Abstract
In the aquatic genus Stuckenia, the wide geographic range of S. pectinata and S. filiformis make them suited for examination of topographic and climatic effects on plant evolution. Using nuclear ITS sequence and ten chloroplast sequences, we conducted comparative phylogeographical analyses to investigate their distribution regions and hybrid zones in China, and compare their phylogeographical patterns and demographical histories. These two species were allopatric in China. S. filiformis occurred only on the Qinghai-Tibet Plateau (QTP), whereas S. pectinata occupied a wide range of habitats. These two species formed hybrid zones on the northeastern edge of QTP. Most of the genetic variance of S. filiformis was between the southern and eastern groups on the QTP, showing a significant phylogeographic structure. The geographical isolations caused by the Nyenchen Tanglha Mountains and the Tanggula Mountains promoted intraspecific diversification of alpine plants on the QTP. This study revealed the lack of phylogeographic structure in S. pectinata, due to the continued gene flow among its distribution regions. The ecological niche modeling showed that the distribution ranges of these two herbaceous species did not contract too much during the glacial period.
Phylogeographical patterns in China did not show an expected contraction-expansion pattern, which is in accordance with the geological records showing that no unified ice sheet had developed in China during the Quaternary1. Instead, intraspecific divergences, regional expansions, and hybridizations between these intraspecific lineages were common during the Quaternary climatic oscillations in China. Hybridizations and introgressions between closely related species were also frequently detected at the hybrid zones of two diverging species with different preferences of the ecological niches2.
China consists of the following five major regions: north China, subtropical (central/east/south) China, southwest China (Yungui Plateau), northwest China and Qinghai–Tibetan Plateau (QTP). In north, subtropical and southwest China, the predominant response of temperate plant species to Quaternary climatic change appeared to be one of range fragmentation, vicariance, and population isolation with limited or no spatial demographic expansion3. In north China, some temperate species migrated to the south, while some species had northern refugia at higher latitudes. Species in the arid northwestern China are often sparsely distributed and ancient. The deserts might have promoted the allopatric divergences of species distributed in this region1.
Species distributions in the western high mountain areas of China were affected by drastic changes in topography and altitude due to the uplift of the QTP4. The QTP is the highest (averaging about 4500 m) and largest (2.5 × 106 km2) plateau on earth5. It started to uplift after the collision between India and Eurasia about 50 million years ago (Ma). However, geological estimates date the start of extensive uplifts within the mid-Miocene (c. 15-10 Ma)4. A large ice-sheet covering the whole QTP has never existed during the Quaternary glacial6. There was phylogeographical evidence for both postglacial re-colonization of the QTP from eastern glacial refugia, and glacial in situ survival on the central platform3.
Few phylogeographical studies in China were conducted on herbaceous aquatic species. Aquatic plants are widespread compared to terrestrial plants. Many aquatic species are globally distributed, such as Ceratophyllum demersum and Stukenia pectinata. Most of the widespread aquatic species excel in asexual reproduction with a variety of clonal propagules. They are also capable of long distance dispersal by seeds and specialized vegetative structures7. The aquatic genus Stuckenia (Potamogetonaceae) is characterized by long stipular sheathes, tubular leaves with air channels bordering the midrib, flexuous peduncles, and subterranean tubers8. The only cosmopolitan species in this genus is S. pectinata (L.) Börner, while S. filiformis (Persoon) Börner is mostly distributed in America and Europe, and extends to Western, Northern and Central Asia9.
Stuckenia species show a wide range of morphological variation. The most reliable characters which separate these two species are the structure of the leaf sheaths and the size of the fruits8. In S. pectinata the margins of the sheaths are free and often overlap, and the fruits are longer than 3.3 mm. S. filiformis, by contrast, has sheaths with margins which are united to form a tube, and fruits which are usually shorter than 3 mm, and do not exceed 3.2 mm10. Their hybrid S. × suecicus has been described by Hollingsworth et al.8 and Preston et al.10. However, because of similar vegetative characters, Stuckenia hybrids are extremely difficult to distinguish from their highly variable parental species. Thus molecular techniques have been used to investigate the nature of some putative Stuckenia hybrids8,10.
Phylogeography is concerned with principles and processes governing the geographical distributions of genealogical lineages, especially those within and among closely related species11. The wide geographical range of S. pectinata and S. filiformis make them suited for examination of topographic and climatic effects on plant evolution and speciation. Herein we report on the comparative phylogeography of S. pectinata and S. filiformis in China. Chloroplast (cp) DNA is maternally inherited in Potamogetonaceae by experimental hybrids12 and is therefore a good marker for tracing range expansion. Internal transcribed spacer (ITS) of nuclear ribosomal DNA is suitable for DNA barcoding in Potamogetonaceae13. So in the present study, nuclear ITS sequence and ten chloroplast sequences were used to examine the phylogeographical pattern of populations of S. pectinata and S. filiformis across their distribution ranges in China.
The specimen records showed that these two species were allopatric in China14. S. pectinata were mainly distributed in the temperate regions and the Yungui Plateau (YGP), whereas its specimens were rare in subtropical China and the QTP. In contrast, S. filiformis were mainly distributed on the QTP. However, its specimens were scarcely found on the high altitudinal and arid northwestern QTP, and were mainly restricted to the middle altitudinal and moist southern and eastern QTP. Our specific objectives were to use DNA barcoding, ecological niche modeling and phylogeographical analysis to (1) investigate the distribution regions of these two species in China and find whether they have sympatric regions and hybrid zones, and (2) compare their phylogeographical patterns and demographical histories as two closely related aquatic species.
Results
Nuclear genotype variation and distribution
The nuclear genotypes of each sampling population were recorded in Supplementary Table S1 and S2. The length of the alignment of ITS sequences of S. filiformis and S. pectinata were 682 bp and 673 bp respectively. A total of 31 base substitutions and two length variants were found between these two species (Supplementary Table S3). Only one ITS genotype was found in S. filiformis, while nine polymorphic sites were detected in S. pectinata defining three homozygous genotypes (AA, BB and CC). Three heterozygous genotypes (AB, AC, BC) with additive pattern of ITS sequences were also generated in S. pectinata by cloning their ITS sequences. Each population of S. filiformis and S. pectinata only had one ITS genotype. All hybrid individuals of S. × suecicus (S. filiformis × S. pectinata) possessed an additive pattern of ITS sequences. When sequences were cloned from them, these hybrid individuals yielded two types of ITS sequences, which were identical to either S. filiformis or S. pectinata.
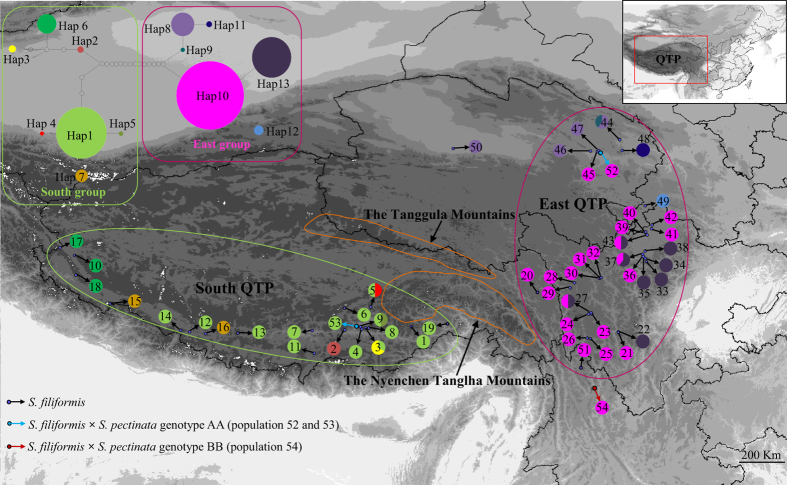
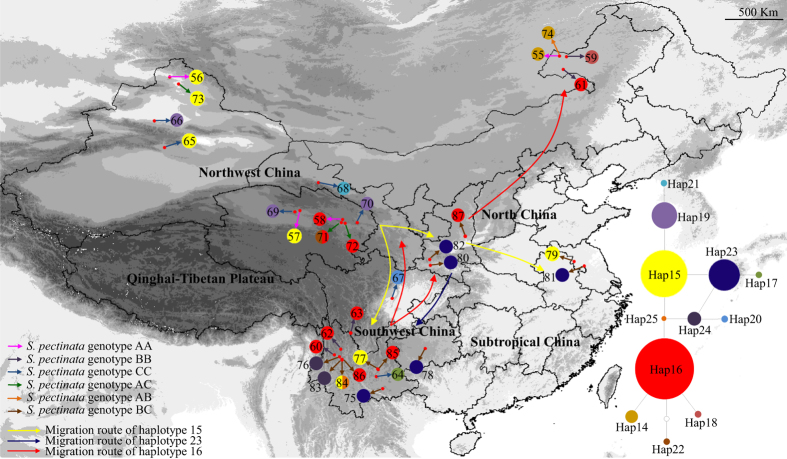
The geographic distributions of nuclear genotypes of S. filiformis and S. pectinata were shown in Figs 1 and 2. Their altitudinal distributions were shown in Supplementary Figs S1 and S2. In China, S. filiformis did not occur outside of the QTP. In accordance with the specimen records, S. filiformis had a discontinuous distribution on the QTP. It scarcely distributed on the high altitudinal and arid northwestern QTP. This species mainly occurred at intermediate altitudes ranging from 3000–4800 m on the southern and eastern plateau. S. pectinata occupied a wide range of habitats in northwest China, north China and southwest China (Fig. 2). On the QTP, S. pectinata occurred only at lower altitudinal northeastern edge of the plateau. This species did not occur in subtropical China. Both the homozygous genotypes (AA, BB and CC) and heterozygous genotypes (AB, AC, BC) of S. pectinata were common in China (Fig. 2). The hybrid S. × suecicus (S. filiformis × S. pectinata) occurred in the northwestern YGP and the northeastern and southern QTP (Fig. 1).
Figure 1. The geographical distribution of S. filiformis populations.
The map is created by DIVA-GIS. Right top is the map of China, indicating the Qinghai-Tibetan Plateau. Sampling locations are marked with population codes. Populations indicated by red and blue arrows are hybrids between S. filiformis and S. pectinata. Pie charts show the proportions of haplotypes within each population. Solid lines encircle SAMOVA-identified groups: green, ‘South group’; and red, ‘East group’. Left top is the network of 13 haplotypes of S. filiformis. The pie size is proportional to the haplotype frequency. Each dot between haplotypes indicates a mutational step.
Figure 2. The geographical distribution of S. pectinata populations.
The map is created by DIVA-GIS. Sampling locations are marked with population codes. Pie charts show the proportions of haplotypes within each population. The nuclear genotypes of S. pectinata are indicated by short arrows of different colors. Long arrows indicate the migration routes of widespread haplotypes. Right bottom is the network of 12 haplotypes of S. pectinata. The pie size is proportional to the haplotype frequency. Each dot between haplotypes indicates a mutational step.
Chloroplast haplotype variation and distribution
Both species were sequenced for the ten cpDNA regions. The total alignment length of the cpDNA sequences was 6819 bp. These two species had 128 polymorphic sites in the combined sequences, including 104 substitutions and 24 indels. For each cpDNA region, the two species had many interspecies variable sites. However, not every sequence was polymorphic within each species. Five cpDNA regions, atpF-atpH, rpl20-rps12, trnD-trnT, trnS-trnG and trnL-trnF, showed polymorphisms in S. filiformis, whereas other five cpDNA regions, trnL-rpl32, trnQ-rpS16, ndhAx1-ndhAx2, rpoB-trnC and psbM-trnD, showed polymorphisms in S. pectinata. Twenty-nine polymorphic sites were found in S. filiformis, while nine substitutions and two indels were found in S. pectinata (Supplementary Table S4 & S5).
Thirteen and 12 haplotypes were revealed in S. filiformis and S. pectinata respectively. Haplotypes in each population of S. filiformis were presented in Supplementary Table S1. Among 51 populations of S. filiformis, 44 were fixed by a single haplotype, while the remaining seven were polymorphic. Haplotypes in the 33 investigated populations of S. pectinata were listed in Supplementary Table S2. Only two populations in the 33 populations of S. pectinata were polymorphic, while the remaining 31 populations were fixed for a single haplotype. The haplotype diversity (Hd) of S. filiformis and S. pectinata were estimated to be 0.797 and 0.776 respectively.
The haplotype distributions of S. filiformis were structured into two geographical regions: the southern and eastern QTP (Fig. 1). Nineteen populations from the southern plateau harboured seven haplotypes (Hap1–7). Thirty populations from the eastern plateau harboured six haplotypes (Hap8-13). The geographical distributions of S. pectinata haplotypes were shown in Fig. 2. Hap15 and Hap16 were the most widespread haplotypes, occurring in northwest, north and southwest China. Hap23 also had a wide spread distribution in north China and southwest China. Some unique haplotypes were found in northwest China (Hap19, 21 & 22), north China (Hap14 & 18) and southwest China (Hap17, 20, 24 & 25).
Ecological niche modeling
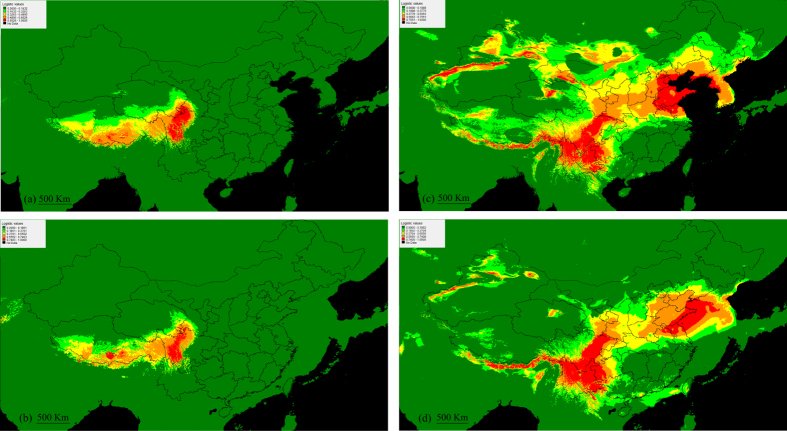
Based on binomial tests of omission, the models produced predictions that were significantly better than random (P < 0.001). The ecological niche models under current climate conditions differed greatly between species (Fig. 3). For both species, the palaeodistribution models showed that the habitats predicted as suitable during the LGM were a little smaller than the current distributions (Fig. 3).
Figure 3. Ecological niche modeling for S. filiformis and S. pectinata in China.
The maps are created by MAXENT and DIVA-GIS. The strength of prediction is indicated according to the key shown. Red and orange areas show strong predictions. (a) Regions in China considered suitable for S. filiformis under current climate conditions. (b) The paleodistribution model for S. filiformis in the LGM (22,000 years ago). (c) Regions in China considered suitable for S. pectinata under current climate conditions. (d) The paleodistribution model for S. pectinata in the LGM (22,000 years ago).
Haplotype network and phylogeny
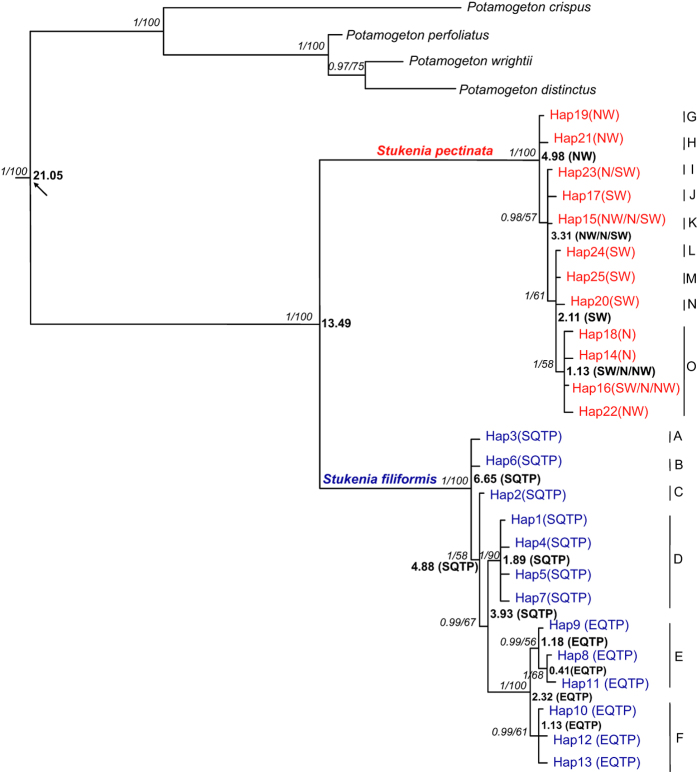
The genealogical relationships among the haplotypes were revealed in the parsimony networks (Figs 1 and 2). Bayesian and maximum likelihood (ML) analyses produced highly consistent tree topologies (Fig. 4). The support values of both Bayesian posterior probability and maximum likelihood bootstrap were annotated in the phylogenetic tree (Fig. 4). Phylogenetic analyses showed that both species were monophyly (Fig. 4). There were six clades in S. filiformis. The first four clades (A, B, C & D) comprised seven haplotypes (Hap1-7) located at the southern plateau, whereas the rest two clades (E & F) comprised six haplotypes (Hap8-13) situated in the eastern plateau. In the haplotype network, these two geographical lineages were separated by 14 steps (Fig. 1). Nine clades were identified in S. pectinata (Fig. 4). Clade G and H included two haplotypes (Hap19 & 21) distributed in northwest China (including part of the northeastern QTP). The rest clades consisted of ten haplotypes distributed in northwest, north and southwest China.
Figure 4. Phylogenetic relationships of haplotypes.
Support values (Bayesian posterior probability /maximum likelihood bootstrap) are next to the nodes. Arrow denotes node where fossil calibration was applied. The divergence times and ancestral areas are shown at nodes. The time scale is in Ma. The distributions were categorized into the following areas: northwest China (NW), north China (N), southwest China (SW), south QTP (SQTP) and east QTP (EQTP).
Divergence time estimation and ancestral area reconstruction
The estimated divergence time between S. filiformis and S. pectinata was 13.49 Ma (Fig. 4). The two geographical lineages of S. filiformis diverged from 3.93 Ma. The reconstructed ancestral areas were shown in Fig. 4. RASP analyses suggested that S. filiformis migrate from the southern plateau (clades A, B, C & D) to the eastern plateau (clades E & F). The reconstruction results showed that S. pectinata migrated from northwest China (clades G & H) to north and southwest China (clades I, J & K). It diversified in southwest China (clades L, M & N), and then dispersed from southwest China to north and northwest China (clade O). The migration routes of the widespread haplotypes (Hap15, 23 &16) were annotated on the distribution map of S. pectinata (Fig. 2).
Population structure and genetic variation
Results of the AMOVA analyses were presented in Supplementary Table S6. About 96.37% of the total variation of S. filiformis was partitioned among populations, whereas just 3.63% was within populations. When populations were grouped according to geographical region, AMOVA revealed that approximately 89% of the total genetic variation was assigned between the southern and eastern QTP, while only 9.11% of variation occurred among populations within these regions. The FST value of S. filiformis was 0.981, and the gene flow (Nm) was 0.01 individual per generation. The AMOVA results indicated that 75.54% of the total genetic variation of S. pectinata occurred among populations and 24.46% within populations. When considering groupings based on geographical range (northwest, north and southwest China), we detected little variation among groups (7.46%). The FST and Nm values of S. pectinata were 0.755 and 0.16 respectively.
Phylogeographical structure
Permutation test of S. filiformis showed that NST (0.982) was significantly higher than GST (0.886; P < 0.05), indicating significant phylogeographical structure across the species’ entire distributional range. Spatial genetic analysis of S. filiformis using SAMOVA revealed the presence of two groups of populations with an FCT value of 0.882. This grouping is mostly consistent with genealogical relationships of haplotypes (Fig. 1). Populations 1–20 comprised the southern plateau group, while the remaining populations 21–51 belonged to the eastern plateau group (Fig. 1). However, within these two groups, NST was not significantly higher than GST (P < 0.05). This result implies that if any phylogeographical structure was present within either group of S. filiformis, it was very weak. The permutation test of S. pectinata showed that NST (0.977) was higher than GST (0.954), but not significantly (P > 0.05), implying a lack of correlation between haplotypes and geographical distribution15. The SAMOVA analysis failed to uncover any reliable groups from populations of S. pectinata.
Demographic history
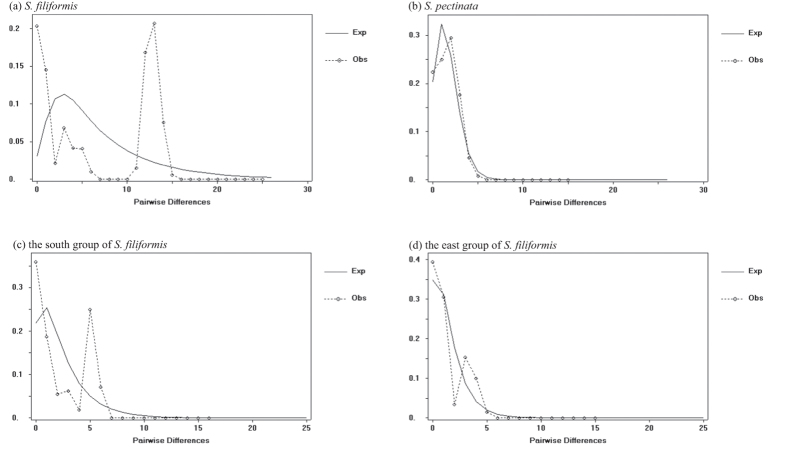
Results of the Fu’s Fs test and Tajima’s D test were presented in Supplementary Table S7. Positive Fu’s Fs value (6.517; P < 0.05) and non-significant Tajima’s D value (2.028; P > 0.05) supported that S. filiformis did not experience rapid range expansion. Its 95% confidence intervals of growth rates included zero (Supplementary Table S7), which indicated that there was little or no growth in S. filiformis. The observed mismatch distributions of S. filiformis also differed from sudden range expansion (Fig. 5). For both geographical groups of S. filiformis, the deviation in mismatch distribution (Fig. 5) and non-significant Tajima’s D and Fu’s Fs values (Supplementary Table S7) indicated that neither group had experienced sudden range expansion.
Figure 5.
Mismatch distributions of the number of pairwise nucleotide differences of S. filiformis (a), S. pectinata (b) and the south and east groups of S. filiformis (c,d). The dashed line represents observed values (Obs) whereas the solid line shows expected values (Exp) under the population growth-decline model. The Y axis is frequency.
The observed mismatch distributions of S. pectinata fitted the population growth-decline model (Fig. 5). However, the tests of neutrality for S. pectinata showed that Fu’s Fs value was slightly negative but not significant (−0.579; P > 0.1) and Tajima’s D value was non-significant (0.2452; P > 0.1). In addition, its 95% confidence intervals of growth rates included zero (Supplementary Table S7). These results indicated that there was some growth in S. pectinata, even though it was not an sudden range expansion. In the three distribution regions of S. pectinata, the results of mismatch distribution analyses (Supplementary Fig. S3) and neutral tests (Supplementary Table S7) provided no evidence of rapid range expansion in these regions.
Discussion
S. pectinatus is the most widespread species of Stuckenia and occurs in all continents of the world. S. filiformis, in contrast, is primarily a circumboreal species of more northerly latitudes8. In this study, DNA barcoding using ITS sequences revealed that only one nuclear genotype was found in S. filiformis, whereas three homozygous genotypes and three heterozygous genotypes were detected in S. pectinata. The geographical distributions of S. filiformis in China showed that this cold-tolerant species had a discontinuous distribution on the QTP (Fig. 1). The ecological niche model (Fig. 3) was consistent with the specimen records14. S. filiformis scarcely distributed on the high altitudinal and arid northwestern QTP, and mainly occurred at intermediate altitudes ranging from 3000–4800 m on the moist southern and eastern plateau. The Nyenchen Tanglha Mountains and the Tanggula Mountains located between the southern and eastern populations of S. filiformis (Fig. 1).
In contrast, S. pectinata occupied a wide range of habitats in temperate regions, whereas in subtropical region it is only found on the Yungui plateau (averaging about 2000 m) and the adjacent hills (Fig. 2). In the QTP, S. pectinata occurred only at altitudes ranging from 2500–3200 m on the northeastern edge of the plateau. The ecological niche model (Fig. 3) and the specimen records showed the same distributions.
These two species could hybridize in sympatric regions, even though their hybrids are sterile and propagate by asexual reproduction8,10. Hybridization between these two species was detected at the hybrid zone of Daotang River (population 52, 3143 m asl) on northeastern QTP, the only region where both species were found. The hybrid S. × suecicus were also found in other areas with only one parental species. In these regions, the disappeared parental species may once disperse to there and leave some relic populations.
In the southern valley of QTP (population 53, 3642 m asl), the hybrid S. × suecicus co-occurred only with S. filiformis. The ecological niche model (Fig. 3) predicted that S. pectinata could live along the southernmost edge of QTP (the Yarlung Tsangpo Grand Canyon in southeastern QTP and the south slope of the Himalayas). These results suggested that some populations of S. pectinata might disperse into the southern valley of QTP along the Yarlung Tsangpo River and hybridize with S. filiformis there. The cpDNA haplotype of this S. × suecicus population (Hap 1) is identical to the southern populations of S. filiformis. Because no S. filiformis population on the northeastern QTP has the same haplotype, this population of S. × suecicus could not be the descendents migrated from the hybrid zone on the northeastern QTP.
In the northwestern YGP (population 54, 2435 m asl), S. × suecicus lived with S. pectinata, while S. filiformis was missing. The postglacial retreat of S. filiformis from the northwest YGP was predicted by the ecological niche model (Fig. 3). The cpDNA haplotype (Hap 10) of this S. × suecicus population was identical to the eastern populations of S. filiformis, and the genotype of its paternal parent (genotype BB) was identical to the northwest YGP populations of S. pectinata. Because no S. pectinata population on the northeastern QTP is genotype BB, this population of S. × suecicus could not be the emigrants from the hybrid zone on the northeastern QTP.
The three homozygous genotypes of S. pectinata were sympatric, which led to the extensive hybridization and introgression among these genotypes (Fig. 2). If S. filiformis and S. pectinata are sympatric, they would hybridize frequently. Allopatric distribution is one of the mechanisms of geographical isolation between species that have incomplete reproductive isolation. It can reduce their hybridization frequency and keep them as two independent species.
Both species had high total genetic diversity at the species level (hT = 0.806 for S. filiformis and 0.876 for S. pectinata), whereas the average haplotype diversity within population was low (hS = 0.092 for S. filiformis and 0.04 for S. pectinata). This is because most of the S. filiformis and S. pectinata populations only contain a single haplotype, which is common in clonal aquatic plants7,8,16. However, the levels of genetic differentiation were different between these two species. Approximately 89% of the total genetic variation in S. filiformis was assigned between the east and south groups. Because of the marked geographical structure, a very high estimate of interpopulation differentiation was recorded (FST = 0.981) in S. filiformis. In contrast, the genetic differentiation was lower (FST = 0.755) in S. pectinata, due to the lack of differentiation among its distribution regions (northwest, north and southwest China). Only 7.46% of the cpDNA variation in S. pectinata was distributed among the three distribution regions. Comparing to the extremely low level of gene flow in S. filiformis (Nm = 0.01), S. pectinata showed a higher level of gene flow (Nm = 0.16).
PERMUT analysis suggested a distinct phylogeographical structure in S. filiformis (GST < NST; P < 0.05). SAMOVA identified that most of the genetic variance was between the south and east groups (Fig. 1). This was largely due to the major differences in haplotype composition between populations on the eastern and southern plateau. Haplotype network also showed that S. filiformis comprises two major lineages. The deep lineages within S. filiformis were dated to occur during the Pliocene (3.93 Ma) far before the LGM. Populations from the southern plateau were found to harbour ancestral haplotypes. Divergence time estimation and ancestral area reconstruction suggested that S. filiformis migrate from the southern plateau to the eastern plateau during the Pliocene (Fig. 4). After this migration, topographic changes on the QTP may have caused the intraspecific divergences through allopatric differentiation. The rising of the Nyenchen Tanglha Mountains and the Tanggula Mountains in the southeastern and central plateau may have blocked the gene flows between these two groups, and triggered the genetic divergence between them.
The Nyenchen Tanglha Mountains is a 700 km long mountain range located in the southeastern QTP17. The rugged and heavily glaciated range counts more than 240 peaks over 6000 m. It contains 7080 glaciers covering an area of 10700 km2. There are 32 glaciers that are over 10 km long17. In central QTP, the Tanggula Mountains locate at the north of Nyenchen Tanglha Mountains. The elevations of its main ridge are above 5000 m, and Peak Geladandong (6621 m) is the tallest in the range. The extremely low temperature on these high mountains and development of glaciers have blocked gene flow between geographically isolated east and south groups of S. filiformis and promoted its deep intraspecific divergence. Because the diverged lineages caused by this geographical isolation have not contact again, intraspecific introgressions between them were not found.
This allopatric divergence was also recovered for another alpine shrub species18. Phylogeographical analysis of Hippophae tibetana showed that it comprised two lineages, distributed in the eastern and southern QTP. The estimated timescale for the deep divergences within this species was 4 Ma if the slow evolutionary rate was adopted. In this study, the two geographical lineages of S. filiformis also diverged from 3.93 Ma. These two studies provided evidence that during the Pliocene geographical isolations caused by high mountains, cold climate and glaciers promoted intraspecific diversification of alpine plants on the QTP.
S. pectinata distributed in northwest China, north China and southwest China. The three homozygous nuclear genotypes regained contact, which led to frequent hybridizations and the share of haplotypes between them. Genotype AA and BB shared Hap16, while genotype AA and CC shared Hap 15. These two haplotypes were the most widespread haplotypes, occurring in northwest, north and southwest China. The heterozygous genotype BC had three unique haplotypes (Hap23, 24 & 25), and Hap23 was widely spread in north China and southwest China.
PERMUT analysis recorded no significant phylogeographical structure in S. pectinata (GST < NST, P > 0.05), which suggested that considerable gene flow occur among the three distribution regions. All the haplotypes of S. pectinata occurred before the LGM, but only the populations from northwest China were found to harbour ancestral haplotypes (Hap 19 & 21). Molecular dating and RASP analyses suggested that their descendent haplotype (Hap15) migrate from northwest China to north China and southwest China during the Pliocene (Fig. 4). After this migration, S. pectinata diversified in the YGP (Hap 20, 24 & 25) during the Pleistocene, and then one new haplotype (Hap 16) migrated backwards from southwest China to north China and northwest China. Followed by this migration, some new haplotypes were formed in northwest China (Hap 22) and north China (Hap 14 & 18). The lack of phylogeographical signal was mostly due to the facts that S. pectinata exhibited a continuous distribution of dominant haplotypes (Hap15, 16 & 23) among its distribution regions, and the gene flows among these regions continued from the Pliocene to the Pleistocene. The lack of geographical isolation among these regions and the ability of long distance dispersal triggered these gene flows among populations of S. pectinata.
Because of arid conditions and complex topography, QTP glaciers were less prominent than other regions in the Northern Hemisphere6. Potential habitats for cold-tolerant species could be found in the plateau during the ice ages19. The palaeodistribution model predicted that S. filiformis maintained in the southern and eastern QTP during the LGM (Fig. 3). At the end of the LGM, we failed to detect the large-scale range expansion of S. filiformis. Mismatch distribution of pairwise difference among haplotypes and Fu’s Fs and Tajima’ D statistic suggested that the whole species and both of the two groups of S. filiformis underwent no interglacial or postglacial rapid range expansion. Some other alpine species experienced the same demographic histories on the QTP18,20,21. The Quaternary glaciations reinforced the geographical isolation by the high mountains between the south and east groups of S. filiformis. After the LGM, they were still isolated by these high mountains. Until now, there is no sign that these two groups have gene flows between them.
The geological records showed that no unified ice sheet had developed in China during the Quaternary Period, so the phylogeographical patterns in China did not show an expansion-contraction pattern at large scales1. The palaeodistribution model predicted that during the LGM S. pectinata persisted in high latitudinal northwest China and north China, and low latitudinal southwest China (Fig. 3). But its distribution range in the northwest and north China has slightly contracted. This is in accordance with the results of the mismatch distribution analysis and the neutrality tests, indicating that there was some growth in S. pectinata, even though the whole species and the three distribution regions of S. pectinata experienced no rapid range expansion. The distribution pattern of S. pectinata differs from large-scale range expansion, in which the haplotype diversity gradually decrease with increasing distance to the southern refugia22. Instead, our result is consistent with demographic history of another widespread herb Clintonia udensis in East Asia, which showed no signature of rapid range expansion23.
This comparative study of Stuckenia species is one of a few studies that have been conducted on widespread herbaceous species in China. It provided evidence that geographical isolations caused by the Nyenchen Tanglha Mountains and the Tanggula Mountains promoted intraspecific diversification of alpine plants on the QTP. It also highlighted the importance of northwest China and southwest China in the evolutionary history of widespread temperate species in China. The populations from northwest China could harbor ancestral haplotypes, and migrate to north China and southwest China (after the uplift of YGP), whereas new haplotypes could diversify in the YGP, and migrate backwards to north China and northwest China. Because the northern part of China and the whole QTP were not covered by large ice sheets during the Quaternary glaciations, some plant species could survive in the north region of China (temperate species) and the QTP (cold-tolerant species) during the glacial period. This comparative study would provide insights into the evolutionary history of plants with similar life histories and distribution patterns in China.
Methods
Population sampling
A total of 87 populations of Stuckenia species were collected throughout the entire range of this genus in China. For each population, 5–10 randomly selected individuals were sampled at intervals of at least 10 m apart to avoid collecting clones. Fresh leaves were immediately dried in silica gel. Voucher specimens were deposited at Wuhan Botanical Garden, Chinese Academy of Sciences. The latitude, longitude, and altitude of each sampling site were recorded in Supplementary Table S1 and S2. Stuckenia was recently elevated from a subgenus in Potamogeton to the rank of genus14. Because we need to use the known fossil ages of Potamogeton for divergence time estimations, we selected four Potamogeton species (P. crispus, P. distinctus, P. wrightii, and P. perfoliatus) as outgroups for phylogenetic analyses.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from silica-gel-dried leaf tissue using a modified CTAB method24. Both ITS1 and ITS2 regions of ITS sequence25 and ten cpDNA regions (trnL-trnF26; trnD-trnT27; rpl20-rps12, trnS-trnG28; rpoB-trnC, psbM-trnD29; trnL-rpl32, trnQ-rpS16, ndhAx1-ndhAx230; atpF-atpH31) were amplified and sequenced for all the samples of both species using universal primers. The procedures for PCR amplification and sequencing were following Du et al.13. If superimposed nucleotides (additive patterns) were found in the chromatograms of ITS sequences, the purified PCR products were cloned into the pUC19 vector (TaKaRa). For each of them, five positive clones were sequenced.
Nuclear genotype and chloroplast haplotype variation and distribution
We used DNASP v.5.0 to extract nuclear genotypes and chloroplast haplotypes32. The sequences of nuclear genotypes and cpDNA haplotypes were deposited in GenBank under accession numbers KP676469 - KP676566. Haplotype diversity (Hd) were calculated using DNASP. The geographical and attitudinal distributions of genotypes and haplotypes were plotted on a relief map of China using DIVA-GIS33.
Ecological niche modeling
The global climate database from WorldClim34 was used for ecological niche modeling under current climate conditions using MAXENT v.3.3.335. Climate estimates for the last glacial maximum (LGM) was provided by the Palaeoclimate Modelling Intercomparison Project Phase II (PMIP2). For each species, a distribution model was generated using 19 bioclimatic parameters for the current climate and the collection localities. The model was then applied to estimate the geographical distribution for each species during the LGM. Binomial tests of omission were conducted by randomly selecting 25% of the occurrence localities as test data and using 10,000 randomly chosen pixels from the study region as random instances35.
Haplotype network and phylogeny
Genealogical relationships among haplotypes were inferred by TCS v.1.2136. Phylogenetic relationships of the two species were estimated by Mrbayes v.3.037. Bayesian analyses were performed over 1.0 × 108 generations under the GTR model with gamma, as inferred from MrModeltest v.2.038. After discarding the first 25% trees as burn in, the remaining trees were used to estimate posterior probability. In addition, phylogenetic relationships were also assessed by maximum likelihood (ML) methods using PhyML v.3.139. The ML analysis was performed using the GTR model with gamma. The robustness of ML tree was tested by 100 bootstrap replicates.
Divergence time estimation and ancestral area reconstruction
BEAST v.1.5.440was used to estimate the divergence times. The partition homogenetity test revealed no character incongruence among all the ten cpDNA intergenic regions, so we used the combined sequences for analysis. The hypothesis of equal substitution rates among species was tested using log-likelihood ratio test in PAUP41, which led to the rejection of the constant molecular clock hypothesis (P < 0.05). Thus, an uncorrelated lognormal model was used to describe the relaxed clock. The root of the tree, which is the split between Potamogeton and Stuckenia, was constrained between 23.03–20.44 Ma based on the known fossils of Potamogeton42,43. Markov Chain Monte Carlo (MCMC) analyses was run for 1.0 × 108 generations under the GTR nucleotide substitution model with gamma. We checked convergence of the stationary distribution using Tracer v.1.5.140, and the effective sample size for each parameter was found to exceed 200. After discarding the first 25% trees as burn in, the samples were summarized in the maximum clade credibility tree using TreeAnnotator v.1.4.840.
We employed the Statistical Dispersal-Vicariance Analysis (S-DIVA) in RASP v.3.0 (Reconstruct Ancestral State in Phylogenies)44 to infer the biogeographical history. The geographical distributions of haplotypes were annotated on the phylogenetic tree. We categorized the distributions into the following areas: northwest China (NW), north China (N), southwest China (SW), south QTP (SQTP) and east QTP (EQTP).
Population structure and genetic variation
Hierarchical analysis of molecular variance (AMOVA) was performed to characterize the population structure and genetic variation using Arlequin v.3.045. To estimate the extent of genetic divergence and gene flow, measures of pairwise genetic differentiation (FST) and gene flow (Nm) among geographical regions were calculated. The Kimura two-parameter model was chosen, and significance of the variance components was tested with 1000 random permutations.
Phylogeographical structure
Two parameters of differentiation, GST (coefficient of genetic variation over all populations) and NST(equivalent coefficient taking into account sequence similarities between haplotypes) were estimated using the program PERMUT15. GST and NST were compared using a permutation test with 1000 permutations. A higher NST than GST indicates the presence of phylogeographical structure with closely related haplotypes being found more often in the same area than less closely related haplotypes15. Then, spatial genetic structures of haplotypes were analysed by spatial analysis of molecular variance using SAMOVA v.1.046 to define groups of populations that are geographically homogenous and maximally differentiated from each other. The configuration with the largest genetic differentiation (FCT) was retained as the best grouping of populations.
Demographic history
To detect historical population dynamics, mismatch distribution was estimated. The observed number of differences between pairs of haplotypes was compared to the theoretical distribution using the population growth-decline model with DNASP32. The demographic expansion was then tested by DNASP using Fu’s Fs test47 and Tajima’s D test48. Significantly negative Fs values indicate recent population expansion following a severe reduction in population size47, and significant D values generally suggest rapid demographic expansions49. We also used LAMARC v.2.150 to estimate the growth rates of the two Stuckenia species. If the estimate of growth rate is positive and big, but the confidence intervals include zero, it’s quite likely that there is in fact little or no growth50. If the intervals exclude zero, that finding is generally reliable50. For the two population groups within S. filiformis (East and South) and the three distribution regions of S. pectinata (Northwest, North and Southwest), we also performed the mismatch distribution analyses, neutrality tests and coalescent simulations.
Additional Information
How to cite this article: Du, Z.-Y. and Wang, Q.-F. Allopatric divergence of Stuckenia filiformis (Potamogetonaceae) on the Qinghai-Tibet Plateau and its comparative phylogeography with S. pectinata in China. Sci. Rep. 6, 20883; doi: 10.1038/srep20883 (2016).
Supplementary Material
Acknowledgments
The authors thank Prof. You-Hao Guo, Jin-Ming Chen, Kuo Liao, Dun Wang, Shu-Ping Gu, Zhi-Cheng Long and Peng Gao for their assistance in sample collection and laboratory work. This study was supported by grants from the National Natural Science Foundation of China (Grant No. 31100175) and the strategic pilot science and technology projects of the Chinese Academy of Sciences (Grant No. XDAO5090305).
Footnotes
Author Contributions Z.Y.D. and Q.F.W. conceived and designed the research. Z.Y.D. performed laboratory experiments, analyzed data and wrote the paper. Q.F.W. revised the manuscript.
References
- Liu J. Q., Sun Y. S., Ge X. J., Gao L. M. & Qiu Y. X. Phylogeographical studies of plants in China: Advances in the past and directions in the future. J. Syst. Evol. 50, 267–275 (2012). [Google Scholar]
- Du F. K. et al. Direction and extent of organelle DNA introgression between two spruce species in the Qinghai-Tibetan Plateau. New Phyto. 192, 1024–1033 (2011). [DOI] [PubMed] [Google Scholar]
- Qiu Y. X., Fu C. X. & Comes H. P. Plant molecular phylogeography in China and adjacent regions: Tracing the genetic imprints of Quaternary climate and environmental change in the world’s most diverse temperate flora. Mol. Phylogenet. Evol. 59, 225–244 (2011). [DOI] [PubMed] [Google Scholar]
- Royden L. H., Burchfiel B. C. & van der Hilst R. D. The geological evolution of the Tibetan plateau. Science 321, 1054–1058 (2008). [DOI] [PubMed] [Google Scholar]
- Zhou S. Z., Wang X. L., Wang J. & Xu L. B. A preliminary study on timing of the oldest Pleistocene glaciation in Qinghai-Tibetan Plateau. Quatern. Int. 154–155, 44–51 (2006). [Google Scholar]
- Seong Y. B., Owen L. A. & Bishop M. P. Quaternary glacial history of the central karakoram. Quaternary Sci. Rev. 26, 3384–3405 (2007). [Google Scholar]
- Philbrick C. T. & Les D. H. Evolution of Aquatic Angiosperm Reproductive Systems: What is the balance between sexual and asexual reproduction in aquatic angiosperms. Bioscience 46, 813–826 (1996). [Google Scholar]
- Hollingsworth P. M., Preston C. D. & Gornall R. J. Isozyme evidence for the parentage and multiple origins of Potamogeton × suecicus (P. pectinatus × P. filiformis, Potamogetonaceae). Plant Syst. Evol. 202, 219–232 (1996). [Google Scholar]
- Wieglet G. & Kaplan Z. An account of the species of Potamogeton L. (Potamogetonaceae). Folia Geobot. 33, 241–316 (1998). [Google Scholar]
- Preston C. D., Hollingsworth P. M. & Gornall R. The distribution and habitat of Potamogeton × suecicus K. Richt. (P. filiformis Pers. × P. pectinatus L.) in the British Isles. Watsonia 22, 329–342 (1999). [Google Scholar]
- Avise J. C. Phylogeography: The History and Formation of Species (Harvard University Press, Cambridge, 2000). [Google Scholar]
- Kaplan Z. & Fehrer J. Comparison of natural and artificial hybridization in Potamogeton. Preslia 78, 303–316 (2006). [Google Scholar]
- Du Z. Y., Qimike A., Yang C. F., Chen J. M. & Wang Q. F. Testing four barcoding markers for species identification of Potamogetonaceae. J. Syst. Evol. 49, 246–251 (2011). [Google Scholar]
- Kaplan Z. A taxonomic revision of Stuckenia (Potamogetonaceae) in Asia, with notes on the diversity and variation of the genus on a worldwide scale. Folia Geobot. 43, 159–234 (2008). [Google Scholar]
- Pons O. & Petit R. J. Measuring and testing genetic differentiation with ordered versus unordered alleles. Genetics 144, 1237–1245 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. M., Du Z. Y., Sun S. S., Gituru R. W. & Wang Q. F. Chloroplast DNA phylogeography reveals repeated range expansion in a widespread aquatic herb Hippuris vulgaris in the Qinghai-Tibetan Plateau and adjacent areas. PLoS One 8, e60948 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V. P., Singh P. & Haritashya U. K. Encyclopedia of snow, ice, and glaciers (Springer, Dordrecht, 2011). [Google Scholar]
- Jia D. R., Liu T. L., Wang L. Y., Zhou D. W. & Liu J. Q. Evolutionary history of an alpine shrub Hippophae tibetana (Elaeagnaceae): allopatric divergence and regional expansion. Biol. J. Linn. Soc. 102, 37–50 (2011). [Google Scholar]
- Wen J., Zhang J. Q., Nie Z. L., Zhong Y. & Sun H. Evolutionary diversifications of plants on the Qinghai-Tibetan Plateau. Front. Genet. 5, 4 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Y. et al. History and evolution of alpine plants endemic to the Qinghai-Tibetan Plateau: Aconitum gymnandrum (Ranunculaceae). Mol. Ecol. 18, 709–721 (2009). [DOI] [PubMed] [Google Scholar]
- Opgenoorth L. et al. Tree endurance on the Tibetan Plateau marks the world’s highest known tree line of the Last Glacial Maximum. New Phyto. 185, 332–342 (2010). [DOI] [PubMed] [Google Scholar]
- Hewitt G. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 (2000). [DOI] [PubMed] [Google Scholar]
- Wang Y. L. et al. Chloroplast DNA phylogeography of clintonia udensis trautv. & mey. (Liliaceae) in East Asia. Mol. Phylogen. Evol. 55, 721–732 (2010). [DOI] [PubMed] [Google Scholar]
- Doyle J. J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 19, 11–15 (1987). [Google Scholar]
- White T. J., Bruns T., Lee S. & Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics in PCR protocols: a guide to methods and applications, 315–322 (Academic Press, New York, 1990). [Google Scholar]
- Taberlet P., Gielly L., Pautou G. & Bouvet J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 17, 1105–1109 (1991). [DOI] [PubMed] [Google Scholar]
- Demesure B., Sodzi N. & Petit R. J. A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol. Ecol. 4, 129–131 (1995). [DOI] [PubMed] [Google Scholar]
- Hamilton M. B. Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol. Ecol. 8, 521–523 (1999). [PubMed] [Google Scholar]
- Shaw J. et al. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 92, 142–166 (2005). [DOI] [PubMed] [Google Scholar]
- Shaw J., Lickey E. B., Schilling E. E. & Small R. L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The tortoise and the hare III. Am. J. Bot. 94, 275–288 (2007). [DOI] [PubMed] [Google Scholar]
- Fazekas A. J. et al. Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS One 3, e2802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P. & Rozas J. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452 (2009). [DOI] [PubMed] [Google Scholar]
- Hijmans R. J. et al. DIVA-GIS Version 7.5: A geographic information system for the analysis of species distribution data. Available at: http://www.diva-gis.org (Accessed: 12th March 2015).
- Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G. & Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- Phillips S. J. et al. Maxent software for species distribution modeling. Available at: http://www.cs.princeton.edu/~schapire/maxent/ (Accessed: 12th March 2015).
- Clement M., Posada D. & Crandall K. A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659 (2000). [DOI] [PubMed] [Google Scholar]
- Ronquist F. & Huelsenbeck J. P. MrBayes Version 3.0: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003). [DOI] [PubMed] [Google Scholar]
- Nylander J. MrModeltest Version 2.0. Available at: https://github.com/nylander/MrModeltest2 (Accessed: 12th March 2015).
- Guindon S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010). [DOI] [PubMed] [Google Scholar]
- Drummond A. J. & Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214–221 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. L. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4. (Sinauer Associates, Sunderland, 2002).
- Teodoridis V. Revision of Potamogeton fossils from the Most Basin and their palaeoecological significance (Early Miocene, Czech Republic). B. Geosci. 82, 409–418 (2007). [Google Scholar]
- Teodoridis V. The Integrated Plant Record vegetation analysis of Early Miocene assemblages from the Most Basin (Czech Republic). Neues Jahrb. Geol. Paläontol. 256, 303–316 (2010). [Google Scholar]
- Yu Y., Harris A. J., Blair C. & He X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogen. Evol. 87, 46–49 (2015). [DOI] [PubMed] [Google Scholar]
- Excoffier L., Laval G. & Schneider S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 1, 47–50 (2005). [PMC free article] [PubMed] [Google Scholar]
- Dupanloup I., Schneider S. & Excoffier L. A simulated annealing approach to define the genetic structure of populations. Mol. Ecol. 11, 2571–2581 (2002). [DOI] [PubMed] [Google Scholar]
- Fu Y. X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 147, 915–925 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123, 585–595 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson R. R. Gene genealogies and the coalescent process in Oxford Surveys in Evolutionary Biology, Vol. 7, 1–44 (Oxford University Press, Oxford, 1990). [Google Scholar]
- Kuhner M. K. LAMARC 2.0: maximum likelihood and Bayesian estimation of population parameters. Bioinformatics 22, 768–770 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.