Abstract
BACKGROUND--The importance of cytokines in the asthmatic inflammatory response is becoming apparent. The aim of this study was to determine whether the non-invasive method of induced sputum combined with the polymerase chain reaction would allow the detection of messenger RNA (mRNA) encoding a range of cytokines on a qualitative basis. METHODS--Four groups were studied comprising 10 normal subjects, six atopic, 10 mild and five moderately severe asthmatic subjects. Sputum was induced by the inhalation of nebulised 3.5% saline and total RNA extracted from the expectorated cells. Expression of cytokine message within induced sputum was examined by reverse transcription and the polymerase chain reaction (PCR) using primers specific for a range of cytokines (IL-1 alpha, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, RANTES, TNF alpha, IFN alpha, IFN gamma). Presence or absence of the signal was determined at 35 and 70 cycles of PCR by electrophoretic size fractionation on ethidium bromide stained agarose gels. RESULTS--Cytokine message was detectable in sputum by this method. All samples showed a positive result for actin control. Analysis of signal for the cytokines in all subjects showed that, at 70 cycles, IL-1, IL-5, IL-8, and TNF alpha were detected in more subjects than would be expected by chance. IL-5 mRNA was detected in more of the asthmatic patients (moderate 80%, mild 40%) than in the atopic subjects (33%), who in turn showed expression of this cytokine in more individuals than nonatopic subjects (10%). CONCLUSIONS--The combination of sputum induction and PCR appears to be a useful, non-invasive tool to explore the chronic inflammation of asthma and possibly other lung disorders. It should enable differences between normal and asthmatic subjects to be identified for future confirmation by quantitative techniques.
Full text
PDF
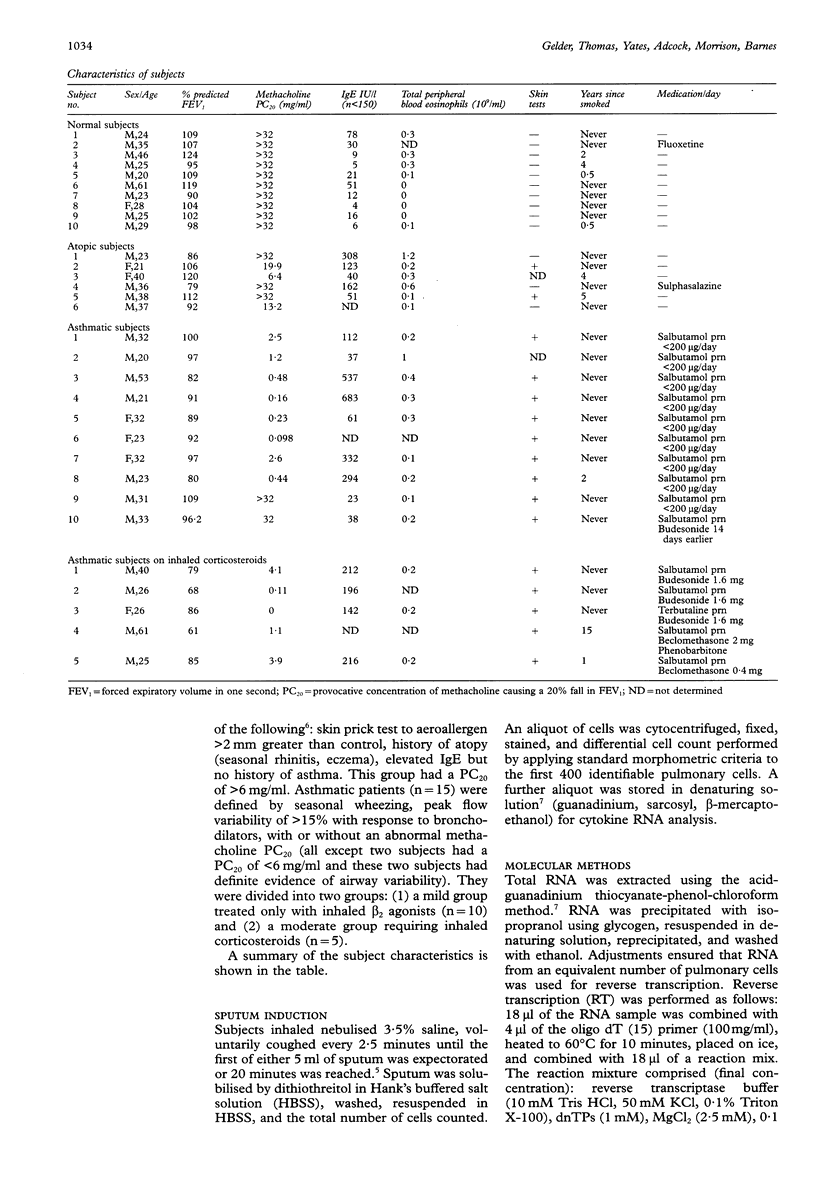
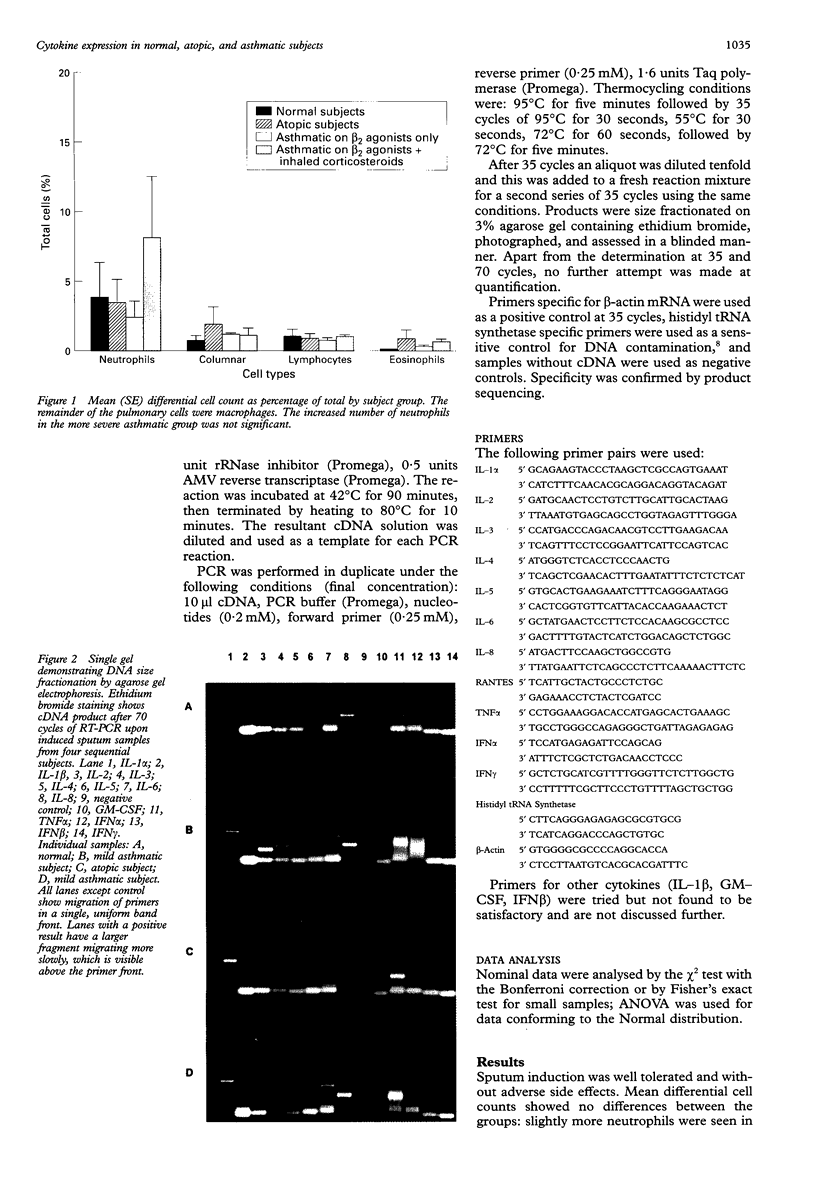


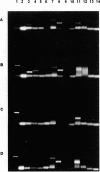
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown P. H., Crompton G. K., Greening A. P. Proinflammatory cytokines in acute asthma. Lancet. 1991 Sep 7;338(8767):590–593. doi: 10.1016/0140-6736(91)90605-o. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cookson W. O., Hopkin J. M. Dominant inheritance of atopic immunoglobulin-E responsiveness. Lancet. 1988 Jan 16;1(8577):86–88. doi: 10.1016/s0140-6736(88)90286-3. [DOI] [PubMed] [Google Scholar]
- Corrochano L. M. A test of human cDNA synthesis by the polymerase chain reaction. Genet Anal Tech Appl. 1991 Jun;8(4):134–135. doi: 10.1016/1050-3862(91)90030-u. [DOI] [PubMed] [Google Scholar]
- Fahy J. V., Liu J., Wong H., Boushey H. A. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993 May;147(5):1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- Fahy J. V., Liu J., Wong H., Boushey H. A. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993 May;147(5):1126–1131. doi: 10.1164/ajrccm/147.5.1126. [DOI] [PubMed] [Google Scholar]
- Hallsworth M. P., Soh C. P., Lane S. J., Arm J. P., Lee T. H. Selective enhancement of GM-CSF, TNF-alpha, IL-1 beta and IL-8 production by monocytes and macrophages of asthmatic subjects. Eur Respir J. 1994 Jun;7(6):1096–1102. [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M. F., Beer D. J. T lymphocytes and cytokine networks in asthma: clinical and therapeutic implications. Adv Intern Med. 1993;38:189–222. [PubMed] [Google Scholar]
- Lynch J. P., 3rd, Standiford T. J., Rolfe M. W., Kunkel S. L., Strieter R. M. Neutrophilic alveolitis in idiopathic pulmonary fibrosis. The role of interleukin-8. Am Rev Respir Dis. 1992 Jun;145(6):1433–1439. doi: 10.1164/ajrccm/145.6.1433. [DOI] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Walker C., Bauer W., Braun R. K., Menz G., Braun P., Schwarz F., Hansel T. T., Villiger B. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med. 1994 Oct;150(4):1038–1048. doi: 10.1164/ajrccm.150.4.7921434. [DOI] [PubMed] [Google Scholar]