Abstract
This study aimed to investigate the dysfunctional ascending/descending pain pathways at the thalamic level in patients with migraine without aura (MWoA) using the effective connectivity analysis of the resting-state functional MRI. Twenty MWoA and 25 matched healthy controls participated in the resting-state functional MRI scans. The directional interactions between the posterior thalamus (PTH) and other brain regions were investigated using the Granger causality analysis and choosing bilateral PTH as two individual seeds. Pearson’s correlation analysis was carried out between the abnormal effective connectivity and the headache duration and pain intensity of MWoA. Compared with healthy controls, MWoA showed decreased inflows to the bilateral PTH from the ventromedial prefrontal cortex and the left precuneus/posterior cingulate cortex, decreased outflow from the left PTH to the ipsilateral dorsomedial prefrontal cortex, and increased inflow to the right PTH from the ipsilateral dorsolateral prefrontal cortex. In addition, the abnormal inflows to the right PTH from the ventromedial prefrontal cortex and the right dorsolateral prefrontal cortex correlated positively with the headache duration and pain intensity, respectively. The abnormal ascending/descending pain pathways between the thalamus and these cortical regions indicate a disrupted pain modulation in affective and sensory domains, which suggests a disequilibrium of pain inhibition and facilitation in MWoA. These findings may help to shed light on the pathophysiologic mechanisms of migraine.
Keywords: effective connectivity, Granger causality analysis, migraine, posterior thalamus, resting-state functional magnetic resonance imaging
Introduction
Migraine is a disabling neurological condition that manifests with attacks of headache, hypersensitivities to visual, auditory, olfactory, and somatosensory stimuli, nausea, and vomiting 1. It affects about 12% of the general population and causes substantial personal and social burden 2. With the help of neuroimaging technology, our perception of migraine has transformed from a vascular into a neurovascular and, most recently, into a central nervous system disorder 3. As such, many neuroimaging studies in migraine have documented structural and functional alterations in a variety of cortical (such as occipital, parietal, and temporal lobes, cingulate cortex, prefrontal cortex, and insula) and subcortical (such as thalamus, amygdala, hippocampus, basal ganglia, and brainstem) pain-processing regions. These areas are often referred to as being part of the ‘pain matrix’, a group of brain regions consistently activated by pain stimulation 3,4.
In addition to a general activation of the trigeminal neurons in the thalamic nuclei in functional imaging studies of migraine, a major network of trigeminovascular-sensitive neurons from the thalamus to widespread regions of the cortex has also been identified 5. The thalamus is a relay region subserving both sensory and motor processing, with nerve fibers not only projecting out to the cerebral cortex in all directions but also receiving feedback information from these multiple cortical areas 6. These functionally distinct and anatomically remote cortical areas that the thalamus projects to are involved in the processing of sensory, cognitive, and affective information arising from the body 7. Therefore, such a thalamocortical network may play a role in the ascending and descending pain pathways responding for the transmission and modulation of nociceptive signals, and explains some of the common disturbances in neurological functions during migraine. However, how the thalamus affects these brain systems and whether it is affected by these brain regions in migraine are poorly understood.
Resting-state effective connectivity and functional connectivity are two effective techniques to address the integration of functionally specialized areas in human brain. Both two methods allow the inference of communication between spatially remote brain regions, whereas effective connectivity additionally allows inferring about the directionality of information transfer within functionally connected networks, utilizing information on time-lagged relationships between brain regions 8. Some resting-state functional connectivity studies in migraine have so far been documented by choosing pain matrix regions such as periaqueductal gray, nucleus cuneiformis, and the affective regions (including the insula, anterior cingulate cortex, and amygdala) as regions of interest (ROIs) (i.e. seeds), and abnormal functional connectivities between these seeds and thalamus have been found 9,10. To date, little is known about whether these reported abnormal functional connectivities are involved in the pain modulation during the migraine process. Therefore, studying the directed influence (effective connectivity) of the thalamus with the other structures can strengthen our understanding of the physiopathologic mechanism in migraine.
In the present study, we sought to investigate the effective connectivity patterns of the bilateral thalamus with the rest of the brain in migraine using resting-state functional MRI (rs-fMRI). Granger causality analysis (GCA) is a special approach to explore such effective connectivity among regions, can drive the deductive network on the basis of a certain hypothetical seed region, and requires no previous knowledge 11. We construct an effective connectivity network associated with the thalamus using the GCA method on rs-fMRI data to test the hypothesis that abnormal ascending/descending pathways at the thalamic level exist in migraine and are involved in the physiopathologic mechanism of migraine.
Participants and methods
Participants
In the present study, 20 right-handed patients with migraine without aura (MWoA) (ages 19–44 years) as well as 25 age-matched, sex-matched, and handedness-matched healthy controls (HC) were recruited. The diagnosis of MWoA was made according to the second edition of the International Classification of Headache Disorder (ICHD-II) 12. The protocol of this study was approved by the Medical Ethics Committee of West China Hospital, Sichuan University, and all participants provided written informed consent. To avoid any possible pharmacological interference, all participants had to be weaned off analgesic drugs 1 week or longer, not receiving preventive treatment, and not used any other drugs for at least 1 month before study participation. Participants had not had a migraine attack at least 72 h before scanning. In addition, patients were excluded if they had a migraine precipitated during the 2-day follow-up. Potential participants were excluded if they had any contraindication to MRI, had a previous brain injury, had a neurologic disorder other than migraine, had a psychiatric disorder other than anxiety or depression, or if they had any acute or chronic pain disorder other than migraine.
All patients were interviewed to determine the demographic features (e.g. age, sex, and education). Additional information including the migraine history [e.g. onset age, frequency and duration of attacks, and pain intensity (evaluated using the visual analogue scale)] and the impact of headache (evaluated with the Migraine Disability Assessment Scale and six-item Headache Impact Test) was collected in participants with migraine by two experienced neurologists. The Allodynia Symptom Checklist-12 (ASC-12) scale was administered to estimate the prevalence and severity of cutaneous allodynia in the migraine population. The yielding score of each participant was placed into one of the four categories: 0–2=no allodynia; 3–5=mild allodynia; 6–8=moderate allodynia; 9 or more=severe allodynia. For all participants, psychiatric assessments including the use of 24-item Hamilton Depression Scale (24-HAMD) and 14-item Hamilton Anxiety Scale (14-HAMA) were also performed to assess depression and anxiety state. Demographic and clinical features between MWoA and HC were determined using an independent-sample t-test or the χ2-test, as appropriate.
Data acquisition and spatial processing
The experiment was conducted on a 3.0-T scanner (Trio Tim; Siemens, Erlangen, Germany) using a 16-channel birdcage head coil and tightly padded clamps were used to minimize head motion. A routine T1-weighted imaging was first obtained and then the rs-fMRI was obtained using an echo-planar imaging sequence with the following protocols: voxel size: 3.75×3.75×5 mm3, TR: 2000 ms, TE: 30 ms, FOV: 240×240 mm2, matrix: 64×64, and slice thickness: 5 mm with no gap. Throughout the scanning, participants were instructed to lie in the scanner supine, relaxed, close their eyes, but remain awake.
The data were preprocessed using SPM8 (The Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The first five time points of the resting-state data were discarded because of instability of the initial MRI signal, leaving 175 time points remaining for further processing. Then, the functional images were slice-timing corrected and realigned to the first volume using a six-parameter rigid body transformation. Images whose head translation exceeded 2 mm or rotation exceeded 2° were excluded. The mean image generated was then spatially normalized into a standard stereotactic space using the Montreal Neurological Institute (MNI) echo-planar imaging template. Computed transformation parameters were applied to all functional images, interpolated to isotropic voxels of 2 mm3, and the resulting images were smoothed using a 4-mm full-width half-maximum isotropic Gaussian kernel. Then, using the Data Processing Assistant for resting-state fMRI (DPARSF) package 13, linear drift was removed. A band-pass frequency filter (0.01<f<0.08 Hz) was then applied to reduce physiological high-frequency noise. To further reduce the effects of confounding factors unlikely to be involved in specific regional correlation, we also removed several sources of spurious variance by linear regression, including six head motion parameters, and average signals from cerebrospinal fluid and white matter according to previous fMRI studies 14.
Granger causality analysis and statistical analysis
We used Granger causality to describe the effective connectivity between the reference time series of the seed regions [left and right posterior thalamus (PTH)] and the time series of each voxel within the whole brain. The coordinates (peak MNI coordinates: the right PTH=3, −14, 6; the left PTH=−6, −21, −3) were obtained on the basis of previous studies that showed structural or functional alterations in PTH in patients with migraine 15,16. Two 6-mm-radius sphere seeds on the basis of the peak coordinates were then designed for GCA. Bivariate first-order coefficient-based voxel-wise GCA was performed using the REST-GCA in the REST toolbox 17. Granger causality estimates the causal effect of the seed region on every other voxel in the brain (X to Y effect), as well as the Y to X effect, the causal effect of every voxel in the brain (Y) on the seed region (X). A positive coefficient from X to Y indicates that activity in region X exerts a causal influence on the activity region Y in the same direction (i.e. positive influence). Similarly, a negative coefficient from X to Y suggests that the activity of region X exerts an opposing directional influence on the activity of region Y (i.e. negative influence). Using this approach, we are able to construct a Granger causal model on the basis of the temporal elements of regional BOLD activity. The voxel-wise GCA maps generated were then transformed into z scores to improve the normality 18. Two-tailed two-sample t-tests were carried out on the causal effects between MWoA and HC with a Gaussian random fields-corrected significance voxel level of P value less than 0.01 and a joint cluster level of P value less than 0.05. Age, sex ratio, and education were used as covariates in the two-sample t-tests.
Pearson’s correlation analysis and statistical analysis
To investigate the association between the headache characteristic (headache duration and pain intensity) and the effective connectivity of bilateral PTH in the MWoA group, the regions showing significantly different (increased or decreased) Granger influences between the MWoA and HC were extracted as ROIs. The mean Granger causality values within these ROIs were correlated against the headache duration and pain intensity of all patients using the Pearson correlation analysis. Statistical analyses were carried out in SPSS (17.0; SPSS Inc., Chicago, Illinois, USA) and the threshold was set at P value less than 0.05.
Results
Demographics and clinical data
The demographic and clinical characteristics of all participants are presented in Table 1. No participant was excluded because of head movement. In all MWoA, the ASC scores were within 0-2 scores (no allodynia). No significant difference was found for sex, age, education, 24-HAMD, or 14-HAMA between MWoA and HC groups.
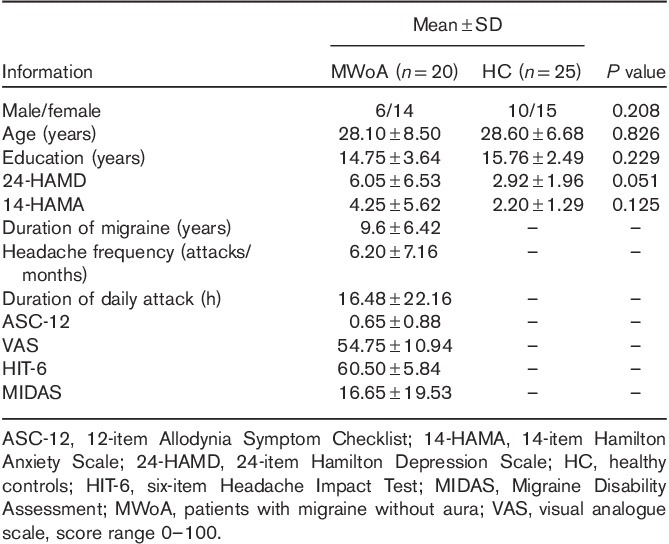
Table 1.
Demographic and clinical characteristics of the study participants
Abnormality for causal influence to and from the left posterior thalamus
Two-sample t-test results of resting-state effective connectivity to the left PTH in MWoA compared with HC are shown in Table 2 and Fig. 1a. The results showed significantly decreased causal inflow to the left PTH from the bilateral ventromedial prefrontal cortex (vmPFC) (including the bilateral anterior cingulate cortex, bilateral olfactory cortex, left caudate nucleus, right rectus gyrus, the left orbital part of the superior frontal gyrus and the middle frontal cortex, and the right orbital part of the medial frontal gyrus), and the ipsilateral precuneus, posterior cingulate cortex (PCC), cuneus, and the middle cingulate cortex in MWoA compared with HC. MWoA also showed decreased outflow from the left PTH to the ipsilateral dorsomedial prefrontal cortex (dmPFC) (including the left supplementary motor area, the superior frontal cortex, and the medial superior frontal gyrus).
Table 2.
Two-sample t-test (voxel-level P<0.01 and cluster-level P<0.05 Gaussian random field corrected) of difference in causal influence to and from the left posterior thalamus in patients with migraine without aura versus healthy controls
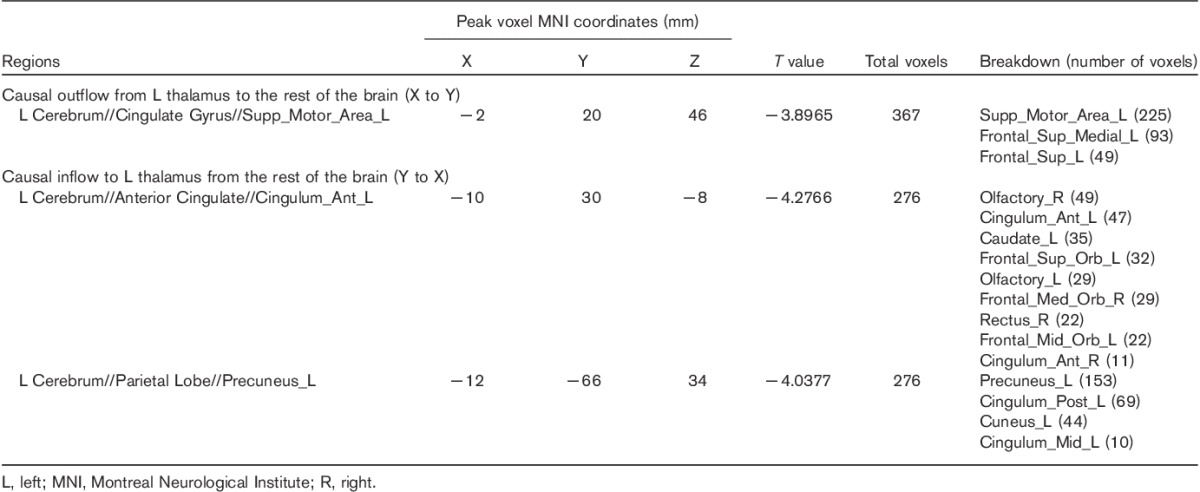
Fig. 1.
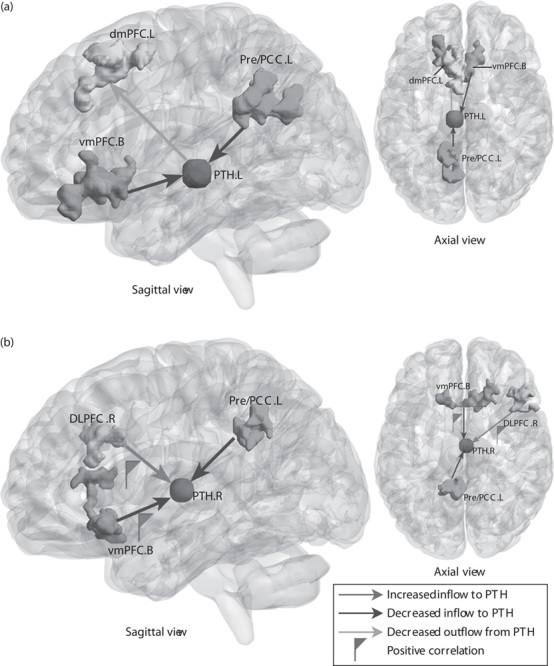
Altered effective connectivity to and from the PTH of MWoA compared with HC (voxel-level P<0.01 and cluster-level P<0.05, GRF corrected). (a) Abnormal effective connectivity pathways associated with the left PTH. (b) Abnormal effective connectivity pathways associated with the right PTH. The red flag represents the positive correlation between the strength of abnormal effective connectivity pathway and headache duration and pain intensity. B, bilateral; DLPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; GRF, Gaussian random field; HC, healthy controls; L, left; MWoA, patients with migraine without aura; PCC, posterior cingulate cortex; Pre, precuneus; PTH, posterior thalamus; R, right; vmPFC, ventromedial prefrontal cortex.
Abnormality for causal influence to and from the right posterior thalamus
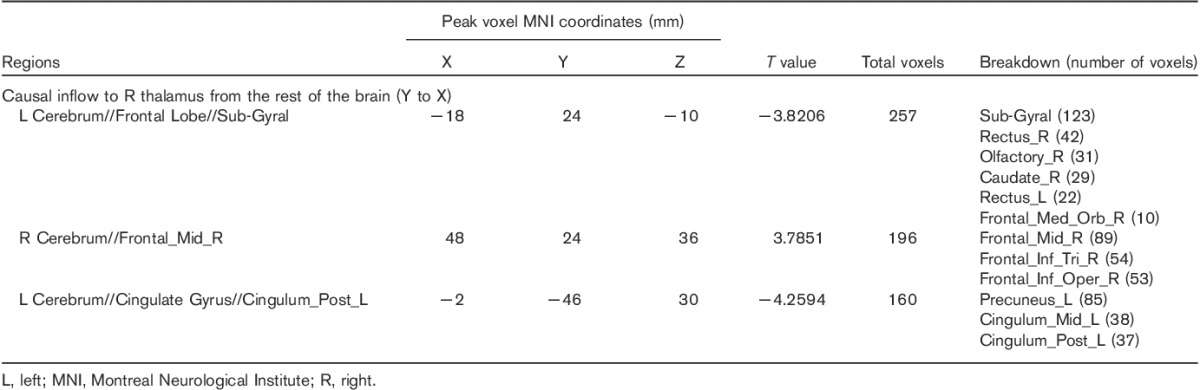
As shown in Table 3 and Fig. 1b, the results showed significantly increased causal inflow from the right dorsolateral prefrontal cortex (DLPFC) (including the right middle frontal gyrus, triangular, and opercular part of the inferior frontal gyrus) to the ipsilateral PTH in MWoA compared with HC. Similar to the left PTH, decreased inflows to the right PTH in MWoA were also found in the bilateral vmPFC (including the bilateral rectus gyrus, right olfactory cortex, and caudate nucleus), and left PCC, precuneus, and middle cingulate cortex. No significant causal outflow from the right PTH to the rest of the brain was found.
Table 3.
Two-sample t-test (voxel-level P<0.01 and cluster-level P<0.05 Gaussian random field corrected) of difference in causal influence to and from the right posterior thalamus in patients with migraine without aura versus healthy controls
Correlations results
Pearson’s correlation analyses showed that the visual analogue scale value (pain intensity) correlated positively with the increased inflow from the right DLPFC to the ipsilateral PTH (r=0.475, P=0.034). In addition, the headache duration correlated positively with the decreased inflow from the bilateral vmPFC to the right PTH (r=0.455, P=0.044).
Discussion
Using an effective connectivity approach to study the interaction of PTH with the rest of the brain in MWoA, we identified altered ascending/descending pathways at the thalamic level mainly involving the prefrontal cortex (including the vmPFC, dmPFC, and DLPFC) and the PCC/precuneus, which are strongly implicated in the affective, cognitive, and perceptual pain processing in migraine. Moreover, we also found a positive correlation between the descending vmPFC–thalamus pathway and the headache duration, as well as between the descending DLPFC–thalamus pathway and pain intensity, which may provide us with a better understanding of the migraine process and progression.
Convergent evidence from functional neuroimaging studies suggests that the dysfunction in the mPFC is associated closely with the cognitive-affective pain dimension in migraine 19,20. The mPFC has widespread anatomical and functional connections with the thalamus 21 and altered functional connectivity between the mPFC and thalamus has commonly been found in migraine studies 10,22. In the present study, we found decreased causal interactions between the mPFC and PTH in MWoA compared with HC, which included decreased inflows from the bilateral vmPFC to both the left and the right PTH and decreased outflow from the left PTH to ipsilateral dmPFC. It is known that the two subdivisions of mPFC (i.e. vmPFC and dmPFC) have a functional differentiation. The dmPFC is involved in the appraisal of negative emotion and detection of emotional conflict, whereas the vmPFC plays a regulatory role with respect to the limbic region in generating emotion responses 23. Therefore, the decreased inflow from the vmPFC to PTH in the present study may indicate that patients with migraine suffered from the dysfunctional affective modulation of pain from mPFC to the thalamus, whereas the decreased outflow from the PTH to dmPFC may suggest a disability of ascending negative emotion appraisal and emotional conflict detection in migraine. In addition, we also found that the decreased inflow from the bilateral vmPFC to the right PTH correlated positively with headache duration in the patients with migraine, which may suggest that the dysfunctional interaction between the vmPFC and thalamus may result from the negative emotional accumulation of repetitive and long-term migraine attacks.
Reduced inflows from the left precuneus/PCC to the bilateral PTH were also found in MWoA compared with HC. The involvement of precuneus/PCC is consistent with previous reports in migraineur studies that show structural 24 and functional 15 abnormalities in these regions. Both the precuneus and the PCC are key nodes of the default mode network, which is the most commonly reported intrinsic connectivity network during resting state and has been implicated in sensation integration, self-relevant, and internally cognitive-attentional dimensions of pain in patients with migraine 25. Moreover, a previous thermal stimuli study had found that the precuneus/PCC showed decreased activation in proportion to perceived pain intensity 26. Therefore, the decreased connectivity from the precuneus/PCC to the thalamus may suggest an impairment in pain integration and dysfunction of pain perception through the descending PCC/precuneus–thalamus pathway.
The results found increased inflow from the right DLPFC (including inferior frontal gyrus and middle frontal gyrus) to ipsilateral PTH in MWoA compared with HC. Activation in the DLPFC is reported commonly in migraine during pain processing 27,28. Brighina et al. 29 have found that repetitive transcranial magnetic stimulation of the DLPFC can exert a bilateral control on the pain system, which supports the role of DLPFC in nociceptive modulation and control, and high-frequency repetitive transcranial magnetic stimulation over the DLPFC has been confirmed as an effective treatment of pain in chronic migraine 30. Thus, we speculate that the increased inflow from the DLPFC to PTH in the present study suggests an abnormal active control on pain perception through the descending DLPFC–thalamus pathway in migraine. Furthermore, we found a positive correlation between the pain intensity and this abnormal effective connectivity pathway. Enhanced activation of the DLPFC has been found in patients with migraine in response to painful heat 27. The hyperactive DLPFC as well as its related connections might provide an objective reference of pain intensity assessment in migraine.
There are several limitations in the present study. First, although the length of disease course, the history of medication use, and other characteristics were possible confounding variables, we could not perform any subgroup comparisons because of the small sample size of the patients. Further studies in a larger population with well-characterized patients with migraine are needed to verify these findings. Second, we only investigated the effective connectivity pathways associated with the PTH. In a future study, more regions implicated in multiple pain processing should be considered as seed regions for further effective connectivity analysis to construct a comprehensive pain circuit model in migraine. Finally, the current report is a cross-sectional study. Whether or not the abnormalities of the cortical–thalamic circuit are altered by headache duration and medication remains unclear and a longitudinal investigation is further needed.
Conclusion
The yielded disrupted ascending/descending pathways between the PTH and cortical regions such as the prefrontal cortex and precuneus/PCC show a dysfunctional pain modulation in sensory and affective domains, which suggests a disequilibrium of pain inhibition and facilitation in MWoA. In addition, the correlation results showed that headache duration and pain intensity probably exert an effect on the altered pain network of MWoA. These findings provide significant implications for our understanding of the pathophysiology of migraine.
Acknowledgements
This study was supported by the National Natural Science Foundation (grant nos 81371536, 81000605, 81500959, 81227002, and 81220108013), the Natural Science Foundation of Guangdong (grant no. S20120200-10867), the Sichuan Province Science and Technology Plan Project (grant no. 2015HH0036), and the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT, Grant No. IRT1272) of China. Dr Zhang and Dr Gong contributed equally as the corresponding authors. Dr. Gong would also like to acknowledge the support from his Changjiang Scholar Professorship Award (Award No. T2014190) of China and the CMB Distinguished Professorship Award (Award No. F510000/ G16916411) administered by the Institute of International Education, USA.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013; 33:629–808. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007; 68:343–349. [DOI] [PubMed] [Google Scholar]
- 3.Schwedt TJ, Dodick DW. Advanced neuroimaging of migraine. Lancet Neurol 2009; 8:560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwedt TJ, Chiang CC, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol 2015; 14:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noseda R, Jakubowski M, Kainz V, Borsook D, Burstein R. Cortical projections of functionally identified thalamic trigeminovascular neurons: implications for migraine headache and its associated symptoms. J Neurosci 2011; 31:14204–14217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrero MT, Barcia C, Navarro JM. Functional anatomy of thalamus and basal ganglia. Childs Nerv Syst 2002; 18:386–404. [DOI] [PubMed] [Google Scholar]
- 7.Noseda R, Kainz V, Borsook D, Burstein R. Neurochemical pathways that converge on thalamic trigeminovascular neurons: potential substrate for modulation of migraine by sleep, food intake, stress and anxiety. PLoS One 2014; 9:e103929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leavitt VM, Wylie G, Genova HM, Chiaravalloti ND, DeLuca J. Altered effective connectivity during performance of an information processing speed task in multiple sclerosis. Mult Scler 2012; 18:409–417. [DOI] [PubMed] [Google Scholar]
- 9.Schwedt TJ, Larson-Prior L, Coalson RS, Nolan T, Mar S, Ances BM, et al. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med 2014; 15:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwedt TJ, Schlaggar BL, Mar S, Nolan T, Coalson RS, Nardos B, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 2013; 53:737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol 2009; 7:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberstein SD, Olesen J, Bousser MG, Diener HC, Dodick D, First M, et al. The International Classification of Headache Disorders, 2nd edition (ICHD-II) – revision of criteria for 8.2 medication-overuse headache. Cephalalgia 2005; 25:460–465. [DOI] [PubMed] [Google Scholar]
- 13.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for ‘Pipeline’ data analysis of resting-state fMRI. Front Syst Neurosci 2010; 4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005; 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Liu J, Dong X, Peng Y, Yuan K, Wu F, et al. Alterations in regional homogeneity assessed by fMRI in patients with migraine without aura stratified by disease duration. J Headache Pain 2013; 14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stankewitz A, Schulz E, May A. Neuronal correlates of impaired habituation in response to repeated trigemino-nociceptive but not to olfactory input in migraineurs: an fMRI study. Cephalalgia 2013; 33:256–265. [DOI] [PubMed] [Google Scholar]
- 17.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS One 2011; 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang ZX, Yan CG, Dong ZY, Huang J, Zang YF. Granger causality analysis implementation on MATLAB: a graphic user interface toolkit for fMRI data processing. J Neurosci Methods 2012; 203:418–426. [DOI] [PubMed] [Google Scholar]
- 19.Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, Seminowicz DA. Altered cognition-related brain activity and interactions with acute pain in migraine. NeuroImage Clin 2015; 7:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin C, Yuan K, Zhao L, Zhao L, Yu D, von Deneen KM, et al. Structural and functional abnormalities in migraine patients without aura. NMR Biomed 2013; 26:58–64. [DOI] [PubMed] [Google Scholar]
- 21.Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol 1984; 227:109–120. [DOI] [PubMed] [Google Scholar]
- 22.Xue T, Yuan K, Cheng P, Zhao L, Zhao L, Yu D, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed 2013; 26:1051–1058. [DOI] [PubMed] [Google Scholar]
- 23.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 2011; 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014; 1:e20.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tessitore A, Russo A, Giordano A, Conte F, Corbo D, De Stefano M, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain 2013; 14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA 2005; 102:12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case–control study of episodic migraine. Cephalalgia 2014; 34:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lirng JF, Chen HC, Fuh JL, Tsai CF, Liang JF, Wang SJ. Increased myo-inositol level in dorsolateral prefrontal cortex in migraine patients with major depression. Cephalalgia 2015; 35:702–709. [DOI] [PubMed] [Google Scholar]
- 29.Brighina F, De Tommaso M, Giglia F, Scalia S, Cosentino G, Puma A, et al. Modulation of pain perception by transcranial magnetic stimulation of left prefrontal cortex. J Headache Pain 2011; 12:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci 2004; 227:67–71. [DOI] [PubMed] [Google Scholar]


