Abstract
Purpose:
To assess the electromechanical properties of human knee articular cartilage with compression-induced streaming potentials for reliability among users and correlation with macroscopic and histological evaluation tools and sulfated glycosaminoglycan (sGAG) content.
Methods:
Streaming potentials are induced in cartilage in response to loading when mobile positive ions in the interstitial fluid temporarily move away from negatively charged proteoglycans. Streaming potential integrals (SPIs) were measured with an indentation probe on femoral condyles of 10 human knee specimens according to a standardized location scheme. Interobserver reliability was measured using an interclass correlation coefficient (ICC). The learning curves of 3 observers were evaluated by regression analysis. At each SPI measurement location the degradation level of the tissue was determined by means of the International Cartilage Repair Society (ICRS) score, Mankin score, and sGAG content.
Results:
The computed ICC was 0.77 (0.70-0.83) indicating good to excellent linear agreement of SPI values among the 3 users. A significant positive linear correlation of the learning index values was observed for 2 of the 3 users. Statistically significant negative correlations between SPI and both ICRS and Mankin scores were observed (r = 0.502, P < 0.001, and r = 0.255, P = 0.02, respectively). No correlation was observed between SPI and sGAG content (r = 0.004, P = 0.973).
Conclusions:
SPI values may be used as a quantitative means of cartilage evaluation with sufficient reliability among users. Due to the significant learning curve, adequate training should be absolved before routine use of the technique.
Keywords: streaming potential integrals (SPI), articular cartilage, osteoarthritis, ICRS/Mankin score
Introduction
Articular cartilage defects are common findings at arthroscopic surgery of the knee joint.1-3 These defects may be the cause of a variety of symptoms, such as swelling, pain, or joint stiffness resulting in functional impairment and the development of osteoarthritis (OA). For focal cartilage defects, the therapeutic options for cartilage repair have been improved in recent years, however with increasing treatment costs in particular for cell-based and matrix-guided techniques.4-6 Nonetheless, success rates are far from being perfect, with patients not being satisfied with the clinical result although imaging and arthroscopic evaluation visually reveals a nicely grafted former defect area.1 For magnetic resonance imaging and arthroscopic evaluation of cartilage defects and repair, various classification systems have been described.1 However, grading depends on the individual experience of the surgeon. Whereas deep cartilage defects down to the subchondral bone and general osteoarthritic changes can usually be easily detected and classified, the classification and therapeutic consequences of early-stage defects remains difficult.7,8 A survey among highly experienced arthroscopic surgeons showed that around 50% of the respondents felt “need for improvement” in differentiation between grade I and grade II lesions, and between grade II and grade III lesions.8 Thus, since simple observations and palpation do not provide reliable quantitative information about the quality of degenerated cartilage and healing grafts, more reliable and sensitive measurement techniques are necessary.
In recent years, several diagnostic tools capable of objective evaluation of the cartilage properties in the early and potentially reversible stages of the disease have been developed to assess the structural, mechanical, and histological changes related to the different grades of cartilage degeneration.9-12 Some of them were designed offering the possibility to assess cartilage defects at arthroscopic surgery.10,13 However, although the demand for an objective and reliable technique to evaluate articular cartilage tissue in clinical practice appears obvious, the use of such devices has not yet gained broader clinical acceptance. Possible reasons for this may be challenges in the usability of these systems, which arise from the necessity of attaining the appropriate sensor orientation relative to cartilage surface as well as the need to perform repeated measurements at the same level of application force.14
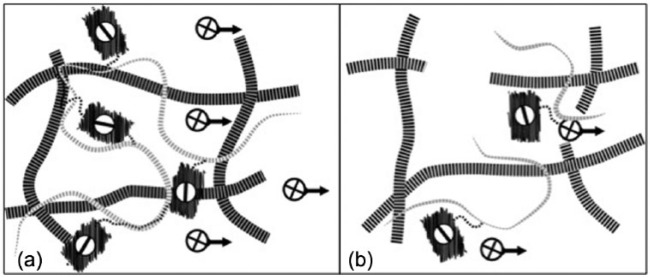
The device investigated herein relies on the fact that streaming potentials are induced in cartilage in response to loading. The extracellular matrix is loaded with negatively charged proteoglycans that are entrapped in the collagen network. As a result of compression loading, mobile positive ions in the interstitial fluid temporarily move away from negatively charged proteoglycans entrapped in the collagen network,15 thus inducing local electrical potential, which are referred to as streaming potentials. In diseased articular cartilage, the collagen network is degraded and there is a loss of proteoglycans leading to abnormal streaming potentials (Fig. 1).16 The hand-held Arthro-BST indentation probe (Biomomentum Inc., Laval, Canada) measures streaming potentials in articular cartilage using microelectrodes located on a hemispherical indenter during a gentle and instantaneous compression.14,16,17 The device is CE marked and available as a diagnostic tool at arthroscopic surgery within the European Economic Area, Canada, and in other countries where CE marking is recognized for medical devices.16 The streaming potential integrals (SPIs) generated by spherical indentation of the cartilage outer surface with the Arthro-BST have been shown to reflect the structural and functional integrity of cartilage with correlation to mechanical properties as well as chemically and mechanically induced degradation of the tissue.14,17-20 A pilot benchtop study of our group confirmed that SPI is a promising surrogate parameter for mechanical properties (modulus and permeability) as well as cartilage degradation scores.17 However, the data were somewhat limited since SPIs were measured and correlated by only one examiner in one cadaver knee specimen.
Figure 1.
(a) Principle of the technique for the measurement of compression-induced streaming potentials of articular cartilage according to the user manual.16 As a result of compression loading, mobile positive ions in the interstitial fluid temporarily move away from negatively charged proteoglycans entrapped in the collagen network, thus inducing local electrical potential, which are referred to as streaming potentials. (b) In diseased articular cartilage, the collagen network is degraded and there is a loss of proteoglycans leading to abnormal streaming potentials.
In this study, multiuser repeating measurements were performed to assess the learning phase for different users in 10 human knee cadaver specimens in vitro and under benchtop measurement configuration. Correlations between SPI and qualitative scores as well as sulfated glycosaminoglycan (sGAG) content of articular cartilage were determined. We hypothesized that there would be a significant learning phase and that SPI values correlate with macro- and microscopic clinically relevant cartilage scoring systems (International Cartilage Repair Society [ICRS]1 and Mankin score21) as well as the sGAG content.
Materials and Methods
Specimen Preparation and SPI Measurements
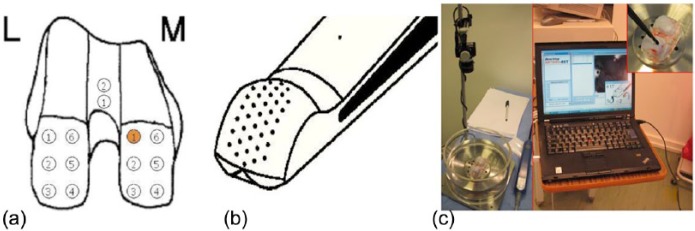
After approval of the local ethics committee (IRB No. 1182-2011), femoral condyles of 10 fresh human cadaver knee specimen (2 male, 3 female, mean age 58 [43-65] years) were dissected and distal femurs were fixed onto a cylindrical platform that was fixed to a testing chamber equipped with a camera (Fig. 2). Measurement points (n = 162) were ink-marked on the trochlea (2-3) and medial/lateral condyles (14) according to a standardized location scheme (Fig. 2). The thus defined measurement locations were evaluated by an experienced board-certified orthopedic surgeon using the ICRS score, an established international standard classification for cartilage degeneration launched in the year 2000 by the International Cartilage Repair Society.1 The score comprises 4 grades of degeneration ranging from 1 to 4 (0 being the healthy cartilage, 1 the first degree of degeneration, and 4 the ultimate degeneration state) as determined by macroscopic examination.
Figure 2.
(a) Location scheme for distal femur with measurement points marked on the anatomical locations for SPI measurement (L, lateral; M, medial). (b) Tip of the Arthro-BST hemispherical indentation probe with an array of microelectrodes for the measurement of streaming potentials. (c) Benchtop setup for the measurements.
The testing chamber was filled with phosphate-buffered saline (pH 7.4) and a minimum of 15 minutes was allowed for equilibration prior to electromechanical mapping. Streaming potentials were measured by spherical indentation of the cartilage outer surface at the ink-marked locations with the Arthro-BST device under benchtop conditions as described earlier17 (Fig. 2). Values were computed by the user interface software for each measurement location (Benchtop Artho-BST, AR299, version 1.0.0.0) as SPIs (mV mm3). SPIs are the result of integration of all streaming potentials measured by the 37 gold-tip microelectrodes to a standardized depth of 150 µm of compression. The spherical tip of the indentation probe (r = 3.05 mm) accommodates an equally distributed array of 37 microelectrodes.10
Histological and Biochemical Evaluation
A total of 162 equal osteochondral cylinders of 6-mm diameter and and 8-mm height were harvested from the marked locations with orthopedic tissue punches (Osteochondral Autograft Transfer System [OATS], Athrex, München, Germany). Eighty-two cylinders were randomized for histological evaluation, and the remaining 80 cylinders were randomized for biochemical evaluation.
General Histology
The cylinders were first fixed in commercial 3.5% formalin for 5 days at room temperature. Then, the cylinders were washed, dehydraded in a graded series of ethanol, embedded in methyl-methacrylate (Technovit 9100 New, Heraeus-Kulzer, Hanau, Germany) according to the manufacturer’s instructions and established protocols.22 The tissue blocks were cut into 5-µm-thick sections using a RM 2155 microtome (Leica, Bensheim, Germany) and placed onto poly-l-lysine-coated glass slides. Slices were pressed and dried for 2 days at 37°C. Before staining, the sections were first deacrylated in xylene (2 × 20 minutes) and 2-methoxyethylacetate (1 × 20 minutes) and then rehydrated in a graded series of ethanol.
Hematoxylin–Eosin Staining
Rehydrated sections were first rinsed in distilled water for 2 minutes, then stained for 6 minutes with Mayer’s hematoxylin (Merck), rinsed in tap water for 10 minutes, then stained with 1% eosin (Merck) for 3 minutes, dehydrated in a graded series of ethanol and mounted in Eukitt (Labonord, Mönchengladbach, Germany).
Safranin-O Staining
Rehydrated sections were incubated for 4 minutes in a solution of 0.1% safranin-O (Sigma, Taufkirchen, Germany), then washed in distilled water, dehydrated in a graded series of ethanol, and mounted in Eukitt.
Mankin Score
Mankin scores were determined for the histological evaluation. Values range from 0 (healthy cartilage) to 14 (severe cartilage destruction), distributed in 4 categories: structure (range 0-6, 0 indicating normal structure and 6 complete disorganization), cells (range 0-3, 0 for normal- and 3 for hypo-cellularity), safranin-O staining (range 0-4, 0 for normal stainability and 4 for no noted dye), and tidemark integrity (range 0-1, 0 for intact tidemark and 1 for blood vessels crossing the tidemark).21
Biochemical Evaluation
The content of sGAG (reported in µg/mL, normalized to a standardized sample surface area of 28.3 mm2) was determined using the commercially available Blyscan Assay (Biocolor, Carrickfergus, UK). Cartilage was separated from the osteochondral cylinders from the subchondral lamella with a scalpel. To extract the sGAGs from the cartilage cylinders, each specimen was dissolved in 1.5-mLmicrocentrifuge tubes in a papain extraction reagent, containing 100 mL of 0.2 M sodium phosphate buffer (pH 6.4) with 0.82 g of sodium acetate, 0.37 g of Na2EDTA, and 80 mg of cysteine HCl mixed with papain suspension. The specimens were incubated for 18 hours in a 65°C water bath according the instruction provided by the assay kit manufacturer. After centrifugation at 10,000g for 10 minutes, the supernatant was used for the actual assay. Adding 1 mL of dye reagent to each tube, a sGAG–dye complex precipitated and the tubes were transferred to the microcentrifuge and spun at 12,000 rpm for 10 minutes. The supernatant was then decanted off and dissociation reagent was added to the remaining pellets and the bound dye was released using a vortex mixer. The specimens were again centrifuged at 12,000 rpm for 5 minutes to remove foam. A total of 200 µL of each tube were transferred to 96 micro well plate and the sGAG content was measured against water and standards (glycosaminoglycan standards supplied with Blyscan assay), with a well plate reader (Synergy 2; BioTek, Bad Friedrichshall, Germany) at 656 nm.
Data Evaluation and Statistical Methods
To validate the methodologies of the measurements, 3 of the authors (observer 1, board-certified orthopedic surgeon; observer 2, engineering student trained in the use of the device17; observer 3, medical student) randomly performed 3 measurements on each marked locations, each blinded to the others’ results. The average SPI value of the 3 measurements was taken for analysis. To determine the interobserver reliability, the interclass correlation coefficient (ICC) and its 95% confidence interval (CI) were calculated based on 2-way, random, single measures with absolute agreement. An ICC >0.75 was considered good to excellent, an ICC >0.40 and <0.75 as fair to good, and an ICC <0.40 as poor. The learning curve (successful measurements vs. total number of measurements) of the 3 observers was determined by regression analysis. Correlation of the average SPI values of the 3 observers with ICRS scores, Mankin scores, and sGAG content were calculated using a 2-sided Pearson’s correlation with a significance level of α = 0.05.
Results
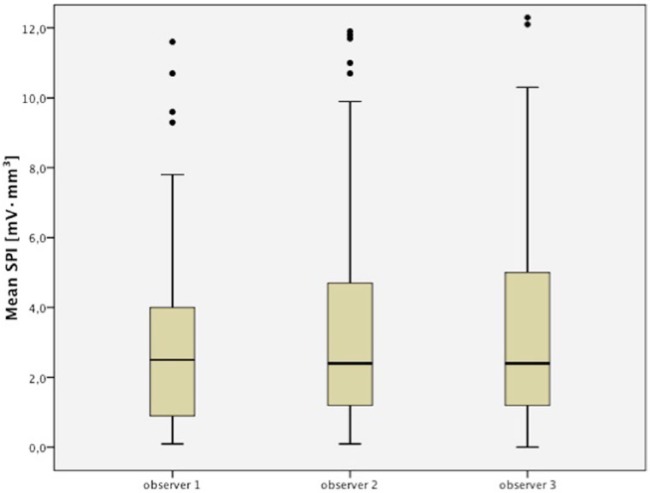
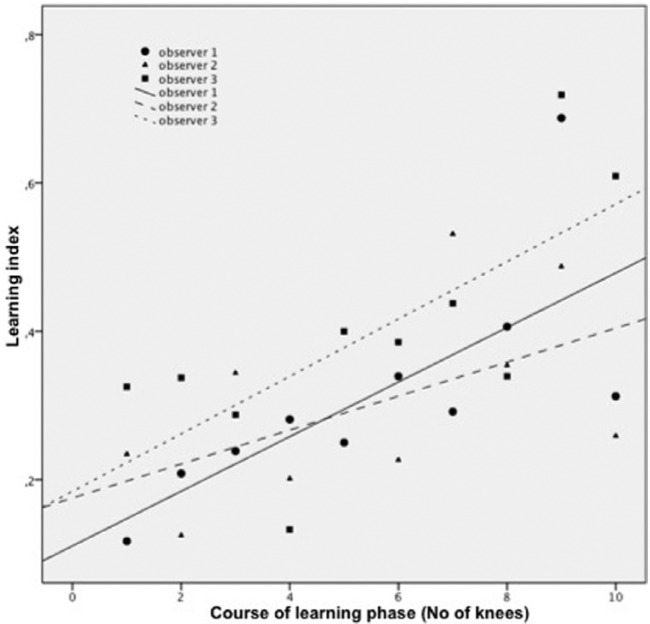
The computed ICC was 0.77 (0.70-0.83), indicating good to excellent linear agreement of SPI values among the 3 users (Fig. 3). However, unsuccessful measurements occurred at several locations for all users (missing values: observer 1, 9.9%; observer 2, 15.8%; observer 3, 8.8%). A significant positive linear correlation of the learning index values was observed for observers 1 and 3 (ro1 = 0.729, P = 0.017; ro3 = 0.711, P = 0.021), whereas the experienced observer merely tended toward significance (ro2 = 0.541, P = 0.106; Fig. 4).
Figure 3.
Box plots displaying the SPI measurements of the 3 observers. The interclass correlation coefficient (ICC) was 0.77 (0.70-0.83) indicating good to excellent linear agreement of streaming potential integral (SPI) values.
Figure 4.
Linear regression between learning index and number of evaluated knees.
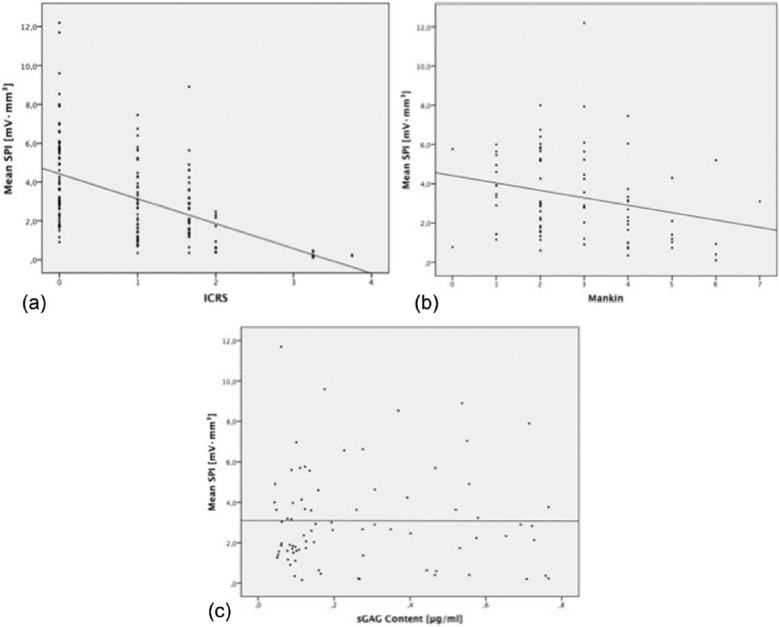
The mean ICRS score was 0.8 ± 0.7 (range 0-3), and the mean Mankin score was 2.8 ± 1.5 (range 0-7). Significant negative correlations between SPI and both ICRS and Mankin scores were observed (r = 0.502, P < 0.001, and r = 0.255, P = 0.02, respectively; Fig. 5a and b) together with a significant positive linear correlation between the ICRS and Mankin scores (r = 0.334, P = 0.002). No significant correlations between sGAG content and SPI were detected (r = 0.004, P = 0.973; Fig. 5c).
Figure 5.
Correlations between streaming potential integral (SPI) values and (a) International Cartilage Repair Society (ICRS) score, (b) Mankin score, and (c) sulfated glycosaminoglycan (sGAG) content.
Discussion
The purpose of this study was to assess the electromechanical properties of human knee articular cartilage with compression-induced streaming potentials for reliability among users and correlation with macroscopic and histological evaluation tools and sGAG content. The most important finding was that the reproducibility of the SPI measurement tool was confirmed through the significant interclass correlation as well as the correlating learning indices between multiple users with consideration of a significant learning curve. Furthermore, linear agreement between the SPI and clinically relevant cartilage scores was observed and thus SPI values may be used as a quantitative means of nondestructive cartilage evaluation.
Standardized methods for evaluating of cartilage defects, cartilage repair tissue, and clinical outcomes of cartilage resurfacing are crucial in both the clinical and research contexts. However, the determination of intraoperative findings and classification with commonly used arthroscopic cartilage lesion classification systems have, for example, shown serious limitations with interobserver agreement ranging from 55% to 100%, mainly depending on the severity of the lesions.23-26 The availability of devices for intraoperative evaluation of articular cartilage is limited; the classification of the lesions is thus typically macroscopic with the aid of a simple probe for indentation. Thus, specific measurements of cartilage properties to differentiate the stages of cartilage degeneration would be desirable. Methods such as histological scoring, biochemical analyses, and biomechanical testing would offer these options but are impossible to use in a clinical setting since they involve destructive processing of the cartilage tissue, are time consuming, and do not represent the entire joint surface. New evaluation tools for intraoperative, at best arthroscopic assessment have been developed in order to facilitate the determination of cartilage properties nondestructively. The hand-held electromechanical device Arthro-BST, which was used in the present study, measures compression-induced electric streaming potentials with its microelectrodes located on the indenter based on fluid–solid phase interactions in the loaded extracellular matrix. Apart from one pilot study from our group published by Abedian et al.,17 the available literature about the Arthro-BST is somewhat related to the inventers of the device.10,14,18-20
In the present study, we demonstrated good to excellent linear agreement of SPI values with an ICC of 0.77 (0.70-0.83), which confirms the reliability of the streaming potential measurements among different users. These results agreed with previous studies using the Arthro-BST, which demonstrated an ICC of 0.87 in a cadaveric female knee27 and ICC of 0.64 in stifle joints of sheep.28 However, a certain learning curve for the use of the device must be considered as shown by the significant positive linear correlation of the learning index values of the users with less experience along with the amount of missing values. The reasons for these observations could be related to known limitations for thin cartilage14 and disruptive factors such as pressure and position of the tip with respect to the cartilage surface, which is being diagnosed especially on curved cartilage surfaces due to the geometry of the indenter. We have observed this in our small cluster of users, which is demonstrated in the learning index as the ratio of the successful measurements to total number of measurements. The closer the learning index to 1 the more proficient the user. However, it could not be determined from our data when the learning curve is completed.
The present study revealed statistically significant negative correlations between SPI and both ICRS (r = 0.502) and Mankin scores (r = 0.255). Although the correlation coefficient was not high, these findings confirm the earlier results of our pilot study (ICRS: r = 0.749; Mankin: r = 0.409). Nevertheless, other in situ evaluation methods such as near infrared spectroscopy (NIRS) have demonstrated comparable findings when correlating the characteristic value (CV) calculated from NIR with a hand-held probe to ICRS scores (r = 0.47)29 and Mankin score (r = 0.55).30 A greater r value was found by Sim et al.14 using the Arthro-BST with a significant correlation between electromechanical QP, a similarly obtained SPI parameter, and the Mankin score (r = 0.73). The QP is a quantitative parameter independent of the velocity of indentation or device orientation and reflects the number of microelectrodes in contact with the articular cartilage and is consequently inversely proportional to SPI.14 It should be considered that the new parameter QP appears to have significant advantages over the SPI including the simplicity of calculation and robustness to noise.14 Hypothetically, the results of the present study might have been improved with better interobserver agreement and correlations to ICRS and Mankin scores if the QP could have been calculated at the time we performed our experiments. Furthermore, our results could have been related and discussed to a reference map that averaged several maps obtained in vitro under camera-registration using visually normal articular surfaces in correlation with ICRS and Mankin scores.16 However, the constitution of “normal cartilage” and thus its defined “normal value” remain unknown and constitute a challenge for future techniques.
Although the decrease of glycosaminoglycan content is typical for severe cartilage lesions,31,32 we could not detect a correlation of SPI values to the sGAG content per wet weight. This observation is different to the weak, but significant negative correlation (r = 0.31, P = 0.0316) of the electromechanical QP with decreasing GAG content per dry weight found by Sim et al.14 Conflicting results concerning the GAG content in correlation to NIRS were demonstrated with a significant correlation (r = 0.58) found in osteoarthritic knee cartilage specimens harvested during total knee arthroplasty,30 but no significant differences in GAG content between intact cartilage (grade 0) and early stages of articular lesions (grade 1) evaluated in fresh medial femoral condyles from female sheep.12 The reasons for these contradictions remain unclear, but might be related to high variability of the GAG content as a function of an inconsistent intra-individual and inter-individual appearance33 and that for instance in early stages of the disease the GAG content can be still normal.12
In conclusion, the findings of this study confirm the agreement between the SPI and clinically relevant cartilage scores ex vivo under benchtop conditions substantiating that the SPI values may be used as a quantitative means of cartilage evaluation with sufficient reliability among users. However, a certain training phase has to be considered in accordance with a significant learning curve. Thus, adequate training should be absolved before routine use of the technique. Since the Arthro-BST has been designed for compatibility with arthroscopy, it could be useful in the evaluation of cartilage quality during surgery and aid in decision making for cartilage repair techniques as well as the assessment of cartilage repair tissue after treatment. However, further research is necessary to prove the applicability and usefulness of the device in vivo.
Footnotes
Acknowledgment and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The local ethics committee provide ethical approval (IRB No. 1182-2011).
References
- 1. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A(Suppl 2):58-69. [DOI] [PubMed] [Google Scholar]
- 2. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 3. Recht MP, Kramer J, Marcelis S, Pathria MN, Trudell D, Haghighi P, et al. Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology. 1993;187(2):473-8. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38(6):1259-71. [DOI] [PubMed] [Google Scholar]
- 5. Gille J, Behrens P, Volpi P, de Girolamo L, Reiss E, Zoch W, et al. Outcome of autologous matrix induced chondrogenesis (AMIC) in cartilage knee surgery: data of the AMIC Registry. Arch Orthop Trauma Surg. 2013;133(1):87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437-41. [DOI] [PubMed] [Google Scholar]
- 7. Kleemann RU, Krocker D, Cedraro A, Tuischer J, Duda GN. Altered cartilage mechanics and histology in knee osteoarthritis: relation to clinical assessment (ICRS Grade). Osteoarthritis Cartilage. 2005;13(11):958-63. [DOI] [PubMed] [Google Scholar]
- 8. Spahn G, Klinger HM, Hofmann GO. How valid is the arthroscopic diagnosis of cartilage lesions? Results of an opinion survey among highly experienced arthroscopic surgeons. Arch Orthop Trauma Surg. 2009;129(8):1117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gelse K, Olk A, Eichhorn S, Swoboda B, Schoene M, Raum K. Quantitative ultrasound biomicroscopy for the analysis of healthy and repair cartilage tissue. Eur Cell Mater. 2010;19:58-71. [DOI] [PubMed] [Google Scholar]
- 10. Quenneville E, Binette JS, Garon M, Legare A, Meunier M, Buschmann MD. Fabrication and characterization of nonplanar microelectrode array circuits for use in arthroscopic diagnosis of cartilage diseases. IEEE Trans Biomed Eng. 2004;51(12):2164-73. [DOI] [PubMed] [Google Scholar]
- 11. Sato M, Ishihara M, Kikuchi M, Mochida J. A diagnostic system for articular cartilage using non-destructive pulsed laser irradiation. Lasers Surg Med. 2011;43(5):421-32. [DOI] [PubMed] [Google Scholar]
- 12. Spahn G, Plettenberg H, Nagel H, Kahl E, Klinger HM, Muckley T, et al. Evaluation of cartilage defects with near-infrared spectroscopy (NIR): an ex vivo study. Med Eng Phys. 2008;30(3):285-92. [DOI] [PubMed] [Google Scholar]
- 13. Spahn G, Klinger HM, Baums M, Hoffmann M, Plettenberg H, Kroker A, et al. Near-infrared spectroscopy for arthroscopic evaluation of cartilage lesions: results of a blinded, prospective, interobserver study. Am J Sports Med. 2010;38(12):2516-21. [DOI] [PubMed] [Google Scholar]
- 14. Sim S, Chevrier A, Garon M, Quenneville E, Yaroshinsky A, Hoemann CD, et al. Non-destructive electromechanical assessment (Arthro-BST) of human articular cartilage correlates with histological scores and biomechanical properties. Osteoarthritis Cartilage. 2014;22(11):1926-35. [DOI] [PubMed] [Google Scholar]
- 15. Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177(3):492-500. [DOI] [PubMed] [Google Scholar]
- 16. Biomomentum. www.biomomentum.com/en/Arthro-BST.html. Accessed June 9, 2015.
- 17. Abedian R, Willbold E, Becher C, Hurschler C. In vitro electro-mechanical characterization of human knee articular cartilage of different degeneration levels: a comparison with ICRS and Mankin scores. J Biomech. 2013;46(7):1328-34. [DOI] [PubMed] [Google Scholar]
- 18. Changoor A, Coutu JP, Garon M, Quenneville E, Hurtig MB, Buschmann MD. Streaming potential-based arthroscopic device is sensitive to cartilage changes immediately post-impact in an equine cartilage injury model. J Biomech Eng. 2011;133(6):061005. [DOI] [PubMed] [Google Scholar]
- 19. Garon M, Legare A, Guardo R, Savard P, Buschmann MD. Streaming potentials maps are spatially resolved indicators of amplitude, frequency and ionic strength dependent responses of articular cartilage to load. J Biomech. 2002;35(2):207-16. [DOI] [PubMed] [Google Scholar]
- 20. Legare A, Garon M, Guardo R, Savard P, Poole AR, Buschmann MD. Detection and analysis of cartilage degeneration by spatially resolved streaming potentials. J Orthop Res. 2002;20(4):819-26. [DOI] [PubMed] [Google Scholar]
- 21. Mankin HJ. Biochemical and metabolic aspects of osteoarthritis. Orthop Clin North Am. 1971;2(1):19-31. [PubMed] [Google Scholar]
- 22. Willbold E, Witte F. Histology and research at the hard tissue-implant interface using Technovit 9100 New embedding technique. Acta Biomater. 2010;6(11):4447-55. [DOI] [PubMed] [Google Scholar]
- 23. Brismar BH, Wredmark T, Movin T, Leandersson J, Svensson O. Observer reliability in the arthroscopic classification of osteoarthritis of the knee. J Bone Joint Surg Br. 2002;84(1):42-7. [DOI] [PubMed] [Google Scholar]
- 24. Hunt N, Sanchez-Ballester J, Pandit R, Thomas R, Strachan R. Chondral lesions of the knee: a new localization method and correlation with associated pathology. Arthroscopy. 2001;17(5):481-90. [DOI] [PubMed] [Google Scholar]
- 25. Javed A, Siddique M, Vaghela M, Hui AC. Interobserver variations in intra-articular evaluation during arthroscopy of the knee. J Bone Joint Surg Br. 2002;84(1):48-9. [DOI] [PubMed] [Google Scholar]
- 26. Jerosch J, Castro WH, de Waal Malefijt MC, Busch M, van Kampen A. Interobserver variation in diagnostic arthroscopy of the knee joint. “How really objective are arthroscopic findings? ” Unfallchirurg. 1997;100(10):782-6. [PubMed] [Google Scholar]
- 27. Garon M, Legare A, Quenneville E, Sims TJ, Hurtig MB, Shive MS, et al. Arthroscopic device measuring streaming potentials reliably indicates functional properties of cartilage. Osteoarthritis Cartilage. 2007;15(Suppl C):143-4. [Google Scholar]
- 28. Changoor A, Quenneville E, Garon M, Cloutier L, Hurtig MB, Buschmann MD. Streaming potential-based arthroscopic device discerns topographical differences in cartilage covered and uncovered by meniscus in ovine stifle joints. Trans Orthop Res Soc. 2007;32:631. [Google Scholar]
- 29. Marticke JK, Hosselbarth A, Hoffmeier KL, Marintschev I, Otto S, Lange M, et al. How do visual, spectroscopic and biomechanical changes of cartilage correlate in osteoarthritic knee joints? Clin Biomech. 2010;25(4):332-40. [DOI] [PubMed] [Google Scholar]
- 30. Pester JK, Stumpfe ST, Steinert S, Marintschev I, Plettenberg HK, Aurich M, et al. Histological, biochemical and spectroscopic changes of articular cartilage in osteoarthritis: is there a chance for spectroscopic evaluation? Z Orthop Unfall. 2014;152(5):469-79. [DOI] [PubMed] [Google Scholar]
- 31. Bonassar LJ, Jeffries KA, Paguio CG, Grodzinsky AJ. Cartilage degradation and associated changes in biochemical and electromechanical properties. Acta Orthop Scand Suppl. 1995;266:38-44. [PubMed] [Google Scholar]
- 32. Franz T, Hasler EM, Hagg R, Weiler C, Jakob RP, Mainil-Varlet P. In situ compressive stiffness, biochemical composition, and structural integrity of articular cartilage of the human knee joint. Osteoarthritis Cartilage. 2001;9(6):582-92. [DOI] [PubMed] [Google Scholar]
- 33. Stumpfe ST, Pester JK, Steinert S, Marintschev I, Plettenberg H, Aurich M, Hofmann GO. Is there a correlation between biophotonical, biochemical, histological, and visual changes in the cartilage of osteoarthritic knee-joints? Muscles Ligaments Tendons J. 2013;3(3):157-65. [PMC free article] [PubMed] [Google Scholar]