Abstract
Sumoylation represents a cascade of enzymatic reactions mediated by SUMO-activating enzyme (SAE1/SAE2 heterodimer), SUMO-conjugating enzyme Ubc9, and SUMO E3 ligases that include five protein inhibitors of activated STATs (PIAS1, PIAS3, PIASy, PIASxα and PIASxβ), and culminates in the formation of an isopeptide bond between the C-terminal glycine of a small ubiquitin-related modifier (SUMO) and the lysine residue of a protein substrate. Conjugation of a SUMO moiety, ranging from 92 (for SUMO-2) to 97 (for SUMO-1) amino acids, not only increases the molecular size but also alters the property and function of the modified protein. Although sumoylation has been observed with many cellular proteins and the majority of transcription factors including the p53 tumor suppressor, this covalent modification is normally detectable only in a small population, often less than 5%, of a given substrate in vivo. This low abundance of SUMO-modified proteins, due to the presence of sentrin/SUMO-specific proteases (SENPs) that actively cleave the reversible SUMO linkage, has posed a challenge to define the biological effect of SUMO in living cells. Nevertheless, the recent development of reconstituted modification and chromatin-dependent transcription assays has provided unique insights into the molecular action of SUMO in modifying protein function. The availability of these reconstitution systems has unraveled the interplay between sumoylation and acetylation in regulating the DNA binding and transcriptional activity of p53 tetramers and further allow the identification of transcriptional corepressors, such as mSin3A, CoREST1/LSD1 and Mi-2/NuRD implicated in SUMO-dependent gene silencing events.
Keywords: p53, SUMO, sumoylation, acetylation, chromatin, posttranslational modification, in vitro transcription
ACF-Based Chromatin Transcription System
In vitro reconstitution of chromatin has been facilitated by the identification of a Drosophila ATP-utilizing chromatin assembly and remodeling factor (ACF).1 The native ACF activity was subsequently reconstituted with two recombinant Drosophila proteins: the ATPase-possessing enzyme, imitation switch (ISWI, 140 kDa), and the Acf1 (180 kDa) accessory factor that contains a bromodomain and two PHD fingers likely for binding respectively to acetylated and methylated nucleosomal histones.2 Reconstitution of chromatin templates with periodic nucleosome arrays was then accomplished by incubating plasmid DNA, in the presence of ATP, with purified native core histones, recombinant ISWI/Acf1 heterodimer, and recombinant Drosophila nucleosome assembly protein 1 (dNAP-1), and used for in vitro transcription typically performed with HeLa nuclear extract that provides all the necessary components for transcription reactions.3 This ACF-based chromatin assembly system is more defined and suitable for transcriptional analysis, compared to the crude Drosophila S190 extract that was originally used for chromatin assembly by many laboratories.4–12 Replacement of dNAP-1 with human NAP-1 (hNAP-1) does not compromise ACF activity for chromatin assembly.13 In fact, positioning of nucleosomes assembled by this modified chromatin assembly system (i.e., native HeLa core histones, recombinant hNAP-1, and reconstituted ISWI/Acf1) faithfully recapitulates the positions of nucleosomes observed in vivo, as illustrated by the comparison of promoter-proximal nucleosome positions mapped on an in vitro-reconstituted chromatin template containing the enhancer-promoter region (~1 kb) derived from human papillomavirus type 18 (HPV-18) with that of endogenous HPV-18 genomes naturally residing in HeLa cells.14 Accordingly, in vitro transcription experiments conducted with this ACF-based chromatin assembly system closely mimics the chromatin environment in living cells.
Histone Acetyltransferase (HAT) Activity is Essential for p53-Dependent Chromatin Transcription in vitro
In reconstituted chromatin transcription assays, HAT activity provided by p300 or CBP is absolutely essential for p53-dependent transcription from chromatin templates reconstituted with either Drosophila S190 extract15 or the purified ACF system.13,16 Other HATs, such as PCAF and GCN5, are not able to substitute for p300/CBP, perhaps due to a lack of protein adaptors mediating their interaction with p53.13 Since HeLa nuclear extract contains a low but limiting amount of p300/CBP and acetyl-CoA (the acetyl group donor), the requirement of acetyl-CoA for p300/CBP HAT activity was demonstrated by the addition of desulfo-CoA, which is a dead-end inhibitor of acetyl-CoA and efficiently blocks p53-dependent chromatin transcription and also p300-mediated acetylation of p53 and nucleosomal core histones.13 That transcription from an internal naked DNA control template devoid of an activator-binding site was not inhibited by the inclusion of desulfo-CoA further indicates that desulfo-CoA serves as a good indicator for monitoring transcription signals specifically from chromatin templates, rather than from residual p53-responsive DNA templates not properly assembled into chromatin.13
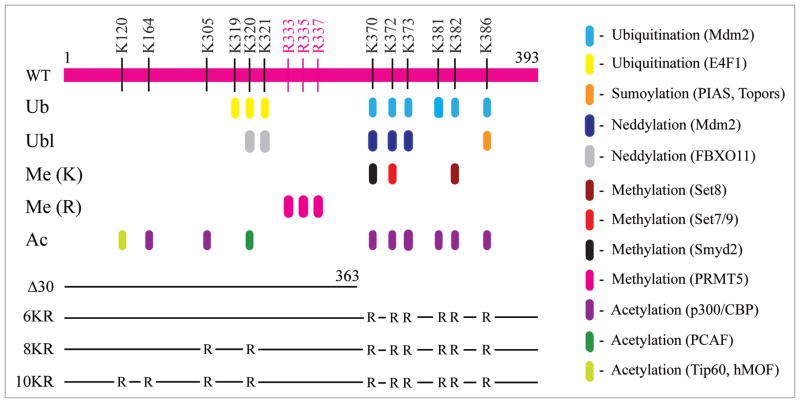
As acetylation of p53 and chromatin was both increased during p53 transactivation, but concurrently decreased in the presence of the HPV-encoded E6 repressor,13 it is important to distinguish which acetylation event (i.e., p53 versus nucleosomal core histones) directly links to p53-regulated gene activation and repression. This distinction is also crucial for our understanding of the functional roles of various types of posttranslational modification occurring on lysine and arginine residues that modulate p53 function in response to stress signals (Fig. 1). With the availability of reconstituted chromatin transcription systems, the relative contribution of p53 acetylation and chromatin acetylation in gene activation was addressed by analyzing the transcriptional activity of four p53 mutants either deleting the regulatory domain in the C-terminal 30 amino acids (Δ30) or containing arginine substitutions in selective lysine residues (see Fig. 1, line drawing). All three lysine mutants (6KR, 8KR and 10KR), which are acetylation-deficient but retain their capability of supporting p300-mediated acetylation of nucleosomal core histones, activate transcription from a completely silenced p53-binding site-containing chromatin template (pWAFMLT) to a level only two-fold less than that seen with the wild-type protein, whereas Δ30 that fails to support p300-mediated acetylation on both p53 and chromatin shows no transcriptional activity.17 This chromatin transcription experiment compellingly demonstrates that acetylation of nucleosomal core histones, but not p53, is directly linked to transcriptional activation, highlighting the importance of epigenetic control in gene regulation, and is consistent with animal experiments revealing a nonessential role of the p53 C-terminal lysine residues during mouse development.18,19 It should be noted that a two-fold difference in transcriptional activity between wild-type and lysine mutants is relatively minor, compared to their ability to trigger a high level of transcription from chromatin with essentially zero activity. Nevertheless, the finding also adds to the ongoing debates regarding the precise role of p53 acetylation.20,21 Since acetylation of p53 does not significantly enhance its DNA/chromatin-binding activity,13,15,22,23 what might be the function of this covalent modification? Earlier studies suggest that acetylation of p53 could help alleviate MDM2-mediated inhibition,23 compete with ubiquitination for modification on the same lysine residues,24 promote cytoplasmic localization of p53,25 or enhance p53-dependent coactivator recruitment and histone acetylation.22,26 Considering that C-terminal lysines of p53 are heavily regulated by different types of posttranslational modification (see Fig. 1), it is likely that acetylation has additional roles yet to be discovered.
Figure 1.
Posttranslation modification of p53 lysine and arginine residues. The specific types of modification occurring at each lysine (K) and arginine (R) residues are indicated by different colors with the enzymes involved included in parenthesis. The position of the amino acid residue in full-length human p53 protein (aa 1–393) is indicated on the top. A line drawing of four p53 mutants, including a C-terminal truncation mutant (Δ30) and three lysine substitution mutants (6KR, 8KR and 10KR), is also shown. Abbreviations used are: Ub, ubiquitin; Ubl, ubiquitin-like; Me (K), methylation on lysine; Me (R), methylation on arginine; Ac, acetylation.
In vitro Reconstitution of p53 Sumoylation
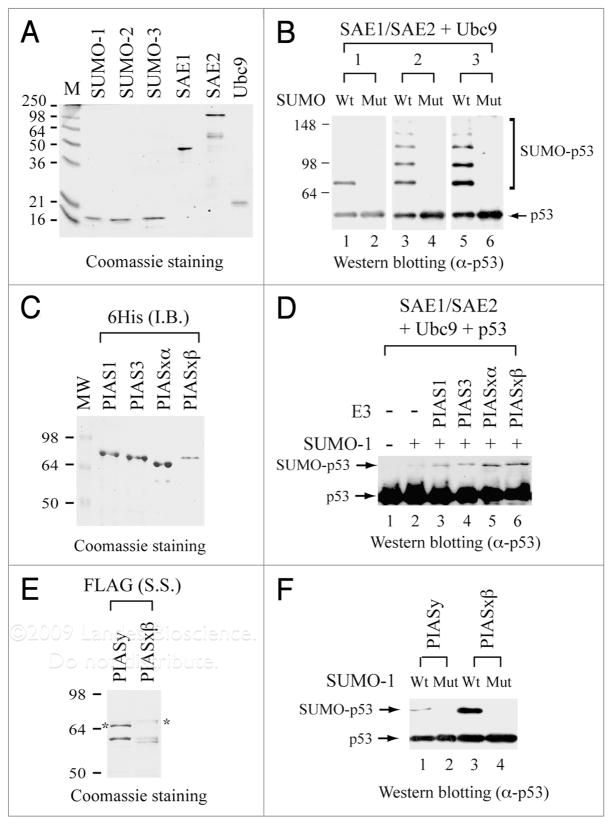
A clue unraveling an additional function of p53 acetylation comes from in vitro-reconstituted sumoylation studies with p53 as the substrate.17 The sumoylation reaction was initially reconstituted with one of the three human SUMO proteins (SUMO-1, SUMO-2 and SUMO-3), in the presence of E1 (SAE1/SAE2 dimer) and E2 (Ubc9) enzymes. All these human proteins were purified respectively from bacteria expressing individual recombinant protein N-terminally tagged with a hexahistidine sequence (Fig. 2A). Mutants of SUMO-1, SUMO-2 and SUMO-3 that change the last amino acid from glycine (G) to alanine (A) in each SUMO protein provide specificity controls for sumoylation reactions. Evidently, FLAG-tagged p53 is sumoylated efficiently by wild-type but not mutant SUMO-1, SUMO-2 and SUMO-3 (Fig. 2B), in which polysumoylated p53 are readily seen with SUMO-2 and SUMO-3 donors. This SUMO E3 ligase-independent reaction occurs when an excess amount of the E2-conjugating enzyme is used for in vitro sumoylation assays. However, SUMO E3 ligases, such as PIAS family proteins, further enhance sumoylation efficiency, substrate selection and subnuclear localization in vivo.27–31 Indeed, four recombinant human PIAS proteins, PIAS1, PIAS3, PIASxα and PIASxβ (Fig. 2C), purified from bacterial inclusion bodies via the N-terminal hexahistidine tag following denaturation-renaturation as described,32 increases the efficiency of p53 sumoylation (Fig. 2D). Recombinant PIASy and PIASxβ proteins (Fig. 2E), purified from respective bacterial sonication supernatant via N-terminal FLAG tagging and peptide elution methods,33 give an even stronger stimulation of p53 sumoylation (Fig. 2F), irrespective of the detection of some degradation products derived from the full-length protein. Accordingly, a large-scale sumoylation reaction, performed with FLAG-tagged SUMO-1 and hexahistidine-tagged p53 and the remaining sumoylation components (SAE1, SAE2, Ubc9, PIASxβ) and followed by a two-step immunoaffinity tag purification scheme, has been successfully employed to purify nearly homogeneous SUMO-1-modified p53 (Su-p53),17 making it possible to define the biochemical and functional effects of sumoylation on p53 binding to DNA/chromatin and regulating its target gene transcription.
Figure 2.
In vitro reconstitution of p53 sumoylation with purified recombinant proteins. (A) Coomassie blue staining of purified human SUMO-1, SUMO-2, SUMO-3, SAE1, SAE2 and Ubc9. All these recombinant proteins are hexahistidine-tagged at their respective N-terminus and purified from bacteria as described.17 Prestained protein size markers (in kDa) are indicated on the left. (B) Detection of sumoylated p53 (SUMO-p53) by immunoblotting. Sumoylation reactions were performed by incubating FLAG-tagged p53 with either wild-type (Wt) or the G-to-A mutant (Mut) of SUMO-1, SUMO-2 and SUMO-3, in the presence of E1 (SAE1/SAE2) and E2 (Ubc9) enzymes as described.17 Antibodies against full-length p53 (Santa Cruz) were used for detection by immunoblotting. (C) Coomassie blue staining of purified human PIAS1, PIAS3, PIASxα and PIASxβ. Each PIAS protein is hexahistidine-tagged at its N-terminus and purified from bacterial inclusion bodies (I.B.) as described.32 (D) PIAS enhances p53 sumoylation. Sumoylation and immunoblotting were performed as described in (B), in the absence (−) or presence (+) of a PIAS protein. (E) Coomassie blue staining of purified human PIASy and PIASxβ. Recombinant PIASy and PIASxβ, each FLAG-tagged at its N-terminus, were purified from bacterial sonication supernatant (S.S.) as described.33 Asterisk indicates the position of the full-length protein. The bands below the asterisk are degradation products from the full-length protein. (F) PIASy and PIASxβ purified from sonication supernatant also enhances p53 sumoylation. Sumoylation and immunoblotting were performed as described in (D) with the inclusion of PIASy or PIASxβ from (E).
Subunit Modification Within a p53 Tetramer
In vitro characterization of purified Su-p53 indicates that sumoylation of a p53 tetramer occurs preferentially on two of the four subunits, even though SUMO conjugation can take place selectively on only one, three or four subunits when SUMO-1 and PIASxβ are present in extremely limiting or excess conditions.17 The unequal accessibility of C-termini within a p53 tetramer indicates that p53 adapts flexible conformations in its regulatory domain (see structural models for p53),34–36 which appears disordered and has non-specific DNA-binding activity.37 In a normal cellular environment, p53 exists predominantly as nuclear tetramers or cytoplasmic dimers, determined in part by its interacting partners and masking/exposure of its nuclear export signal in the oligomeric state.25,38–40 Once formed, p53 tetramers are stable with an ultraslow dissociation rate to dimers (t1/2 = 2.3 hr at 20°C).41 Thus, the DNA/chromatin binding and transcription/enzymatic assays conducted in reconstituted cell-free systems generally reflect the functional properties of a p53 tetramer, consistent with the fact that tetrameric p53 is the main substrate for posttranslational modification by phosphorylation,42 acetylation43,44 and sumoylation.17
Sumoylation Inhibits p53-Dependent Chromatin Transcription and Prevents the Free Form of p53 from Binding to DNA and Chromatin
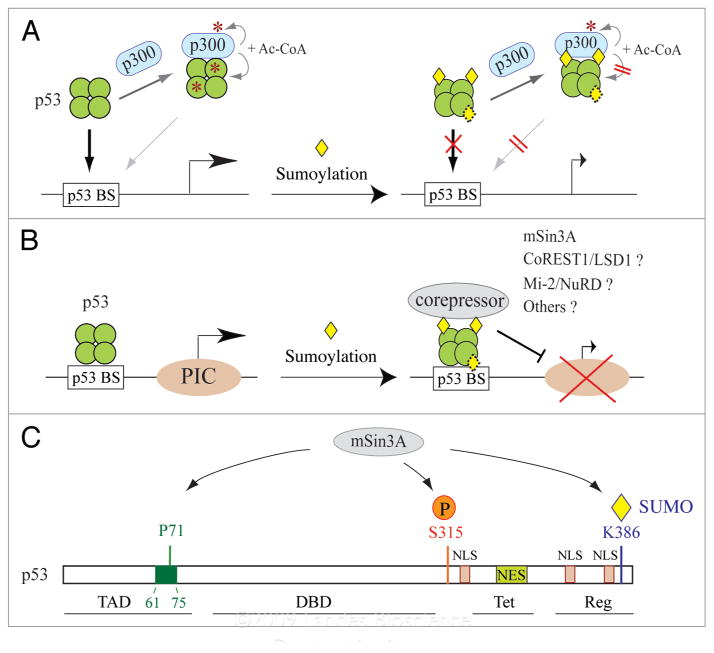
When examined in the reconstituted ACF chromatin assembly-coupled transcription system, Su-p53 fails to activate transcription from pWAFMLT chromatin, suggesting that sumoylation of p53 inhibits p53-dependent transcription. Although Su-p53 still interacts with p300 as efficiently as unmodified p53, it is no longer acetylated by p300 and is incapable of supporting p300-mediated acetylation of chromatin. In vitro chromatin immunoprecipitation (ChIP) and electrophoretic mobility shift assay (EMSA) further indicate that Su-p53 is unable to bind its cognate sequence in both DNA and chromatin. The loss of Su-p53 DNA binding activity is partially restored when SUMO is removed from Su-p53 following SENP1 treatment. These results17 (Fig. 3A) illustrate several important points regarding the molecular action of sumoylation: (1) sumoylation of p53 blocks its subsequent acetylation by p300, likely due to steric hindrance of the bulky SUMO moiety preventing p300 access to lysine residues adjacent to the K386 sumoylation site (see Fig. 1); (2) the loss of Su-p53 transcription activity is attributed to its inability to bind DNA, suggesting that SUMO conjugation at the C-terminal regulatory domain may induce acetylation-independent allosteric changes of its central DNA-binding domain without impairing its tetramerization ability; (3) the “partial” restoration of p53 DNA-binding activity following a “complete” removal of the SUMO conjugate leads to the discovery of an isopeptidase-independent function of SENP1 in impeding p53 binding to DNA, as illustrated by EMSA;17 and (4) sumoylation likely plays a more active role in inhibiting p300-mediated acetylation of chromatin when p53 is tethered on DNA (see below), since p300 autoacetylation and interaction with Su-p53 remain undiminished.
Figure 3.
Effect of sumoylation on p53 binding to DNA and interaction with p300 and mSin3A. (A) Model for SUMO-inhibited DNA binding by the free form of p53 tetramers. (B) Model for SUMO-dependent recruitment of transcriptional corepressors by DNA-bound p53. (C) Association of mSin3A with p53 depends on multiple types of interactions with different regions of p53 and also on phosphorylation of serine 315 (S315) and sumoylation at K386. Abbreviations used for distinct p53 domains are: TAD, transactivation domain (aa 1–92); DBD, DNA-binding domain (aa 102–292); Tet, tetramerization domain (aa 326–356); Reg, regulatory domain (aa 363–393); NES, nuclear expert signal (aa 340–351); NLS, nuclear localization signal (aa 316–325, aa 369–375 and aa 379–384). The green box indicates a proline-rich region (aa 61–75), previously shown to bind mSin3A.65
These findings are crucial for proper interpretation of contradictory data in previous studies showing that sumoylation either enhances45,46 or has no effect47 on p53 transcription activity, based primarily on transient transfection and reporter gene assays in cultured cells for measuring reporter activity derived from less than 5% of modified p53 in the cell.48 As sumoylation may potentially regulate subcellular localization and nuclear distribution of p53 as well as p53’s association with other cellular partners before reaching its target genes,27–31 the reporter gene assay tends to be influenced by the cellular environment when a given assay is conducted. Moreover, it is important to note that many transcriptional components, besides p53, are likewise sumoylated and their regulatory properties are altered by their sumoylation status. Examples include p300,49–51 CBP,52 MDM2,53 MDMX,54 HDAC155,56 and SIRT1,57 that are directly involved in p53 target gene transcription. Sumoylation on any of these p53 coregulators may positively or negatively influence p53 transcription activity. An additional point to remember is that enzymatic cofactors implicated in p53 modification may have regulatory function independently of their catalytic activity, as seen with PIAS,29,31,58 SENP117 and p300.59,60 Hence, results based on transiently induced gene expression in cultured cells need to be carefully interpreted with proper controls of these potential variables. All in all, the finding that sumoylation negatively regulates p53 DNA binding and transcriptional activity is also consistent with SUMO-mediated inhibition of the transcriptional activity of the other p53 family members, such as p63α and p73α, that share similar domain organizations as p53, thus providing a uniform effect of SUMO conjugation in regulating the transcriptional activity of p53 family proteins.61
Sumoylation on DNA-Bound p53 Provides a Protein Mark for the Recruitment of Transcriptional Corepressors
Additional mechanistic insights, based on the use of immobilized DNA templates for sequential modification (i.e., sumoylation followed by acetylation, and vice versa) and DNA-binding assays with either free or DNA-bound p53 tetramers, have also been revealed.17 First, sumoylation indeed blocks ensuing acetylation, but acetylation does not obstruct subsequent sumoylation. This unidirectional effect is consistent with the spatial accommodation of a small chemical (i.e., acetyl) group at K382 and/or K373 without imposing a significant barrier for SUMO conjugatoin at K386. The result further indicates that K386 is not a major acetylation site for p300, even though acetylated K386 is detectable by mass spectrometric analysis in tricostatin A/nicotinamide-treated H1299 cells cotransfected with p53 and CBP expression plasmids.23 Importantly, double acetyl/SUMO-modified p53 binds DNA as efficiently as the unmodified protein, suggesting that acetylation is able to overcome SUMO-inhibited p53 binding to DNA perhaps by inducing a distinct conformation of p53 competent for DNA binding. In this way, a new function of p53 acetylation has been uncovered from these in vitro reconstitution studies. Second, SUMO conjugation on only one subunit of a p53 tetramer is sufficient to block its DNA-binding activity, suggesting that sumoylation is very effective in preventing the free form of p53 from binding to DNA. Third, DNA-bound p53 is still subject to sumoylation at the same lysine residue (i.e., K386) as seen in solution. Yet, the SUMO conjugate fails to dislodge p53 from DNA. This finding implies that sumoylation of prebound p53 likely plays a more active role in recruiting transcriptional corepressors to inhibit p53 target gene transcription. Indeed, the association of a cellular corepressor, mSin3A, but not p300 HAT, with DNA-bound p53 is dramatically increased when SUMO-1 is conjugated to p53.
The SUMO-1-dependent recruitment of mSin3A provides a rationale why a small amount of p53 molecules modified in vivo, presumably representing mostly DNA-bound p53, is able to affect transcription. The recruitment of mSin3A by SUMO-1-conjugated p53 parallels a recent result showing that the CoREST1 corepressor binds directly to SUMO-2/3, rather than SUMO-1, immobilized on GST beads.62 In addition, several chromatin-associated corepressors, including Mi-2 and other components of the nucleosome remodeling and deacetylase (NuRD) complex have been identified by a genome-wide RNA interference screen in Drosophila cells to associate specifically with SUMO-conjugated Sp3, whose activity was measured by a SUMO-dependent reporter assay.63 It is interesting to note that CoREST1 and Mi-2/NuRD both associate with lysine-specific demethylase 1 (LSD1),62,64 suggesting that LSD1 has the potential to bridge CoREST1 and Mi-2/NuRD complexes for association with SUMO-2/3-conjugated proteins. Taken together, these findings suggest that paralog-specific recruitment of transcriptional corepressors and/or chromatin-modifying complexes (Fig. 3B) likely account for SUMO-dependent silencing of transcription mediated by many transcription factors and cofactors. With respect to p53-dependent transcription, it would be interesting to see whether SUMO-2/3-conjugated p53 recruits other transcriptional corepressors distinct from mSin3A in a SUMO-2/3-specific manner.
The SUMO-dependent recruitment of mSin3A to DNA-bound p53 is in agreement with a previous study indicating that mSin3A-interacting p53 represents less than 10% of total p53 and may occur on promoter-associated p53.65 The association with mSin3A helps stabilize p53 on target genes65 and is crucial for p53-mediated repression of pluripotency-associated gene expression, such as AFP66 and Nanog.67 Although SUMO-1 conjugation at K386 is important for p53 interaction with mSin3A, other regions of p53, particularly proline 71 in the mSin3A-binding surface (aa 61–75)65 and phosphorylation at serine 315,67 also seem necessary for forming a stable mSin3A-p53 core complex on p53 target genes (Fig. 3C). Clearly, sumoylation of p53 coordinates with other types of posttranslational modification to regulate p53 DNA binding and transcriptional activity. The molecular insights revealed from reconstitution studies are invaluable for proper elucidation of the biological and functional assays conducted in vivo and in cultured cells.
Acknowledgments
We thank Elizabeth E. Johnson for assistance in performing some of the sumoylation assays shown in Figure 2. The research conducted in Dr. Chiang’s laboratory is currently supported by grants CA103867 and CA124760 from the National Institutes of Health in the United States. This work is Report CSCN #052 from University of Texas Southwestern Medical Center Simmons Comprehensive Cancer Center.
References
- 1.Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90:145–55. doi: 10.1016/s0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 2.Ito T, Levenstein ME, Fyodorov DV, Kutach AK, Kobayashi R, Kadonaga JT. ACF consists of two subunits, Acf1 and ISWI, that function cooperatively in the ATP-dependent catalysis of chromatin assembly. Genes Dev. 1999;13:1529–39. doi: 10.1101/gad.13.12.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang W, Nordeen SK, Kadonaga JT. Transcriptional analysis of chromatin assembled with purified ACF and dNAP1 reveals that acetyl-CoA is required for preinitiation complex assembly. J Biol Chem. 2000;275:39819–22. doi: 10.1074/jbc.C000713200. [DOI] [PubMed] [Google Scholar]
- 4.Kamakaka RT, Bulger M, Kadonaga JT. Potentiation of RNA polymerase II transcription by Gal4-VP16 during but not after DNA replication and chromatin assembly. Genes Dev. 1993;7:1779–95. doi: 10.1101/gad.7.9.1779. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong JA, Emerson BM. NF-E2 disrupts chromatin structure at human β-globin locus control region hypersensitive site 2 in vitro. Mol Cell Biol. 1996;16:5634–44. doi: 10.1128/mcb.16.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pazin MJ, Sheridan PL, Cannon K, Cao Z, Keck JG, Kadonaga JT, Jones KA. NFκB-mediated chromatin reconfiguration and transcriptional activation of the HIV-1 enhancer in vitro. Genes Dev. 1996;10:37–49. doi: 10.1101/gad.10.1.37. [DOI] [PubMed] [Google Scholar]
- 7.Näär AM, Beaurang PA, Robinson KM, Oliner JD, Avizonis D, Scheek S, et al. Chromatin, TAFs, and a novel multiprotein coactivator are required for synergistic activation by Sp1 and SREBP-1a in vitro. Genes Dev. 1998;12:3020–31. doi: 10.1101/gad.12.19.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92:105–16. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- 9.Kraus WL, Manning ET, Kadonaga JT. Biochemical analysis of distinct activation functions in p300 that enhance transcription initiation with chromatin templates. Mol Cell Biol. 1999;19:8123–35. doi: 10.1128/mcb.19.12.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu S-Y, Thomas MC, Hou SY, Likhite V, Chiang C-M. Isolation of mouse TFIID and functional characterization of TBP and TFIID in mediating estrogen receptor and chromatin transcription. J Biol Chem. 1999;274:23480–90. doi: 10.1074/jbc.274.33.23480. [DOI] [PubMed] [Google Scholar]
- 11.Dilworth FJ, Fromental-Ramain C, Remboutsika E, Benecke A, Chambon P. Ligand-dependent activation of transcription in vitro by retinoic acid receptor α/retinoid X receptor α heterodimers that mimics transactivation by retinoids in vivo. Proc Natl Acad Sci USA. 1999;96:1995–2000. doi: 10.1073/pnas.96.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Kadam S, Emerson BM, Bieker JJ. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Krüppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol Cell Biol. 2001;21:2413–22. doi: 10.1128/MCB.21.7.2413-2422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas MC, Chiang C-M. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17:251–64. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Wu S-Y, Lee AY, Hou SY, Kemper JK, Erdjument-Bromage H, Tempst P, Chiang C-M. Brd4 links chromatin targeting to HPV transcriptional silencing. Genes Dev. 2006;20:2383–96. doi: 10.1101/gad.1448206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espinosa JM, Emerson BM. Transcriptional regulation by p53 through intrinsic DNA/chromatin binding and site-directed cofactor recruitment. Mol Cell. 2001;8:57–69. doi: 10.1016/s1097-2765(01)00283-0. [DOI] [PubMed] [Google Scholar]
- 16.An W, Kim J, Roeder RG. Ordered cooperative functions of PRMT1, p300 and CARM1 in transcriptional activation by p53. Cell. 2004;117:735–48. doi: 10.1016/j.cell.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Wu S-Y, Chiang C-M. Crosstalk between sumoylation and acetylation regulates p53-dependent chromatin transcription and DNA binding. EMBO J. 2009;28:1246–59. doi: 10.1038/emboj.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng L, Lin T, Uranishi H, Gu W, Xu Y. Functional analysis of the roles of posttranslational modifications at the p53 C terminus in regulating p53 stability and activity. Mol Cell Biol. 2005;25:5389–95. doi: 10.1128/MCB.25.13.5389-5395.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummel KA, Lee CJ, Toledo F, Wahl GM. The C-terminal lysines fine-tune P53 stress responses in a mouse model but are not required for stability control or transactivation. Proc Natl Acad Sci USA. 2005;102:10188–93. doi: 10.1073/pnas.0503068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barlev NA, Liu L, Chehab NH, Mansfield K, Harris KG, Halazonetis TD, Berger SL. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell. 2001;8:1243–54. doi: 10.1016/s1097-2765(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 23.Tang Y, Zhao W, Chen Y, Zhao Y, Gu W. Acetylation is indispensable for p53 activation. Cell. 2008;133:612–26. doi: 10.1016/j.cell.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, Yao T-P. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. EMBO J. 2002;21:6236–45. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawaguchi Y, Ito A, Appella E, Yao T-P. Charge modification at multiple C-terminal lysine residues regulates p53 oligomerization and its nucleus-cytoplasm trafficking. J Biol Chem. 2006;281:1394–400. doi: 10.1074/jbc.M505772200. [DOI] [PubMed] [Google Scholar]
- 26.Li AG, Piluso LG, Cai X, Gadd BJ, Ladurner AG, Liu X. An acetylation switch in p53 mediates holo-TFIID recruitment. Mol Cell. 2007;28:408–21. doi: 10.1016/j.molcel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–82. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 28.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 29.Sharrocks AD. PIAS proteins and transcriptional regulation—more than just SUMO E3 ligases? Genes Dev. 2006;20:754–8. doi: 10.1101/gad.1421006. [DOI] [PubMed] [Google Scholar]
- 30.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–56. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 31.Liu B, Shuai K. Regulation of the sumoylation system in gene expression. Curr Opin Cell Biol. 2008;20:288–93. doi: 10.1016/j.ceb.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W-M, Lee AY, Chiang C-M. One-step affinity tag purification of full-length recombinant human AP-1 complexes from bacterial inclusion bodies using a polycistronic expression system. Protein Expr Purif. 2008;59:144–52. doi: 10.1016/j.pep.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiang C-M, Roeder RG. Expression and purification of general transcription factors by FLAG epitope-tagging and peptide elution. Pept Res. 1993;6:62–4. [PubMed] [Google Scholar]
- 34.Kitayner M, Rozenberg H, Kessler N, Rabinovich D, Shaulov L, Haran TE, Shakked Z. Structural basis of DNA recognition by p53 tetramers. Mol Cell. 2006;22:741–53. doi: 10.1016/j.molcel.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 35.Okorokov AL, Sherman MB, Plisson C, Grinkevich V, Sigmundsson K, Selivanova G, et al. The structure of p53 tumour suppressor protein reveals the basis for its functional plasticity. EMBO J. 2006;25:5191–200. doi: 10.1038/sj.emboj.7601382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tidow H, Melero R, Mylonas E, Freund SM, Grossmann JG, Carazo JM, et al. Quaternary structures of tumor suppressor p53 and a specific p53 DNA complex. Proc Natl Acad Sci USA. 2007;104:12324–9. doi: 10.1073/pnas.0705069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKinney K, Mattia M, Gottifredi V, Prives C. p53 linear diffusion along DNA requires its C terminus. Mol Cell. 2004;16:413–24. doi: 10.1016/j.molcel.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Stommel JM, Marchenko ND, Jimenez GS, Moll UM, Hope TJ, Wahl GM. A leucine-rich nuclear export signal in the p53 tetramerization domain: regulation of subcellular localization and p53 activity by NES masking. EMBO J. 1999;18:1660–72. doi: 10.1093/emboj/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter S, Bischof O, Dejean A, Vousden KH. C-terminal modifications regulate MDM2 dissociation and nuclear export of p53. Nat Cell Biol. 2007;9:428–35. doi: 10.1038/ncb1562. [DOI] [PubMed] [Google Scholar]
- 40.van Dieck J, Fernandez-Fernandez MR, Veprintsev DB, Fersht AR. Modulation of the oligomerization state of p53 by differential binding of proteins of the S100 family to p53 monomers and tetramers. J Biol Chem. 2009;284:13804–11. doi: 10.1074/jbc.M901351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Natan E, Hirschberg D, Morgner N, Robinson CV, Fersht AR. Ultraslow oligomerization equilibria of p53 and its implications. Proc Natl Acad Sci USA. 2009;106:14327–32. doi: 10.1073/pnas.0907840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shieh S-Y, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, Ser20, requires tetramerization. EMBO J. 1999;18:1815–23. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cešková P, Chichger H, Wallace M, Vojtesek B, Hupp TR. On the mechanism of sequence-specific DNA-dependent acetylation of p53: the acetylation motif is exposed upon DNA binding. J Mol Biol. 2006;357:442–56. doi: 10.1016/j.jmb.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 44.Itahana Y, Ke H, Zhang Y. p53 Oligomerization is essential for its C-terminal lysine acetylation. J Biol Chem. 2009;284:5158–64. doi: 10.1074/jbc.M805696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez MS, Desterro JM, Lain S, Midgley CA, Lane DP, Hay RT. SUMO-1 modification activates the transcriptional response of p53. EMBO J. 1999;18:6455–61. doi: 10.1093/emboj/18.22.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gostissa M, Hengstermann A, Fogal V, Sandy P, Schwarz SE, Scheffner M, Del Sal G. Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J. 1999;18:6462–71. doi: 10.1093/emboj/18.22.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwek SS, Derry J, Tyner AL, Shen Z, Gudkov AV. Functional analysis and intracellular localization of p53 modified by SUMO-1. Oncogene. 2001;20:2587–99. doi: 10.1038/sj.onc.1204362. [DOI] [PubMed] [Google Scholar]
- 48.Melchior F, Hengst L. SUMO-1 and p53. Cell Cycle. 2002;1:245–9. [PubMed] [Google Scholar]
- 49.Girdwood D, Bumpass D, Vaughan OA, Thain A, Anderson LA, Snowden AW, et al. p300 transcriptional repression is mediated by SUMO modification. Mol Cell. 2003;11:1043–54. doi: 10.1016/s1097-2765(03)00141-2. [DOI] [PubMed] [Google Scholar]
- 50.Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–76. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 51.Huang C, Han Y, Wang Y, Sun X, Yan S, Yeh ET, et al. SENP3 is responsible for HIF-1 transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009;28:2748–62. doi: 10.1038/emboj.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuo H-Y, Chang C-C, Jeng J-C, Hu H-M, Lin D-Y, Maul GG, et al. SUMO modification negatively modulates the transcriptional activity of CREB-binding protein via the recruitment of Daxx. Proc Natl Acad Sci USA. 2005;102:16973–8. doi: 10.1073/pnas.0504460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xirodimas DP, Chisholm J, Desterro JM, Lane DP, Hay RT. p14ARF promotes accumulation of SUMO-1 conjugated (H)Mdm2. FEBS Lett. 2002;528:207–11. doi: 10.1016/s0014-5793(02)03310-0. [DOI] [PubMed] [Google Scholar]
- 54.Pan Y, Chen J. Modification of MDMX by sumoylation. Biochem Biophys Res Commun. 2005;332:702–9. doi: 10.1016/j.bbrc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 55.David G, Neptune MA, DePinho RA. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J Biol Chem. 2002;277:23658–63. doi: 10.1074/jbc.M203690200. [DOI] [PubMed] [Google Scholar]
- 56.Cheng J, Wang D, Wang Z, Yeh ET. SENP1 enhances androgen receptor-dependent transcription through desumoylation of histone deacetylase 1. Mol Cell Biol. 2004;24:6021–8. doi: 10.1128/MCB.24.13.6021-6028.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Fu W, Chen J, Olashaw N, Zhang X, Nicosia SV, et al. SIRT1 sumoylation regulates its deacetylase activity and cellular response to genotoxic stress. Nat Cell Biol. 2007;9:1253–62. doi: 10.1038/ncb1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palvimo JJ. PIAS proteins as regulators of small ubiquitin-related modifier (SUMO) modifications and transcription. Biochem Soc Trans. 2007;35:1405–8. doi: 10.1042/BST0351405. [DOI] [PubMed] [Google Scholar]
- 59.Zeng X, Lee H, Zhang Q, Lu H. p300 does not require its acetylase activity to stimulate p73 function. J Biol Chem. 2001;276:48–52. doi: 10.1074/jbc.C000722200. [DOI] [PubMed] [Google Scholar]
- 60.Harton JA, Zika E, Ting JP. The histone acetyltransferase domains of CREB-binding protein (CBP) and p300/CBP-associated factor are not necessary for cooperativity with the class II transactivator. J Biol Chem. 2001;276:38715–20. doi: 10.1074/jbc.M106652200. [DOI] [PubMed] [Google Scholar]
- 61.Stehmeier P, Muller S. Regulation of p53 family members by the ubiquitin-like SUMO system. DNA Repair. 2009;8:491–8. doi: 10.1016/j.dnarep.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 62.Ouyang J, Shi Y, Valin A, Xuan Y, Gill G. Direct binding of CoREST1 to SUMO-2/3 contributes to gene-specific repression by the LSD1/CoREST1/HDAC complex. Mol Cell. 2009;34:145–54. doi: 10.1016/j.molcel.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stielow B, Sapetschnig A, Krüger I, Kunert N, Brehm A, Boutros M, Suske G. Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol Cell. 2008;29:742–54. doi: 10.1016/j.molcel.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–72. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 65.Zilfou JT, Hoffman WH, Sank M, George DL, Murphy M. The corepressor mSin3a interacts with the proline-rich domain of p53 and protects p53 from proteasome-mediated degradation. Mol Cell Biol. 2001;21:3974–85. doi: 10.1128/MCB.21.12.3974-3985.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilkinson DS, Tsai WW, Schumacher MA, Barton MC. Chromatin-bound p53 anchors activated Smads and the mSin3A corepressor to confer transforming-growth-factor-beta-mediated transcription repression. Mol Cell Biol. 2008;28:1988–98. doi: 10.1128/MCB.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin T, Chao C, Saito S, Mazur SJ, Murphy ME, Appella E, Xu Y. p53 induces differentiation of mouse embryonic stem cells by suppressing Nanog expression. Nat Cell Biol. 2005;7:165–71. doi: 10.1038/ncb1211. [DOI] [PubMed] [Google Scholar]