table 1 for AEDs). AED information was available for all 17 pediatric patients, with 1 patient not taking any AEDs and 6 on more than one medication. Controls had IQ > 70 and no history of developmental, learning, neurological, or psychiatric disorders.
Table 1.
Seizure Characteristics and FMRI Task Performance.
| Epilepsy Characteristics |
AGE GROUPS | ||
|---|---|---|---|
| ADULT | PEDIATRIC | ||
| Localization (left focus) | 15 temporal, 3 frontal, and 2 fronto-temporal (1 temporal and parasagittal) | 4 temporal, 3 frontal, 1 fronto-temporal, 2 parietal, 2 temporo-parietal, 1 occipital, and 4 lobe undetermined | |
| Seizure Duration (mean) | 11.1 years (±7.1; range=3–33 years) | 3.4 years (±2.8; range=0.4–10.4 years) | |
| Age of Onset | 15.0 years of age (±5.1; range=5–24 years) | 6.5 years (±2.2; range=1.0–9.0 years) | |
| AEDs* | Carbamazepine: n=2, clonazepam: n=1, gabapentin: n=2, lamotrigine: n=5, levetiracitam: n=6; lorazepam: n=1, oxcarbazepine: n=3, phenytoin: n=2, pregabalin: n=1, topiramate: n=3, valproate: n=1, zonisamide: n=2 |
Carbamazepine: n=2, clonazepam: n=1, diazepam: n=2, lamotrigine: n=4, levetiracitam: n=3; lorazepam: n=1, oxcarbazepine: n=7, phenobarbital: n=1, topiramate: n=1, valproate: n=2, zonisamide: n=1 |
|
| PEDIATRIC | |||
| fMRI Task Performance | Patients | Controls | |
| Overall Accuracy (Percent)* | 61% (±28) (n=17) | 76% (±23) (n=48) | |
Seizure characteristics for pediatric and adult groups. Mean and standard deviations of FMRI Task Performance for the pediatric group only.
Overall accuracy was different between groups (p= 0.04), but was not related to MTL lateralization [overall or for either group (patients, controls) separately].
The study was approved by the Combined Neurosciences Institutional Review Board of National Institutes of Health and the Institutional Review Board of Children’s National Medical Center. Adult participants and parents of pediatric participants provided informed consent; all minors provided assent.
MRI
Pediatric patients and TD controls were scanned on a 3.0 Tesla Siemens Magnetom Trio scanner (Erlangen, Germany), and adult patients and TD controls were scanned on a 3.0 Tesla General Electric scanner (General Electric Medical Systems, Milwaukee, WI) using echoplanar imaging (EPI) blood oxygen level dependent (BOLD) techniques. All participants underwent EPI BOLD fMRI during an age-adjusted language task (auditory description decision task) known to activate inferior frontal gyrus (Broca’s area). Details of acquisition methods and experimental paradigm were previously described2,20, and acquisition methods are reviewed in Supplemental Materials.
We used a block design composed of five epoch cycles; each cycle consisted of an experimental condition that alternated with a control condition and each hemicycle lasted 30 seconds. Total scan time was five minutes. Individual stimuli were presented every three seconds (pediatric) or two second (adults) for a total of 10 per block (pediatric) and 15 per block (adult). Our previous analyses show no differences between scanners using these paradigms and acquisition parameters24,25. Tasks were presented via E-Prime software version 1.1 (Psychology Software Tools, Inc., Pittsburgh, PA). Auditory stimuli were digitized and presented via pneumatic earphones. Responses were performed via fiber-optic push button (MRA, Inc., Washington, PA) response recorded by PC in E-Prime.
Experimental Paradigm
Auditory description decision task
For the experimental condition, the participant hears an auditory clue (5–6 word sentences) that describes and names an object (e.g., “A long yellow fruit is a banana”) and pushes a button when the description accurately describes the object. Seventy percent of items are correct targets and 30% are foils. The task is designed to provide in-scanner monitoring of performance by requiring a semantic decision identified by button press response. Task performance was evaluated by the overall accuracy for the task (true positives divided by the sum of the number of correct answers possible and the number of commissions, multiplied by 100); these data were only available for the pediatric groups (Table 1). The control condition consists of reverse speech with tone identification where the participant pushes a button when the tone follows reverse speech (70% have tones, 30% are foils). The task was adjusted by age-level with different versions of the tasks developed based on word frequency normative data derived from children’s reading materials26.
Image processing and Regions of Interest
Preprocessing and group analyses were performed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK) using MATLAB (Version 8.1 Mathworks, Inc., Sherborn, MA). Regions of interest (ROIs) for bilateral MTL included hippocampi and parahippocampal gyri4,18, based on the Anatomical Atlas Library in the Wake Forest PickAtlas (Wake Forest University School of Medicine, Winston-Salem, NC). To categorize each subject’s language patterns traditional language regions were used as ROIs: Broca’s area (inferior frontal gyrus) and Wernicke’s area (Brodmann’s areas: 21, 22, 39, dilated) using the Wake Forest PickAtlas27. All images were temporally filtered (high-pass filter: 128s).
The MTL is a small region subject to artifact, signal loss, and field distortion28. To improve detection of MTL activation we reduced smoothing to 5mm full width at half maximum Gaussian kernel after functional images were spatially normalized to MNI standard anatomical space; we used the same amount of smoothing for bilateral MTL ROIs to ensure accurate matching with underlying voxels. Data for language analysis (Broca’s and Wernicke’s) were smoothed using the typical 8 mm kernel. We were conservative in ROI application; rather than applying ROIs at design level, which improves ROI activation by restricting first-level statistical analysis to that area (but interferes with smoothness estimation), we applied ROIs (MTL, Broca’s, or Wernicke’s) to whole brain activation t-maps calculated without restrictions. As we planned to compare MTL and language (Broca’s, Wernicke’s) lateralization, this allowed for application of ROIs at the same analysis point.
As the small size and location of the MTL increase motion vulnerability we employed an individualized approach to explaining motion-related variance (motion fingerprint)29. This motion fingerprint (6 total traces: 3 traces plus their shifted versions) was entered into first-level statistical analyses as covariates of no interest. Participants were excluded if movement exceeded 3 mm (~1 voxel size) total displacement (TotD; common criterion). To be rigorous, we examined results with and without participants demonstrating TotD< 3, but scan-to-scan (STS) displacement ≥ 1 mm. See Supplemental Materials for more specifics about TotD and STS.
Determination of Language Lateralization and MTL Lateralization
Lateralization indices (LIs) were calculated for each participant’s MTL and language areas (Broca’s, Wernicke’s). We categorized lateralization as left, right, or bilateral based on a commonly-used value of 0.2030. Using the LI Toolbox bootstrap method (implemented in SPM8)31 ROIs were individually categorized as left lateralized if LI ≥ 0.20, bilateral if LI < |0.20|, or right if LI ≤ -0.20. Only individuals with activation clusters of 5 voxels or greater (k ≥ 5) were used in MTL lateralization analyses.
Data analyses
Overall, we examined MTL activation during a language task and then investigated if MTL lateralization differed by clinical (epilepsy vs. controls) or age group (adult vs. pediatric). When appropriate, age was examined as a linear variable. We explored relationships between MTL and language lateralization categorically (left, bilateral, right) and linearly (using individual LIs). Categorical analyses allow for characterization of lateralization based on common, clinically useful thresholds; linear analyses may be differentially sensitive due to increased variance providing better understanding of variable relationships.
We first established group level MTL activation, then individual level activation. As individual level activation is less robust, particularly for the MTL, we examined activation rates within bilateral MTL ROIs at various thresholds (0.05, FDR; 0.001, 0.01, 0.05, uncorrected). We used ANOVA to determine if average MTL LI values differed by those thresholds. Categorical agreement (left, right, bilateral) between MTL and language (Broca’s, Wernicke’s) lateralization for each age group was tested using Cohen’s kappa coefficient. Linear MTL lateralization was assessed using two ANCOVA models with clinical (epilepsy vs. controls) and age group (adult vs. pediatric) as between-group factors. One ANCOVA had Broca’s LI as the covariate and the other Wernicke’s LI, to separately examine impacts of each language area LI on MTL LI. We examined age linearly with Pearson’s correlation to investigate the relationship between age and MTL LI, and linear regression models to predict MTL LI from Wernicke’s and Broca’s LI for each age group.
RESULTS
MTL Activation on a Group Level
Adult
The combined activation map for controls and patients demonstrated MTL activation (p = 0.05, FWE; k ≥ 5 voxels). Controls showed MTL activation (p = 0.05, FWE; k ≥ 5 voxels), but patients only at more liberal thresholds. No activation clusters were evident when comparing patients and controls (p = 0.05, FWE).
Pediatric
Controls and patients displayed MTL activation (p = 0.05, FWE; k ≥ 5 voxels), with no activation differences between groups (p = 0.05, FWE). Controls demonstrated MTL activation (p = 0.05, FWE; k ≥ 5 voxels), but patients only showed MTL activation at more liberal thresholds.
MTL Activation on an Individual Level
The majority of adult and pediatric controls and patients demonstrated individual MTL activation (k ≥ 5) at the most lenient threshold (0.05, uncorrected); however, rates decreased at more stringent thresholds (Table 2). Despite different proportions of individual MTL activation by threshold, MTL LIs were not significantly affected by activation thresholds for either age or clinical group (Table 2). To account for possible movement effects, we examined results with more stringent exclusion criteria (excluding scan-to-scan displacement ≥ 1 mm); results were stable (Table 2). As a result, for the remaining analyses participants were only excluded if movement exceeded 3 mm (~1 voxel size) total displacement.
Table 2.
MTL Activation and Lateralization.
| AGE GROUPS | MTL: Thresholds (k ≥ 5) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.05, FDR | 0.001* | 0.01* | 0.05* | |||||
| ADULT: | Activation | Mean LI | Activation | Mean LI | Activation | Mean LI | Activation | Mean LI |
| Control (n=57) | 46% (26) | 0.49 (±0.29) | 44% (25) | 0.49 (±0.30) | 68% (39) | 0.41 (±0.35) | 86% (49) | 0.42 (±0.39) |
| Control (STS<1) (n=54) | 46% (25) | 0.49 (±0.30) | 44% (24) | 0.49 (±0.30) | 69% (37) | 0.42 (±0.35) | 85% (46) | 0.42 (±0.39) |
| Patients (n=20) | 55% (11) | 0.44 (±0.25) | 35% (7) | 0.39 (±030) | 75% (15) | 0.42 (±0.43) | 90% (18) | 0.35 (±0.50) |
| Patients (STS<1) (n=17) | 59% (10) | 0.51 (±0.14) | 35% (6) | 0.49 (±0.16) | 82% (14) | 0.46 (±0.41) | 94% (16) | 0.40 (±0.51) |
| PEDIATRIC: | ||||||||
| Controls (n=48) | 25% (12) | 0.22 (±0.40) | 31% (15) | 0.27 (±0.37) | 60% (29) | 0.27 (±0.33) | 94% (45) | 0.18 (±0.35) |
| Control (STS<1) (n=28) | 25% (7) | 0.28 (±0.42) | 25% (7) | 0.28 (±0.42) | 57% (16) | 0.29 (±0.33) | 93% (26) | 0.18 (±0.37) |
| Patients (n=17) | 24% (4) | 0.21 (±0.06) | 18% (3) | 0.20 (±0.08) | 77% (13) | 0.16 (±0.42) | 100% (17) | 0.09 (±0.42) |
| Patients (STS<1) (n=9) | 33% (3) | 0.19 (±0.05) | 22% (2) | 0.17 (±0.05) | 67% (6) | 0.26 (±0.22) | 100% (9) | 0.11 (±0.35) |
Thresholds are uncorrected
Percentages of participants demonstrating MTL activation at different thresholds and mean LIs for each activation threshold for adult and pediatric groups.
MTL, mesial temporal lobe; STS, scan-to-scan displacement; k, cluster size; LI, lateralization index.
MTL Lateralization
As MTL LIs were similar regardless of activation thresholds, we included all individuals demonstrating MTL activation (k ≥ 5) at the most lenient threshold (0.05, uncorrected) for the following LI analyses.
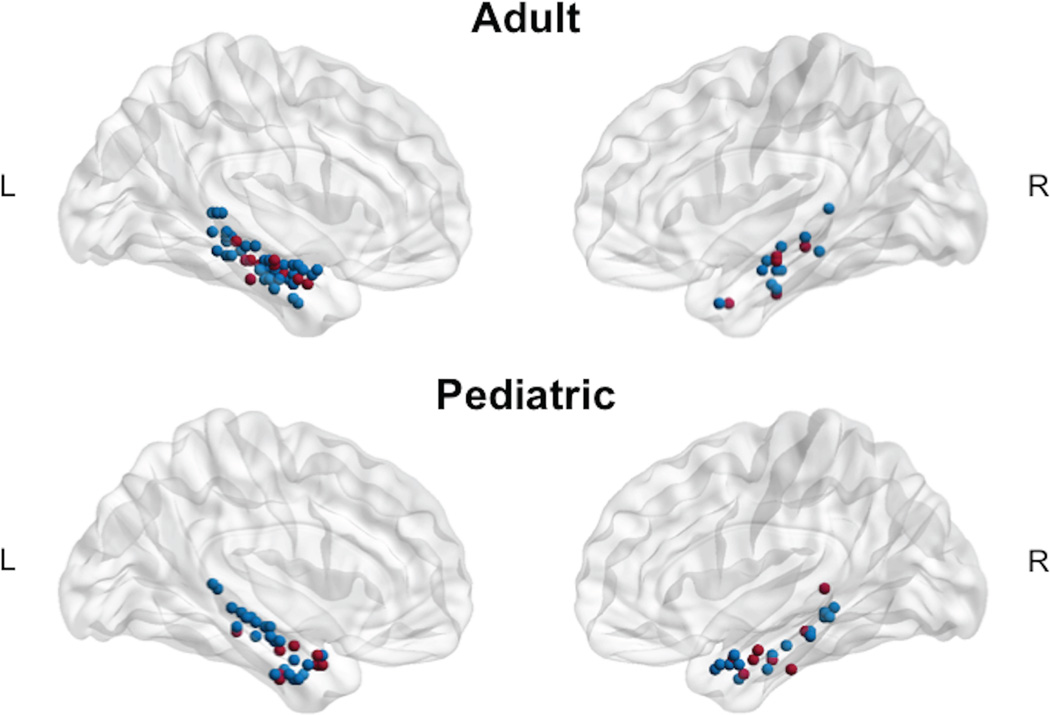
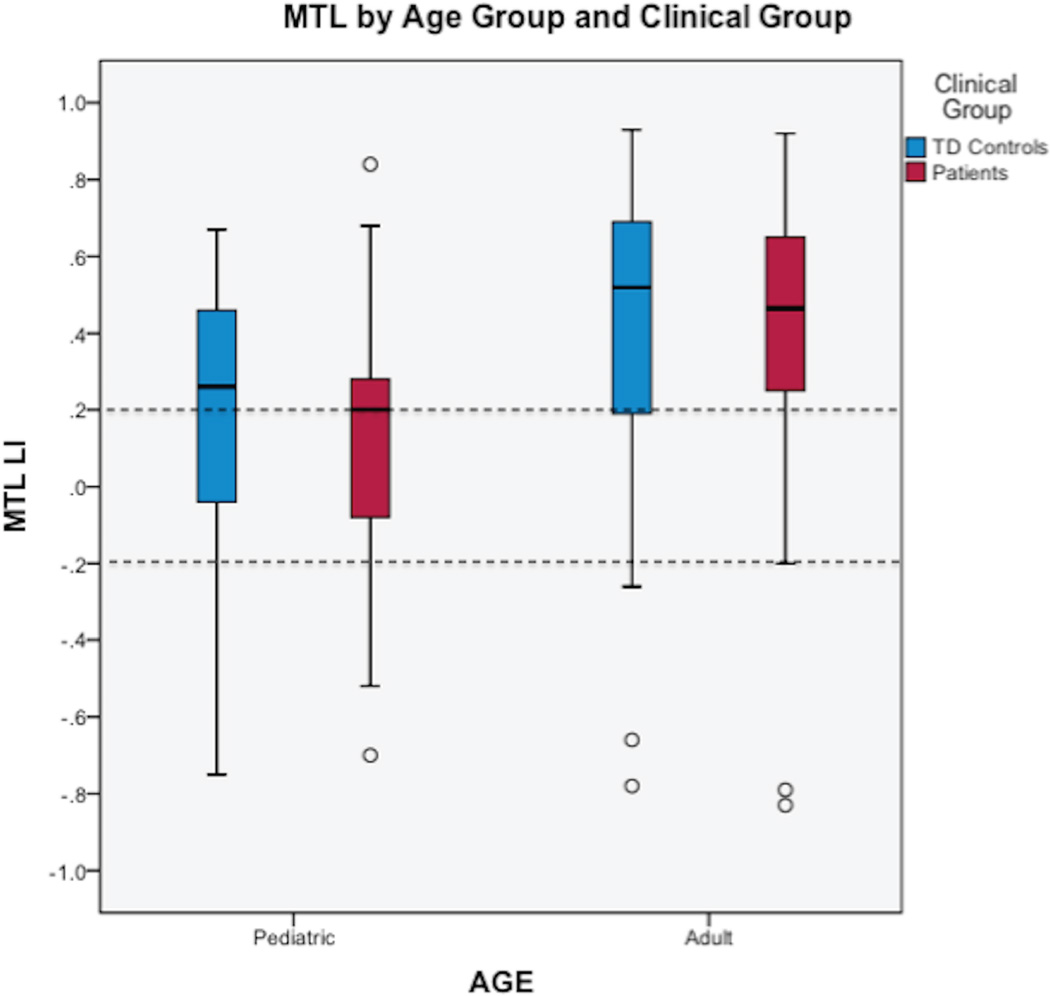
We found a main effect of age group for both ANCOVAs (covariate Wernicke’s LI: F (1,124) = 8.67, p < 0.001; covariate Broca’s LI: F (1,124) = 6.80, p = 0.004) demonstrating more left-lateralized LI in adults than children (Figure 1). No significant differences were found between controls and patients with epilepsy and there was no clinical by age group interaction. For adults, MTL LI was left lateralized overall (control mean MTL LI = 0.42, SD = 0.39; epilepsy mean MTL LI = 0.35, SD = 0.50; Figure 2). Categorically, 25% (17 of 67) of adults had right or bilateral MTL lateralization. For children, MTL LI was bilateral overall (control mean MTL LI = 0.18, SD=0.35; epilepsy mean MTL LI = 0.09, SD = 0.42; Figure 2). Categorically, 45% (28 of 62) had right or bilateral MTL lateralization. Each individual’s peak voxel within the bilateral MTL ROIs illustrates that adults have more left-lateralized activation than children (Figure 1). Greater MTL LI was positively correlated with age for the pediatric and adult samples combined (r = 0.21, p = 0.02).
Figure 1. Peak MTL Activation.
The peak voxel in the MTL is depicted for each individual in each age group (adult and pediatric) if cluster size was 5 voxels or greater (k ≥ 5). Activation peaks depicted in blue represent TD Controls and red represent Epilepsy. MTL, mesial temporal lobe.
Figure 2. MTL Lateralization Index.
Graph of MTL lateralization in the pediatric and adult samples by clinical group. Dotted lines signify common a priori cut-offs of 0.20 and −0.20 that correspond to lateralization categories (left if LI ≥ 0.20, bilateral if LI < |0.20|, or right if LI ≤ −0.20). MTL, mesial temporal lobe; LI, lateralization index.
MTL Lateralization related to Language Lateralization: Categorical Analysis
As expected, language lateralization was primarily left lateralized for adults and children in both temporal (Wernicke’s) and frontal (Broca’s) areas; however, the relationship with MTL lateralization varied by age group.
Adult
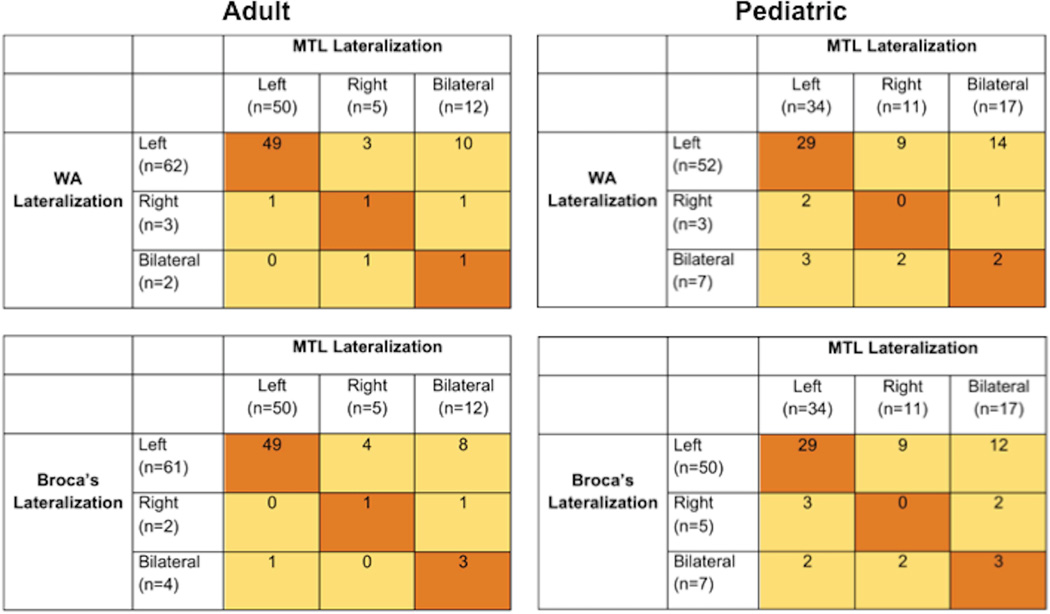
Patients and controls with left-lateralized temporal (Wernicke’s) language showed mostly left-lateralized MTL activation; those with right/bilateral Wernicke’s lateralization (n = 5) had variable MTL activation patterns (Figure 3). Similarly, those with left-lateralized frontal (Broca’s) language showed left lateralized MTL activation, while those with right/bilateral Broca’s lateralization (n=6) had variable MTL activation (Figure 3). We found agreement between categorical lateralization of Wernicke’s and MTL ROIs (p = 0.005; Cohen’s kappa = 0.21), and between Broca’s and MTL ROIs (p < 0.001, Cohen’s kappa = 0.32).
Figure 3. MTL LI related to Wernicke’s (WA) and Broca’s lateralization for pediatric and adult samples.
Areas colored in orange represent consistent lateralization between WA or Broca’s and MTL; areas in yellow are inconsistent. As seen in these tables, language LI (WA and Broca’s) and MTL lateralization was more consistent for adults than children.
Pediatric
Patients and controls showed variable MTL lateralization patterns across categories of Wernicke’s and Broca’s language lateralization; we did not find significant agreement between categorical lateralization of Wernicke’s and MTL (Figure 3) nor Broca’s and MTL using Cohen’s kappa.
MTL Lateralization related to Language Lateralization: Linear Analysis
Overall, Wernicke’s and Broca’s LI were both related to MTL LI across age and clinical groups [covariate Wernicke’s LI: F (1,124) = 8.72, p = 0.004; covariate Broca’s LI: F (1,124) = 12.92, p < 0.001]; however, the relationship between language LI and MTL LI varied by age group.
Adult
The linear regression model including Wernicke’s LI and Broca’s LI accounted for 18.7% of the variance in MTL LI, F (2,64) = 7.34, p = 0.001 (Table 3).
Table 3.
Regression Analyses Predicting MTL LI.
|
ADULT: MTL LI |
||||||
| Predictor | b | SE B | β | t | ΔF | ΔR2 |
| 7.34*** | 0.19 | |||||
| Wernicke’s LI | 0.34 | 0.20 | 0.27 | 1.74 | ||
| Broca’s LI | 0.25 | 0.20 | 0.20 | 1.28 | ||
|
PEDIATRIC: MTL LI |
||||||
| Predictor | b | SE B | β | t | ΔF | ΔR2 |
| 1.47 | 0.05 | |||||
| Wernicke’s LI | −0.09 | 0.17 | −0.08 | −0.52 | ||
| Broca’s LI | 0.26 | 0.16 | 0.25 | 1.65 | ||
p ≤ .05,
p ≤ .01,
p ≤ .001
Results of the linear regression models predicting MTL LI from language LI (Wernicke’s and Broca’s) for adults and children. Both models presented passed tests for multicollinearity.
Pediatric
The linear regression model including Wernicke’s LI and Broca’s LI was not significant (Table 3).
DISCUSSION
We found group and individual MTL activation during language fMRI in adults and children with epilepsy, as well as controls. MTL activation was more left lateralized with increasing age. Our results suggest a developmental shift in the relationship between MTL and language lateralization. Adults showed relatively homogeneous left-lateralized MTL activation that was related to language lateralization, consonant with the concept of material specificity of memory function in adulthood. Our study is the first to provide neuroimaging evidence showing that children have a more variable distribution of MTL lateralization, which is unrelated to language LI. Our results offer important insight into MTL lateralization across development. Overall, MTL activation becomes more left lateralized with age, eventually mirroring language lateralization.
Our results reinforce other empirical evidence of the MTL’s role in language processing. Specifically, studies suggest MTL functioning affects language ability10,11 and hippocampal pathology affects language lateralization13,14,17, intrahemispheric reorganization of language15, and pre- and post-operative language functioning12. Further, approximately 25% of patients with mesial temporal sclerosis have atypical language dominance14.
We found that language lateralization predicted MTL lateralization for adults, which parallels another fMRI study where patients with left-lateralized language showed increased left MTL lateralization for a verbal memory task and increased right MTL lateralization for visual memory, while the opposite occurred for patients with right-dominant language23. In contrast, for children in our study, neither posterior nor anterior language lateralization was predictive of MTL lateralization.
Our results suggest less lateralization of MTL function in children than adults. The majority of neuropsychological studies suggest children with epilepsy do not show pre-surgical lateralizing memory impairments33,34. One TLE study demonstrated adults and adolescents, but not children, showed laterality differences in memory performance35. The decreased MTL lateralization we found in children may explain the lack of material-specific memory deficits in TLE children compared to adults. We propose that children initially engage both MTLs, with specialization for specific types of material (verbal-left MTL, visual-right MTL) occurring later in development. This follows research demonstrating increased left lateralization for language20 and right lateralization for visuospatial functions with age36.
In typical development, structural and functional changes occur along the hippocampal longitudinal axis37,38. Functionally, episodic recall is associated with posterior hippocampal activity in children, and anteriorly in adults38. Our results suggest this hippocampal/parahippocampal activation may also be lateralized differently across development. This lateralization difference may result from neuroanatomical changes throughout development, different processing strategies (e.g., decreased visual imagery) across the age span, or a combination of both factors. Future studies are necessary to evaluate these hypotheses.
Our results have implications for improving assessment of surgical risk. Selective anterior temporal resection is the most effective treatment for intractable TLE39, but given the MTL’s critical role in language and memory, resection can have negative cognitive effects. Dominant temporal resections may lead to post-operative naming and verbal memory decline in adults40. Post-operative memory outcomes may be predicted by activation in the hippocampus to be resected (hippocampal adequacy) or activation in the non-resected hippocampus (hippocampal reserve)5. TLE adults with greater ipsilateral than contralateral MTL activation on pre-operative fMRI memory demonstrate greater memory decline following temporal resection, supporting hippocampal adequacy3,5. Language fMRI also helps predict post-operative memory outcome in TLE adults8,9, with outcomes correlating better with fMRI language lateralization than hippocampal lateralization during scene-encoding9. Our results may explain the utility of language fMRI to predict post-operative verbal memory outcome, suggesting it represents MTL activation during language tasks.
Children with epilepsy do not show material-specific memory impairments pre-33,34 or post-surgery1, suggesting children, unlike adults, are less reliant on the dominant MTL for memory functioning. Although we used an indirect measure (language fMRI), our results support this hypothesis; children with epilepsy may have less post-operative memory deficit risk due to contralateral MTL activation. These findings posit a developmental shift in lateralization of MTL function, and thus a switch in prediction of post-operative outcome from hippocampal reserve to hippocampal adequacy across development.
We found that adults with left focal epilepsy demonstrated left-lateralized MTL activation similar to TD adults, which is discrepant from previous memory fMRI studies showing greater activation in the MTL contralateral to seizure foci in TLE4,6. One language fMRI study (using a single-word category decision task) also found that individuals with right TLE displayed stronger contralateral hippocampal, parahippocampal, and collateral sulcus activation compared to left TLE7. Discrepant findings may be due to use of fMRI tasks which burden language/memory networks differently; for example, we did not use an explicit memory task and our language task (auditory description decision) varies from the single-word category decision task previously described7. Alternatively, our sample of left focal epilepsy was not limited to TLE; 81% of adult patients and 24% of pediatric patients had TLE. Adult patients in our sample had TLE without evidence of hippocampal atrophy, which could also account for the discrepancies from previous memory fMRI studies. However, different TLE distributions in our sample are not a likely factor as developmental MTL lateralization differences were also evident in TD adult and pediatric samples.
Limitations
The MTL is small and subject to artifact; therefore, accurate activation estimation is difficult. Decreased activation may be due to reduced MTL function or acquisition difficulty. The number of activated voxels in the MTL may be low, making it difficult to accurately assess lateralization. However, our methods—adjusting smoothing level for MTL activation data and ROIs, and stringent movement criteria—improved activation assessment. New acquisition methods, such as fieldmaps, fast image acquisition, thin slices, and reversing phase encoding direction may reduce MTL distortion and signal loss28. Further, due to our hypothesis regarding MTL activation during language fMRI, we employed an ROI approach as opposed to an exploratory whole-brain search. As memory and language functioning involves a distributed network of brain regions, this limits our ability to comment on other brain regions that might be involved in these cognitive processes.
All children were scanned on a 3.0 Tesla Siemens Magnetom and adults on a 3.0 Tesla GE scanner using slightly different parameters. Previous analyses showed no differences between scanners using these paradigms and acquisition parameters24,25. The Siemens scanner has potential for better spatial resolution, while the GE may allow for greater MTL activation given better temporal resolution. However, neither variable should affect MTL LI. Task accuracy was only available for pediatric participants and not adults. While it is theoretically possible that task accuracy differences between the age groups may explain some of the developmental MTL lateralization differences, we have previously shown that accuracy on this auditory description decision task does not correlate with lateralization in children20. We do not have a reason to suspect this would be different for adults. Further, the two age groups (adult vs. pediatric) have different seizure foci, with more TLE in the adult compared to the pediatric group. The difference in seizure foci may explain some of the discrepancy in results between age groups; however, given that the majority of adults had left TLE and we expect greater MTL activation contralateral to seizure foci4,6, our results showing greater left-lateralized MTL activation in the left focus adult group further supports our hypothesis. This study involved patients with left focal epilepsy due to the clinical selection bias; future studies should include patients with right focal epilepsy.
A pragmatic objective of this study was to ascertain if a single fMRI task could yield presurgical MTL activation helpful in understanding both language and memory function. Thus, future research will examine if this MTL lateralization during language fMRI predicts post-operative language and memory ability. Finally, we expect stronger individual MTL activation with explicit memory paradigms, where MTL activation can be measured directly. Thus, our results need to be confirmed by memory fMRI paradigms.
Conclusions
Differences exist between children and adults for the effects of epilepsy and temporal resection on memory networks. Our results provide insight into understanding MTL lateralization across development. Adults demonstrated homogeneous left-lateralized MTL activation during a language task, while children were more heterogeneous in their MTL lateralization patterns. Developmental differences in MTL lateralization and relationships with language lateralization may represent a shift in MTL lateralization across development. Contralateral MTL activation may explain better memory performance following surgery in children compared to adults, supporting hippocampal reserve in pediatric epilepsy. Better understanding of MTL function and development will minimize potential adverse effects of temporal resection by helping to establish the optimal developmental window for surgery.
Supplementary Material
KEY POINTS.
Language fMRI may be used to elicit group and individual MTL activation.
Adults demonstrated homogeneous left-lateralized MTL activation during language fMRI, while children were more heterogeneous.
Our results suggest a developmental shift in lateralization of MTL function, with increased left lateralization across the age span.
Contralateral MTL activation may explain why children have better memory outcomes following resection compared to adults.
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (NIH) [5T32HD046388-08 to L.N.S]; the National Institutes of Neurological Disorders and Stroke, NIH[5K23NS065121-01A2 to M.M.B., R01NS44280 to W.D.G.]; National Center for Research Resources, NIH [M01RR020359]; Epilepsy Foundation of America; Intellectual and Developmental Disabilities Research Center, Children’s National Health SystemGrant [HD040677-07]; Children’s National Health System Clinical and Translational Science Award (CTSA). We thank the patients and controls who participated in the study and acknowledge the valuable contributions of L. Gilbert Vezina, M.D. and Nadia Biassou, M.D.
Dr. Sepeta reports grants from Epilepsy Foundation of America and NICHD, Dr. Berl reports grants from NINDS, and Dr. Wilke reports grants from H.W. & J. Hector Foundation, Weinheim, Germany (M66), during the conduct of the study, Dr. Gaillard reports receiving grant support from NIH, NSF, PCORI, American Epilepsy Society, Epilepsy Foundation, CURE, and Infantile Epilepsy Research Foundation (funded by Lundbeck), sits on the editorial board of Epilepsia, and holds stock with spouse from Pfizer (>$10,000), Siemens (>$10,000), General Electric (>$10,000), and receives funds related to patient care of patients with epilepsy.
Footnotes
We confirm that we have read Epilepsia’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflicts of Interest
The remaining authors have no conflicts of interest to report.
REFERENCES
- 1.Lah S. Neuropsychological outcome following focal cortical removal for intractable epilepsy in children. Epilepsy Behav. 2004;5:804–817. doi: 10.1016/j.yebeh.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Berl MM, Zimmaro LA, Khan OI, et al. Characterization of atypical language activation patterns in focal epilepsy. Ann Neurol. 2014;75:33–42. doi: 10.1002/ana.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonelli SB, Powell RH, Yogarajah M, et al. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain. 2010;133:1186–1199. doi: 10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golby AJ, Poldrack RA, Illes J, et al. Memory lateralization in medial temporal lobe epilepsy assessed by functional MRI. Epilepsia. 2002;43:855–863. doi: 10.1046/j.1528-1157.2002.20501.x. [DOI] [PubMed] [Google Scholar]
- 5.Rabin ML. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–2298. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- 6.Richardson MP, Strange BA, Duncan JS, et al. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003;20:S112–S119. doi: 10.1016/j.neuroimage.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Bellgowan PSF, Binder JR, Swanson SJ, et al. Side of seizure focus predicts left medial temporal lobe activation during verbal encoding. Neurology. 1998;51:479–484. doi: 10.1212/wnl.51.2.479. [DOI] [PubMed] [Google Scholar]
- 8.Binder JR, Sabsevitz DS, Swanson SJ, et al. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–1394. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Binder J, Swanson S, Sabsevitz D, et al. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy_Language lateralization versus hippocampal activation asymmetry. Epilepsia. 2010;51:618–626. doi: 10.1111/j.1528-1167.2009.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alessio A, Bonilha L, Rorden C, et al. Memory and language impairments and their relationships to hippocampal and perirhinal cortex damage in patients with medial temporal lobe epilepsy. Epilepsy Behav. 2006;8:593–600. doi: 10.1016/j.yebeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Bonelli SB, Powell R, Thompson PJ, et al. Hippocampal activation correlates with visual confrontation naming: fMRI findings in controls and patients with temporal lobe epilepsy. Epilepsy Res. 2011;95:246–254. doi: 10.1016/j.eplepsyres.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies KG, Bell BD, Bush AJ, et al. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–419. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 13.Duke ES, Tesfaye M, Berl MM, et al. The effect of seizure focus on regional language processing areas. Epilepsia. 2012;53:1044–1050. doi: 10.1111/j.1528-1167.2012.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard WD, Berl MM, Moore EN, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- 15.Hamberger M, Seidel W, Goodman R, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007;130:2942–2950. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- 16.Liegeois F, Connelly A, Cross H, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- 17.Weber B. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2005;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- 18.Golby AJ, Poldrack RA, Brewer JB, et al. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001;124:1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- 19.Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 20.Berl MM, Mayo J, Parks EN, et al. Regional differences in the developmental trajectory of lateralization of the language network. Hum Brain Mapp. 2014;35:270–284. doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland SK, Plante E, Weber Byars A, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- 22.Chiu C-YP, Schmithorst VJ, Brown RD, et al. Making memories: a cross-sectional investigation of episodic memory encoding in childhood using FMRI. Dev Neuropsychol. 2006;29:321–340. doi: 10.1207/s15326942dn2902_3. [DOI] [PubMed] [Google Scholar]
- 23.Weber B, Fliessbach K, Lange N, et al. Material-specific memory processing is related to language dominance. Neuroimage. 2007;37:611–617. doi: 10.1016/j.neuroimage.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 24.You X, Adjouadi M, Guillen MR, et al. Sub-patterns of language network reorganization in pediatric localization related epilepsy: A multisite study. Hum brain mapp. 2011;32:784–799. doi: 10.1002/hbm.21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You X, Adjouadi M, Wang J, et al. A decisional space for fMRI pattern separation using the principal component analysis—A comparative study of language networks in pediatric epilepsy. Hum brain mapp. 2012;34:2330–2342. doi: 10.1002/hbm.22069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carroll JB, Davies P, Richman B. The American heritage word frequency book. Boston: Houghton Mifflin; 1971. [Google Scholar]
- 27.Mbwana J, Berl MM, Ritzl EK, et al. Limitations to plasticity of language network reorganization in localization related epilepsy. Brain. 2009;132:347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olman CA, Davachi L, Inati S. Distortion and signal loss in medial temporal lobe. PLoS One. 2009;4:e8160. doi: 10.1371/journal.pone.0008160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilke M. An alternative approach towards assessing and accounting for individual motion in fMRI timeseries. Neuroimage. 2012;59:2062–2072. doi: 10.1016/j.neuroimage.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 30.Seghier ML. Laterality index in functional MRI: methodological issues. Magn Reson Imaging. 2008;26:594–601. doi: 10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Berl MM, Balsamo LM, Xu B, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez LM, Anderson VA, Wood SJ, et al. The localization and lateralization of memory deficits in children with temporal lobe epilepsy. Epilepsia. 2007;48:124–132. doi: 10.1111/j.1528-1167.2006.00907.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith ML, Elliott IM, Lach L. Cognitive skills in children with intractable epilepsy: comparison of surgical and nonsurgical candidates. Epilepsia. 2002;43:631–637. doi: 10.1046/j.1528-1157.2002.26101.x. [DOI] [PubMed] [Google Scholar]
- 35.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–2830. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- 36.Everts R, Lidzba K, Wilke M, et al. Strengthening of laterality of verbal and visuospatial functions during childhood and adolescence. Hum Brain Mapp. 2009;30:473–483. doi: 10.1002/hbm.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gogtay N, Nugent TF, Herman DH, et al. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- 38.Demaster DM, Ghetti S. Developmental differences in hippocampal and cortical contributions to episodic retrieval. Cortex. 2013;49:1482–1493. doi: 10.1016/j.cortex.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Wyllie E, Comair YG, Kotagal P, et al. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740–748. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- 40.Hamberger MJ, Seidel WT, McKhann GM, et al. Hippocampal removal affects visual but not auditory naming. Neurology. 2010;74:1488–1493. doi: 10.1212/WNL.0b013e3181dd40f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.