Summary
Inflammation instigated by interleukin (IL)‐17‐producing cells is central to the development and pathogenesis of several human autoimmune diseases and animal models of autoimmunity. The expansion of IL‐17‐producing cells from healthy donors is reportedly promoted by mesenchymal stem cells derived from fetal bone marrow. In the present study, human umbilical cord‐derived mesenchymal stem cells (hUC‐MSCs) were examined for their effects on lymphocytes from healthy donors and from patients with systemic lupus erythematosus (SLE). Significantly higher levels of IL‐17 were produced when CD4+ T cells from healthy donors were co‐cultured with hUC‐MSCs than those that were cultured alone. Blocking experiments identified that this effect might be mediated partially through prostaglandin E2 (PGE2) and IL‐1β, without IL‐23 involvement. We then co‐cultured hUC‐MSCs with human CD4+ T cells from systemic lupus erythematosus patients. Ex‐vivo inductions of IL‐17 by hUC‐MSCs in stimulated lymphocytes were significantly higher in SLE patients than in healthy donors. This effect was not observed for IL‐23. Taken together, our results represent that hUC‐MSCs can promote the IL‐17 production from CD4+ T cells in both healthy donor and SLE patients. PGE2 and IL‐1β might also be partially involved in the promotive effect of hUC‐MSCs.
Keywords: hUC‐MSCs, IL‐17, prostaglandin E2, SLE
Introduction
Mesenchymal stem cells (MSCs) are multi‐potent stem cells that can be isolated from a number of different tissues, including bone marrow, peripheral blood 1, cord blood 2, trabecular bone 3, adipose tissue 4 and synovium 5. Various studies have shown that MSCs have unique immunoregulatory properties and the ability to treat several kinds of autoimmune disease, including systemic lupus erythematosus (SLE). Its immunosuppression has been observed with regard to modulating the balance of T helper type 1 (Th1)/Th2 or regulatory T cells (Treg)/Th17, which are the subsets of CD4+ T cells per functional categories. There have been some reports that differently derived MSCs can promote the subset productions of Th2 and Treg in the balance of Th1/Th2 and Treg/Th17, respectively 6, 7, 8. Inconsistently, in 2009, bone marrow (BM)‐MSCs were shown to promote the expansion of Th17 cells 9. However, little is known about the immune properties of MSCs derived from human umbilical cords (hUC‐MSCs).
Interleukin (IL)‐17 was described in 1995–96 as a proinflammatory cytokine that was produced by Th17 cells, which were distinct from Th1 or Th2 10, 11. Accumulating evidence has since described the differentiation pathway of Th17 cells as using IL‐23, transforming growth factor (TGF)‐β, IL‐6, IL‐1β and prostaglandin E2 (PGE2) 12, 13, 14, 15, 16. With the discovery of Th17 cells, a better understanding has become available regarding the physiopathology of a number of chronic inflammatory diseases 17, such as spondyloarthritis, rheumatoid arthritis 18, asthma 19, multiple sclerosis 20, systemic lupus erythematosus (SLE) 21 and psoriasis 22. In some studies, increased levels of IL‐17 and IL‐23 have been detected in the serum of SLE patients 23, 24.
In the present study, we co‐cultured hUC‐MSCs with human CD4+ T cells from SLE patients or healthy donors. We have demonstrated for the first time, to our knowledge, that hUC‐MSCs increased the production of IL‐17. In addition to IL‐1β, PGE2 might partially promote the production of IL‐17.
Materials and methods
Ex‐vivo induction of cytokines
Human peripheral blood mononuclear cells (hPBMCs) were isolated by Ficoll‐Paque (Axis‐Shield, Dundee, UK) density gradient centrifugation (density 1·077 ± 0·002 at 2200 rpm/min × 20 min) from the venous blood of healthy volunteers and SLE patients. A subpopulation of CD4+ T cells was purified by using relevant magnetic MicroBead kits (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. The purity of the isolated cells was more than 95%. hPBMCs (1 × 105) were then incubated with phytohaemagglutinin (PHA) (Sigma, St Louis, MO, USA), while CD4+ T cells (1 × 105) were incubated with anti‐CD3/CD28 Dynabeads (Invitrogen, Carlsbad, CA, USA) in the presence or absence of hUC‐MSCs at 37°C in a 5% CO2 atmosphere. In another series of experiments, we added indicating inhibitors (10 µM indomethacin: Biosource, Rochdale, UK; 1 µg/ml IL‐1RA: R&D Systems, Minneapolis, MN, USA; 10 µg/ml anti‐IL‐6 antibody: Biolegend, San Diego, CA, USA; anti‐TGF‐β antibody (clone 2G7 in ascitic fluid at 1 : 20 dilution) was kindly provided by D. Fradelizi) to stimulated hUC‐MSCs/CD4+ T cells. After incubation, the cell‐free supernatant of the ex‐vivo culture was collected and kept frozen at −80°C until assayed for cytokine concentrations by enzyme‐linked immunosorbent assay (ELISA).
Quantification of cytokines by ELISA
Concentrations of IL‐23 and IL‐17 in plasma and ex‐vivo culture supernatant were measured by ELISA. PGE2 was assayed using an ELISA kit from Cayman Chemicals. Interferon (IFN)‐γ, IL‐4 and TGF‐β were obtained from Jingmei Biotech Co. Ltd (PR China).
Flow cytometry
After 3 days of culture, CD4+ T cells were harvested and restimulated for another 5 h with 25 ng/ml phorbol myristate acetate (PMA) and 1 mg/ml ionomycin in the presence of GolgiStop. Upon fixation and permeabilization with Cytofix/Cytoperm (Becton Dickinson, San Jose, CA, USA), cells were labelled with anti‐IFN‐γ fluorescein isothiocyanate (FITC) and anti‐IL‐17 phycoerythrin (PE) monoclonal antibodies (mAb). In another experiment the cells were labelled with anti‐CD4 PE and anti‐CD25 FITC mAb without additional stimulation to indicate Treg cells. All the antibodies were purchased from eBioscience (San Diego, CA, USA) and the flow cytometry analysis was performed using the Becton Dickinson fluorescence activated cell sorter (FACS)Calibur using CellQuest software (Becton Dickinson).
Quantitative analysis of mRNA expression
Total RNA was extracted with TRIzol (Life Technologies, Carlsbad, CA, USA) and then used to synthesize cDNA using murine leukaemia virus reverse transcriptase (MLV RT) (Life Technologies), following the manufacturer's protocol. Polymerase chain reaction (PCR) cycling conditions were: predenature at 95°C for 10 min, denature at 95°C for 15 s and extension at 60°C for 1 min, followed by a final single peak‐melting curve program. The ratio was calculated according to the formula: ratio = 2–ddCt (ddCT = mean Ct gene – mean Ct housekeeping). The primer sequences were as follows: hypoxanthine guanine phosphoribosyl transferase (HPRT) forward: 5′‐TGACACTGGCAAAACAATGCA‐3′ and reverse: 5′‐GTCCTTTTCACCAGCAAGCT‐3′; retinoic acid receptor‐related orphan receptor C (RORC) forward: 5′‐ TTTTCCGAGGATGAGATTGC‐3′ and reverse: 5′‐CTTTCCACATGCTGGCTACA‐3′.
SLE patients
Twelve SLE patients (ten females, two males) were recruited at the Beijing Hospital. Diagnosis of SLE was established according to the 1982 revised American Rheumatism Association criteria (ARA) 25. Active lupus patients were identified according to the SLE Activity Index (SLEDAI) score 26 and informed consent, as specified by the Declaration of Helsinki, was obtained from all participants.
Statistical analysis
The results were analysed using the GraphPad Prism software package version 4 (GraphPad Software Inc., San Diego, CA, USA). Data are presented as mean ± standard error of the mean (s.e.m.). The two‐tailed Student's t‐test was used to determine the significance and P < 0·05 was considered statistically significant.
Results
hUC‐MSCs promote the production of IL‐17 from hPBMCs/CD4+ T cells of healthy donors
To study the effects of hUC‐MSCs on the production of IL‐17 from hPBMCs/CD4+ T cells, hPBMCs were incubated with PHA and were then co‐cultured with hUC‐MSCs. Our findings demonstrated that the hUC‐MSCs promoted the production of IL‐17 from hPBMCs in a dose‐dependent manner. The best ratio was 2 : 1 (hPBMCs : hUC‐MSCs) in our co‐culture system. IL‐17 production was increased slightly when non‐fractionated hPBMCs were activated by PHA (2·18 ± 0·77 versus 17·47 ± 3·81, hUC‐MSCs only: 2·43 ± 0·88; P < 0·05), (Fig. 1a). Subsequent experiments therefore used hPBMCs or CD4+ T cells co‐cultured with hUC‐MSCs at the ratio of 2 : 1 (hPBMCs or CD4+ T cells : hUC‐MSCs). The hUC‐MSCs showed promotive effects on hPBMCs from different donors (Fig. 1b). Furthermore, these promotive effects appeared to be a consistent and stable feature of hUC‐MSCs, because similar effects were observed with hUC‐MSCs prepared from eight different donors and with different numbers of cell passages (data not shown).
Figure 1.
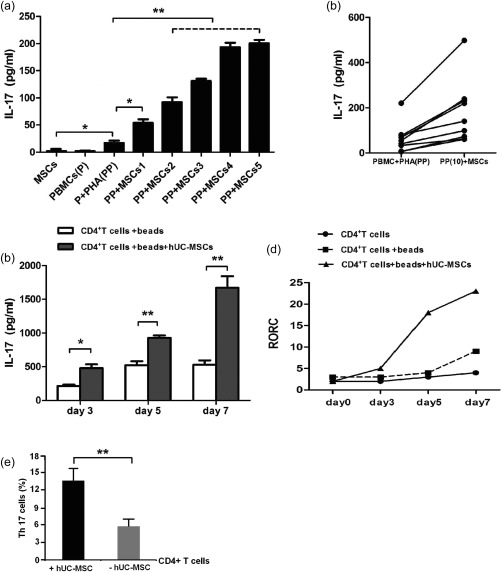
Human umbilical cord‐derived mesenchymal stem cells (hUC‐MSCs) promote interleukin (IL)−17 release by human peripheral blood mononuclear cells (hPBMCs)/CD4+ T cells. (a) hPBMCs (1 × 105) were stimulated with 10 μg/ml phytohaemagglutinin (PHA), with or without different numbers of hUC‐MSCs. (MSCs1: 5 × 103; MSCs2: 1 × 104; MSCs3: 2 × 104; MSCs4: 5 × 104; MSCs5: 1 × 105). The data are expressed as the mean ± standard error of the mean (s.e.m.) from three different experiments, each performed in triplicate. *P < 0·05, **P < 0·01. (b) PHA‐activated‐hPBMCs from 10 donors were co‐cultured with one source of hUC‐MSCs from eight donors were co‐cultured with one source of PHA‐activated hPBMCs. IL‐17 concentrations in each culture were measured by enzyme‐linked immunosorbent assay (ELISA) and the data represent the mean of single experiments, each performed in triplicate. (c) IL‐17 secretion is shown from CD4+ T cells stimulated in the presence or absence of hUC‐MSCs for different days. (d) Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis of RAR‐related orphan receptor C (RORC) transcripts in anti‐CD3/CD28 Dynabeads‐stimulated CD4+ T cells in the presence or absence of hUC‐MSCs for different days. The data are expressed as the mean ± s.e.m. from three different experiments, each performed in triplicate. *P < 0·05, **P < 0·01. (e) Flow cytometry analysis of percentages of T helper type 17 (Th17) cells in the presence or absence of hUC‐MSCs for 3 days of culture. All data were excluded from the autofluorescence and were analysed based on four repeat experiments. **P < 0·01.
To assess whether hUC‐MSCs acted directly on T cells or indirectly through accessory cells, we activated freshly sorted CD4+ T cells by anti‐CD3/CD28 Dynabeads at different culture times in the absence of accessory. IL‐17 levels were almost tripled when CD4+ T cells were co‐cultured with hUC‐MSCs for 7 days, while these levels doubled in other co‐culture systems (Fig. 1c).
Recently, retinoid‐related orphan receptor gamma t (RORγt) has been reported as a crucial transcription factor that regulates IL‐17 expression and Th17 differentiation in mouse and human 15. We further evaluated mRNA levels for retinoic acid receptor‐related orphan receptor C (RORC). As shown in Fig. 1d, RORC mRNA levels were slightly higher in isolated CD4+ T cells in response to anti‐CD3/CD28 Dynabeads than in an unstimulated culture at day 7 (2·10 ± 0·72 versus 8·53 ± 1·02; P < 0·01). However, it was increased significantly by the addition of hUC‐MSCs at day 5 (7·77 ± 0·79 versus 19·07 ± 1·91; P < 0·01) or at day 7 (8·53 ± 1·02 versus 22·10 ± 1·86; P < 0·01).
We further used flow cytometry to analyse the percentages of Th17 cells when CD4+ T cells were co‐cultured with or without hUC‐MSCs. After 3 days of culture, CD4+ T cells were further restimulated for PMA and ionomycin, and were labelled with anti‐IFN‐γ and anti‐IL‐17 fluorescence. Meanwhile, in another experiment, the cells were labelled with CD4CD25high without restimulation. We found that the percentage of Th17 cells in hUC‐MSCs/CD4+ T cells activated by anti‐CD3/CD28 Dynabeads was significantly higher than that in activated‐CD4+ T cell cultures (13·67 ± 3·79% versus 5·82 ± 1·53%, P < 0·01). The flow cytometry data suggest that hUC‐MSCs promote the expansion of Th17 cells (Fig. 1e).
PGE2 and IL‐1β might promote the augmentation of Th17 cells mediated by hUC‐MSCs
PGE2 is an important cytokine involved in promotion of Th17 cell differentiation 27. Cell co‐culture experiments were performed to investigate effects of some important soluble factors on Th17 cell augmentation mediated by hUC‐MSCs. As in Fig. 2a, very low levels of PGE2 were produced by hUC‐MSCs or CD4+T cells when they were cultured separately. In co‐cultures of hUC‐MSCs and CD4+T cells stimulated with anti‐CD3/CD28 Dynabeads, PGE2 was increased dramatically. In the presence of the COX inhibitor indomethacin (indo), the IL‐17 production was decreased (1564·83 ± 235·3 versus 829·0 ± 91·43; P < 0·05), as was RORC expression (20·67 ± 2·74 versus 10·47 ± 2·01; P < 0·05) in CD4+ T cells (Fig. 2b). When CD4+ T cells stimulated with anti‐CD3/CD28 Dynabeads were co‐cultured with hUC‐MSCs in the presence of anti‐IL‐6 antibody or anti‐TGF‐β antibody, no effects were observed on IL‐17 production. In contrast, IL‐1βRA decreased the production of IL‐17 (Fig. 2c). Furthermore, exogenous PGE2 and IL‐23 have been reported to synergize in inducing IL‐17 27, 28, while we did not detect the difference of IL‐23 in the supernatant of co‐culture system (data not shown), and no significant difference (P > 0·05) was found in IL‐17 production between activated CD4+ T cells/hUC‐MSCs in the presence of IL‐23 (1429 ± 166·6) and absence of IL‐23 (1374 ± 208·3) (Fig. 2d). Thus, PGE2 and IL‐1β appeared to be involved in the expansion of Th17 cells mediated by hUC‐MSCs, but this response did not require IL‐23.
Figure 2.
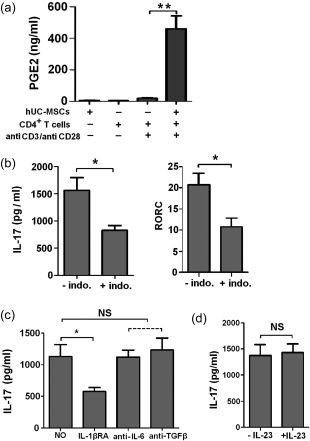
Human umbilical cord‐derived mesenchymal stem cells (hUC‐MSCs) promote interleukin (IL)−17 release by CD4+ T cells partly through prostaglandin E2 (PGE2). (a) Expression levels of PGE2 compared with CD4+ T cells and CD4+ T cells/hUC‐MSCs. (b) Activated CD4+ T cells were co‐cultured with hUC‐MSCs in the presence or absence of 10 μM indomethacin (indo). Cytokines were measured in the supernatant and transcripts for RAR‐related orphan receptor C (RORC) were determined by real‐time polymerase chain reaction (PCR) in culture. (c) Activated CD4+ T cells were co‐cultured with hUC‐MSCs in the presence or absence of indicating neutralizing antibodies. (d) Detection of IL‐17 secretion in hUC‐MSCs/CD4+ co‐cultures in the presence or absence of IL‐23 by enzyme‐linked immunosorbent assay (ELISA). All the data are expressed as the mean ± standard error of the mean (s.e.m.) from three different experiments, each performed in triplicate. *P < 0·05; **P < 0·01.
hUC‐MSCs might decrease the production of Th1 and increase the production of Treg
To investigate the effect of hUC‐MSCs on the production of Th1 and Treg, ELISA was performed to detect the corresponding cytokines from the CD4+ T cells/hUC‐MSCs co‐culture supernatant. We observed that addition of hUC‐MSCs to cultures of CD4+T cells activated with anti‐CD3/CD28 Dynabeads resulted in decreased IFN‐γ (46·63 ± 4·27 versus 9·23 ± 1·70; P < 0·01) and enhanced TGF‐β production (333·3 ± 43·72 versus 938·3 ± 187·5; P < 0·05) (Fig. 3a). Furthermore, we detected the percentages of Th1 and Treg separately using flow cytometry. These data were consistent with the data from ELISA analysis (Th1: 17·40 ± 2·07 versus 5·71 ± 2·92%, P < 0·01; Treg: 11·35 ± 2·05 versus 24·49 ± 4·17%, P < 0·05) (Fig. 3b).
Figure 3.
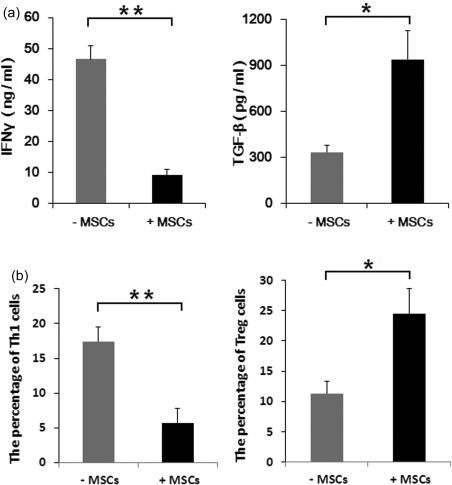
The different effect of human umbilical cord‐derived mesenchymal stem cells (hUC‐MSCs) on CD4+ T cells. CD4+ T cells were stimulated with anti‐CD3/CD28 beads in the presence or absence of hUC‐MSCs. (a) Interferon (IFN)‐γ and transforming growth factor (TGF)‐β were measured in the supernatant. (b) Flow cytometry analysis of T helper type 1 (Th1) cells and regulatory T cells (Treg) in CD4+ T cell cultures and hUC‐MSC/CD4+ co‐cultures. All the data are expressed as the mean ± standard error of the mean (s.e.m.) from three different experiments, each performed in triplicate. *P < 0·05; **P < 0·01.
hUC‐MSCs promote IL‐17 production but not IL‐23 production in hPBMCs of SLE patients
Plasma IL‐23 and IL‐17 concentrations were reported to be significantly higher in SLE patients than in healthy individuals. Using ELISA, we also detected concentrations of IL‐23 and IL‐17 from healthy donors and SLE patients. As shown in Fig. 4a, the level of IL‐17 secretion in all SLE patients (37·4 ± 18·1) was higher than that of the control group (1·99 ± 0·85) (P < 0·05). Plasma IL‐23 levels were significantly different between patients and healthy donors (81·70 ± 15·37 versus 0·56 ± 0·11; P < 0·01). Furthermore, we investigated the ex‐vivo production of IL‐17 and IL‐23 from CD4+ T cells co‐cultured with hUC‐MSCs activated with anti‐CD3/CD28 Dynabeads in the patient cohort. hUC‐MSCs induced the release of IL‐17 (P < 0·05) (Fig. 4b) but not IL‐23 (P > 0·05) (Fig. 4c) in activated CD4+ T cells in SLE patients than the healthy donors.
Figure 4.
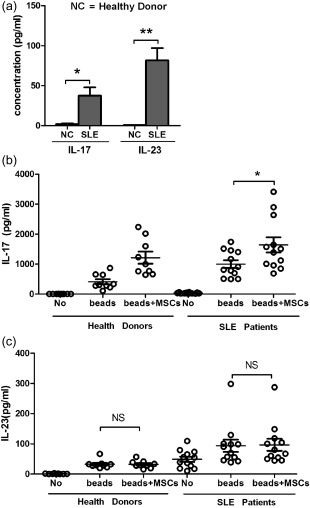
Human umbilical cord‐derived mesenchymal stem cells (hUC‐MSCs) promote interleukin (IL)−17 release by CD4+ T cells from systemic lupus erythematosus (SLE) patients. (a) The concentration of IL‐17 and IL‐23 in the plasma of SLE patients and the NC (healthy donors) group. Ex‐vivo production of IL‐17 (b) and IL‐23 (c) from anti‐CD3/CD28‐activated CD4+ T cells of all SLE (n = 12) and NC healthy donors (n = 9) groups in the presence or absence of hUC‐MSCs.
Discussion
For years, Th1 cells have been considered to be the major mediators of inflammation in most autoimmune syndromes. However, with the discovery of the Th17 subtype of CD4+ T cells, it is now evident that Th17 cells are the primary drivers of these autoimmune disease responses 18. In the case of lupus, many studies have examined the potential for a role for T cell‐derived cytokines in its pathogenesis. IL‐17 levels have been reported as significantly elevated in the serum of lupus patients compared with healthy donors 29, and have been implicated in at least some lupus mouse models 30.
More recently, fetal bone marrow‐derived mesenchymal stem cells (FBM‐MSCs) have been reported to promote the expansion of Th17 cells from CD4+ T cells 9. As a promising candidate for many potential clinical applications, hUC‐MSCs appear to directly suppress T cell activation 31, 32, but the effect of hUC‐MSCs on the differentiation of human CD4+ T cells is still unknown. In the current study, hUC‐MSCs also promoted Th17 production from CD4+ T cells. Because recent evidence indicates that transplantation of human MSCs can inhibit significantly autoimmune progression in Murphy Roths Large/lymphoproliferation (MRL/lpr) mice, it was of interest to determine if a similar effect would be observed on the function of Th17 cells from SLE patients.
Previous studies have reported elevated plasma levels of IL‐17 in SLE patients 33. In contrast, Kurasawa et al. reported no elevation of IL‐17 in the serum of a cohort of 24 Japanese lupus patients 34. Our current results revealed a clearly higher level of IL‐17 secretion in SLE patients than that in healthy donors. Our data also showed that hUC‐MSCs promoted IL‐17 production in activated CD4+ T cells from SLE patients.
A recent study reported that IL‐23 was essential for Th17 differentiation and expressing IL‐17 in the process mediated by BM‐MSCs. IL‐23 is also required for disease pathogenesis in many models of autoimmunity, and appears to play a role in the exacerbation of SLE symptoms 35. Roles for IL‐23 are thought to possibly involve a Th17 effector function as well as recruitment of Th17 to sites of inflammation. We observed no significant increases in ex‐vivo production of IL‐23 in activated CD4+ T cells from either SLE patients or healthy donors cultured in the presence of hUC‐MSCs. Our results suggested that the IL‐23 signal might not be involved in the production of IL‐17 mediated by hUC‐MSCs.
In‐vitro PGE2 specifically increases the frequency of CD4+ T cells producing IL‐17 28. The proinflammatory effect of PGE2 in experimental inflammatory bowel disease/collagen‐induced arthritis in mice is also mediated through the IL‐23/IL‐17 axis 36, 37. In the current study, the level of PGE2 was increased by approximately 30‐fold when hUC‐MSCs were co‐cultured with CD4+ T cells stimulated with anti‐CD3/CD28 Dynabeads. The possibility therefore exists that hUC‐MSCs induce IL‐17 production and RORγt expression in activated CD4+ T cells through the secretion of PGE2. Moreover, in a reciprocal manner, the production of PGE2 by MSCs was observed to be increased strongly by soluble factors produced by Th17 cells 38. Guo and co‐workers have suggested that FBM‐MSCs might promote the expansion of Th17 cells partly through increasing expression of IL‐6 and IL‐1 9. The role of TGF‐β in the induction of IL‐17 in human CD4+ T cells is controversial 39, 40. Our blocking studies showed that IL‐1β, but not IL‐6/TGF‐β, was essential for the production of IL‐17 mediated by hUC‐MSCs. These controversial effects may due to the diverse types of MSCs and the complicated inflammatory environment in vivo.
General points are that the immunomodulatary capacity of MSCs such as inducing Th17 differentiating from naive cells and further secreting IL‐17 is not intrinsic. These effects of MSCs on the inflammatory cytokine‐production profile were dependent in part upon their secretion of soluble proinflammatory factors. Combined with the activity of TGF‐β, Ι L‐1β and IL‐21 have been reportedly involved in the MSC‐mediated process of Th17 differentiation and IL‐17 production. These cytokines modulated IL‐17 production by inducing the expression of a key lineage‐specific transcription factor, RORγt 39, 41.
Naive CD4+ T cells are believed to differentiate into Th17 cells, but also two other possible lineages, Th1 and Treg. In our study, hUC‐MSCs suppressed significantly the expansion of Th1 cells and promoted the expansion of Treg cells, which is consistent with a previous report 6.
MSC effects might be highly dependent upon the timing of MSC administration 42. We assumed that, during the early phase, hUC‐MSCs promoted Th17 cell production, followed by conversion of the Th17 into Treg cells. However, the extent of how many Th17 could be converted into Treg cells might be impacted by the co‐culture period time, such as the long‐term MSC survival with T cells in vivo, or some additional soluble factors from the complicated in vivo environment, or some additives used, such as mycophenolate mofetil 43. As discussed above, the key to success of using MSCs might be the timing of the MSC administration and the additives used in combination with MSCs.
Various studies have demonstrated that MSCs have the ability to mediate immunomodulatory function in vitro and in vivo, with beneficial effects observed in animal models and clinical studies in disease such as graft‐versus‐host disease (GVHD), autoimmune diseases, bone, cartilage and cardiovascular diseases 44, 45. Some other studies have also reported that MSCs alone accelerated allograft rejection 43, 46, which prompts us to explore further how to optimize the use of MSCs.
In conclusion, this study reports for the first time that hUC‐MSCs can promote the production of IL‐17 from hPBMCs of SLE patients. This finding provides some controversial effects of BM‐MSCs reported recently, and gives a new insight into the potential immunomodulative function of MSC‐based clinical studies. This is the first report, to our knowledge, demonstrating that PGE2 is also critical for IL‐17 production by hUC‐MSCs. Given that serum abundance of IL‐17 has been correlated with SLE disease activity, clinical treatments using hUC‐MSCs in SLE may require more careful research.
Disclosure
None declared.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81201729, No. 81300433), Beijing Municipal Natural Science Foundation (No. 7144211) and Specialized Research Fund for the Doctoral Program of Higher Education (No. 20131107120008).
References
- 1. Zvaifler NJ, Marinova‐Mutafchieva L, Adams G et al Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res 2000; 2:477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol 2004; 18:893–908. [DOI] [PubMed] [Google Scholar]
- 3. Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone‐derived cells. J Orthop Res 2002; 20:1060–9. [DOI] [PubMed] [Google Scholar]
- 4. Pittenger MF, Mackay AM, Beck SC et al Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143–7. [DOI] [PubMed] [Google Scholar]
- 5. De Bari C, Dell'Accio F, Luyten FP. Human periosteum‐derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum 2001; 44:85–95. [DOI] [PubMed] [Google Scholar]
- 6. Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105:1815–22. [DOI] [PubMed] [Google Scholar]
- 7. Luz‐Crawford P, Kurte M, Bravo‐Alegria J et al Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther 2013; 4:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Ren S, Qu X, Ge C, Cheng K, Zhao RC. Mesenchymal stem cells inhibit Th17 cells differentiation via IFN‐gamma‐mediated SOCS3 activation. Immunol Res 2015; 61:219–29. [DOI] [PubMed] [Google Scholar]
- 9. Guo Z, Zheng C, Chen Z et al Fetal BM‐derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur J Immunol 2009; 39:2840–9. [DOI] [PubMed] [Google Scholar]
- 10. Park H, Li Z, Yang XO et al A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005; 6:1133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harrington LE, Hatton RD, Mangan PR et al Interleukin 17‐producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6:1123–32. [DOI] [PubMed] [Google Scholar]
- 12. Hasan M, Neumann B, Haupeltshofer S et al Activation of TGF‐beta‐induced non‐Smad signaling pathways during Th17 differentiation. Immunol Cell Biol 2015; 93:662–72. [DOI] [PubMed] [Google Scholar]
- 13. Yang L, Anderson DE, Baecher‐Allan C et al IL‐21 and TGF‐beta are required for differentiation of human T(H)17 cells. Nature 2008; 454:350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo W, Luo C, Wang C et al Suppression of human and mouse Th17 differentiation and autoimmunity by an endogenous interleukin 23 receptor cytokine‐binding homology region. Int J Biochem Cell Biol 2014; 55:304–10. [DOI] [PubMed] [Google Scholar]
- 15. Volpe E, Servant N, Zollinger R et al A critical function for transforming growth factor‐beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)‐17 responses. Nat Immunol 2008; 9:650–7. [DOI] [PubMed] [Google Scholar]
- 16. Napolitani G, Acosta‐Rodriguez EV, Lanzavecchia A, Sallusto F. Prostaglandin E2 enhances Th17 responses via modulation of IL‐17 and IFN‐gamma production by memory CD4+ T cells. Eur J Immunol 2009; 39:1301–12. [DOI] [PubMed] [Google Scholar]
- 17. Singh RP, Hasan S, Sharma S et al Th17 cells in inflammation and autoimmunity. Autoimmun Rev 2014; 13:1174–81. [DOI] [PubMed] [Google Scholar]
- 18. Lubberts, E. The IL‐23‐IL‐17 axis in inflammatory arthritis. Nat Rev Rheumatol 2015; 11:415–29. [DOI] [PubMed] [Google Scholar]
- 19. Kim YM, Kim YS, Jeon SG, Kim YK. Immunopathogenesis of allergic asthma: more than the th2 hypothesis. Allergy Asthma Immunol Res 2013; 5:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Becher B, Segal BM. T(H)17 cytokines in autoimmune neuro‐inflammation. Curr Opin Immunol 2011; 23:707–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL‐17 promotes murine lupus. J Immunol 2014; 193:540–3. [DOI] [PubMed] [Google Scholar]
- 22. Guenova E, Skabytska Y, Hoetzenecker W et al IL‐4 abrogates T(H)17 cell‐mediated inflammation by selective silencing of IL‐23 in antigen‐presenting cells. Proc Natl Acad Sci USA 2015; 112:2163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin JC, Baeten DL, Josien R. Emerging role of IL‐17 and Th17 cells in systemic lupus erythematosus. Clin Immunol 2014; 154:1–12. [DOI] [PubMed] [Google Scholar]
- 24. Shah K, Lee WW, Lee SH et al Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther 2010; 12:R53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tan EM, Cohen AS, Fries JF et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–7. [DOI] [PubMed] [Google Scholar]
- 26. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992; 35:630–40. [DOI] [PubMed] [Google Scholar]
- 27. Duffy MM, Pindjakova J, Hanley SA et al Mesenchymal stem cell inhibition of T‐helper 17 cell‐ differentiation is triggered by cell–cell contact and mediated by prostaglandin E2 via the EP4 receptor. Eur J Immunol 2011; 41:2840–51. [DOI] [PubMed] [Google Scholar]
- 28. Chizzolini C, Chicheportiche R, Alvarez M et al Prostaglandin E2 synergistically with interleukin‐23 favors human Th17 expansion. Blood 2008; 112:3696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Garrett‐Sinha LA, John S, Gaffen SL. IL‐17 and the Th17 lineage in systemic lupus erythematosus. Curr Opin Rheumatol 2008; 20:519–25. [DOI] [PubMed] [Google Scholar]
- 30. Hsu HC, Yang P, Wang J et al Interleukin 17‐producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol 2008; 9:166–75. [DOI] [PubMed] [Google Scholar]
- 31. Weiss ML, Anderson C, Medicetty S et al Immune properties of human umbilical cord Wharton's jelly‐derived cells. Stem Cells 2008; 26:2865–74. [DOI] [PubMed] [Google Scholar]
- 32. Li L, Liu S, Xu Y et al Human umbilical cord‐derived mesenchymal stem cells downregulate inflammatory responses by shifting the Treg/Th17 profile in experimental colitis. Pharmacology 2013; 92:257–64. [DOI] [PubMed] [Google Scholar]
- 33. Wong CK, Ho CY, Li EK, Lam CW. Elevation of proinflammatory cytokine (IL‐18, IL‐17, IL‐12) and Th2 cytokine (IL‐4) concentrations in patients with systemic lupus erythematosus. Lupus 2000; 9:589–93. [DOI] [PubMed] [Google Scholar]
- 34. Kurasawa K, Hirose K, Sano H et al Increased interleukin‐17 production in patients with systemic sclerosis. Arthritis Rheum 2000; 43:2455–63. [DOI] [PubMed] [Google Scholar]
- 35. Puwipirom H, Hirankarn N, Sodsai P, Avihingsanon Y, Wongpiyabovorn J, Palaga T. Increased interleukin‐23 receptor(+) T cells in peripheral blood mononuclear cells of patients with systemic lupus erythematosus. Arthritis Res Ther 2010; 12:R215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheibanie AF, Yen JH, Khayrullina T et al The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL‐23–>IL‐17 axis. J Immunol 2007; 178:8138–47. [DOI] [PubMed] [Google Scholar]
- 37. Sheibanie AF, Khayrullina T, Safadi FF, Ganea D. Prostaglandin E2 exacerbates collagen‐induced arthritis in mice through the inflammatory interleukin‐23/interleukin‐17 axis. Arthritis Rheum 2007; 56:2608–19. [DOI] [PubMed] [Google Scholar]
- 38. Ghannam S, Pene J, Moquet‐Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol 2010; 185:302–12. [DOI] [PubMed] [Google Scholar]
- 39. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)‐17 cells requires transforming growth factor‐beta and induction of the nuclear receptor RORgammat. Nat Immunol 2008; 9:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu HP, Cao AT, Feng T et al TGF‐beta converts Th1 cells into Th17 cells through stimulation of Runx1 expression. Eur J Immunol 2015; 45:1010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ivanov II, McKenzie BS, Zhou L et al The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell 2006; 126:1121–33. [DOI] [PubMed] [Google Scholar]
- 42. Obermajer N, Popp FC, Soeder Y et al Conversion of Th17 into IL‐17A(neg) regulatory T cells: a novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell‐supported minimized immunosuppressive therapy. J Immunol 2014; 193:4988–99. [DOI] [PubMed] [Google Scholar]
- 43. Eggenhofer E, Steinmann JF, Renner P et al Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol 2011; 24:157–63. [DOI] [PubMed] [Google Scholar]
- 44. Castro‐Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res 2015; 2015:394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nery AA, Nascimento IC, Glaser T, Bassaneze V, Krieger JE, Ulrich H. Human mesenchymal stem cells: from immunophenotyping by flow cytometry to clinical applications. Cytometry A 2013; 83:48–61. [DOI] [PubMed] [Google Scholar]
- 46. Ge W, Jiang J, Baroja ML et al Infusion of mesenchymal stem cells and rapamycin synergize to attenuate alloimmune responses and promote cardiac allograft tolerance. Am J Transplant 2009; 9:1760–72. [DOI] [PubMed] [Google Scholar]


