Abstract
IMPORTANCE
Antibiotics disrupt human microbiota and have been associated with several pediatric autoimmune diseases. Psoriasis activity has been linked to group A streptococcal and viral infections.
OBJECTIVE
To determine whether antibiotic exposure and infections are independently associated with incident psoriasis in children.
DESIGN, SETTING, AND PARTICIPANTS
This nested case-control study used data from the Health Improvement Network database, a population-representative electronic health records database from the United Kingdom, from June 27, 1994, through January 15, 2013. Data were analyzed from September 17, 2014, through August 12, 2015. Children aged 1 to 15 years with newly diagnosed psoriasis (n = 845) were compared with age- and sex-matched controls (n = 8450) randomly chosen at the time of psoriasis diagnosis from general practices with at least one case, excluding children with immunodeficiency, inflammatory bowel disease, and juvenile arthritis.
EXPOSURES
Systemic antibacterial prescriptions and infections of the skin and other sites within 2 years before psoriasis diagnosis.
MAIN OUTCOMES AND MEASURES
Incident psoriasis as determined by validated diagnostic codes. The association of antibiotic exposure and infections with incident psoriasis was determined by conditional logistic regression, adjusting for confounders.
RESULTS
After adjusting for matching, country, socioeconomic deprivation, outpatient visits, and infections within the past 2 years, antibiotic exposure in the last 2 years was weakly associated with incident psoriasis (adjusted odds ratio [aOR], 1.2; 95% CI, 1.0–1.5). The associations for infections of skin (aOR, 1.5; 95% CI, 1.2–1.7) and other sites (aOR, 1.3; 95% CI, 1.1–1.6) were similar. Untreated nonskin infections (aOR, 1.5; 95% CI, 1.3–1.8) but not antibiotic-treated nonskin infections (aOR, 1.1; 95% CI, 0.9–1.4) were associated with psoriasis. Results were similar when using a lifetime exposure window. Different classes of antibiotics and age of first antibiotic exposure were also not associated with psoriasis. The findings did not substantively change when excluding periods of varying length before diagnosis.
CONCLUSIONS AND RELEVANCE
Infections are associated with the development of pediatric psoriasis, but antibiotics do not appear to contribute substantially to that risk.
Psoriasis is a chronic autoimmune disease that affects approximately 4 in 1000 children.1,2 The pathogenesis of psoriasis is incompletely understood but likely involves genetic, immunologic, and environmental factors.3 Infections may contribute to the development of psoriasis. For instance, individuals with guttate psoriasis frequently have antecedent group A streptococcal infection,4,5 even though antistreptococcal treatment does not change psoriatic outcomes.6 Various viruses have been linked to the development or worsening of psoriatic disease.7–9
Medications compose another class of reported triggers and exacerbating factors for psoriasis. Among drugs linked to psoriasis are antibiotics, including tetracyclines, mostly on the basis of older case reports and one survey-based study that lacked rigorous methods.10–12 More recent studies13–15 have found an association between childhood antibiotic use and 2 related pediatric autoimmune diseases: inflammatory bowel disease (IBD) and juvenile idiopathic arthritis (JIA). Antibiotics disrupt human microbiota, and microbial disturbance has been implicated in the pathogenesis of pediatric IBD and JIA.16,17 Intestinal microbial disturbance has also been recently observed in adults with psoriasis and psoriatic arthritis compared with unaffected adults.18 Furthermore, psoriatic lesions contain abnormal bacterial populations compared with healthy skin, raising the hypothesis that cutaneous microbial disturbance may contribute to psoriasis pathogenesis.19 No study has examined the connection between antibiotic use and psoriasis in a large pediatric population. We hypothesized that antibiotic use and infections were independently associated with the development of childhood psoriasis in a dose- and time-dependent manner.
Methods
Study Design and Data Source
We performed a nested case-control study using The Health Improvement Network (THIN), a population-representative electronic health records database from more than 550 general practices across the United Kingdom,20 using methods similar to our previous study on antibiotics and JIA.15 Nested case-control designs efficiently produce unbiased estimates of incidence rate ratios.21 THIN contains anonymized patient data on demographics, diagnoses, referrals, and outpatient prescriptions by general practitioners (GPs) collected during routine primary care. This study of anonymized data was exempted by the University of Pennsylvania Institutional Review Board and approved by THIN’s scientific review committee. This study included data from June 27, 1994, through January 15, 2013. THIN has been validated for pharmacoepidemiologic research in several diseases,22 including psoriasis in adults.2 Data were analyzed from September 17, 2014, through August 12, 2015.
Participant Selection
Eligible participants were 1 to 15 years old and registered within 3 months after birth to capture lifetime outpatient prescriptions. Cases were defined by the first psoriasis diagnosis using an established list of diagnostic Read codes (analogous to International Classification of Diseases, Ninth Revision, codes).2 We used secondary case definitions to improve diagnostic specificity, consisting of the psoriatic code plus (1) psoriatic medication (eTable 1 in the Supplement) or (2) dermatology referral. Children with prior IBD, immunodeficiency, or JIA were excluded.
We matched each case at the time of diagnosis to 10 controls by age and sex without prior psoriasis or exclusion diagnosis, using incidence density sampling. Controls were randomly selected from practices with at least one child diagnosed as having psoriasis.
Exposure and Covariate Data
The primary exposures were systemic antibacterial prescriptions and infections from THIN registration to the index date (defined below). We categorized infections as skin related, which early psoriasis might mimic, or not skin related, using Read codes (Table 1). We classified infections as treated if antibiotics were prescribed within 1 week. Additional analyses categorized antibiotics by class and spectrum of coverage (Table 1).13 Nonbacterial antimicrobials (eg, antiviral, antimalarial) were analyzed for comparison.
Table 1.
Characteristics of the Study Populationa
| Characteristic | Cases (n = 845) | Controls (n = 8450) | P Valueb |
|---|---|---|---|
| Demographics | |||
| Female sex | 465 (55.0) | 4650 (55.0) | …c |
| Age, median (IQR), y | 6 (4–8) | 6 (4–8) | … |
| Age category, y | |||
| 1–5 | 366 (43.3) | 3660 (43.3) | … |
| 6–10 | 367 (44.4) | 3670 (44.4) | |
| 11–15 | 112 (13.3) | 1120 (13.3) | |
| Country of origin | |||
| England | 660 (78.1) | 7189 (85.1) | <.001d |
| Northern Ireland | 62 (7.3) | 325 (3.9) | |
| Scotland | 78 (9.2) | 545 (6.5) | |
| Wales | 45 (5.3) | 391 (4.6) | |
| Townsend indexe | |||
| 1 | 179 (21.2) | 2157 (25.5) | <.001d |
| 2 | 161 (19.1) | 1657 (19.6) | |
| 3 | 159 (18.8) | 1596 (18.9) | |
| 4 | 173 (20.5) | 1578 (18.7) | |
| 5 | 146 (17.3) | 1186 (14.0) | |
| Missing | 27 (3.2) | 276 (3.3) | |
| Comorbidities | |||
| Personal autoimmunityf | 4 (0.5) | 16 (0.2) | |
| Celiac disease | 0 | 8 (0.1) | .10g |
| Thyroid disease | 1 (0.1) | 4 (<0.1) | |
| Type 1 diabetes mellitus | 3 (0.4) | 5 (0.1) | |
| Any infectionf | 754 (89.2) | 7185 (85.0) | <.001 |
| Any skin infectionf | 475 (56.2) | 3838 (45.4) | <.001 |
| External genitourinary | 65 (7.7) | 444 (5.3) | .003 |
| Herpes simplex | 18 (2.1) | 114 (1.4) | .07 |
| Molluscum contagiosum | 6.2 (7.3) | 516 (6.1) | .15 |
| Tinea | 67 (7.9) | 341 (4.0) | <.001 |
| Wart | 90 (10.7) | 609 (7.2) | <.001 |
| Varicella | 194 (23.0) | 1679 (19.9) | .03 |
| Other viral exanthem | 50 (5.9) | 311 (3.7) | .001 |
| Other skin and soft-tissue infection | 249 (29.5) | 1943 (23.0) | <.001 |
| Any other infectionf | 736 (87.1) | 6946 (82.2) | <.001 |
| Upper respiratory tract | 674 (79.8) | 6317 (74.8) | <.001 |
| Lower respiratory tract | 327 (38.7) | 2399 (28.4) | <.001 |
| Gastrointestinal | 163 (19.3) | 1504 (17.8) | .28 |
| Urinary tract | 52 (6.2) | 395 (4.7) | .05 |
| Other | 355 (42.0) | 3108 (36.8) | .002 |
| No. of infections, median (IQR) | 5 (2–10) | 4 (1–8) | <.001 |
| Antibiotic Exposures | |||
| Any antibiotic prescribed | 710 (84.0) | 6343 (75.1) | <.001 |
| Antianaerobic antibioticsf | 683 (80.8) | 6099 (72.2) | <.001 |
| Penicillins | 677 (80.1) | 6056 (71.7) | <.001 |
| Broad-spectrum penicillins | 109 (12.9) | 667 (7.9) | <.001 |
| Metronidazole | 4 (0.5) | 48 (0.6) | .73 |
| Clindamycin | 0 | 0 | … |
| Other antianaerobich | 0 | 5 (0.1) | … |
| Nonantianaerobic antibioticsf | 354 (41.9) | 2827 (33.5) | <.001 |
| Cephalosporins | 136 (16.1) | 946 (11.2) | <.001 |
| Macrolides | 254 (30.1) | 1988 (23.5) | <.001 |
| Sulfonamides | 107 (12.7) | 764 (9.0) | <.001 |
| Other nonantianaerobich | 6 (0.7) | 34 (0.4) | .20 |
| Antimalarial | 13 (1.5) | 88 (1.0) | .19 |
| Other antimicrobial exposurei | 72 (8.5) | 591 (7.0) | .10 |
| Maternal Variables | |||
| Maternal autoimmunityf | 150 (19.7) | 671 (8.8) | <.001 |
| Arthritis | 15 (2.0) | 35 (0.5) | <.001 |
| Celiac disease | 3 (0.4) | 18 (0.2) | .40 |
| Connective tissue disease | 2 (0.3) | 14 (0.2) | .72 |
| Diabetes | 2 (0.3) | 25 (0.3) | .81 |
| Inflammatory bowel disease | 12 (1.6) | 53 (0.7) | .01 |
| Multiple sclerosis | 1 (0.1) | 10 (0.1) | … |
| Psoriasis | 101 (13.3) | 270 (3.5) | <.001 |
| Thyroid disease | 26 (3.4) | 248 (3.2) | .81 |
| Uveitis | 8 (1.1) | 33 (0.4) | .04 |
| Maternal smoking | 285 (33.7) | 2364 (28.0) | .001 |
| Missing maternal smoking data | 96 (11.4) | 1067 (12.6) | … |
| Missing maternal data | 83 (9.8) | 804 (9.5) | … |
| Other Variables | |||
| Cesarean delivery | 119 (14.1) | 1224 (14.5) | .77 |
| Missing delivery data | 358 (42.4) | 3360 (39.8) | … |
| Hospitalizationf | 104 (12.3) | 863 (10.2) | .06 |
| Infection | 46 (5.4) | 347 (4.1) | .07 |
| Other | 71 (8.4) | 626 (7.4) | .29 |
| No. of outpatient visits in last 2 years,j median (IQR) | 5 (2–9) | 3 (0–7) | <.001 |
Abbreviation: IQR, interquartile range.
Data are presented as number (percentage) unless otherwise indicated.
All P values were obtained from univariable conditional logistic regression models.
Ellipses indicate data not applicable.
Overall χ2 test P value.
Geography-based deprivation index; higher index score means more deprived.
Some participants had more than one type.
Comparison of main category.
Other antianaerobic antibiotics include tetracyclines, glycopeptides (oral vancomycin), carbapenems, and cefoxitin (all other cephalosporins categorized as nonantianaerobic); other nonantianaerobic antibiotics include fluoroquinolones and all other antibiotic classes.
Other antimicrobial agents, including antifungal, antiviral, and antimycobacterial drugs.
Total outpatient visits per year for 2-year period starting 3 years before psoriasis diagnosis.
We anticipated protopathic bias whereby early psoriasis symptoms would sometimes be treated as skin infections. To exclude the most likely period of psoriasis misdiagnosis from analyses, we chose the primary index date empirically at the time before psoriasis diagnosis when GPs began reporting more dermatitis and/or skin lesions in cases relative to controls.
Potential confounders were demographic variables, comorbidities, maternal autoimmunity, and other clinical factors, such as hospitalization and number of outpatient visits within 2 years before the index date (Table 1). We matched participants to their mothers using an algorithm described previously.15
Statistical Analysis
Using conditional logistic regression to account for matching, we estimated the association of antibiotic prescription and history of infection (stratified as skin and nonskin) with psoriasis by using odds ratios (ORs) with 95% CIs. Multivariable models included all 3 primary exposure variables and initially all covariates associated with psoriasis in univariable analysis with P < .20. In multivariable models, we retained those variables that were independently associated with psoriasis (P < .05) or changed the OR for antibiotic exposure by 10% or more. We compared adjusted ORs (aORs) of variables within the same model using linear combination to determine whether they significantly differed from each other. We omitted variables with 10% or more missing data from multivariable models. The primary analysis examined antibiotics prescribed within 2 years before the index date because microbial changes may sometimes persist for more than 1 year after antibiotic exposure.23 This time window also accommodated uncertainty about preclinical psoriasis onset.
To distinguish further between the effects of antibiotics and infections, additional models compared the associations of treated and untreated infections. We specifically evaluated antibiotic use for upper respiratory tract infections because these infections frequently do not require antibiotic use. Thus, they offer the opportunity to examine people with similar infections but treated and not treated with antibiotics. Secondary analyses studied antibiotic coverage and drug class, the timing of first and last antibiotics prescribed, and lifetime exposure windows.
We performed multiple sensitivity analyses. We examined assumptions about antibiotic timing and protopathic bias by moving the index date from 0 to 36 months before diagnosis. Because THIN lacks inpatient medication data, we repeated analyses assuming antibiotics were received during hospitalizations within 1 week of infections. Additional analyses focused on cases with 2 or more psoriasis Read codes for improved diagnostic predictive value.2 Considering possible confounding by local practice patterns and unmeasured environmental factors, we repeated analyses after matching cases and controls by practice. To further consider confounding from infection, we compared the rate of infections between cases and controls not prescribed antibiotics.
We used STATA/IC statistical software, version 12.1 (StataCorp), for all analyses. Hypothesis tests were 2-sided with a type I error of 0.05.
Results
Characteristics of the Study Population
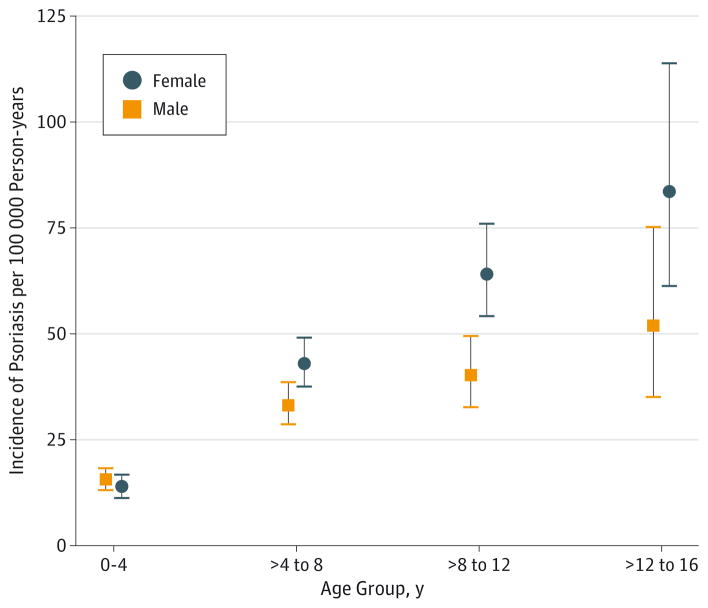
We identified 845 eligible cases among 894 children with psoriasis in a population of 454 463 children followed up for 3.1 million person-years. Guttate psoriasis was the most common variant reported (18.9%) although most cases had nonspecific psoriasis diagnoses (eTable 1 in the Supplement). Psoriasis incidence was 29 per 100 000 person-years, with increasing incidence and female predominance at older ages (Figure). Cases more likely lived in northern countries (P < .001) and in areas with more socioeconomic deprivation (P < .001) (Table 1). Cases more commonly had a history of infections of skin and other sites, as well as more prior infections than controls (P < .001). Cases also had more outpatient visits within 2 years of the index date (defined below) (P < .001). Mothers of cases more likely had autoimmune diseases (P < .001), particularly psoriasis; maternal psoriasis was strongly associated with psoriasis (unadjusted OR, 4.0; 95% CI, 3.2–5.1). Tobacco use, but not cesarean delivery, was more common among cases’ mothers, but the data were missing for 11.4% and 42.4% of cases and 12.6% and 39.8% of controls, respectively.
Figure.
Incidence of Psoriasis by Age and Sex in the Source Population
The incidence of pediatric psoriasis in the study’s source population in The Health Improvement Network (THIN) is presented across 4 age groups stratified by sex. Error bars indicate 95% CIs.
The GPs noted more dermatitis and/or skin lesions among cases starting 12 months before psoriasis diagnosis, a trend accelerating 4 months before diagnosis (eFigure 1 in the Supplement). In contrast, monthly reporting of dermatitis and/or skin lesions for controls did not change during the same period. On the basis of these trends, we selected a primary index date 12 months before psoriasis diagnosis, excluding subsequent antibiotic prescriptions and infections to limit capturing treatment of early psoriasis as infection.
Association of Antibiotics, Infections, and Psoriasis
Receipt of an antibiotic prescription within 2 years of the index date was strongly associated with psoriasis in unadjusted models (OR, 1.9; 95% CI, 1.6–2.2). However, after adjusting for prior infection and number of outpatient visits within 2 years (both strong confounders), country, and socioeconomic status, antibiotic exposure was only weakly associated with psoriasis (aOR, 1.2; 95% CI, 1.0–1.5) (Table 2). The associations of prior infections with psoriasis were similar in the same model (skin infection: aOR, 1.5; 95% CI, 1.2–1.7; nonskin infection: aOR, 1.3; 95% CI, 1.1–1.6).When considering the time-frame back to birth, the association of antibiotic exposure with psoriasis was similar to that of skin infections. The association between antibiotics and psoriasis was similar in those with and without prior infection (eTable 2 in the Supplement). Additional adjustment for maternal history yielded similar results (eTable 2 in the Supplement). Treated and untreated skin infections had associations with psoriasis of equal magnitude (Table 3). In contrast, untreated non-skin infections in the last 2 years were more strongly associated with psoriasis than treated infections (P = .02 for comparison of aORs) (Table 3).
Table 2.
Association of Antibiotic Prescriptions and Infections With Psoriasis
| Variable | Exposed Cases (n = 818) | Exposed Controls (n = 7913) | Unadjusted OR | Adjusted ORa (95% CI) | P Value |
|---|---|---|---|---|---|
| Primary analysis: 2-y exposure window | |||||
| Any antibiotic prescription | 453 | 3273 | 1.9 | 1.2 (1.0–1.5) | .05 |
| Any skin infectionb | 248 | 1531 | 1.8 | 1.5 (1.2–1.7) | <.001 |
| Any other infectionc | 466 | 3438 | 1.9 | 1.3 (1.1–1.6) | .005 |
| Secondary analysis: lifetime exposure window | |||||
| Any antibiotic prescription | 686 | 5946 | 2.1 | 1.5 (1.2–1.9) | .002 |
| Any skin infectionb | 465 | 3616 | 1.7 | 1.4 (1.2–1.7) | <.001 |
| Any other infectionc | 712 | 6515 | 1.6 | 1.0 (0.8–1.3) | .86 |
Abbreviation: OR, odds ratio.
Models adjusted for antibiotics, skin infections, nonskin infections, matching, country, deprivation score, and number of outpatient visits.
Includes external genitourinary, herpes simplex, molluscum contagiosum, tinea, varicella, other acute viral exanthems, warts, and other skin or soft-tissue infections.
Includes upper and lower respiratory tract, gastrointestinal tract, urinary tract, and all other infections.
Table 3.
Association of Treated and Untreated Infections with Psoriasis
| Exposure Window | Exposed Cases (n = 818) | Exposed Controls (n = 7913) | Unadjusted OR | Adjusted ORa (95% CI) | P Value |
|---|---|---|---|---|---|
| 2 Years | |||||
| Skin infection,b treated | 84 | 495 | 1.7 | 1.4 (1.1–1.8) | .02 |
| Skin infection,b untreated | 190 | 1184 | 1.7 | 1.4 (1.2–1.7) | <.001 |
| Other infection,c treated | 293 | 2103 | 1.6 | 1.1 (0.9–1.4) | .18 |
| Other infection,c untreated | 366 | 2471 | 2.0 | 1.5 (1.3–1.8) | <.001 |
| Upper respiratory tract infections | |||||
| Any, treated | 241 | 1755 | 1.5 | 1.1 (0.9–1.3) | .39 |
| Any, untreated | 263 | 1816 | 1.7 | 1.3 (1.1–1.5) | .008 |
| Lifetime | |||||
| Skin infection,b treated | 216 | 1534 | 1.5 | 1.3 (1.1–1.5) | .009 |
| Skin infection,b untreated | 293 | 3011 | 1.6 | 1.3 (1.1–1.6) | .001 |
| Other infection,c treated | 573 | 4782 | 1.6 | 1.2 (1.0–1.5) | .03 |
| Other infection,c untreated | 664 | 5888 | 1.6 | 1.2 (1.0–1.5) | .11 |
| Upper respiratory tract infections | |||||
| Any, treated | 510 | 4239 | 1.5 | 1.2 (1.02–1.4) | .03 |
| Any, untreated | 559 | 4937 | 1.4 | 1.1 (0.9–1.3) | .36 |
Abbreviation: OR, odds ratio.
Models adjusted for antibiotic-treated infections, untreated infections, matching, country, deprivation score, and number of outpatient visits.
Includes external genitourinary, herpes simplex, molluscum contagiosum, tinea, varicella, other acute viral exanthems, warts, and other skin or soft-tissue infections.
Includes upper and lower respiratory tract, gastrointestinal tract, urinary tract, and all other infections.
Secondary analyses of cases with documented treatment for psoriasis produced similar results to the primary analyses (eTable 2 in the Supplement). Analyses limited to cases referred to dermatologists (n = 201) yielded estimates that suggested a slightly stronger association of antibiotic exposure and skin infections with psoriasis compared with the primary analysis; however, aORs for antibiotics and both skin and nonskin infections did not differ significantly from one another (eTable 2 in the Supplement).
When considering exposure timing, cases were as likely to have recent antibiotic prescriptions as untreated skin or nonskin infections during the same interval (Table 4). Age of first antibiotic exposure did not relate to psoriasis diagnosis (P = .39). Analyses of antibiotic coverage and specific drug classes resembled those for overall antibiotic exposure, with weak or no associations with psoriasis (eTable 2 in the Supplement). Nonbacterial antimicrobials were not associated with psoriasis.
Table 4.
Effect of Timing of Last Exposure on the Association With Psoriasis
| Timing of Last Exposure Before Psoriasis Diagnosis (mo) | Any Antibiotic
|
Any Untreated Skin Infectiona
|
Any Untreated Other Infectionb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposed Cases (n = 818) | Exposed Controls (n = 7913) | Adjusted OR (95% CI)c | P Value | Exposed Cases (n = 818) | Exposed Controls (n = 7913) | Adjusted OR (95% CI)c | P Value | Exposed Cases (n = 818) | Exposed Controls (n = 7913) | Adjusted OR (95% CI)c | P Value | |
| Unexposed (reference) | 132 | 1967 | 426 | 4902 | 155 | 2037 | ||||||
|
| ||||||||||||
| >24 | 233 | 2673 | 1.3 (1.02–1.7) | .03 | 202 | 1827 | 1.2 (1.01–1.5) | .04 | 302 | 3423 | 1.0 (0.8–1.3) | .80 |
|
| ||||||||||||
| 12–24 | 131 | 1088 | 1.6 (1.2–2.2) | .001 | 80 | 536 | 1.5 (1.2–2.0) | .003 | 141 | 1005 | 1.6 (1.2–2.1) | .001 |
|
| ||||||||||||
| 6–12 | 110 | 822 | 1.9 (1.4–2.5) | <.001 | 45 | 297 | 1.5 (1.1–2.1) | .02 | 108 | 611 | 2.0 (1.5–2.7) | <.001 |
|
| ||||||||||||
| 0–6 | 212 | 1363 | 2.1 (1.6–2.7) | <.001 | 65 | 351 | 1.8 (1.4–2.5) | <.001 | 112 | 837 | 1.5 (1.1–1.9) | .007 |
Abbreviation: OR, odds ratio.
Includes external genitourinary, herpes simplex, molluscum contagiosum, tinea, varicella, other acute viral exanthems, warts, and other skin or soft-tissue infections.
Includes upper and lower respiratory tract, gastrointestinal tract, urinary tract, and all other infections.
Models adjusted for timing of last antibiotic course, skin infection, nonskin infection, matching, country, and deprivation score.
Sensitivity Analyses
When the index date moved from 4 to 36 months before psoriasis diagnosis, the effects of antibiotics remained comparable to skin and nonskin infections, although nonskin infections were not associated with psoriasis when the index date was the date of diagnosis (eFigure 2 in the Supplement). With each study period considered, the effects of untreated infections were similar to or greater than the effects of treated infections. Of note, the ORs for treated and untreated skin infections increased as the index date moved toward psoriasis diagnosis, possibly reflecting antibiotic treatment of early psoriasis as possible infection (protopathic bias). Findings were similar when assuming antibiotic receipt during hospitalization for infection, when analyzing cases with 2 or more psoriasis codes, and when comparing cases and controls matched by practice (eTable 3 in the Supplement). Skin infections were associated with psoriasis in unexposed participants (aOR, 1.5; 95% CI, 1.1–2.1; P = .02). When analyzing the original and practice- matched data sets jointly to protect against type I error, no infection type was consistently associated with psoriasis. In exploratory analyses using the date of psoriasis diagnosis as the index date, antibiotic-treated pharyngitis was associated with guttate psoriasis (aOR, 1.9; 95% CI, 1.0–3.6) but not other psoriasis variants (aOR, 1.0; 95 % CI, 0.7–1.4). In the same models, antibiotic-untreated pharyngitis was not associated with guttate or nonguttate psoriasis.
Discussion
We found that antibiotics were no more strongly associated with newly diagnosed psoriasis in children than infections managed without antibiotics. These findings were consistent across multiple analyses of various types of infections, drug classes, and exposure windows. Among our many analyses, only those limited to cases referred to dermatologists revealed a slightly stronger effect of antibiotics compared with infections. However, the imprecise estimates from this small model (<25% of the overall cohort) did not significantly differ from one another. Overall, our findings support prior literature that infections may play a role in the development of psoriasis5,7,9 but suggest that antibiotics do not substantially, independently contribute to this risk.
Previous studies10–12 linking psoriasis and antibiotics (particularly tetracyclines) were either uncontrolled case reports or observational studies in adults based on self-report, a design susceptible to recall bias. Few children in our cohort were prescribed tetracyclines, limiting our ability to study this antibiotic class. However, our results suggest that an apparent association between antibiotics and psoriasis more likely reflects confounding from infections, at least in children. Older literature analogously suggested a link between pediatric asthma and antibiotic use,24 but more recent studies25,26 implicate respiratory tract infections and familial risk factors as principal drivers of this association. In our study, adjustment for maternal history of psoriasis and other autoimmune diseases did not change results.
Using analogous methods, we and others have previously reported associations between childhood antibiotic exposure and pediatric autoimmune diseases that overlap clinically with psoriasis, namely, IBD and JIA.13–15 One proposed explanation for those findings is antibiotic-induced disruption of microbiota.27 Microbial disruption has been reported in pediatric IBD and 1 form of JIA.16,17 Cesarean delivery is another potential source of early-life microbial disturbance.28 A recent population-based study29 suggested that cesarean delivery was a risk factor for certain pediatric autoimmune diseases, including IBD and JIA, but not for psoriasis. Those findings parallel our own in suggesting that factors disrupting microbiota may not play the same role in psoriasis pathogenesis as in clinically related pediatric autoimmune diseases.
Other studies5,9 have suggested that infections, including group A streptococcus and viruses, may trigger psoriasis. We found that untreated infections not affecting the skin, presumably viral infections, were more strongly associated with psoriasis than antibiotic-treated infections. Although respiratory tract infections have long been described in connection with psoriasis in children, our case-control design was more rigorous than many previous studies30–32 whose conclusions were based on patient and family report in uncontrolled case series and single-arm cohorts. Our data also support previous research linking group A pharyngitis specifically with guttate psoriasis.4,5 In addition, skin infections were associated with psoriasis in our study. It is unclear to what extent this finding reflects misdiagnosis of early psoriasis as infection, misdiagnosis of an infection-related skin diseaseas psoriasis (eg, molluscum contagiosum dermatitis, pityriasis rosea), or whether skin infections may trigger psoriasis in children. Previous work19,33 has found different microbiota in psoriatic lesions compared with healthy skin, including increased abundance of streptococcal and staphylococcal species. The functional significance of these differences remains unclear; some hypothesize that abnormal cutaneous immune responses to skin-dwelling bacteria lead to psoriatic disease.34 Another potential mechanism linking infections and psoriasis are endogenous cutaneous antimicrobial molecules that trigger loss of immune tolerance and production of proinflammatory cytokines.35 Genetic differences may also explain how some children may develop psoriasis after infections. Individuals with psoriasis triggered or worsened after upper respiratory tract infections more likely carried interleukin 20 polymorphisms in one study.36 Another study found that young children with psoriasis more commonly had interleukin 22 promoter variants associated with higher circulating levels of this proinflammatory cytokine important in epithelial host defense.37
An alternative explanation for an association between infections and pediatric psoriasis is that altered immunity in children with psoriasis could render them more susceptible to infections earlier in life. Severe psoriasis has been observed in people with advanced human immunodeficiency virus disease and other immunodeficiencies.8,38 We noted a decreasing association between nonskin infections and psoriasis as the index date moved to the date of diagnosis, arguing against the hypothesis of disease-associated susceptibility to infection. Ascertainment bias, whereby an unrelated infection brings a person’s psoriasis to medical attention, was unlikely to have explained the results because our primary analyses excluded infections within 1 year before GPs diagnosed psoriasis.
Obesity may disproportionately affect individuals with psoriasis.39,40 Some evidence suggests that obesity may predispose children to psoriasis,41 although the pathophysiology of this association is poorly understood. Of interest, antibiotic exposure and microbial disruption may play a role in the development of obesity in children.42 If obesity were on the causal pathway between microbial disturbance and psoriasis, antibiotics could theoretically play a role in the development of psoriasis during a longer time horizon in susceptible individuals. We did not investigate the association of antibiotics, obesity, and psoriasis in this study because most children lacked body mass index data.
Other environmental factors have been suggested as linked to psoriasis in children. In one report,43 children with newly diagnosed psoriasis had an increased risk of tobacco exposure. Smoking has been more clearly associated with the development of psoriasis in adults.44 Our study suggested a possible association with maternal smoking although we did not evaluate this in detail because of missing data. Sunlight exposure could also relate to psoriasis risk by stimulating the production of vitamin D, which is an important treatment for psoriasis.45 In our study, those living in more northern countries of the United Kingdom were more likely to develop psoriasis, which could reflect the risk from lower levels of natural sunlight exposure and endogenous vitamin D production.
Our study has several strengths. The age- and sex-related incidence of psoriasis with female predominance in our cohort was consistent with previous population-based studies in children.46,47 Use of secondary case definitions to improve diagnostic specificity yielded consistent results. In addition, our findings were robust to multiple secondary and sensitivity analyses designed to test study assumptions and to consider potential sources of bias.
This study also has certain limitations. Several factors may have biased results toward the null: evaluation of antibiotic prescriptions rather than dispensing and consumption data, diagnostic misclassification of psoriasis by GPs, and adjustment for clinic visits if this variable were on the causal pathway between antibiotics and psoriasis. However, even unadjusted effects of antibiotics were similar to those of infections, and infections may also be underreported in a clinical database because not all infections come to medical attention. Psoriasis has been validated in THIN but only in adults.2 Because most children in this study were 10 years or younger, we were unable to examine the association between antibiotics and psoriasis in older children and adults, to whom our findings may not apply. Findings from the United Kingdom may also not generalize to other countries with distinct ethnic populations. We also could not evaluate the association between antibiotics and pediatric psoriatic arthritis because we excluded children with preexisting JIA.
Conclusions
Our study reveals that infections are associated with the development of pediatric psoriasis in a large general pediatric population, but antibiotics do not appear to contribute independently to disease risk. Infections may play a role in triggering psoriatic disease in children, perhaps through alterations in skin microbiota or exaggerated immunologic responses. Although psoriasis in children shares clinical and genetic features with IBD and JIA, which have been associated with antibiotic use, the mechanisms underlying the initiation of these autoimmune diseases may differ.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants T32-GM075766 (National Research Service Award, clinical pharmacoepidemiology training grant) (Drs Strom and Horton), F32-AR066461 (National Research Service Award, individual fellowship) (Dr Horton), K08-DK095951 (Dr Scott), and K24-DK078228 (Dr Lewis) from the National Institutes of Health.
Footnotes
Author Contributions: Dr Horton had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Horton, Scott, Rose, Lewis, Strom.
Acquisition, analysis, or interpretation of data: Horton, Scott, Haynes, Putt, Lewis, Strom.
Drafting of the manuscript: Horton.
Critical revision of the manuscript for important intellectual content: Horton, Scott, Haynes, Rose, Lewis, Strom.
Statistical analysis: Horton.
Obtained funding: Horton, Scott, Lewis, Strom.
Administrative, technical, or material support: Haynes.
Study supervision: Rose, Strom.
Conflict of Interest Disclosures: Dr Scott reported receiving research funding from Takeda. Dr Haynes reported working for HealthCore, a wholly owned subsidiary of Anthem. Dr Lewis reported serving as a consultant for the following antibiotic manufacturers: AbbVie, AstraZeneca, Janssen Pharmaceuticals, Medimmune, Merck, Takeda, and Shire. He reported serving on a data and safety monitoring board for clinical trials (unrelated to antibiotics) sponsored by Pfizer, another antibiotic manufacturer. He also reported serving as a consultant for Nestle Health Science and Rebiotix, companies studying therapies for intestinal health. Dr Strom reported consulting for the following antibiotic manufacturers: Abbott, AbbVie, AstraZeneca, Bayer, Bristol-Myers Squibb, GSK, Lundbeck, Novartis, Otsuka, Pfizer, Roche, Sanofi, Takeda, and Teva. No other disclosures were reported.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Additional Contributions: Joel Gelfand, MD, MSCE, provided helpful insight and suggestions, and Molly Collins, MD, critically reviewed the manuscript. No compensation was provided.
Supplemental content at jamadermatology.com
References
- 1.Matusiewicz D, Koerber A, Schadendorf D, Wasem J, Neumann A. Childhood psoriasis: an analysis of German health insurance data. Pediatr Dermatol. 2014;31(1):8–13. doi: 10.1111/pde.12205. [DOI] [PubMed] [Google Scholar]
- 2.Seminara NM, Abuabara K, Shin DB, et al. Validity of The Health Improvement Network (THIN) for the study of psoriasis. Br J Dermatol. 2011;164(3):602–609. doi: 10.1111/j.1365-2133.2010.10134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan C, Korman NJ, Gelfand JM, et al. Research gaps in psoriasis: opportunities for future studies. J AmAcad Dermatol. 2014;70(1):146–167. doi: 10.1016/j.jaad.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 4.Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30(4):424–428. doi: 10.1111/pde.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Arch Dermatol. 1992;128(1):39–42. [PubMed] [Google Scholar]
- 6.Krishnamurthy K, Walker A, Gropper CA, Hoffman C. To treat or not to treat? Management of guttate psoriasis and pityriasis rosea in patients with evidence of group A Streptococcal infection. J Drugs Dermatol. 2010;9(3):241–250. [PubMed] [Google Scholar]
- 7.Weitz M, Kiessling C, Friedrich M, et al. Persistent CMV infection correlates with disease activity and dominates the phenotype of peripheral CD8+ T cells in psoriasis. Exp Dermatol. 2011;20(7):561–567. doi: 10.1111/j.1600-0625.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- 8.Johnson TM, Duvic M, Rapini RP, Rios A. AIDS exacerbates psoriasis. N Engl J Med. 1985;313(22):1415. [PubMed] [Google Scholar]
- 9.Seetharam KA, Sridevi K. Chikungunya infection: a new trigger for psoriasis. J Dermatol. 2011;38(10):1033–1034. doi: 10.1111/j.1346-8138.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsankov N, Botev–Zlatkov N, Lazarova AZ, Kostova M, Popova L, Tonev S. Psoriasis and drugs: influence of tetracyclines on the course of psoriasis. J AmAcad Dermatol. 1988;19(4):629–632. doi: 10.1016/s0190-9622(88)70216-9. [DOI] [PubMed] [Google Scholar]
- 11.Tsankov NK, Vassileva SV, Lazarova AZ, Berowa NV, Botev-Zlatov N. Onset of psoriasis coincident with tetracycline therapy. Australas J Dermatol. 1988;29(2):111–112. doi: 10.1111/j.1440-0960.1988.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz M, Seidenbaum M, Weinrauch L. Penicillin-induced generalized pustular psoriasis. J AmAcad Dermatol. 1987;17(5 pt 2):918–920. doi: 10.1016/s0190-9622(87)70281-3. [DOI] [PubMed] [Google Scholar]
- 13.Kronman MP, Zaoutis TE, Haynes K, Feng R, Coffin SE. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics. 2012;130(4):e794–e803. doi: 10.1542/peds.2011-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvonen M, Virta LJ, Pokka T, Kroger L, Vahasalo P. Repeated exposure to antibiotics in infancy: a predisposing factor for juvenile idiopathic arthritis or a sign of this group’s greater susceptibility to infections? J Rheumatol. 2015;42(3):521–526. doi: 10.3899/jrheum.140348. [DOI] [PubMed] [Google Scholar]
- 15.Horton DB, Scott FI, Haynes K, et al. Antibiotic exposure and juvenile idiopathic arthritis: a case-control study. Pediatrics. 2015;136(2):e333–e343. doi: 10.1542/peds.2015-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoll ML, Kumar R, Morrow CD, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16(6):486. doi: 10.1186/s13075-014-0486-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwiertz A, Jacobi M, Frick JS, Richter M, Rusch K, Kohler H. Microbiota in pediatric inflammatory bowel disease. J Pediatr. 2010;157(2):240–244. e1. doi: 10.1016/j.jpeds.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 18.Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes the altered gut microbiota in patients with psoriatic arthritis, resembling dysbiosis in inflammatory bowel disease. Arthritis Rheumatol. 2015;67(1):128–139. doi: 10.1002/art.38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alekseyenko AV, Perez-Perez GI, De Souza A, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1(1):31–31. doi: 10.1186/2049-2618-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blak BT, Thompson M, Dattani H, Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19(4):251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 21.Austin PC, Anderson GM, Cigsar C, Gruneir A. Comparing the cohort design and the nested case-control design in the presence of both time-invariant and time-dependent treatment and competing risks: bias and precision. Pharmacoepidemiol Drug Saf. 2012;21(7):714–724. doi: 10.1002/pds.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JD, Schinnar R, Bilker WB, Wang X, Strom BL. Validation studies of the health improvement network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf. 2007;16(4):393–401. doi: 10.1002/pds.1335. [DOI] [PubMed] [Google Scholar]
- 23.Jernberg C, Lofmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1(1):56–66. doi: 10.1038/ismej.2007.3. [DOI] [PubMed] [Google Scholar]
- 24.Marra F, Lynd L, Coombes M, et al. Does antibiotic exposure during infancy lead to development of asthma? a systematic review and metaanalysis. Chest. 2006;129(3):610–618. doi: 10.1378/chest.129.3.610. [DOI] [PubMed] [Google Scholar]
- 25.Mai XM, Kull I, Wickman M, Bergstrom A. Antibiotic use in early life and development of allergic diseases: respiratory infection as the explanation. Clin Exp Allergy. 2010;40(8):1230–1237. doi: 10.1111/j.1365-2222.2010.03532.x. [DOI] [PubMed] [Google Scholar]
- 26.Ortqvist AK, Lundholm C, Kieler H, et al. Antibiotics in fetal and early life and subsequent childhood asthma: nationwide population based study with sibling analysis. BMJ. 2014;349:g6979. doi: 10.1136/bmj.g6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vangay P, Ward T, Gerber JS, Knights D. Antibiotics, pediatric dysbiosis, and disease. Cell Host Microbe. 2015;17(5):553–564. doi: 10.1016/j.chom.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakobsson HE, Abrahamsson TR, Jenmalm MC, et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. 2014;63(4):559–566. doi: 10.1136/gutjnl-2012-303249. [DOI] [PubMed] [Google Scholar]
- 29.Sevelsted A, Stokholm J, Bonnelykke K, Bisgaard H. Cesarean section and chronic immune disorders. Pediatrics. 2015;135(1):e92–e98. doi: 10.1542/peds.2014-0596. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Lin Y, Liu HJ, Huang CZ, Feng AP, Li JW. Childhood psoriasis: a study of 137 cases from central China. World J Pediatr. 2010;6(3):260–264. doi: 10.1007/s12519-010-0213-0. [DOI] [PubMed] [Google Scholar]
- 31.Nyfors A, Lemholt K. Psoriasis in children: a short review and a survey of 245 cases. Br J Dermatol. 1975;92(4):437–442. doi: 10.1111/j.1365-2133.1975.tb03105.x. [DOI] [PubMed] [Google Scholar]
- 32.Kumar B, Jain R, Sandhu K, Kaur I, Handa S. Epidemiology of childhood psoriasis: a study of 419 patients from northern India. Int J Dermatol. 2004;43(9):654–658. doi: 10.1111/j.1365-4632.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- 33.Gao Z, Tseng CH, Strober BE, Pei Z, Blaser MJ. Substantial alterations of the cutaneous bacterial biota in psoriatic lesions. PLoS One. 2008;3(7):e2719. doi: 10.1371/journal.pone.0002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fry L, Baker BS, Powles AV, Fahlen A, Engstrand L. Is chronic plaque psoriasis triggered by microbiota in the skin? Br J Dermatol. 2013;169(1):47–52. doi: 10.1111/bjd.12322. [DOI] [PubMed] [Google Scholar]
- 35.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 36.Chen XY, Jin LW, Chen YW, et al. The association between the IL-20-1723C→G allele on the 1q chromosome and psoriasis triggered or exacerbated by an upper respiratory tract infection in the Chinese Han population. Dermatology. 2011;222(1):24–30. doi: 10.1159/000320772. [DOI] [PubMed] [Google Scholar]
- 37.Nikamo P, Cheuk S, Lysell J, et al. Genetic variants of the IL22 promoter associate to onset of psoriasis before puberty and increased IL-22 production in T cells. J Invest Dermatol. 2014;134(6):1535–1541. doi: 10.1038/jid.2014.5. [DOI] [PubMed] [Google Scholar]
- 38.Baroudjian B, Viguier M, Battistella M, et al. Psoriasis associated with idiopathic CD4+ T-cell lymphopenia: a regulatory T-cell defect? Br J Dermatol. 2014;171(1):186–189. doi: 10.1111/bjd.12922. [DOI] [PubMed] [Google Scholar]
- 39.Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176. doi: 10.1001/jamadermatol.2013.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schafer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–636. doi: 10.1111/j.1365-2133.2009.09593.x. [DOI] [PubMed] [Google Scholar]
- 41.Becker L, Tom WL, Eshagh K, Benjamin LT, Paller AS. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150(5):573–574. doi: 10.1001/jamadermatol.2014.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168(11):1063–1069. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 43.Ozden MG, Tekin NS, Gurer MA, et al. Environmental risk factors in pediatric psoriasis: a multicenter case-control study. Pediatr Dermatol. 2011;28(3):306–312. doi: 10.1111/j.1525-1470.2011.01408.x. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong AW, Harskamp CT, Dhillon JS, Armstrong EJ. Psoriasis and smoking: a systematic review and meta-analysis. Br J Dermatol. 2014;170(2):304–314. doi: 10.1111/bjd.12670. [DOI] [PubMed] [Google Scholar]
- 45.Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54(4):383–392. doi: 10.1111/ijd.12790. [DOI] [PubMed] [Google Scholar]
- 46.Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J AmAcad Dermatol. 2010;62(6):979–987. doi: 10.1016/j.jaad.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. doi: 10.1001/archderm.141.12.1537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.