Abstract
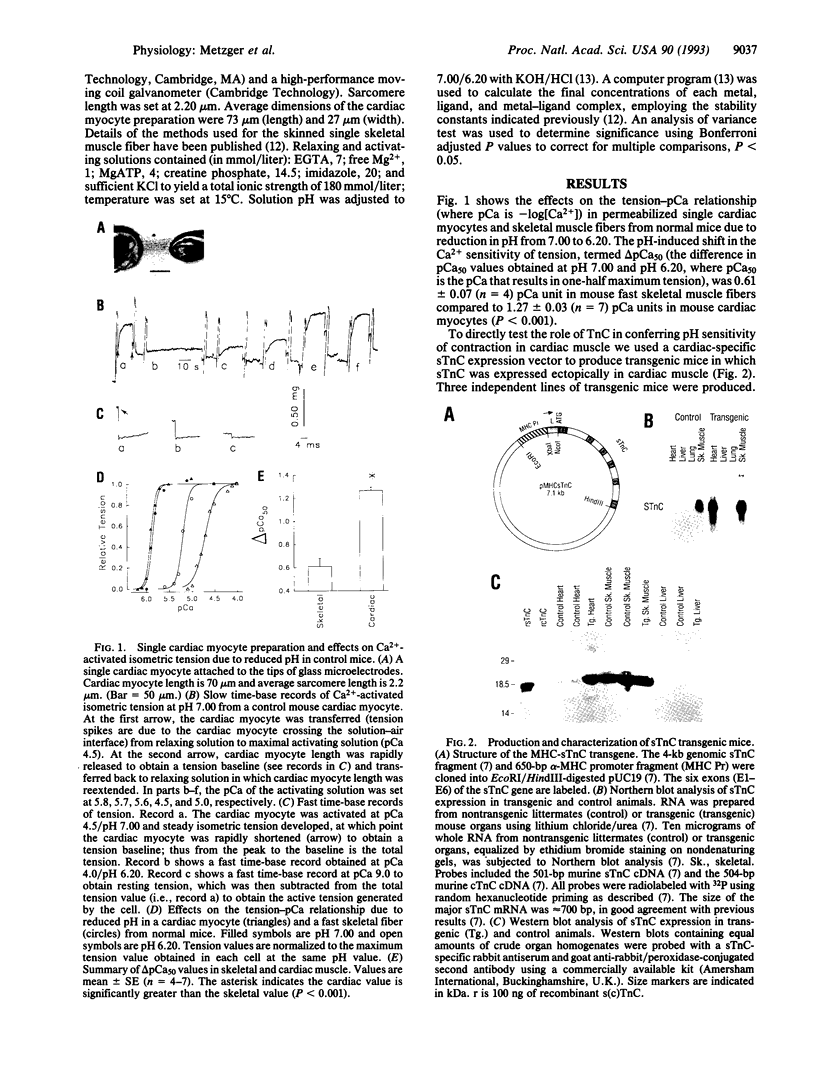
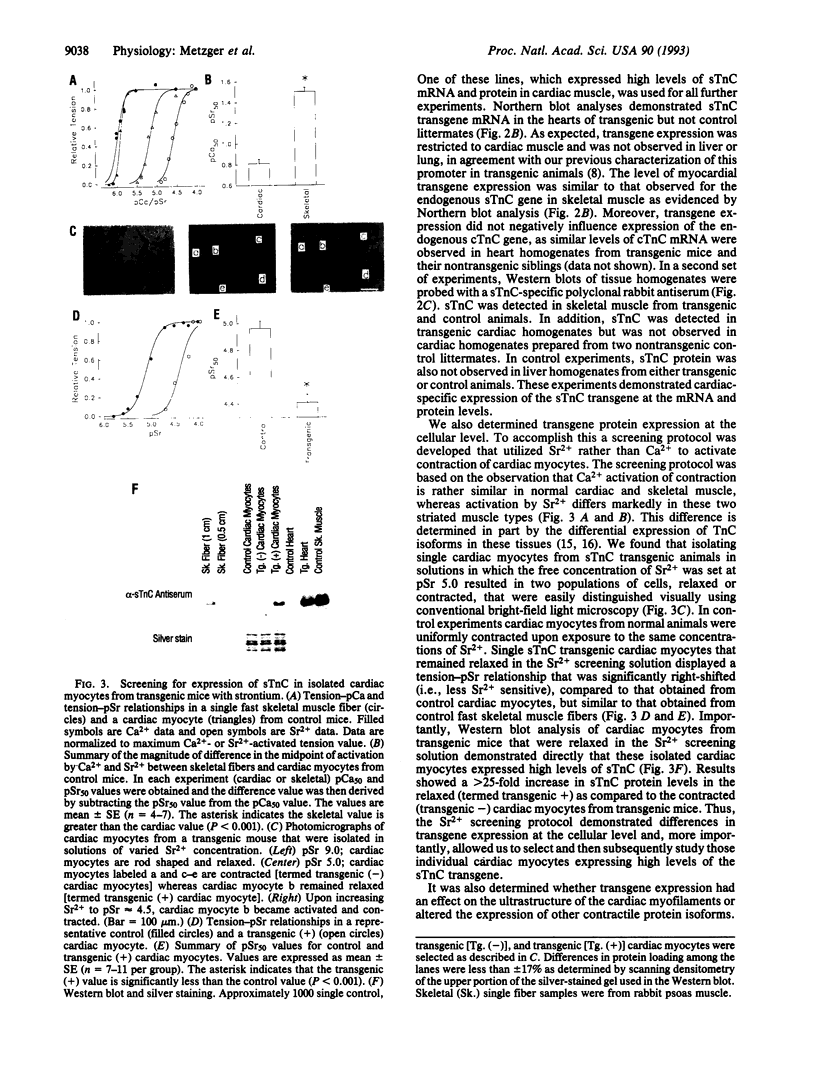
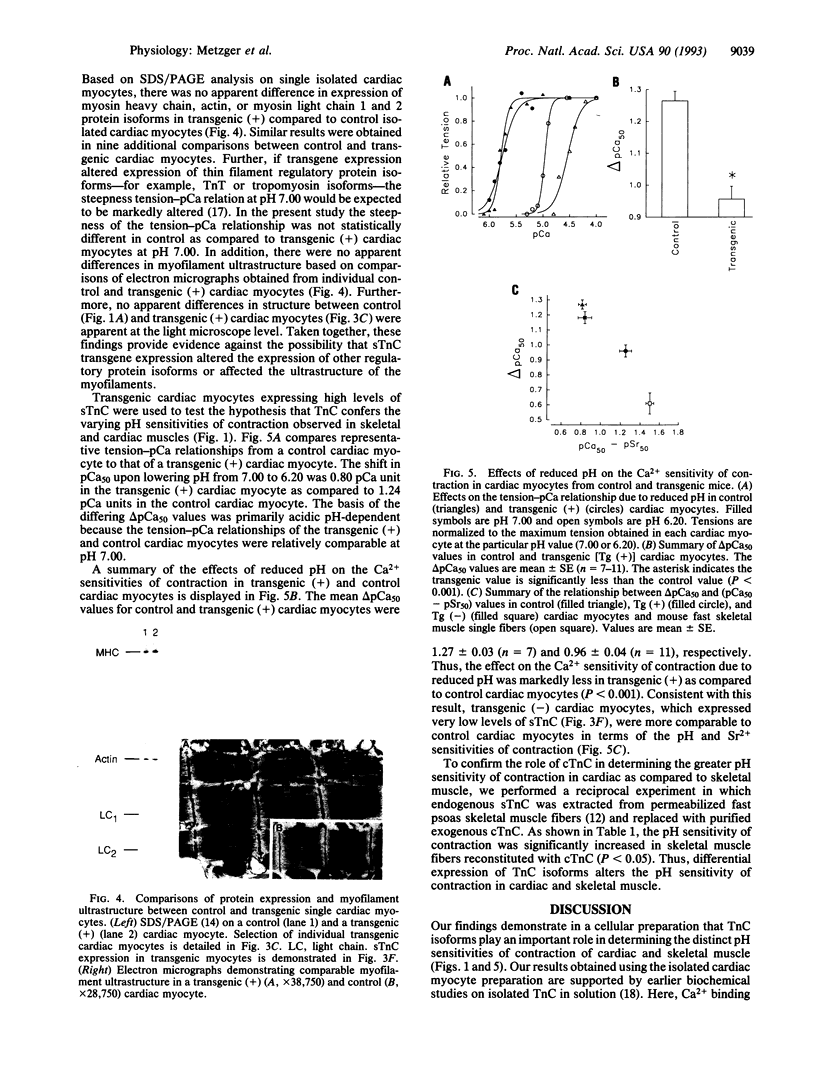
Depressed contractile function plays a primary role in the pathophysiology of acute myocardial ischemia. Intracellular acidification is an important factor underlying the inhibition of force production in the ischemic myocardium. The effect of acidosis to depress contractility is markedly greater in cardiac as compared to skeletal muscle; however, the molecular basis of this difference in sensitivity to acidosis is not clearly understood. In this report, we describe transgenic mice that express the fast skeletal isoform of troponin C (sTnC) in cardiac muscle. In permeabilized single cardiac myocytes the shift in the midpoint of the tension-pCa relationship (i.e., pCa50, where pCa is -log[Ca2+]) due to lowering pH from 7.00 to 6.20 was 1.27 +/- 0.03 (n = 7) pCa units in control cardiac TnC (cTnC) expressing myocytes and 0.96 +/- 0.04 (n = 11) pCa unit in transgenic cardiac myocytes (P < 0.001). The effect of pH to alter maximum Ca(2+)-activated tension was unchanged by TnC isoforms in these cardiac myocytes. In a reciprocal experiment, contractile sensitivity to acidosis was increased in fast skeletal muscle fibers following extraction of endogenous sTnC and reconstitution with purified cTnC in vitro. Our findings demonstrate that TnC plays an important role in determining the profound sensitivity of cardiac muscle to acidosis and identify cTnC as a target for therapeutic interventions designed to modify ischemia-induced myocardial contractile dysfunction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu A., Scordilis S. P., Sonnenblick E. H., Gulati J. The control of myocardial contraction with skeletal fast muscle troponin C. J Biol Chem. 1987 Apr 25;262(12):5815–5822. [PubMed] [Google Scholar]
- Bailey I. A., Williams S. R., Radda G. K., Gadian D. G. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem J. 1981 Apr 15;196(1):171–178. doi: 10.1042/bj1960171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremel R. D., Weber A. Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol. 1972 Jul 26;238(82):97–101. doi: 10.1038/newbio238097a0. [DOI] [PubMed] [Google Scholar]
- Donaldson S. K., Hermansen L., Bolles L. Differential, direct effects of H+ on Ca2+ -activated force of skinned fibers from the soleus, cardiac and adductor magnus muscles of rabbits. Pflugers Arch. 1978 Aug 25;376(1):55–65. doi: 10.1007/BF00585248. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell W. H. On the Tonicity of the Heart and Blood Vessels. J Physiol. 1880 Aug;3(1):48–92.16. doi: 10.1113/jphysiol.1880.sp000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Gulati J., Babu A. Effect of acidosis on Ca2+ sensitivity of skinned cardiac muscle with troponin C exchange. Implications for myocardial ischemia. FEBS Lett. 1989 Mar 13;245(1-2):279–282. doi: 10.1016/0014-5793(89)80237-6. [DOI] [PubMed] [Google Scholar]
- Hoar P. E., Potter J. D., Kerrick W. G. Skinned ventricular fibres: troponin C extraction is species-dependent and its replacement with skeletal troponin C changes Sr2+ activation properties. J Muscle Res Cell Motil. 1988 Apr;9(2):165–173. doi: 10.1007/BF01773738. [DOI] [PubMed] [Google Scholar]
- Katz E. B., Steinhelper M. E., Delcarpio J. B., Daud A. I., Claycomb W. C., Field L. J. Cardiomyocyte proliferation in mice expressing alpha-cardiac myosin heavy chain-SV40 T-antigen transgenes. Am J Physiol. 1992 Jun;262(6 Pt 2):H1867–H1876. doi: 10.1152/ajpheart.1992.262.6.H1867. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. Mechanisms of acute ischemic contractile failure of the heart. Role of intracellular calcium. J Clin Invest. 1991 Aug;88(2):361–367. doi: 10.1172/JCI115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. F., Ball K., Gao L. Z., Kumar P., Solaro R. J. Identification and functional significance of troponin I isoforms in neonatal rat heart myofibrils. Circ Res. 1991 Nov;69(5):1244–1252. doi: 10.1161/01.res.69.5.1244. [DOI] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J Physiol. 1990 Sep;428:737–750. doi: 10.1113/jphysiol.1990.sp018238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Greater hydrogen ion-induced depression of tension and velocity in skinned single fibres of rat fast than slow muscles. J Physiol. 1987 Dec;393:727–742. doi: 10.1113/jphysiol.1987.sp016850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. M., Moss R. L. Kinetics of a Ca(2+)-sensitive cross-bridge state transition in skeletal muscle fibers. Effects due to variations in thin filament activation by extraction of troponin C. J Gen Physiol. 1991 Aug;98(2):233–248. doi: 10.1085/jgp.98.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmacek M. S., Leiden J. M. Structure, function, and regulation of troponin C. Circulation. 1991 Sep;84(3):991–1003. doi: 10.1161/01.cir.84.3.991. [DOI] [PubMed] [Google Scholar]
- Schachat F. H., Diamond M. S., Brandt P. W. Effect of different troponin T-tropomyosin combinations on thin filament activation. J Mol Biol. 1987 Dec 5;198(3):551–554. doi: 10.1016/0022-2836(87)90300-7. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., el-Saleh S. C., Kentish J. C. Ca2+, pH and the regulation of cardiac myofilament force and ATPase activity. Mol Cell Biochem. 1989 Sep 7;89(2):163–167. doi: 10.1007/BF00220770. [DOI] [PubMed] [Google Scholar]
- Steinhelper M. E., Cochrane K. L., Field L. J. Hypotension in transgenic mice expressing atrial natriuretic factor fusion genes. Hypertension. 1990 Sep;16(3):301–307. doi: 10.1161/01.hyp.16.3.301. [DOI] [PubMed] [Google Scholar]
- Sweitzer N. K., Moss R. L. The effect of altered temperature on Ca2(+)-sensitive force in permeabilized myocardium and skeletal muscle. Evidence for force dependence of thin filament activation. J Gen Physiol. 1990 Dec;96(6):1221–1245. doi: 10.1085/jgp.96.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Zhan Q., Tao T., Gergely J. pH-dependent structural transition in rabbit skeletal troponin C. J Biol Chem. 1987 Jul 15;262(20):9636–9640. [PubMed] [Google Scholar]
- Zot A. S., Potter J. D. Reciprocal coupling between troponin C and myosin crossbridge attachment. Biochemistry. 1989 Aug 8;28(16):6751–6756. doi: 10.1021/bi00442a031. [DOI] [PubMed] [Google Scholar]