Abstract
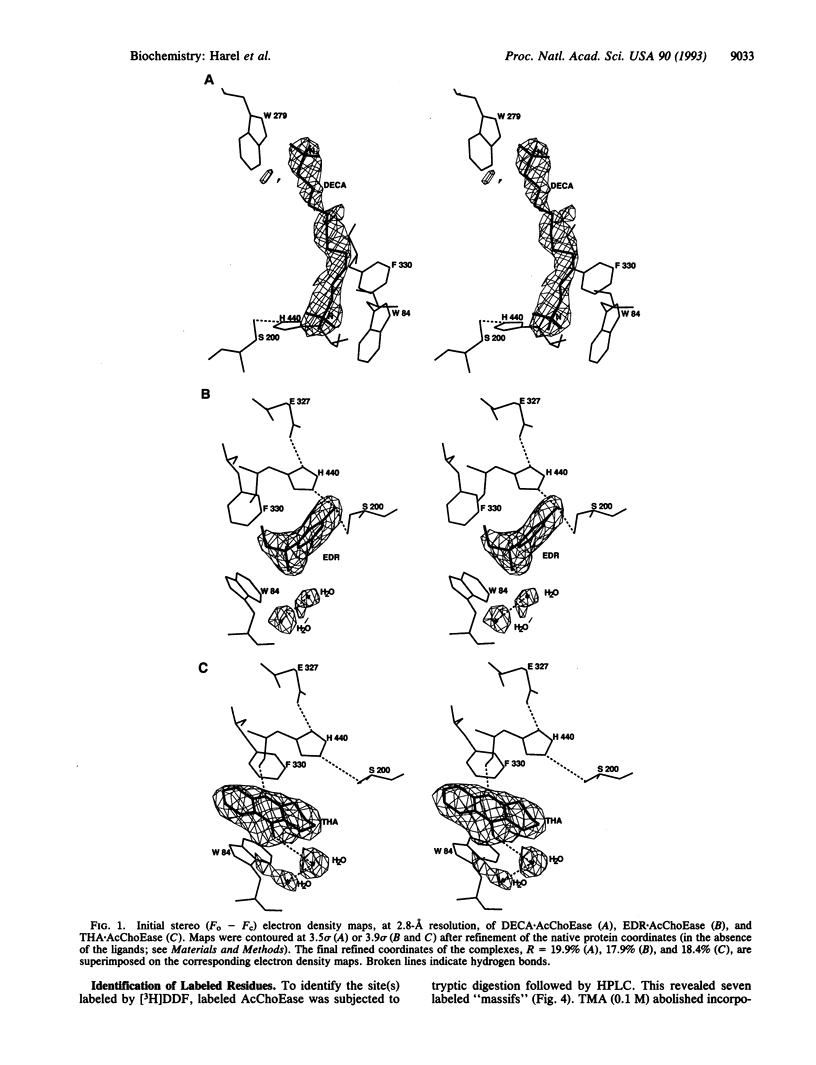
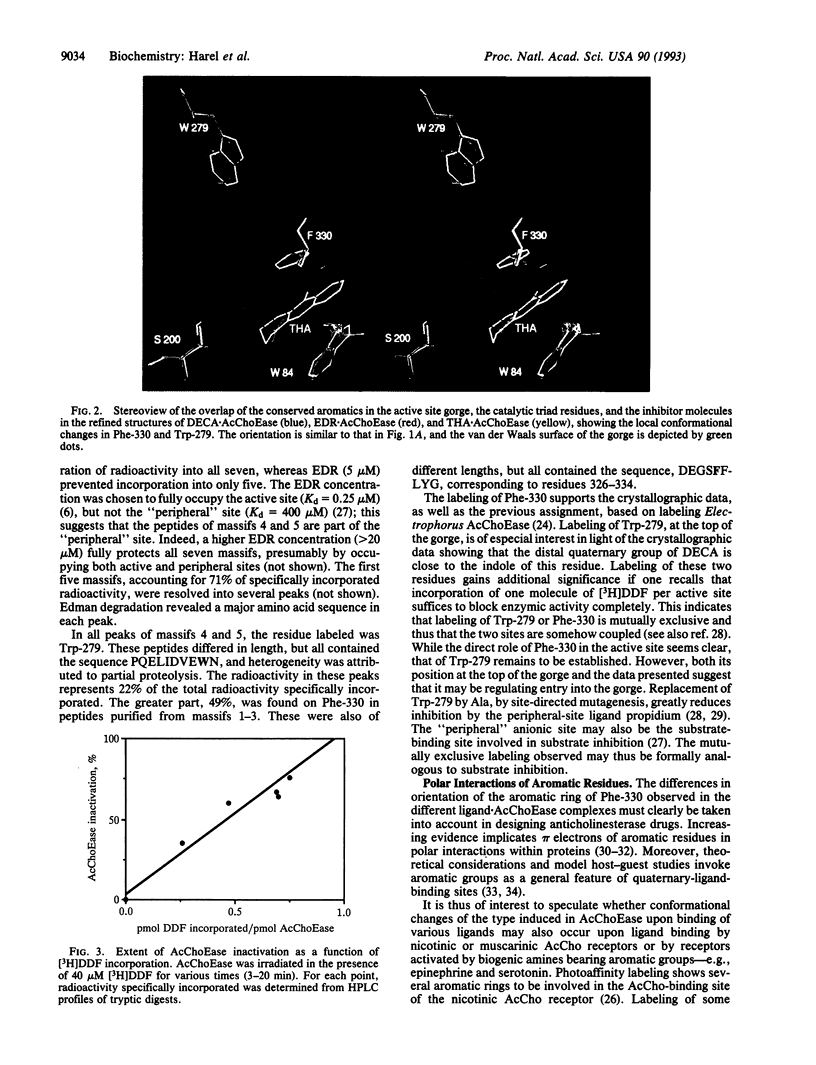
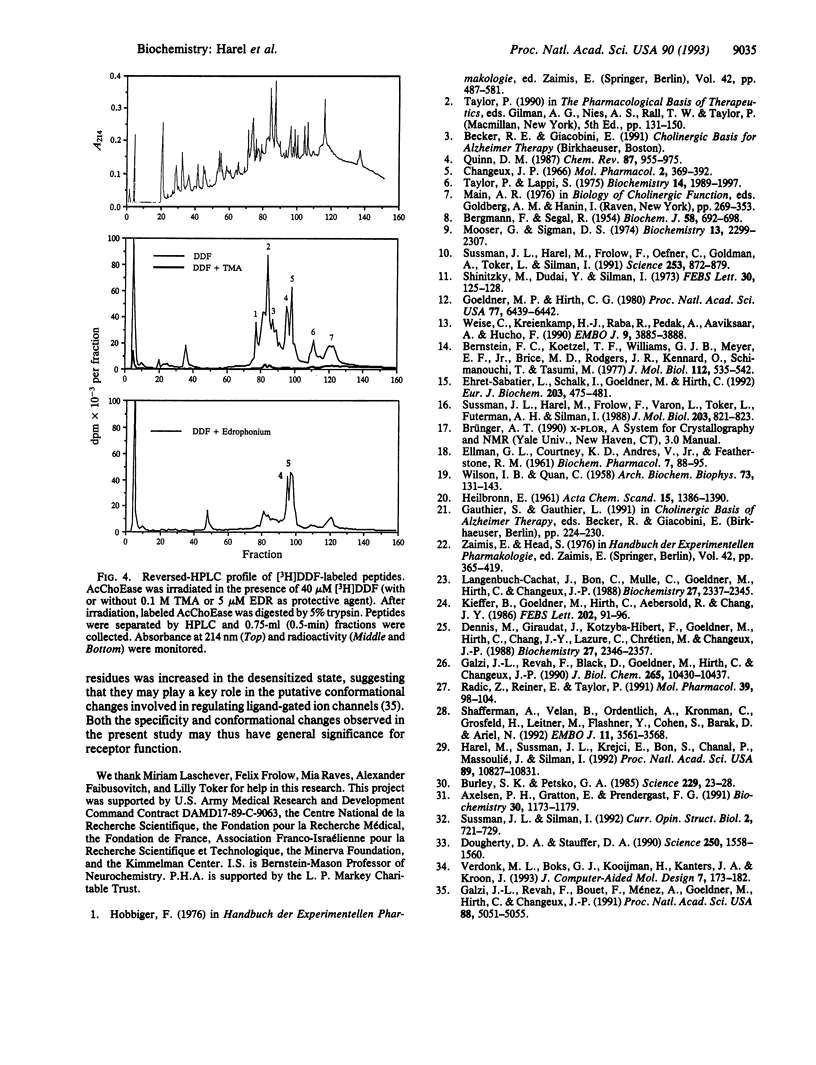
Binding sites of Torpedo acetylcholinesterase (EC 3.1.1.7) for quaternary ligands were investigated by x-ray crystallography and photoaffinity labeling. Crystal structures of complexes with ligands were determined at 2.8-A resolution. In a complex with edrophonium, and quaternary nitrogen of the ligand interacts with the indole of Trp-84, and its m-hydroxyl displays bifurcated hydrogen bonding to two members of the catalytic triad, Ser-200 and His-440. In a complex with tacrine, the acridine is stacked against the indole of Trp-84. The bisquaternary ligand decamethonium is oriented along the narrow gorge leading to the active site; one quaternary group is apposed to the indole of Trp-84 and the other to that of Trp-279, near the top of the gorge. The only major conformational difference between the three complexes is in the orientation of the phenyl ring of Phe-330. In the decamethonium complex it lies parallel to the surface of the gorge; in the other two complexes it is positioned to make contact with the bound ligand. This close interaction was confirmed by photoaffinity labelling by the photosensitive probe 3H-labeled p-(N,N-dimethylamino)benzenediazonium fluoroborate, which labeled, predominantly, Phe-330 within the active site. Labeling of Trp-279 was also observed. One mole of label is incorporated per mole of AcChoEase inactivated, indicating that labeling of Trp-279 and that of Phe-330 are mutually exclusive. The structural and chemical data, together, show the important role of aromatic groups as binding sites for quaternary ligands, and they provide complementary evidence assigning Trp-84 and Phe-330 to the "anionic" subsite of the active site and Trp-279 to the "peripheral" anionic site.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsen P. H., Gratton E., Prendergast F. G. Experimentally verifying molecular dynamics simulations through fluorescence anisotropy measurements. Biochemistry. 1991 Feb 5;30(5):1173–1179. doi: 10.1021/bi00219a002. [DOI] [PubMed] [Google Scholar]
- BERGMANN F., SEGAL R. The relationship of quaternary ammonium salts to the anionic sites of true and pseudo cholinesterase. Biochem J. 1954 Dec;58(4):692–698. doi: 10.1042/bj0580692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Aromatic-aromatic interaction: a mechanism of protein structure stabilization. Science. 1985 Jul 5;229(4708):23–28. doi: 10.1126/science.3892686. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol Pharmacol. 1966 Sep;2(5):369–392. [PubMed] [Google Scholar]
- Dennis M., Giraudat J., Kotzyba-Hibert F., Goeldner M., Hirth C., Chang J. Y., Lazure C., Chrétien M., Changeux J. P. Amino acids of the Torpedo marmorata acetylcholine receptor alpha subunit labeled by a photoaffinity ligand for the acetylcholine binding site. Biochemistry. 1988 Apr 5;27(7):2346–2357. doi: 10.1021/bi00407a016. [DOI] [PubMed] [Google Scholar]
- Dougherty D. A., Stauffer D. A. Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science. 1990 Dec 14;250(4987):1558–1560. doi: 10.1126/science.2274786. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ehret-Sabatier L., Schalk I., Goeldner M., Hirth C. Photoaffinity labelling of cholinesterases. Discrimination between active and peripheral sites. Eur J Biochem. 1992 Feb 1;203(3):475–481. doi: 10.1111/j.1432-1033.1992.tb16572.x. [DOI] [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Black D., Goeldner M., Hirth C., Changeux J. P. Identification of a novel amino acid alpha-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. J Biol Chem. 1990 Jun 25;265(18):10430–10437. [PubMed] [Google Scholar]
- Galzi J. L., Revah F., Bouet F., Ménez A., Goeldner M., Hirth C., Changeux J. P. Allosteric transitions of the acetylcholine receptor probed at the amino acid level with a photolabile cholinergic ligand. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5051–5055. doi: 10.1073/pnas.88.11.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner M. P., Hirth C. G. Specific photoaffinity labeling induced by energy transfer: application to irreversible inhibition of acetylcholinesterase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6439–6442. doi: 10.1073/pnas.77.11.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel M., Sussman J. L., Krejci E., Bon S., Chanal P., Massoulié J., Silman I. Conversion of acetylcholinesterase to butyrylcholinesterase: modeling and mutagenesis. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10827–10831. doi: 10.1073/pnas.89.22.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbuch-Cachat J., Bon C., Mulle C., Goeldner M., Hirth C., Changeux J. P. Photoaffinity labeling of the acetylcholine binding sites on the nicotinic receptor by an aryldiazonium derivative. Biochemistry. 1988 Apr 5;27(7):2337–2345. doi: 10.1021/bi00407a015. [DOI] [PubMed] [Google Scholar]
- Mooser G., Sigman D. S. Ligand binding properties of acetylcholinesterase determined with fluorescent probes. Biochemistry. 1974 May 21;13(11):2299–2307. doi: 10.1021/bi00708a010. [DOI] [PubMed] [Google Scholar]
- Radić Z., Reiner E., Taylor P. Role of the peripheral anionic site on acetylcholinesterase: inhibition by substrates and coumarin derivatives. Mol Pharmacol. 1991 Jan;39(1):98–104. [PubMed] [Google Scholar]
- Shafferman A., Velan B., Ordentlich A., Kronman C., Grosfeld H., Leitner M., Flashner Y., Cohen S., Barak D., Ariel N. Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center. EMBO J. 1992 Oct;11(10):3561–3568. doi: 10.1002/j.1460-2075.1992.tb05439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky Meir, Dudai Yadin, Silman Israel. Spectral evidence for the presence of tryptophan in the binding site of acetylcholinesterase. FEBS Lett. 1973 Feb 15;30(1):125–128. doi: 10.1016/0014-5793(73)80633-7. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Oefner C., Goldman A., Toker L., Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- Sussman J. L., Harel M., Frolow F., Varon L., Toker L., Futerman A. H., Silman I. Purification and crystallization of a dimeric form of acetylcholinesterase from Torpedo californica subsequent to solubilization with phosphatidylinositol-specific phospholipase C. J Mol Biol. 1988 Oct 5;203(3):821–823. doi: 10.1016/0022-2836(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Taylor P., Lappi S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry. 1975 May 6;14(9):1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- Verdonk M. L., Boks G. J., Kooijman H., Kanters J. A., Kroon J. Stereochemistry of charged nitrogen-aromatic interactions and its involvement in ligand-receptor binding. J Comput Aided Mol Des. 1993 Apr;7(2):173–182. doi: 10.1007/BF00126443. [DOI] [PubMed] [Google Scholar]
- WILSON I. B., QUAN C. Acetylcholinesterase studies on molecular complementariness. Arch Biochem Biophys. 1958 Jan;73(1):131–143. doi: 10.1016/0003-9861(58)90248-0. [DOI] [PubMed] [Google Scholar]
- Weise C., Kreienkamp H. J., Raba R., Pedak A., Aaviksaar A., Hucho F. Anionic subsites of the acetylcholinesterase from Torpedo californica: affinity labelling with the cationic reagent N,N-dimethyl-2-phenyl-aziridinium. EMBO J. 1990 Dec;9(12):3885–3888. doi: 10.1002/j.1460-2075.1990.tb07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]