Abstract
Fear-related behaviors are prone to relapse following extinction. We tested in humans a compound extinction design (“deepened extinction”) shown in animal studies to reduce post-extinction fear recovery. Adult subjects underwent fear conditioning to a visual and an auditory conditioned stimulus (CSA and CSB, respectively) separately paired with an electric shock. The target CS (CSA) was extinguished alone followed by compound presentations of the extinguished CSA and nonextinguished CSB. Recovery of conditioned skin conductance responses to CSA was reduced 24 h after compound extinction, as compared with a group who received an equal number of extinction trials to the CSA alone.
The inability to control or regulate emotional expression in the face of nonthreatening stimuli is a hallmark of many anxiety disorders. A growing body of clinical translational research is beginning to characterize abnormalities associated with the control of fear expression in anxiety disorders by utilizing fear conditioning and experimental extinction protocols (Milad and Quirk 2012; Vervliet et al. 2013). Clinical translational research benefits from decades of work in laboratory animals, which reveal the ways conditioned fear returns following extinction training. As extinction forms the basis for effective therapeutic interventions like exposure therapy, techniques that enhance extinction have value for improving the control of fear and arousal in clinical anxiety. Importantly, studies in laboratory animals show how extinction can be modulated through the use of innovative behavioral techniques (Craske et al. 2014; Fitzgerald et al. 2014; Myskiw et al. 2014). Here, we translated to humans one such behavioral technique previously developed in animal models, known as deepened extinction (Rescorla 2006).
Deepened extinction is an emerging procedure based on maximizing surprise in order to promote the loss of associative value of conditioned stimuli (CS) that no longer predict unconditioned stimuli (US). The technique involves an initial acquisition phase in which multiple CSs (e.g., a tone and a light) are separately paired with the same US (e.g., an electric shock), an isolated extinction phase, and a compound CS extinction phase. The compound CS can be either a combination of two previously extinguished CSs (Hendry 1982) or a compound of a previously extinguished CS (the target) with a nonextinguished one (Reberg 1972). In both versions, the compound CS presentation restores conditioned responding following CS alone extinction trials, providing evidence that the compound presentation generates renewed expectation of the US. Both versions also diminished fear recovery in laboratory animals, providing further evidence for deepened extinction (Leung et al. 2012). In line with prediction error models of associative learning (Rescorla and Wagner 1972), the unexpected absence of the US deepens the loss of associative value for the extinguished CS below the level attained during CS alone extinction trials.
Experiments in rats have demonstrated the effectiveness of deepened extinction in reducing post-extinction fear recovery (Rescorla 2006; Leung et al. 2012; McConnell et al. 2013), recovery of lever press responding (Janak and Corbit 2011) and recovery of cocaine seeking (Kearns et al. 2012; Kearns and Weiss 2012). There are clinical examples of multiple cue exposure in humans as well; for example, therapies that initially expose a patient with spider phobia to different spiders in isolation, followed by multiple spiders at the same time (for review, see Craske et al. 2014). Leung et al. (2012), directly compared two forms of deepened extinction, and found that the Reberg (1972) method of combining an extinguished CS with a nonextinguished CS resulted in stronger inhibition of the conditioned response than the Hendry (1982) method of combining multiple extinguished CSs.
Although deepened extinction is well supported by associative learning models and laboratory research in rats, empirical research on deepened extinction in humans is extremely limited. Recently, Culver et al. (2014) provided evidence that the Hendry (1982) method of extinguishing two CSs in isolation prior to compound extinction diminished post-extinction recovery and reinstatement following unsignaled presentations of an aversive human scream (US). Here, we used intermodal cues (visual and auditory) and a potentially stronger US (i.e., electrical shock) to explore the effect of compound extinction with an extinguished and nonextinguished cue (Reberg 1972) on spontaneous recovery and reinstatement in humans, which animal models demonstrate is a more robust procedure to deepen extinction (Leung et al. 2012).
Ninety-six healthy adult volunteers provided written informed consent in accordance with the New York University Committee on Activities Involving Human Subjects and were compensated monetarily for their participation. For a variety of reasons, measurable electrodermal responses cannot be obtained from some individuals, i.e., nonresponders (Dawson et al. 2007). Because our primary dependent measure was the skin conductance response (SCR), we conducted a preconditioning SCR test to exclude individuals who exhibited a total lack of measurable electrodermal activity from participating any further in the study (N = 21). The SCR test involved a deep breath while the experimenter monitored psychophysiological recordings for a deflection in SCRs. An additional eight subjects were excluded from analysis due to technical problems (N = 3) or failure to follow instructions (N = 5). Finally, as spontaneous recovery is predicated on subjects having initially acquired and extinguished conditioned fear prior to recovery test, and deepened extinction assumes both successful extinction of a CS in isolation and successful extinction of the compound CS, failure to exhibit successful fear acquisition and extinction (in both extinction blocks) were exclusionary criteria. This resulted in an additional 16 subjects being removed from analysis for failure to exhibit either successful acquisition (N = 8) or extinction in either extinction block (N = 8) as defined below. The final sample included 51 subjects (age range = 18–36, mean age = 22.88 yr, SD = 3.86, 26 females). Subjects were randomly assigned to either a Deepened Extinction group (DE; N = 25, 8 females) or to a Control Group that underwent standard extinction procedures (CTL; N = 26, 18 females). Subjects wore headphones throughout the entire experiment on both days (Sennheiser HD-280 PRO).
The experiment began with a short habituation phase that included one presentation of each CS to reduce initial orienting responses (data not reported). The first trial of each experimental block was always a CS− trial that was discarded from analysis to account for initial orienting responses (or dishabituation) at the start of each block (see also Schiller et al. 2010). The paradigm occurred over 2 d (Table 1). On Day 1, subjects underwent a partial reinforcement simple discriminatory fear conditioning procedure (Acquisition) using three CSs: two colored squares (blue or green), one paired with the shock (CSA), and one never paired with the shock (CS−), and a tone paired with the shock (CSB, 500 Hz, <70 decibels). The acquisition phase occurred over two blocks (Acquisitions 1 and 2), and each block included six nonreinforced trials of each CS, along with an additional four CSA and four CSB trials paired with shock (40% CS–US pairing rate).
Table 1.
Experimental design
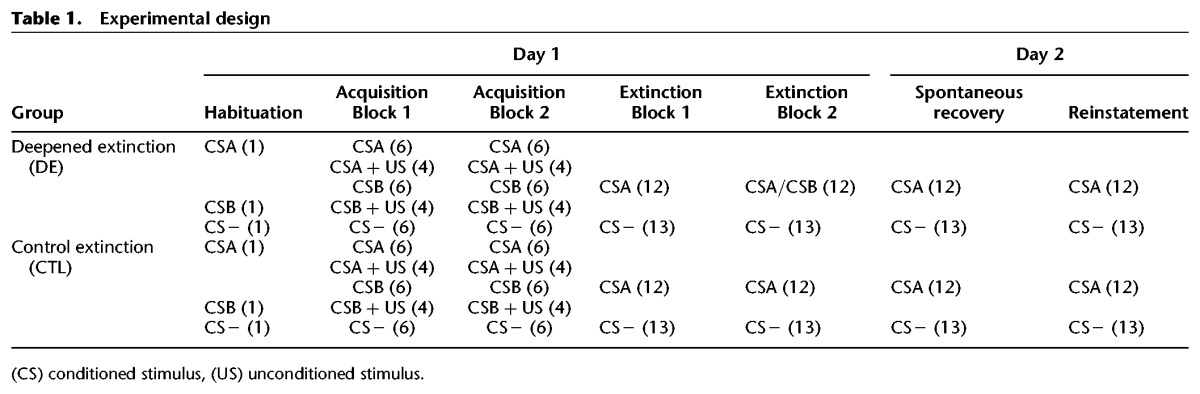
Following acquisition, both groups underwent an extinction training phase. Extinction training occurred over two blocks (Extinctions 1 and 2), and each block included 12 CSA and 13 CS− trials in the absence of the US. The CSB (tone) was not presented during Extinction 1, and this block was identical for both groups. For the CTL group, the second block of extinction (Extinction 2) was identical to Extinction 1 and included 12 CSA and 13 CS− trials. In contrast, the DE group received 12 compound presentations of CSA/CSB (simultaneous 6-sec audio–visual presentation) along with 13 CS− trials during Extinction 2. Twenty-four hours later, subjects in both groups returned for a test of spontaneous recovery. The spontaneous recovery test included 12 CSA and 13 CS− presentations in the absence of the US. A test of reinstatement followed the spontaneous recovery test, and involved three unsignaled shocks followed 15 sec later by 12 CSA and 13 CS− presentations.
Each CS was presented for 6 sec followed by an 8-sec inter-trial interval that included a crosshair on a blank background. The color of CSA and CS− were counterbalanced between subjects as blue or green, and trial order was counterbalanced between subjects. The presentation of each CS was pseudorandomized so that no more than two of the same stimuli occurred in a row. Subjects were not informed of the CS–US contingencies at any point of the experiment, but were told to pay attention and try to learn the association between the stimuli and the shock.
Subjects who did not show evidence of conditioning to CSA and CSB during acquisition, extinction to CSA during Extinction 1, and extinction to CSA (for CTL) or CSA/CSB (for DE) during Extinction 2 were excluded from all reported analyses. Successful fear conditioning was defined as a mean SCR difference score of CSA minus CS− >0.1 microsiemens (µS), and CSB minus CS− >0.1 µS. In addition, subjects exhibiting an SCR difference score >0.1 µS on the last two trials of Extinctions 1 or 2 were removed from analysis (Steinfurth et al. 2014). These criteria ensured that results from spontaneous recovery focused exclusively on subjects who evinced successful learning and extinction on Day 1.
The shock was a 200-msec electrical pulse delivered to the right wrist using a Grass Technologies stimulator. Shocks were calibrated for each subject using an ascending staircase procedure to reach a level deemed “highly uncomfortable, but not painful.” SCRs were collected from the hypothenar eminence of the left palmar surface (MP-100 BIOPAC system), and scored according to criteria described in detail elsewhere (Dunsmoor et al. 2015). SCRs were extracted using an automated software package (Autonomate) executed in Matlab (Green et al. 2014). Analysis of SCRs focused on early (first two) and late (last two) trials for each block.
On each trial, subjects rated the likelihood of receiving the shock using a three-alternative forced choice scale (1 = no risk; 2 = moderate risk; 3 = high risk), based on Lissek et al. (2008). Due to an error in the Extinction two scripts, 18 subjects (7 from DE group) did not have their expectancy rating data recorded for that block. The χ2 analysis reported below was carried with the remaining subjects (DE: N = 18; CTL: N = 15).
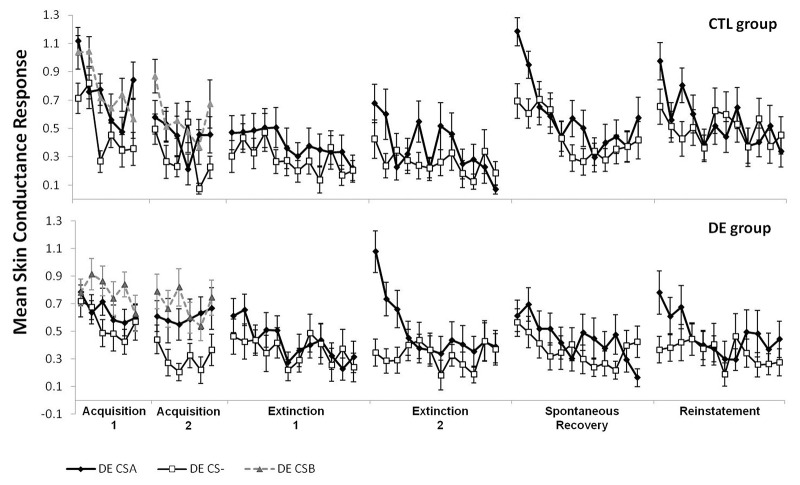
Results from late Acquisition 2 SCRs using a two-way ANOVA revealed a main effect of CS (F(2,98) = 15.89, P < 0.001; Fig. 1), but no effect of Group (P = 0.29) and no CS × Group interaction (P = 0.93). As expected from our exclusionary criteria, the Tukey post hoc showed heightened SCRs to CSA versus CS− (P < 0.001) and CSB versus CS− (P < 0.001), with no difference between CSA and CSB (P = 0.97), during late Acquisition 2. These results confirm that both groups successfully acquired conditioned SCRs to CSA and CSB.
Figure 1.
Mean trial by trial skin conductance response of each CS (excluding the CSAs and CSBs paired with the shock) for both experimental groups (CTL, upper, N = 26, and DE, lower, N = 25). Error bars represent standard error.
In the following analyses, we used the conditioned response (CR), defined as the mean differential SCR response (i.e., CSA minus CS−; or CSA/CSB minus CS−), to compare the groups (Fig. 2). A two-way ANOVA with repeated measures comparing the conditioned responses from late Acquisition 2, late Extinction 1, and late Extinction 2 blocks revealed a significant main effect of Block (F(2,98) = 12.06, P < 0.001), but no effect of Group (P = 0.83) and no Group × Block interaction (P = 0.33). The Tukey post hoc showed a higher conditioned response in late Acquisition 2 compared with late Extinction 1 (P < 0.001) and late Extinction 2 (P < 0.001). These results confirm successful extinction of conditioned response by late trials of Extinctions 1 and 2 in both groups.
Figure 2.
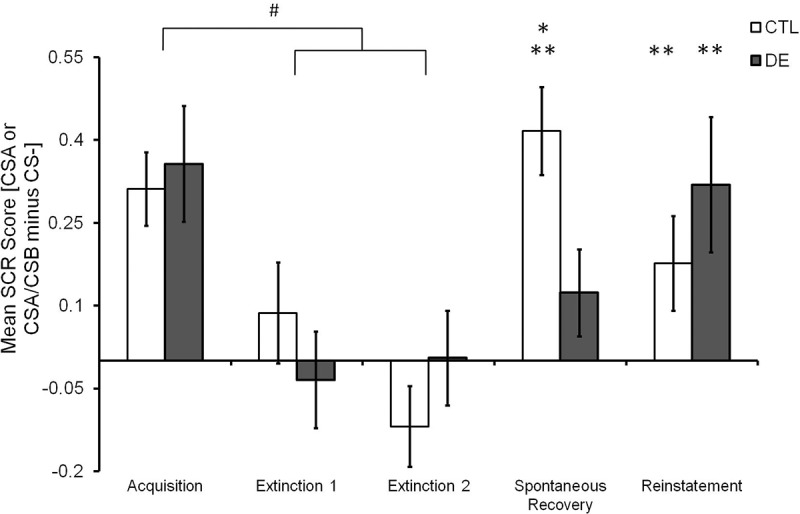
Effect of compound extinction of an extinguished CS on spontaneous recovery. Mean differential SCR score (CSA or CSA/CSB minus CS−) during late Acquisition, late Extinction 1 and 2, early Recall and early Reinstatement test of the experimental groups (CTL, N = 26, and DE, N = 25). Acquisition in both groups was significantly higher than in both Extinction blocks. Spontaneous Recovery was found in the CTL group but not in DE group, as shown by comparison of Recall with Extinction 2. Error bars represent standard error, “*” shows difference between CTL and DE groups, P < 0.05, “**” shows difference between Recall and Extinction 2 in the same group, P < 0.05, “#” shows difference between Acquisition and Extinction, P < 0.01.
Animal studies on deepened extinction show that compound stimulus presentation temporarily enhances conditioned responding following CS alone extinction trials (Rescorla 2006; Leung et al. 2012). We therefore compared the conditioned responses from the first trial of Extinction 2 to address whether compound stimulus extinction increased responding in the DE versus the CTL group. Similar to animal studies, conditioned responses to compound stimuli presentation in the DE group (mean ± SE = 0.731 ± 0.145) were heightened compared with continued CSA alone presentation in the CTL group (mean ± SE = 0.252 ± 0.139), as revealed by a t-test (t(49) = 2.377, P = 0.021). Heightened conditioned responses to compound stimuli suggest renewed anticipation for receiving the US, which is in accordance with associative learning models for deepening extinction (Rescorla 2006).
Spontaneous recovery on Day 2 was assessed by comparing the conditioned response between late trials from Extinction 2 and early trials from Spontaneous Recovery. There was a main effect of Phase (F(1,49) = 20.68, P < 0.001), and a Group × Phase interaction (F(1,49) = 8,43, P = 0.006), but no main effect of Group (P = 0.33). The Tukey post hoc revealed higher conditioned responses in the CTL group versus the DE group during early Spontaneous Recovery (P = 0.050). Further, the CTL group showed a higher conditioned response in the early Spontaneous Recovery trials compared with the late Extinction 2 trials (P < 0.001), whereas the DE group did not show any difference in conditioned responses between these two time points (P = 0.66).
Reinstatement was assessed in the same manner as spontaneous recovery. The ANOVA of conditioned responses from late the Extinction 2 and early Reinstatement showed a main effect of Phase (F(1,49) = 6.020, P = 0.049) accounting for a general higher conditioned response in early Reinstatement compared with Extinction 2, but there was no Group effect (P = 0.283) or Group × Phase interaction (P = 0.854). Thus, deepened extinction did not affect reinstatement, despite its effect on spontaneous recovery (Fig. 2).
The analysis of the shock expectancy data examined associations (differential ratings) between groups and shock expectancy categories using χ2. During late Acquisition 2, there was no significant association between group and shock expectancy for CSA (χ2(2,N = 51) = 0.377, P = 0.865) or CSB (χ2(2,N = 49) = 2.149, P = 0.391). Likewise, during Extinction there was no association between groups and shock expectancy ratings of the CSA or CSA/CSB compound (late Extinction 1: χ2(2,N = 51) = 2.195, P = 0.346; late Extinction 2: χ2(2,N = 32) = 3.441, P = 0.236). In contrast, during early Spontaneous Recovery, the DE group was significantly associated with “no risk” in CSA ratings (χ2(2,N = 51) = 6.020, P = 0.049), suggesting that deepened extinction resulted in reduced shock expectancy as compared with standard extinction. During early reinstatement, there was no significant association between group and shock expectancy ratings for CSA (χ2(2,N = 51) = 4.212, P = 0.122).
The present results provide evidence that compound stimulus extinction following CS alone extinction trials reduced 24-h recovery of SCRs and shock expectancy in humans. According to error-correction models of associative learning (Rescorla and Wagner 1972), extinction to CSs alone followed by compound extinction decreases associative strength below the level achieved by continued extinction to the CSs alone (Rescorla 2006). This result is derived from the renewed expectation of the US built on the summed residual associative strength of the extinguished CSs. In this way, the increased prediction for the US contributes to the error term generated by the US omission. Despite the well predicted increased extinction learning by compound extinction, fear recovery is not accounted in associative learning models (see McConnell and Miller 2014), and the process by which deepened extinction prevents fear recovery is not entirely clear.
Laboratory animal studies have detailed two methods by which extinguished CSs can summate to increase expectation of the US: extinguishing multiple CSs in isolation followed by compound stimulus presentation (Hendry 1982), or extinguishing a target CS alone followed by a compound of the extinguished and nonextinguished CSs (Reberg 1972). Recently, Culver et al. (2014) provided evidence of deepened extinction using the Hendry (1982) method in human fear conditioning with an aversive human scream as US. Associative learning accounts of deepened extinction indicate that combining a target CS with a nonextinguished CS should generate an even greater prediction error, thereby contributing to further decreases in associative strength for the target CS (Leung et al. 2012).
Animal studies of compound extinction provide evidence that the technique not only reduces post-extinction recovery, but converts the target CS to a net inhibitor that takes on the properties of a safety signal. For example, using the Reberg (1972) method of combining an extinguished CS and nonextinguished CS, Leung et al. (2012) showed that the target CS reduced CRs when added with another fear conditioned CS (i.e., summation) and impaired acquisition when combined with a novel CS (i.e., retardation). Importantly, continued extinction to a CS in isolation does not convert a CS into a net inhibitor with similar properties (Leung et al. 2012). Thus, deepened extinction is an especially powerful technique that appears to overcome some of the limits to standard extinction, which is highly prone to relapse (Bouton 2002). Future research in humans should directly compare the two methods of deepened extinction described here and elsewhere, and investigate whether combining an extinguished and nonextinguished CS during extinction converts the target into a safety signal.
The present findings replicate and extend evidence of deepened extinction in humans (Culver et al. 2014). It is important to note, however, that earlier fear extinction studies in humans found the opposite effect of combining CSs during extinction (Lovibond et al. 2000; Vervliet et al. 2007). A critical distinction between this and earlier studies is the use of CS− alone extinction prior to compound extinction, allowing the target CS to lose associative value on its own. Additionally, the use of intermodal CSs (auditory and visual) may have promoted elemental, rather than configural, processing of the cues. More specifically, a potential issue of including a concurrent excitor is that subjects may treat the compound as a new configural cue, rather than a combination of two elements (Pearce 1987), therefore preventing extinction to the target CS. A visual-auditory compound, following CS− alone trials, may have increased the chance that subjects treated each element of the compound independently, leading to deepened, rather than impaired extinction learning.
Despite reduced spontaneous recovery following compound extinction, we found no evidence of reduced reinstatement, in contrast to a recent finding in humans (Culver et al. 2014). One possible interpretation of the inconsistency between studies is that reinstatement was tested here following spontaneous recovery test. Therefore, subjects in both groups were re-extinguished to the CS prior to reinstatement, limiting the opportunity to see a selective effect of deepened extinction, per se. At a broader level, however, contrary findings contribute to inconsistencies in the human fear reinstatement literature (Haaker et al. 2014). Reinstatement in humans is methodologically challenging for a number of reasons, and one important factor that may determine the effectiveness of reinstatement is the nature of the US (Haaker et al. 2014). For instance, prior studies have shown electric shocks to be rated as more unpleasant and induce larger CRs than a human scream US (Glenn et al. 2012), used in Culver et al. (2014). Further, participants may habituate to an auditory US faster, which may result in weaker conditioning that is less susceptible to reinstatement effects.
One limitation of this—as well as other recent studies of deepened extinction (Rescorla 2006; Kearns et al. 2012; Leung et al. 2012)—is that the effect of compound extinction on the nontarget CS (CSB) was not assessed. Future studies could also incorporate an additional control condition in which CSB has no prior association with the US, in order to further clarify the role of error-correction mechanisms putatively responsible for deepened extinction. Finally, we used partial reinforcement in the present study, but whether deepened extinction is sensitive to the CS–US pairing rate at the time of initial learning should be explored.
There is continued interest in innovative behavioral techniques to prevent the return of fear, motivated in large part by continued reliance on conditioning-based models of clinical anxiety (Mineka and Zinbarg 2006; Dymond et al. 2014; Kindt 2014). The deepened extinction technique adds to the growing arsenal of nonpharmacological techniques to improve upon standard models of extinction in humans, including extinction training during the reconsolidation time window (Schiller et al. 2010; Agren 2014), extinction under multiple contexts (Shiban et al. 2013; Dunsmoor et al. 2014), and replacing the aversive US with a novel nonaversive outcome (Dunsmoor et al. 2015). Adapting, and even combining, these approaches from the laboratory into clinical treatments could improve outcomes for therapies based on associative learning.
Acknowledgments
We thank Sandra F.L. Armstrong, Catherine Stevenson and Jackie Reitzes for the technical support during the execution of the experiments. This study was supported by NIH RO1 MH097085 to E.A.P. J.E.D. is supported by NIMH K99MH106719. C.A.O.C. is supported by BEPE FAPESP 2013/10903-0.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.039479.115.
References
- Agren T. 2014. Human reconsolidation: a reactivation and update. Brain Res Bull 105: 70–82. [DOI] [PubMed] [Google Scholar]
- Bouton ME. 2002. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry 52: 976–986. [DOI] [PubMed] [Google Scholar]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. 2014. Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 58: 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver NC, Vervliet B, Craske MG. 2014. Compound extinction: using the Rescorla–Wagner model to maximize exposure therapy effects for anxiety disorders. Clin Psychol Sci 3: 335–348. [Google Scholar]
- Dawson ME, Schell AM, Filion DL. 2007. The electrodermal system. In Handbook of psychophysiology (ed. Cacioppo IT, Tassinary LG, Berntson GG), pp. 159–181. Cambridge University Press, Cambridge, UK. [Google Scholar]
- Dunsmoor JE, Ahs F, Zielinski DJ, LaBar KS. 2014. Extinction in multiple virtual reality contexts diminishes fear reinstatement in humans. Neurobiol Learn Mem 113: 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Campese VD, Ceceli AO, LeDoux JE, Phelps EA. 2015. Novelty-facilitated extinction: providing a novel outcome in place of an expected threat diminishes recovery of defensive responses. Biol Psychiatry 78: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D. 2014. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav Ther. 10.1016/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. 2014. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull 105: 46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Lieberman L, Hajcak G. 2012. Comparing electric shock and a fearful screaming face as unconditioned stimuli for fear learning. Int J Psychophysiol 86: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SR, Kragel PA, Fecteau ME, LaBar KS. 2014. Development and validation of an unsupervised scoring system (Autonomate) for skin conductance response analysis. Int J Psychophysiol 91: 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Golkar A, Hermans D, Lonsdorf TB. 2014. A review on human reinstatement studies: an overview and methodological challenges. Learn Mem 21: 424–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry J. 1982. Summation of undetected excitation following extinction of the CER. Anim Learn Behav 10: 476–482. [Google Scholar]
- Janak PH, Corbit LH. 2011. Deepened extinction following compound stimulus presentation: noradrenergic modulation. Learn Mem 18: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Weiss SJ. 2012. Extinguished cocaine cues increase drug seeking when presented simultaneously with a non-extinguished cocaine cue. Drug Alcohol Depend 121: 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns DN, Tunstall BJ, Weiss SJ. 2012. Deepened extinction of cocaine cues. Drug Alcohol Depend 124: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M. 2014. A behavioural neuroscience perspective on the aetiology and treatment of anxiety disorders. Behav Res Ther 62: 24–36. [DOI] [PubMed] [Google Scholar]
- Leung HT, Reeks LM, Westbrook RF. 2012. Two ways to deepen extinction and the difference between them. J Exp Psychol Anim Behav Process 38: 394–406. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. 2008. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther 46: 678–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond PF, Davis NR, O'Flaherty AS. 2000. Protection from extinction in human fear conditioning. Behav Res Ther 38: 967–983. [DOI] [PubMed] [Google Scholar]
- McConnell BL, Miller RR. 2014. Associative accounts of recovery-from-extinction effects. Learn Motiv 46: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell BL, Miguez G, Miller RR. 2013. Extinction with multiple excitors. Learn Behav 41: 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. 2012. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 63: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. 2006. A contemporary learning theory perspective on the etiology of anxiety disorders: it's not what you thought it was. Am Psychol 61: 10–26. [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Izquierdo I, Furini CR. 2014. Modulation of the extinction of fear learning. Brain Res Bull 105: 61–69. [DOI] [PubMed] [Google Scholar]
- Pearce JM. 1987. A model for stimulus generalization in Pavlovian conditioning. Psychol Rev 94: 61–73. [PubMed] [Google Scholar]
- Reberg D. 1972. Compound tests for excitation in early acquisition and after prolonged extinction of conditioned suppression. Learn Motiv 3: 246–258. [Google Scholar]
- Rescorla RA. 2006. Deepened extinction from compound stimulus presentation. J Exp Psychol Anim Behav Process 32: 135–144. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. 1972. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In Classical conditioning II: current research and theory (ed. Black AH, Prokasy WF), pp. 64–99. Appleton-Century-Crofts, New York. [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiban Y, Pauli P, Mühlberger A. 2013. Effect of multiple context exposure on renewal in spider phobia. Behav Res Ther 51: 68–74. [DOI] [PubMed] [Google Scholar]
- Steinfurth EC, Kanen JW, Raio CM, Clem RL, Huganir RL, Phelps EA. 2014. Young and old Pavlovian fear memories can be modified with extinction training during reconsolidation in humans. Learn Mem 21: 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Hermans D, Eelen P. 2007. Concurrent excitors limit the extinction of conditioned fear in humans. Behav Res Ther 45: 375–383. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Craske MG, Hermans D. 2013. Fear extinction and relapse: state of the art. Annu Rev Clin Psychol 9: 215–248. [DOI] [PubMed] [Google Scholar]